Article ID: CJ-14-1195
Article ID: CJ-14-1195
Background: Heart failure (HF) is a leading cause of hospitalization throughout the world, and the mortality rate remains elevated. HF is frequently complicated by acute kidney injury (AKI), worsening the patient’s prognosis. There have been no studies evaluating the role that endothelial glycocalyx damage plays in HF patients and its association with AKI and mortality.
Methods and Results: We measured several endothelial biomarkers in 201 consecutive patients with acute decompensated HF (ADHF) during emergency department (ED) admission. In-hospital mortality, AKI development and 6-month mortality rates were assessed. ADHF patients with worsening renal function had higher levels of syndecan-1 but not those patients with stable chronic kidney disease. Syndecan-1 levels during ED admission were predictive for AKI during the hospital stay (AUC 0.741, P<0.001) and had an even better discriminatory capacity in more severe AKI (AUC 0.812, P<0.001). Additionally, after adjusting for several confounding factors, including biomarkers of endothelial function and endothelial cell activation, syndecan-1 remained associated with in-hospital mortality rates. On a Cox multivariate analysis regression, syndecan-1 was associated with 6-month mortality rates.
Conclusions: The concentration of syndecan-1, a marker of glycocalyx damage measured during ED admission, is valuable in assessing the risk of developing AKI and in-hospital mortality. Its association with mortality is strong after 6-month follow-up.
Heart failure (HF) is a lethal disease and one of the most common reasons for hospitalization in developed countries.1–3 Despite modern therapy, the prognosis of patients with HF remains poor. Although in-hospital mortality rates have been reduced over the past 2 decades, the 30-day cumulative mortality rate remains approximately 6–7%.4,5
Editorial p????
HF patients constitute a heterogeneous population because they frequently suffer from comorbidities, which are not only prevalent but also pose an excess mortality risk and can evolve to acute complications during hospital stay. Renal impairment, one of the most important comorbidity factors, plays a crucial role in the pathophysiology of HF and also increases the mortality rate.6,7 Acute, chronic or acute-on-chronic kidney disease (CKD) can complicate HF with ominous prognostic implications.
In both HF and renal disease, there is endothelial dysfunction,8–10 which appears to have a bidirectional relationship in both conditions. Endothelial function is impaired in patients with renal and cardiac dysfunction, affecting HF progression and the pathophysiology of acute kidney injury (AKI).11 Moreover, endothelial activation biomarkers, such as vascular cell adhesion molecule-1, have recently been associated with HF patient mortality rates.12
However, glycocalyx, an approximately 1-μm thick carbohydrate-rich structure with antiadhesive and anticoagulant properties that protects the endothelium and maintains vascular barrier function, has been scarcely studied in HF patients. Only 1 study evaluated glycocalyx damage in stable HF patients. The roles of glycocalyx include maintenance of the vascular permeability barrier, mediation of shear stress-dependent nitric oxide (NO) production and housing of vascular protective enzymes (eg, superoxide dismutase), as well as a wide array of coagulation inhibition factors, such as antithrombin, the protein C system and tissue factor pathway inhibitor. Glycocalyx also modulates the inflammatory response by preventing leukocyte adhesion and by binding numerous ligands, including chemokines, cytokines and growth factors.13,14 Many of the functions of glycocalyx are related to the pathophysiologic processes involved in the development of AKI and can also contribute to increased morbidity and mortality.15 Syndecan-1 is a biomarker of endothelial glycocalyx damage and it has been shown to be increased in patients suffering acute myocardial infarction.16 However, its role as a biomarker in HF patients is unknown, especially in patients with acute decompensated HF (ADHF). Additionally, there have been no studies evaluating glycocalyx integrity in patients with newly recognized cardiorenal syndromes.
In the present study, we aimed to investigate the level of endothelial glycocalyx damage in patients admitted to the emergency department (ED) because of ADHF. For that purpose, we measured the plasma level of syndecan-1 and assessed its association with acute or chronic kidney disease as well as its capacity to predict short- and long-term mortality rates.
This study was performed in a reference hospital in Fortaleza, Brazil. The hospital specializes in cardiopulmonary diseases and is used by an estimated population of 3 million people. The study was performed in the ED between April 2013 and September 2013. The patients were enrolled after the attending physician requested their hospital stay. After the patients provided informed consent, a blood sample was collected immediately after ED admission and no more than 2 h of any therapeutic approach. Patients with a clinical diagnosis of HF consistent with the Framingham clinical criteria17 and with a diagnosis of ADHF at presentation to the ED were initially considered. We excluded patients with endstage renal disease undergoing maintenance dialysis therapy, patients with a previous renal transplantation, patients with admission serum creatinine (SCr) levels >4 mg/dl, or patients with hospital stay <24 h.
The estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI equation.18 Baseline kidney function was defined as the mean of all outpatient SCr levels in the 6 months before hospitalization. If not available, we used the lowest SCr level during the hospital stay and follow-up. Patients with eGFR <60 ml/min/1.73 m2 were considered as having chronic kidney disease (CKD).
AKI was defined according to the creatinine-based Kidney Disease Improving Global Outcome (KDIGO) criteria.19 Briefly, AKI stage 1 included acute SCr increases ≥0.3 mg/dl and up to 200% above the stable baseline SCr, whereas values of SCr in the range of 200–300% greater than the stable baseline level were considered as AKI stage 2. AKI stage 3 entailed acute increases of SCr to more than 300% (>3-fold), as well as SCr values ≥4 mg/dl while showing an acute increase of at least 0.5 mg/dl. Patients with an AKI diagnosis at admission but who recovered to a maximum of 0.2 mg/dl above their baseline SCr after 72 h of ED admission were considered as having hemodynamic renal dysfunction and were not considered as having AKI.
The following parameters were collected in the ED: age, sex, pre-admission functional status by New York Heart Association (NYHA) classification, previous history of diabetes mellitus, arterial hypertension, chronic pulmonary obstructive disease and drug prescription history. During the hospital stay, patients were evaluated daily and the laboratory data were collected. The length of hospital stay (LOS) and in-hospital mortality rates were recorded. After being discharged from the hospital, patients were followed up for 6 months to assess late mortality rates. The Institutional Ethical Committee approved all the procedures in this study.
Laboratory MeasurementsAt the time of ED presentation, EDTA tubes were used to collect blood and urine samples that were immediately processed and frozen at −80℃ for later measurement of B-type natriuretic peptide (BNP), syndecan-1 and high-sensitivity C-reactive protein (hsCRP) in the blood. Also, 2 other renal biomarkers previously studied in cardiorenal syndrome20,21 were measured: urine kidney injury molecule-1 (KIM-1), and plasma neutrophil gelatinase-associated lipocalin (NGAL). Finally, we calculated the fractional excretion of sodium (FENa) according to the formula: (UNa×Scr)/(Ucr×SNa)×100. The MCP-1 value was corrected by urinary creatinine.
Syndecan-1 was measured as a biomarker of endothelial glycocalyx injury (Abcam, Cambridge, MA, USA). The detection range for syndecan-1 is 8–256 ng/ml and the intra-assay coefficient of variation is 6.2%. Intercellular adhesion molecule-1 (ICAM-1), a marker of endothelial cell activation, was measured using a commercially available enzyme-linked immunosorbent assay kit (Life Technologies Brasil, São Paulo, Brazil). We utilized the Griess diazotization reaction to spectrophotometrically detect nitrite formed by the spontaneous oxidation of NO under physiological conditions.22 The Griess reaction can be used to analyze nitrate via its catalytic reduction to nitrite, and total NO (NOx) levels were measured spectrophotometrically. The BNP levels were measured in venous blood samples with a commercially available kit (Elecsys proBNP; Roche Diagnostics GmbH, Mannheim, Germany). Finally, hsCRP was evaluated by the nephelometry method.
Statistical AnalysisAnalysis of the data was performed using SPSS 19.0 for Windows (Chicago, IL, USA). All of the variables were tested for normal distribution using the Kolmogorov-Smirnov test. Normally or near-normally distributed variables are reported as the means with standard deviations. Categorical data are reported as proportions and compared using Fisher’s exact test. The ability of ED admission levels of syndecan-1 in predicting AKI and in-hospital mortality rates was assessed using the area under the receiver-operator characteristic (AUROC) curve. Regarding syndecan-1, low and high levels were defined according to the highest Youden index, which was calculated as [1−(1−sensitivity)+(1−specificity)]. After controlling for colinearity, we applied a multiple insertion of the covariates to determine the adjusted odds ratio (OR) for syndecan-1 level while using AKI and hospital mortality rates as the dependent variable. For evaluating the effects of syndecan-1 levels upon admission on long-term outcomes, Kaplan-Meier survival curves with log-rank tests and Cox regression analyses based on a multivariate approach were used. Data are presented as the OR or hazard ratio, with 95% confidence intervals (CI). A P value <0.05 was considered to be statistically significant for all comparisons.
After exclusion criteria, 201 ADHF patients (de novo ADHF: n=36; CHF decompensation: n=165) remained in the final analysis (Figure 1). The baseline demographic and ED admission biochemical characteristics are presented in Table 1. The mean age was 64.2±13.5 years, mean calculated ejection fraction (EF) was 41.5±14.4% and the BNP was 1,690±1,204 pg/ml at admission.
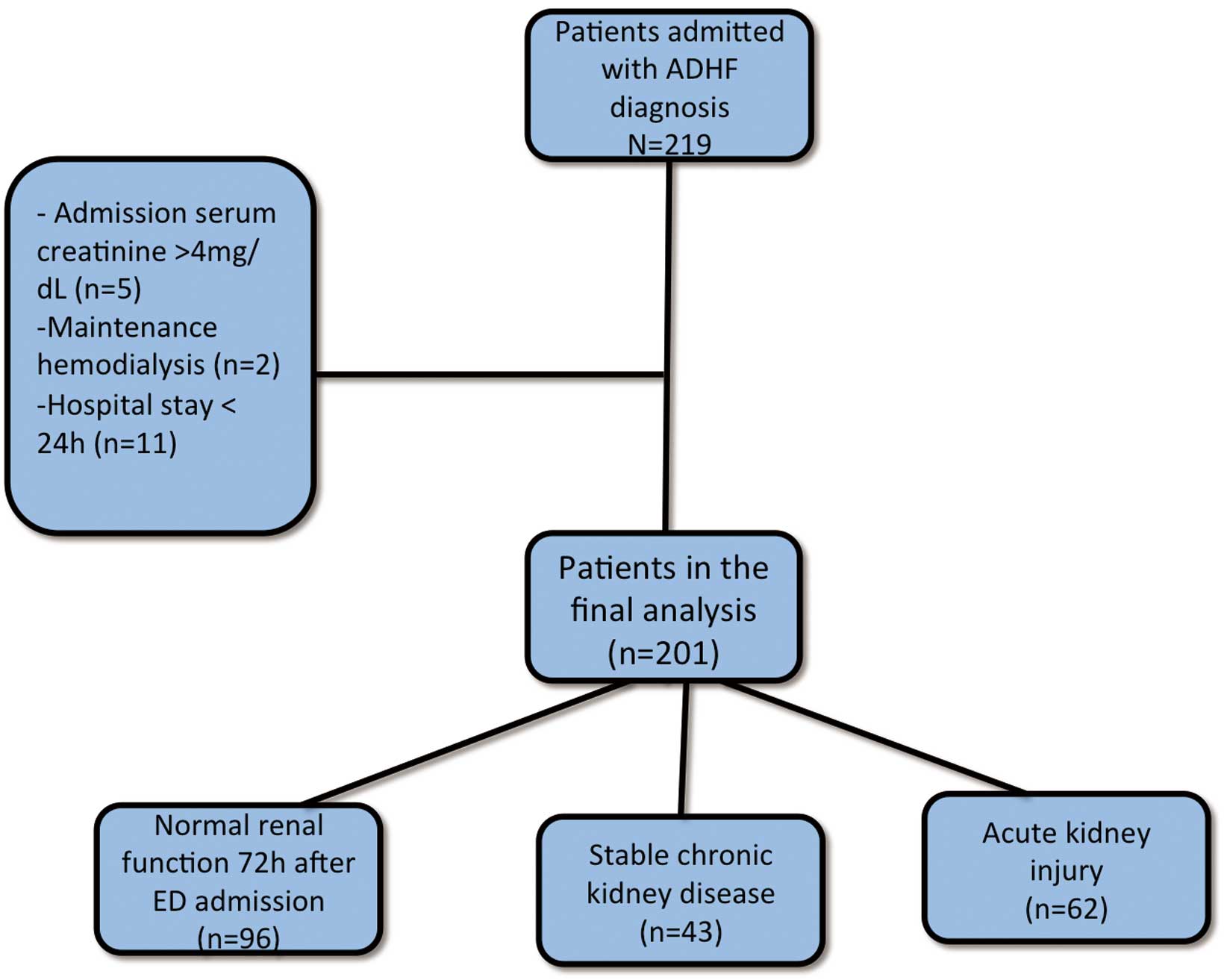
Flow chart of patients with acute decompensated heart failure (ADHF). ED, emergency department.
| All patients (n=201) |
Syndecan <125 ng/ml (n=143) |
Syndecan >125 ng/ml (n=58) |
P value | |
|---|---|---|---|---|
| Age (years) | 64.2±13.5 | 64.1±13.2 | 64.5±14.3 | 0.338 |
| Male n (%) | 109 (54.2) | 80 (56.3) | 29 (50.0) | 0.414 |
| Diabetes mellitus (%) | 46 (22.9) | 30 (21.0) | 16 (27.6) | 0.355 |
| LVEF (%) | 41.5±14.4 | 41.7±14.0 | 41.1±15.3 | 0.520 |
| NYHA class (%) | 0.406 | |||
| 1/2 | 16 (8.0) | 14 (9.8) | 2 (3.4) | 0.159 |
| 3/4 | 184 (92.0) | 128 (90.1) | 56 (96.6) | |
| Sodium (mEq/L) | 135.9±5.0 | 136.6±4.3 | 134.4±6.0 | 0.016 |
| eGFR (ml/min/1.73 m2) | 64.9±22.6 | 66.2±20.8 | 62.0±26.3 | 0.226 |
| CKD n (%) | 79 (39.9) | 54 (38.0) | 25 (43.1) | 0.553 |
| Syndecan (ng/ml) | 133.7±195.1 | 39.5±31.3 | 364.6±232.5 | <0.001 |
| hsCRP(mg/dl) | 2.54±3.6 | 1.5±2.0 | 3.9±4.7 | 0.005 |
| ICAM-1 (ng/ml) | 423.6±128.1 | 411.3±109.3 | 473.5±148.7 | 0.485 |
| NOx (μmol/L) | 9.8±2.0 | 9.8±2.1 | 9.7±1.9 | 0.978 |
| BNP (pg/ml) | 1,690±1,204 | 1,719±1,145 | 1,640.5±1,049 | 0.957 |
| Hospital LOS | 8.4±6.5 | 7.8±6.7 | 9.9±6.2 | 0.080 |
| Hospital mortality, n (%) | 10 (5) | 3 (2.1) | 7 (12.1) | 0.003 |
| 6-month mortality, n (%) | 25 (12.5) | 12 (8.5) | 13 (22.4) | 0.007 |
ADHF, acute decompensated heart failure; BNP, B-type natriuretic peptide; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; LOS, length of stay; LVEF, left ventricular ejection fraction; NOx, total nitric oxide; NYHA, New York heart association functional classification.
Taking into account all patients, 79 (39.9%) had previous CKD and 43 patients (21.4%) maintained stable CKD during hospital stay; 62 patients (37.8%) developed AKI during their hospital stay (Figure 1, Table 2). The majority of patients had AKI stage 1 (n=49), and only 2 had AKI stage 3. Another group of 14 patients was admitted with elevated SCr at the time of their ED admission but recovered renal function within less than 72 h. The mean hospital LOS was 8.7±4.9 days, and the in-hospital mortality rate was 5.0%.
| Normal renal function (n=96) |
Stable CKD (n=43) |
AKI (n=62) |
P value | |
|---|---|---|---|---|
| Age (years) | 63.5±13.6 | 64.7±13.2 | 64.9±13.8 | 0.787 |
| Male n (%) | 56 (58.9) | 20 (46.5) | 33 (53.2) | 0.386 |
| LVEF (%) | 42.6±13.4 | 42.5±15.5 | 41.0±14.0 | 0.765 |
| NYHA class (%) | 0.406 | |||
| 1/2 | 11 (11.6) | 03 (7.0) | 2 (3.2) | |
| 3/4 | 84 (88.4) | 40 (93.0) | 60 (96.8) | |
| Sodium (mEq/L) | 136.7±4.0 | 136.4±4.7 | 134.6±6.2 | 0.028* |
| eGFR (ml/min/1.73 m2) | 77.2±20.1 | 47.5±11.8 | 58.5±21.6 | <0.001# |
| Syndecan (ng/ml) | 91.4±42.9 | 98.1±65.6 | 248.7±165.6 | <0.001* |
| hsCRP(mg/dl) | 2.6±2.1 | 2.7±2.2 | 2.9±2.3 | 0.700 |
| NOx (μmol/L) | 9.3±4.4 | 10.9±5.3 | 9.6±5.7 | 0.217 |
| BNP(pg/ml) | 1,819±1,034 | 1,729±1,257 | 1,495±945 | 0.171 |
| Urinary KIM-1 (ng/mgCr) | 3.7±2.8 | 4.6±3.2 | 4.4±3.6 | 0.205 |
| Plasma NGAL (ng/ml) | 108.3±82.2 | 119.6±94.1 | 142.3±92.6 | 0.062 |
| EFNa (%) | 2.7±1.9 | 2.9±2.2 | 2.8±2.0 | 0.855 |
*P<0.01 for AKI vs. other groups; #P<0.01 for normal renal function vs. other groups. AKI, acute kidney injury; FENa, fractional excretion of sodium; KIM-1, kidney injury molecule 1; NGAL, neutrophil gelatinase-associated lipocalin. Other abbreviations as in Table 1.
ADHF patients had a mean level of serum syndecan-1 on ED admission of 133.7±95.0 ng/ml. This value was higher in the patients with a higher pre-admission NYHA classification (Class III/IV) (140.3±80.1 vs. 58.4±37.7 ng/ml, P=0.002) compared with NYHA Class I/II. No significant correlation was verified between syndecan-1 and BNP (r=0.074, P=0.281), but hsCRP had a direct correlation with syndecan-1 (r=0.367, P<0.001). Regarding other markers of endothelium, there were no significant correlations with ICAM-1 (r=0.123, P=0.111) or NOx (r=−0.037, P=0.468). In relation to other renal biomarkers, syndecan-1 correlated only marginally with admission GFR (r=−0.142, P=0.053). There was no correlation between syndecan-1 with urinary KIM-1 (r=0.14, P=0.891), plasma NGAL (r=0.002, P=0.993) or FENa (r=−0.093, P=0.188).
Syndecan-1 and AKI vs. Stable CKDThe highest level of syndecan-1 was observed in the patients who developed AKI during their hospital stay (mean 248.7±165.6 ng/ml). There was no significant difference between patients with stable CKD and those with normal renal function (Figure 2). Additionally, there was a stepwise increase in syndecan-1 level according to AKI severity. Patients with AKI class 2/3 (n=13) had higher syndecan-1 levels than patients with AKI class 1 (n=49) (443.2±281.1 vs. 178.9±100.8 ng/ml, P<0.001).

Mean syndecan-1 level according to renal function in patients with acute decompensated heart failure (ADHF). P<0.05 for ADHF with AKI vs. others. AKI, acute kidney injury.
To assess the discriminative ability of syndecan-1 level in predicting AKI occurrence, ROC curves were generated. The AUC for developing AKI during a hospital stay was 0.741 (95% CI 0.669–0.812, P<0.001), as shown in Figure 3. The results improved only when higher grades of AKI severity (AKI grade ≥2) were considered (AUC 0.840, 95% CI 0.733–0.948, P<0.001). Comparison of performance between syndecan-1 and known renal biomarkers is shown in Table S1.

Discriminative ability of syndecan-1 level in predicting acute kidney injury. AUC, area under the curve.
Even after adjusting for other variables associated with AKI occurrence (age, admission SCr and BNP levels, left ventricular ejection fraction (LVEF), diabetes, hypertension and admission sodium), syndecan-1 remained associated with AKI (OR 1.405 with 95% CI 1.240–1.670 per each 100 ng/ml, P<0.001).
Syndecan-1 and Hospital Mortality RatesIn the univariate analysis, syndecan-1 level, as a continuous variable, was significantly related to hospital mortality (OR 1.461 with 95% CI 1.256–1.677 per each 100 ng/ml, P<0.001). After adjusting for age and sex (model 1) and for age, sex, previous CKD, admission serum sodium levels, EF, ICAM-1, NOx, BNP and AKI severity (model 2), syndecan-1 level remained a significant predictor of hospital mortality rate (Table 3).
| Univariate analysis | Model 1 | Model 2 | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Syndecan-1 (per each 100 ng/ml) | 1.461 (1.256–1.677) | 1.389 (1.162–1.614) | 1.388 (1.166–1.711) |
Model 1: adjusts for age, sex. Model 2 adjusts for covariate in model 1 and previous CKD, admission serum sodium, LVEF, intercellular adhesion molecule-1, nitrite, BNP and AKI severity. CI, confidence intervals; OR, odds ratio. Other abbreviations as in Tables 1,2.
Moreover, syndecan-1 levels upon ED admission had a good discriminative ability to predict hospital mortality rates (AUC 0.788 95% CI 0.673–0.903, P<0.001). We determined the cut-off level of syndecan-1 that provides the best discriminatory capacity and found it to be 125 ng/ml. There was no significant difference between patients with high or low syndecan-1 levels when considering NYHA functional level as well as LVEF and BNP level. However, patients with syndecan-1 levels >125 ng/ml had higher hsCRP levels and low serum levels of sodium. Additionally, there was a trend towards higher hospital LOS in patients with syndecan-1 levels >125 ng/ml (9.9±6.2 vs. 7.8±6.7 days, P=0.080) and a significant higher in-hospital mortality rate (Table 1).
Syndecan-1 and Long-Term Mortality RatesPatients with high syndecan-1 levels had higher 6-month mortality rates (22.4 vs. 8.5%, P=0.007). Using the same cut-off value for the in-hospital mortality rates, Kaplan-Meyer analyses also showed a significant separation of survival curves for patients with low and/or high levels of syndecan-1 for 6-month survival studies (Figure 4). To further explore syndecan-1, measured on ED as a long-term prognostic marker, we performed a multivariate Cox regression analysis including age, sex, markers of HF severity (NYHA, LVEF), inflammatory status (hsCRP), and BNP as well as presence of CKD. Of these variables, serum hemoglobin, BNP, hsCRP and syndecan-1 were independently associated with a high risk of 6-month mortality (Table 4).

Kaplan-Meyer survival curves for long-term outcome (6 months) according to syndecan-1 level.
| Univariate analysis | Model 1 | Model 2 | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age (per year) | – | 1.009 (0.979–1.040) | 1.005 (0.968–1.043) |
| Sex (male) | – | 1.626 (0.724–3.653) | 1.749 (0.635–4.817) |
| Previous CKD | – | – | 1.603 (0.568–4.521) |
| LVEF (per percentage) | – | – | 0.959 (0.915–1.005) |
| NYHA (per class) | – | – | 1.899 (0.799–4.509) |
| Admission hemoglobin (per g/dl) | – | – | 0.794 (0.654–0.963) |
| Admission sodium (per mEq/L) | – | – | 1.009 (0.922–1.104) |
| BNP (per 100 pg/ml) | – | – | 1.034 (1.010–1.059) |
| hsCRP (per mg/dl) | – | – | 1.435 (1.192–1.729) |
| NOx (per μmol/L) | – | – | 0.994 (0.958–1.030) |
| ICAM-1 (per 10 ng/ml) | – | – | 1.006 (0.962–1.258) |
| Syndecan-1 (per 100 ng/ml) | 1.283 (1.150–1.429) | 1.270 (1.146–1.412) | 1.262 (1.079–1.464) |
| AKI during hospital stay | – | – | 3.154 (1.394–12.757) |
HR, hazard ratio. Other abbreviations as in Tables 1–3.
In the present study, we evaluated an endothelial glycocalyx damage biomarker (syndecan-1) for the first time in ADHF patients on admission to the ED. The syndecan-1 level had a good discriminatory capacity for predicting acute worsening renal function and in-hospital mortality rates. Moreover, after adjusting for other confounding factors, syndecan-1 remained associated with long-term mortality.
It has already been well established that endothelial function is important in the long-term prognosis of HF patients. Impaired endothelium-dependent vasodilation reduces myocardial perfusion and increases ventricular afterload with ominous consequences on cardiac remodeling.23 In the setting of ADHF, there is activation of endothelial cells,11 but little is known about the prognostic significance.24,25 To the best of our knowledge, there have been no studies investigating the role of endothelial glycocalyx in patients presenting to the ED because of ADHF.
Recently, Tromp et al26 studied the value of syndecan-1 measured before hospital discharge, when patients were stabilized after an ADHF admission. The authors reported that syndecan-1 was associated with poor outcome only in HF patients with preserved EF, suggesting syndecan-1 is also an important marker of cardiac fibrosis. As stated before, endothelial and glycocalyx damage in the acute setting is probably so different from when the patient is in a stable condition (eg, because of sympathetic activation),16 it is difficult to compare the findings of the aforementioned study with our data.
The first important finding in the present study was that ADHF patients had an increase in syndecan-1 levels compared with the control group. Syndecan-1 can be elevated in patients with chronic diseases (eg, diabetes mellitus27) or acute processes (ischemic-reperfusion injury,28 septic shock29 and post-cardiac arrest syndrome30). An inflammatory state and adrenergic activation are present in HF patients and can contribute to glycocalyx damage.16 In the present study, we disclosed an association between the inflammatory state and glycocalyx damage through a positive and significant correlation between syndecan-1 and CRP. In addition to the inflammatory state, high levels of syndecan-1 in ADHF can also be explained by the elevation of BNP levels observed in these patients. In experimental models, natriuretic peptides augment the level of endothelium glycocalyx biomarkers (including syndecan-1) and increase endothelium permeability.31 Although degradation of the mouse endothelial glycocalyx increases the number of leukocytes adhered to the vessel walls,32 we did not find any correlation between syndecan-1 and other biomarkers of endothelial activation and function (eg, ICAM-1 and NOx).
Previous studies have reported that patients under maintenance hemodialysis and those with very advanced CKD (mean eGFR 8 ml/min/1.73 m2) had increased syndecan-1 levels.33 Although we disclosed no significant increase in patients with stable CKD, our data are not necessarily contradictory to previous findings. Our CKD patients only had a moderate decline in renal function (mean eGFR 47 ml/min/1.73 m2). In the cited study, and in accordance with our findings, patients with moderate kidney impairment after renal transplantation (eGFR 22 ml/min/1.73 m2) presented with similar syndecan-1 levels when compared with controls.33
More interesting is that the syndecan-1 level measured on ED admission has value as a predictor of acute worsening renal function during hospital stay, even after adjusting for other risk factors for AKI in this population, as suggested in other studies.34 This finding merits consideration for 2 reasons: (1) the importance of glycocalyx damage on the pathophysiology of cardiorenal syndrome and (2) the use of syndecan-1 as a biomarker for the early detection of AKI.
Concerning the role that glycocalyx shedding plays in AKI pathophysiology, it is known that endothelium damage and activation is an important step in sepsis and ischemic-mediated AKI, especially in its extension phase.15 As glycocalyx integrity is important in activating antithrombin III, regulating endothelial permeability and preventing leukocyte interaction, we speculate that its damage is able to initiate/participate in at least 2 pathophysiologic processes during AKI development. The first process potentially leads to microthrombi formation, and the second process potentially favors inflammatory cell migration.
Syndecan-1 also emerges as a potential early biomarker of AKI. Even when measured on ED admission, it was able to predict a worse renal function outcome during the hospital stay. Of note, patients who recovered their renal function 72 h after ED admission had syndecan-1 levels comparable to those of patients with stable renal function. This suggests that syndecan-1 is able to differentiate renal structural lesions from hemodynamic impairment only, but this finding requires more research. The discriminatory capacity of syndecan-1 in predicting AKI could be comparable with other renal-specific biomarkers, such as NGAL and KIM-1,35 especially when considering the capacity of syndecan-1 in predicting severity of AKI.
It has been already demonstrated that accumulation of syndecan-1 in CKD patients is not related to a possibly reduced renal clearance.36 It has been suggested that syndecan-1 levels in these patients are related to active refurbishment and on-going damage to the endothelial glycocalyx. Because in the present patients the syndecan-1 levels were already increased at ED admission before AKI development in many cases, our data supports the conclusion that syndecan-1 is not increased because of reduced renal clearance. Moreover, because syndecan-1 increases before renal function worsens, glycocalyx damage occurs prior to the decline in renal function, reinforcing the importance of glycocalyx damage on the pathophysiology of cardiorenal syndrome. Preventing glycocalyx damage in these patients may be a possible intervention for preventing AKI.
We also measured 2 renal biomarkers to evaluate their discriminatory capacity in predicting AKI. First, plasma NGAL was chosen because it demonstrated a higher discriminatory capacity in another study,37 although those findings have not been confirmed.21 Also, we measured urinary KIM-1, a successful biomarker used in other AKI etiologies,38 but not in ADHF.20 In comparison with both, syndecan-1 showed better performance. The superiority of syndecan-1 in relation to renal biomarkers can be explained by urinary biomarkers being generally lower in patients who present with ADHF than is reported in the literature for AKI, which suggests that AKI in ADHF is likely related to other causes rather than structural tubular damage.39
Regarding mortality rates, this is the first study to demonstrate that a glycocalyx biomarker is associated with short- and long-term mortality rates in ADHF patients. We found that syndecan-1 levels were associated with mortality rates, even after controlling for several important variables. For example, all the variables, except tricuspid regurgitation, in the ADHF/NT-proBNP risk score40 were included in our model in addition to hsCRP and other endothelial markers.
Some studies suggest that endothelial dysfunction is associated with HF progression and long-term mortality in HF.41 Concerning the prognostic significance of ICAM-1 in HF patients, the literature is controversial.24,25 In our data, we did not find any independent association between ICAM-1 or NOx levels with short- or long-term mortality rates.
Study LimitationsFirst, it was performed in only a single center. Second, because of the technical difficulty in performing flow-mediated dilatation methods in ED patients taking several vasodilator drugs, we accessed endothelial function only through a NOx measurement, which is controversial.42,43 Finally, we cannot extrapolate syndecan-1 as a marker for mortality in stable HF patients.
In conclusion, measurement of syndecan-1, a marker of glycocalyx damage, on ED admission is a valuable tool for assessing the risk of developing AKI and in-hospital mortality. Its association with mortality is strong, even after a 6-month follow-up period.
A.B.L. and A.M.C.M are recipients of a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The funding sources had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of Interest: None.
Supplementary File 1
Table S1. Diagnostic accuracy of biomarkers for the assessment of AKI during hospitalization
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-1195