2025 Volume 89 Issue 5 Pages 629-637
2025 Volume 89 Issue 5 Pages 629-637
Background: The relationship between cumulative non-high-density lipoprotein-cholesterol (cum-non-HDL-C) and the risk of new-onset arterial stiffness has not been characterized.
Methods and Results: A total of 6,852 participants with 3 consecutive measurements of total cholesterol and HDL-C and a baseline brachial-ankle pulse wave velocity (baPWV) <1,400 cm/s during 2010–2011, 2012–2013, and 2014–2015 were included. The cum-non-HDL-C concentrations were determined using time weighting, and the participants were grouped: G1 <130 mg/dL, G2 130–159 mg/dL, G3 160–189 mg/dL, and G4 ≥190 mg/dL. Cox models were used to characterize the relationships between cum-non-HDL-C and arterial stiffness by calculating hazard ratios (HRs) and 95% confidence intervals (CIs). Arterial stiffness (baPWV ≥1,800 cm/s) was present in 327 (4.77%) participants over a median follow-up period of 7.7 (interquartile range 7.2–8.2) years. After adjustment for multiple confounders, G2–4 had adjusted HRs (95% CIs) of 1.12 (0.85, 1.48), 1.45 (1.05, 1.99), and 2.52 (1.69, 3.74), respectively (P=0.0004), vs. G1. The adjusted HRs (95% CIs) for exposures of 2, 4, and 6 years were 1.17 (0.87, 1.58), 1.46 (1.96, 2.01), and 1.67 (1.14, 2.44), respectively (P=0.0029), vs. 0 years. Restricted cubic spline analysis revealed a linear dose–response relationship between cum-non-HDL-C and arterial stiffness risk.
Conclusions: A high cum-non-HDL-C concentration and prolonged exposure to this increase the risk of arterial stiffness. The monitoring and maintenance of appropriate cum-non-HDL-C may reduce the risk of arterial stiffness.
Cardiovascular disease (CVD) is the leading cause of death among residents of China, accounting for 40% of total deaths.1,2 Arterial stiffness is a chronic condition characterized by the gradual hardening and narrowing of arterial walls, eventually leading to the obstruction of blood flow.3,4 Previous studies have shown that arterial stiffness is a significant risk factor for CVD and a predictor of future CVD events, such as acute myocardial infarction, stroke, and heart failure.5–7 The etiology and pathogenesis of arterial stiffness involve various factors, such as abnormal lipid metabolism, hypertension, diabetes, smoking, lack of exercise, and an unhealthy diet.5,6,8–10 These risk factors can lead to endothelial cell damage, vascular intimal sclerosis, and ultimately the development and progression of arterial stiffness.11 Brachial-ankle pulse wave velocity (baPWV) is a non-invasive measure that reflects arterial stiffness,5,7 but given that arterial stiffness is a progressive condition, there is a need to validate biomarkers that can be used for the early identification of high-risk patients and the development of appropriate preventive strategies.
Dysregulation of lipid metabolism is an important risk factor for arterial stiffness.12–17 Previous studies have shown that non-high-density lipoprotein-cholesterol (non-HDL-C) is not only a risk factor for coronary artery disease and heart failure,18 but also that the combination of non-HDL-C and flow-mediated dilation measurements helps predict cardiovascular prognosis in patients with coronary artery disease.19 In particular, a cross-sectional study showed that non-HDL-C more closely correlated with arterial stiffness than triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), and HDL-C,14 and another showed that even with LDL-C concentration <70 mg/dL, non-HDL-C was associated with arterial stiffness.15 However, most of those previous studies have been cross-sectional in nature,14,15,20 relatively small in size,13,14,20 only enrolled participants from specific populations,12,13,20–23 and have typically relied on single measurements of the non-HDL-C concentration.12,13,20 Furthermore, lipid concentrations fluctuate and measurements are prone to interference from other substances,14 whereas arterial stiffness is a chronic pathological process.5 However, to date, there have been few longitudinal studies regarding the relationship between cumulative non-HDL-C (cum-non-HDL-C) and arterial stiffness. Therefore, in the present study, we aimed to expand upon previous research findings and characterize the longitudinal relationship between cum-non-HDL-C and arterial stiffness, assessed using baPWV.
The participants were recruited from the Kailuan cohort, which has been described in detail previously.24,25 Individuals from the Kailuan community were enrolled between 2006 and 2007, when they completed questionnaires and underwent physical examination and laboratory assessments, and were subsequently followed up every 2 years. After the third survey, conducted in 2010, the vascular health of a subcohort was evaluated in a nested study, using baPWV to assess arterial wall health.25
Participants were included in the present study on the basis of the following criteria: participation in health examinations during 2010–2011, 2012–2013, and 2014–2015, completion of the initial baPWV examination, completion of ≥1 subsequent baPWV examination during the follow-up period, and a complete set of baseline data regarding total cholesterol (TC) and HDL-C concentrations. Strategies and design of the current study are shown in Figure 1A. Participants who were excluded did not complete health examinations during 2010–2011, 2012–2013, and 2014–2015, had missing baseline TC or HDL-C data, or had a baseline baPWV ≥1,400 cm/s. Ultimately, 6,852 participants remained after applying the specific inclusion and exclusion criteria shown in Figure 1B. The study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kailuan General Hospital (approval no. 200605). Furthermore, written informed consent was given by all the participants.

(A) Strategies and design of the current study. Health examinations in the Kailuan Study were conducted approximately every 2 years. For the current study, participants’ non-HDL-C data were collected in 2010–2011, 2012–2013, and 2014–2015. For the survival analysis of arterial stiffness outcome, the cumulative exposure period was from 2010–2011 to 2014–2015. At the end of Visit_2014–2015, the participants were followed up through December 31, 2022. Baseline characteristics were based on the information in Visit_2014–2015. (B) Flow chart for the inclusion of the 6,852 participants in the study. baPWV, brachial-ankle pulse wave velocity; HDL-C, high-density lipoprotein-cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein-cholesterol; TC, total cholesterol.
Data Collection
We collected patients’ demographic data (sex, age, educational level), medical history (e.g., hypertension, diabetes, and CVD), history of medication and lifestyle information of the participants using structured questionnaires.26 The definitions of body mass index (BMI), hypertension, diabetes, smokers, drinkers, and physical exercise were referenced from previously published articles.27,28
Participants were instructed to fast for 8–12 h, after which venous blood samples were collected from a cubital vein. After centrifugation, serum samples were collected and used for biochemical testing. The biochemical indices measured included the TC, HDL-C, LDL-C, TG, fasting plasma glucose, high-sensitivity C-reactive protein (hs-CRP), creatinine, and uric acid concentrations. Biochemical analyses were conducted using a Hitachi 7600 automated biochemical analyzer (Hitachi, Tokyo, Japan). The instructions provided in the reagent kits were strictly followed, and the analyses were performed by trained laboratory technicians.29
Measurement of baPWVThe baPWV was measured using a BP-203 RPE III networked arterial stiffness measurement device (Omron, Kyoto, Japan), according to previously described methods.7,25,30,31 Each participant underwent 2 measurements with a 5-min interval, and the second value obtained was recorded. The higher of the left and right baPWV values was used in the analyses.31,32
Definition of cum-non-HDL-CThe formula used to calculate the time-weighted cum-non-HDL-C was as follows: cum-non-HDL-C = [(non-HDL-C2010 + non-HDL-C2012) / 2 × (Visit time2012 − Visit time2010) + (non-HDL-C2012 + non-HDL-C2014) / 2 × (Visit time2014 − Visit time2012)] / (Visit time2014 − Visit time2010)].33,34 Visit time2010, Visit time2012, and Visit time2014 represent the dates of follow-up during the years 2010, 2012, and 2014, respectively. According to the 2018 American College of Cardiology (ACC) and American Heart Association (AHA) Lipid Management Guidelines,35 and the 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Dyslipidemia Management Guidelines,36 the participants were placed into 4 groups: G1, cum-non-HDL-C <130 mg/dL; G2, 130 mg/dL ≤cum-non-HDL-C <160 mg/dL; G3, 160 mg/dL ≤cum-non-HDL-C <190 mg/dL; and G4, with cum-non-HDL-C ≥190 mg/dL.
Definition of the Duration of Exposure to cum-non-HDL-CAccording to the guidelines,35,36 during the health examinations conducted during 2010, 2012, and 2014, 3 values of cum-non-HDL-C were calculated, and the participants were categorized according to the number of times these values were ≥160 mg/dL. Zero occurrences corresponded to a duration of exposure of 0 years, 1 occurrence corresponded to a duration of 2 years, 2 occurrences corresponded to a duration of 4 years, and 3 occurrences corresponded to a duration of 6 years.33
OutcomesWe used the 2014 health examination as the starting point for the follow-up period and the presence of arterial stiffness as the endpoint event. Arterial stiffness was defined, as in previous studies, using a baPWV ≥1,800 cm/s.7,31 For the participants who underwent ≥2 baPWV measurements during the follow-up period, the timing of the first baPWV measurement ≥1,800 cm/s was recorded. The end of follow-up for participants in whom the endpoint did not occur was recorded as December 31, 2022.
Statistical AnalysisData analysis was conducted using SAS 9.4 statistical software (Cary, NC, USA). Normally distributed continuous variables are presented as mean±standard deviation, and between-group comparisons were performed using one-way analysis of variance (ANOVA). Skewed continuous variables are presented as median and interquartile range (IQR), and between-group comparisons were conducted using the Kruskal-Wallis test. Categorical data are presented as relative numbers or percentages, and between-group comparisons were made using the chi-square test. Missing data for baseline covariates were imputed using multiple imputation.
The Kaplan-Meier method was used to calculate the cumulative incidence of arterial stiffness for the various cum-non-HDL-C groups, and comparisons were made using the log-rank test. After having checked that the proportional hazards assumption had been met, Cox proportional hazards regression models were used to characterize the relationships of cum-non-HDL-C and high cum-non-HDL-C exposure duration with the risk of arterial stiffness. Model 1 was unadjusted; Model 2 was adjusted for age and sex; Model 3 was adjusted for the variables in Model 2, plus HDL-C, BMI, hs-CRP, estimated glomerular filtration rate, smoking status, alcohol consumption habit, physical exercise habit, diabetes mellitus, hypertension, use of antihypertensive drugs, hypoglycemic drugs or lipid-lowering drugs; and Model 4 was adjusted for the variables in Model 3, plus non-HDL-C at baseline. After adjustment for these potential confounders, restricted cubic splines were used to describe the dose–response relationship between cum-non-HDL-C and the risk of arterial stiffness. We next compared the ability of non-HDL-C2010, non-HDL-C2012, non-HDL-C2014, and cum-non-HDL-C to predict arterial stiffness risk in the constructed model. Subgroup analyses were then conducted on the basis of age, sex, hypertension, current smoking, and physical activity. Finally, to verify the robustness of the results, sensitivity analyses were performed by (1) excluding participants who developed arterial stiffness within 2 years, (2) excluding participants using antihypertensive, lipid-lowering, or hypoglycemic drugs, and (3) using baPWV ≥1,400 cm/s as the endpoint event, and then repeating the Cox analysis. We compared the contributions of cum-LDL-C and cum-TG-rich lipoprotein-cholesterol (TRL-rich-C, cum-non-HDL-C minus the cumulative LDL) to arterial stiffness in the Cox model. P<0.05 (two-tailed) was considered to represent statistical significance.
We studied 6,852 participants with a mean age of 48.72±9.19 years, of whom 49.10% were male. The baseline clinical and biochemical characteristics of the participants, categorized according to cum-non-HDL-C, are shown in Table 1. Compared with the other 3 groups, the participants in the cum-non-HDL-C ≥190 mg/dL group were older, more likely to be male, had higher blood pressure, a poorer metabolic profile (FPG, TG, LDL-C, non-HDL-C, and hs-CRP), were more likely to have hypertension, and more likely to consume alcohol and smoke.
Baseline Characteristics of Study Participants by Different Groups of cum-non-HDL-C
| Variables | Overall | G1 (<130 mg/dL) |
G2 (130–159 mg/dL) |
G3 (160–189 mg/dL) |
G4 (≥190 mg/dL) |
P value |
|---|---|---|---|---|---|---|
| Participants, N | 6,852 | 3,648 | 2,070 | 850 | 284 | |
| Age (years) | 48.72±9.19 | 46.98±8.75 | 49.61±9.08 | 52.54±9.15 | 53.26±9.68 | <0.01 |
| Male, N (%) | 3,362 (49.1) | 1,571 (43.1) | 1,129 (54.5) | 487 (57.3) | 175 (61.6) | <0.01 |
| BMI (kg/m2) | 24.47±3.47 | 23.80±3.35 | 25.00±3.41 | 25.68±3.57 | 25.55±3.31 | <0.01 |
| SBP (mmHg) | 122.21±15.27 | 120.00±14.65 | 123.81±15.04 | 126.03±16.34 | 127.58±16.38 | <0.01 |
| DBP (mmHg) | 77.61±9.97 | 76.14±9.60 | 78.60±9.79 | 80.48±10.83 | 80.57±9.84 | <0.01 |
| TC (mg/dL) | 193.70±37.47 | 172.79±25.41 | 205.32±26.54 | 231.14±28.62 | 265.50±45.38 | <0.01 |
| HDL-C (mg/dL) | 52.39±13.02 | 53.80±12.85 | 51.27±12.56 | 50.03±13.69 | 49.48±14.28 | <0.01 |
| LDL-C (mg/dL) | 100.48±29.26 | 88.35±22.58 | 109.07±25.95 | 119.99±29.95 | 135.24±41.78 | <0.01 |
| TG (mg/dL)* | 42.35 (28.88–65.84) |
34.27 (24.64–50.44) |
48.51 (34.65–70.84) |
64.30 (45.05–95.10) |
81.81 (55.06–127.63) |
<0.01 |
| non-HDL-C2010 (mg/dL) | 130.66±35.62 | 110.18±24.39 | 141.94±22.43 | 176.53±25.31 | 209.21±42.68 | <0.01 |
| non-HDL-C2012 (mg/dL) | 131.87±36.08 | 107.40±19.55 | 144.65±16.49 | 176.16±17.44 | 210.45±31.72 | <0.01 |
| non-HDL-C2014 (mg/dL) | 131.31±36.76 | 108.99±23.03 | 144.05±23.58 | 171.11±25.90 | 216.02±42.89 | <0.01 |
| cum-non-HDL-C (mg/dL) | 131.21±31.49 | 109.31±15.47 | 143.58±8.43 | 174.19±8.22 | 212.64±24.68 | <0.01 |
| baPWV (cm/s) | 1,156.91±163.22 | 1,133.22±154.62 | 1,167.95±151.81 | 1,202.73±160.21 | 1,243.44±141.21 | <0.01 |
| FPG (mmol/L) | 5.36±1.61 | 5.21±1.23 | 5.44±2.03 | 5.63±1.51 | 5.91±2.32 | <0.01 |
| hs-CRP (mg/L)* | 0.91 (0.40–1.90) | 0.80 (0.35–1.70) | 1.00 (0.44–2.10) | 1.17 (0.55–2.36) | 1.20 (0.56–2.76) | <0.01 |
| eGFR (mL/min/1.73 m2) | 103.99±16.21 | 105.62±15.97 | 102.94±16.16 | 100.60±16.40 | 100.74±16.52 | <0.01 |
| Hypertension, N (%) | 1,459 (21.3) | 559 (15.3) | 424 (20.5) | 331 (39.1) | 145 (51.1) | <0.01 |
| Diabetes mellitus, N (%) | 387 (5.65) | 117 (3.21) | 140 (6.76) | 98 (11.5) | 32 (11.3) | <0.01 |
| Antihypertensive drugs, N (%) | 692 (10.1) | 251 (6.88) | 251 (12.1) | 143 (16.8) | 47 (16.5) | <0.01 |
| Hypoglycemic drugs, N (%) | 140 (2.04) | 41 (1.12) | 42 (2.03) | 41 (4.82) | 16 (5.63) | <0.01 |
| Lipid-lowering drugs, N (%) | 39 (0.57) | 8 (0.22) | 15 (0.72) | 12 (1.41) | 4 (1.41) | <0.01 |
| Physical activity, N (%) | 755 (11.0) | 354 (9.70) | 242 (11.7) | 118 (13.9) | 41 (14.4) | <0.01 |
| Education, N (%) | 4,116 (60.1) | 2,282 (62.6) | 1,207 (58.3) | 473 (55.6) | 154 (54.2) | <0.01 |
| Current drinker, N (%) | 2,105 (30.7) | 957 (26.2) | 707 (34.2) | 327 (38.5) | 114 (40.1) | <0.01 |
| Current smoking, N (%) | 1,877 (27.4) | 828 (22.7) | 634 (30.6) | 306 (36.0) | 109 (38.4) | <0.01 |
*P value, comparison of baseline characteristics between different cum-non-HDL-C groups. Age, BMI, SBP, DBP, TC, HDL-C, LDL-C, non-HDL-C, cum-non-HDL-C, baPWV, FPG and eGFR expressed as mean±SD. TG and hs-CRP expressed as median (IQR). baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; cum-non-HDL-C, cumulative non-high-density lipoprotein-cholesterol; DBP, diastolic blood pressure; Education, senior high school or above; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein-cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein-cholesterol; non-HDL-C, non-high-density lipoprotein-cholesterol; TC, total cholesterol; TG, triglyceride; SBP, systolic blood pressure.
Relationship Between cum-non-HDL-C and the Risk of Arterial Stiffness
During a median follow-up period of 7.7 years (IQR 7.2, 8.2), 327 (4.77%) participants developed arterial stiffness. The incidence density of arterial stiffness increased across the cum-non-HDL-C groups. The incidence densities were 4.33, 6.25, 10.99, and 16.95/1,000 person-years for the <130 mg/dL, 130–159 mg/dL, 160–189 mg/dL, and ≥190 mg/dL groups, respectively (Table 2) (log-rank test, P<0.01; Figure 2A). After adjustment for potential confounding factors in Model 3, compared with the cum-non-HDL-C <130 mg/dL group, the risk of arterial stiffness gradually increased from the 130–159 mg/dL group to the 160–189 mg/dL group and to the ≥190 mg/dL group, with adjusted hazard ratios (HRs) (95% confidence intervals [CIs]) of 1.12 (0.85, 1.48), 1.45 (1.05, 1.99), and 2.52 (1.69, 3.74) (P=0.0004), respectively (Table 2). In Model 4, after further adjustment for the baseline non-HDL-C concentration, the adjusted HR (95% CI) for the cum-non-HDL-C ≥190 mg/dL group vs. the cum-non-HDL-C <130 mg/dL group was 2.22 (1.33, 3.69) (P=0.0016). Furthermore, restrictive cubic spline analysis demonstrated a linear dose-response relationship between cum-non-HDL-C and the risk of developing arterial stiffness (Figure 3).
Association of cum-non-HDL-C and Exposure Duration to cum-non-HDL-C With Arterial Stiffness
| Cases/overall | Incidence density, per 1,000 person-years |
Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| cum-non-HDL-C | ||||||
| G1 (<130 mg/dL) | 121/3,648 | 4.33 | 1.00 | 1.00 | 1.00 | 1.00 |
| G2 (130–159 mg/dL) | 99/2,070 | 6.25 | 1.46 (1.12, 1.90) | 1.16 (0.90, 1.51) | 1.12 (0.85, 1.48) | 1.07 (0.79, 1.44) |
| G3 (160–189 mg/dL) | 71/850 | 10.99 | 2.59 (1.94, 3.46) | 1.59 (1.19, 2.14) | 1.45 (1.05, 1.99) | 1.34 (0.92, 1.98) |
| G4 (≥190 mg/dL) | 36/284 | 16.95 | 4.45 (3.14, 6.31) | 2.69 (1.90, 3.83) | 2.52 (1.69, 3.74) | 2.22 (1.33, 3.69) |
| P trend | <0.0001 | <0.0001 | 0.0004 | 0.0016 | ||
| Exposure duration of cum-non-HDL-C | ||||||
| G1 (0 year) | 158/4,456 | 4.63 | 1.00 | 1.00 | 1.00 | 1.00 |
| G2 (2 years) | 67/1,239 | 7.07 | 1.56 (1.18, 2.05) | 1.30 (0.98, 1.72) | 1.17 (0.87, 1.58) | 1.07 (0.77, 1.48) |
| G3 (4 years) | 62/716 | 11.35 | 2.46 (1.83, 3.30) | 1.62 (1.21, 2.17) | 1.46 (1.96, 2.01) | 1.26 (0.86, 1.83) |
| G4 (6 years) | 40/441 | 12.03 | 2.82 (2.03, 3.93) | 1.68 (1.21, 2.35) | 1.67 (1.14, 2.44) | 1.37 (1.09, 1.85) |
| P trend | <0.0001 | <0.0001 | 0.0029 | 0.0102 | ||
Model 1: Unadjusted. Model 2: adjusted for age and sex. Model 3: included variables in model 1 plus HDL-C, BMI, hs-CRP, eGFR, smoking status, alcohol consumption habit, physical exercise habit, diabetes mellitus, hypertension, and the use of antihypertensive, hypoglycemic or lipid-lowering drugs. Model 4: included variables in model 3 plus non-HDL-C at baseline. Abbreviations as in Table 1.
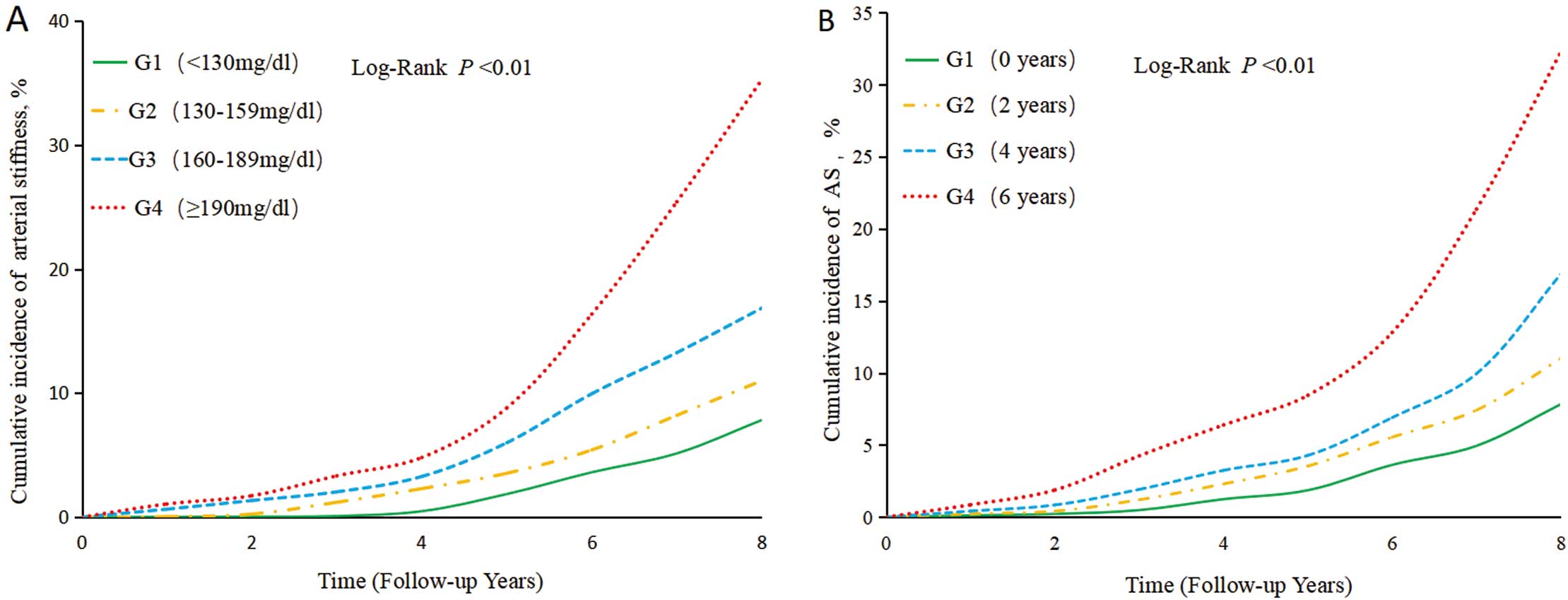
Kaplan-Meier analyses of the risk of arterial stiffness. (A) Kaplan-Meier analysis of the incidence of arterial stiffness according to cumulative non-high-density lipoprotein-cholesterol (cum-non-HDL-C). (B) Kaplan-Meier analysis of the incidence of arterial stiffness according to the duration of exposure to cum-non-HDL-C.

Relationship of cumulative non-high-density lipoprotein-cholesterol (cum-non-HDL-C) with the risk of arterial stiffness, according to restricted cubic splines analysis. Cox regression models with restricted cubic splines were fitted to the data with 3 knots at the 10th, 50th, and 90th percentiles of cum-non-HDL-C concentrations. The solid lines represent the point estimates of the relationships of cum-non-HDL-C with the risk of arterial stiffness, and the shaded areas represent the 95% confidence interval (CI) estimates. The covariates included in the model were age, sex, HDL-C, body mass index, high-sensitivity-C-reactive protein, estimated glomerular filtration rate, smoking status, alcohol consumption habits, physical exercise, diabetes mellitus, hypertension, and the use of antihypertensive hypoglycemic or lipid-lowering drugs.
Relationship Between the Duration of Exposure to cum-non-HDL-C and the Risk of Arterial Stiffness
Regarding the duration of exposure to cum-non-HDL-C, the incidence densities of arterial stiffness for the 0-year, 2-year, 4-year, and 6-year exposure groups were 4.63, 7.07, 11.35, and 12.03/1,000 person-years, respectively (Table 2). The cumulative incidences of arterial stiffness for the group were compared using the log-rank test, and all the differences between groups were statistically significant (P<0.01; Figure 2B). Compared with the 0-year group of high cum-non-HDL-C exposure duration, the risk of incidence gradually increased across the 2-, 4-, and 6-year groups, with adjusted HRs (95% CIs) of 1.17 (0.87, 1.58), 1.46 (1.96, 2.01), and 1.67 (1.14, 2.44), respectively (P=0.0029, Table 2). After further adjustment for baseline non-HDL-C in Model 4, the adjusted HR (95% CI) for the 6-year group vs. the 0-year group of exposure to high cum-non-HDL-C was 1.37 (1.09, 1.85) (P=0.0102, Table 2).
Comparison of the Ability of Non-HDL-C2010, Non-HDL-C2012, Non-HDL-C2014, and cum-non-HDL-C to Predict the Risk of Arterial StiffnessThe C-index values for non-HDL-C in 2010, non-HDL-C in 2012, non-HDL-C in 2014, and cum-non-HDL-C were 0.8199, 0.8207,0.8211 and 0.9220, respectively; the net reclassification index values were 4.58%, 5.22%, 2.74%, and 16.53%, respectively; and the integrated discrimination improvement values were 0.27%, 0.30%, 0.16%, and 1.58%; respectively (Supplementary Table 1). Calculation of cum-non-HDL-C demonstrated significantly higher predictive ability for the risk of arterial stiffness than non-HDL-C2010, non-HDL-C2012 or non-HDL-C2014.
Results of the Stratified and Sensitivity AnalysesStratified analyses revealed interactions of cum-non-HDL-C with smoking status and physical activity, but not with age, sex, or hypertension. The risk of arterial stiffness was higher in smokers than non-smokers, and higher in inactive participants than in active participants. Among the middle-aged and older participants, men, smokers, non-smokers, and inactive participants, high cum-non-HDL-C was associated with a higher risk of arterial stiffness. However, there were no statistically significant differences in the risk of arterial stiffness between the cum-non-HDL-C groups in younger participants, women, or active participants (Table 3).
Stratified Analysis of Association of cum-non-HDL-C and Arterial Stiffness in Groups of Participants
| Event/total | cum-non-HDL-C | P trend | P interaction | ||||
|---|---|---|---|---|---|---|---|
| G1 (<130 mg/dL) |
G2 (130–159 mg/dL) |
G3 (160–189 mg/dL) |
G4 (≥190 mg/dL) |
||||
| Age | 0.6117 | ||||||
| <45 years | 28/2,316 | 1.00 | 1.14 (0.48, 2.65) | 1.15 (0.49, 3.05) | 1.84 (0.37, 4.94) | 0.8142 | |
| ≥45 years | 299/4,536 | 1.00 | 1.09 (0.81, 1.45) | 1.44 (1.13, 2.04) | 2.61 (1.73, 3.94) | <0.0001 | |
| Sex | 0.4725 | ||||||
| Female | 112/3,490 | 1.00 | 0.87 (0.54, 1.41) | 0.76 (0.41, 1.39) | 0.95 (0.40, 2.25) | 0.9270 | |
| Male | 215/3,362 | 1.00 | 1.16 (0.87, 1.68) | 1.59 (1.09, 2.33) | 3.20 (2.04, 5.05) | <0.0001 | |
| Hypertension | 0.4459 | ||||||
| Yes | 183/1,459 | 1.00 | 1.09 (0.75, 1.59) | 1.49 (1.23, 1.86) | 2.28 (1.37, 3.78) | 0.0039 | |
| No | 144/5,393 | 1.00 | 1.07 (0.71, 1.61) | 1.40 (0.84, 2.33) | 2.62 (1.34, 5.01) | 0.0126 | |
| Current smoking | 0.0442 | ||||||
| Yes | 167/1,877 | 1.00 | 1.07 (0.69, 1.68) | 1.45 (1.19, 1.79) | 3.60 (2.06, 6.28) | 0.0001 | |
| No | 160/4,975 | 1.00 | 1.09 (0.76, 1.54) | 1.30 (0.86, 1.98) | 1.76 (0.98, 3.15) | 0.0614 | |
| Physical activity | 0.0416 | ||||||
| Active | 53/755 | 1.00 | 0.78 (0.35, 1.71) | 1.16 (0.46, 2.90) | 1.33 (0.38, 4.64) | 0.5935 | |
| Inactive | 274/6,097 | 1.00 | 1.12 (0.83, 1.51) | 1.46 (1.04, 2.07) | 2.77 (1.80, 4.26) | <0.0001 | |
Model adjusted for age, sex, HDL-C, BMI, hs-CRP, eGFR, smoking status, alcohol consumption habit, physical exercise habit, hypertension, diabetes mellitus, and the use of antihypertensive, hypoglycemic or lipid-lowering drugs. Abbreviations as in Table 1.
To reduce the potential for misinterpretation because of reverse causation and the use of antihypertensive, hypoglycemic, or lipid-lowering medication, we repeated the Cox regression analysis after excluding participants who developed arterial sclerosis within 2 years (n=45) and those who were taking antihypertensive, hypoglycemic, or lipid-lowering drugs (n=811). The results were consistent: high cum-non-HDL-C was associated with a higher risk of arterial stiffness, and longer exposure to a high cum-non-HDL-C increased this risk. In addition, we conducted a sensitivity analysis with baPWV ≥1,400 cm/s as the threshold used to define arterial stiffness, and obtained results that were consistent with the main findings (Supplementary Table 2). The results showed that cum-TRL-rich-C has a greater effect on arterial stiffness than cum-LDL-C (Supplementary Table 3).
In the present study, we identified a significant association between cum-non-HDL-C and baPWV, as a quantitative index of arterial stiffness. A high cum-non-HDL-C value was associated with a higher risk of arterial stiffness, independent of conventional risk factors. Moreover, a long exposure to a high cum-non-HDL-C was also found to be associated with a higher risk of arterial stiffness.
A previous study showed that non-HDL-C is a risk factor for CVD and that its value as a predictor of the incidence of, and death associated with, CVD is superior to that of LDL-C.37,38 In addition, the relationship between non-HDL-C and the progression of arterial pathology has been studied.39 Vallée et al. showed that the correlation of non-HDL-C concentration with arterial stiffness is significantly closer than with conventional lipid parameters such as TG, LDL-C, and HDL-C.14 A cross-sectional study conducted in China showed that, compared with participants with LDL-C <70 mg/dL and non-HDL-C <100 mg/dL, those with non-HDL-C ≥100 mg/dL had a 66% higher risk of arterial stiffness.15 Another cohort study showed that, compared with individuals in the lowest quartiles of non-HDL-C, those in the highest quartiles had a 26% higher risk of arterial stiffness.12 However, those studies were limited by factors such as a cross-sectional design,14,15,20 small sample size,13,14,20 short duration of follow-up,12 and enrolling specific patient populations.13,20
Because lipid parameters can be influenced by lifestyle, diet, and medication, and are subject to substantial fluctuations,40 we used a time-weighted cumulative approach in the present study, and calculated the cum-non-HDL-C using 3 consecutive measurements.41 This method better represents the lipid status of individuals over a period of time,41 and our study showed that cum-non-HDL-C is more effective in predicting arterial stiffness. In the present study, we followed 6,852 participants for a median 7.7 years, and found that, compared with participants with non-HDL-C <130 mg/dL, those with cum-non-HDL-C ≥190 mg/dL had a 1.52-fold higher risk of developing arterial stiffness. Furthermore, even after adjustment for their baseline non-HDL-C concentration, they had a 1.22-fold higher risk. In addition, given that arterial stiffness is a chronic progressive condition,5 the cumulative exposure to high non-HDL-C concentrations may also be a risk factor for arterial stiffness. We found that, compared with the group with 0 years of exposure to a high cum-non-HDL-C, the group with 6 years of exposure had a 67% higher risk of developing arterial stiffness, which implies that early interventions following the identification of a high non-HDL-C concentration are important to prevent the development of arterial stiffness. These findings add to the limited available research evidence regarding the association between non-HDL-C and arterial stiffness, and may aid with the early identification of individuals who are at high risk of arterial stiffness, and therefore with the implementation of appropriate strategies for the prevention of CVD.
In the stratified analysis, we found that high cum-non-HDL-C interacted with smoking status and exercise habit. Smokers had a higher risk of arterial stiffness than non-smokers, and inactive individuals had a higher risk than those who were active. These findings suggests that quitting smoking and maintaining an active lifestyle may help reduce the risk of developing arterial stiffness. In the sensitivity analysis, we also used baPWV ≥1,400 cm/s as the endpoint, and obtained results that were consistent with the main results. Thus, whether baPWV ≥1,400 cm/s or ≥1,800 cm/s is used as the threshold for the definition of arterial stiffness, cum-non-HDL-C is significantly associated with arterial stiffness. In addition, sensitivity analyses in which individuals taking antihypertensive, hypoglycemic, or lipid-lowering drugs were excluded yielded similar results. Given the suboptimal health status of many people, characterized by smoking and a lack of physical exercise, the maintenance of low cum-non-HDL-C, cessation of smoking, and participation in physical exercise should reduce the risk of arterial stiffness.42,43 We also found that cum-TRL-rich-C has a greater effect on arterial stiffness than cum-LDL-C, suggesting that interventions targeting TG may be more effective in preventing arterial stiffness.
The mechanism underpinning the association between high cum-non-HDL-C and the risk of arterial stiffness remains unclear. Potential mechanisms include cholesterol accumulation, the inflammatory response, insulin resistance, lipid peroxidation, and platelet activation.44–46 Cholesterol accumulation in the vessel wall leads to the formation of oxidized LDL, triggering inflammation and plaque formation.47 The oxidized lipids induce cell apoptosis and an inflammatory response, and cytokines and growth factors can affect plaque formation and stability.44,48 Platelet activation exacerbates vascular damage and thrombus formation, leading to arterial stiffness.44,48 Finally, insulin resistance causes greater lipid deposition, thereby promoting arterial stiffness.49
Study LimitationsThis is the first longitudinal study to explore the relationship between cum-non-HDL-C and arterial stiffness in a relatively large sample size with a long follow-up period. However, there were several limitations to the study. Firstly, the participants were all from the Kailuan community, so the findings may not be applicable to other ethnic groups. Secondly, owing to the lack of cfPWV measurements, we were unable to compare the findings with those of studies in which cfPWV was used as an index of arterial stiffness. Thirdly, the proportion of individuals receiving lipid-lowering therapy was relatively low, which prevented us from performing adequate statistical analysis to evaluate the effect of lipid-lowering therapy on arterial stiffness. Fourthly, although a large number of demographic and clinical variables were adjusted for in the statistical models, there may still have been some residual confounding or unmeasured confounding factors, such as the performance of specific types of exercise (individual or team sports, aerobic or anaerobic exercise), and dietary variations (consumption of salt and fats), which may have affected the results. However, we adjusted for numerous potential confounding factors in the Cox regression models and conducted appropriate sensitivity analyses, and therefore the results should be reliable.
In conclusion, a high cum-non-HDL-C increased the risk of arterial stiffness. Furthermore, prolonged exposure to a high cum-non-HDL-C also increased the risk of arterial stiffness. These results indicate that the monitoring and maintenance of appropriate non-HDL-C concentrations should reduce the risk of arterial stiffness.
We sincerely express our gratitude to all parties in the Kailuan Study, as well as members of Kailuan General Hospital and its affiliated hospitals.
This study was supported by 2023 Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (No. STKJ2023003), Shantou Medical Health Science and Technology Plan (Nos. 210624106490813 and 230509206496513).
None.
The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (Approval Number: 2006-05). All participants agreed to take part in the study and provided informed written consent.
Data will be made available upon reasonable request.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-24-0921