Article ID: CJ-14-1422
Article ID: CJ-14-1422
Background: We aimed to evaluate whether specific monocyte subsets could serve as surrogate markers of disease activity in cardiac sarcoidosis (CS) evaluated by 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET).
Methods and Results: We studied 28 patients with CS (8 men; mean age: 61±9 years) diagnosed according to consensus criteria. We divided the patients into 2 groups: known CS receiving corticosteroid therapy (Rx(+); n=13) and new-onset CS (Rx(−); n=15), and analyzed 3 distinct monocyte subsets (CD14+CD16−, CD14++CD16+, and CD14+−CD16+). Monocyte subsets were also analyzed in 10 Rx(−) patients before and 12 weeks after starting corticosteroid therapy. Inflammatory activity was quantified by 18F-FDG PET using the coefficient of variation (COV) of the standardized uptake value (SUV). The proportion of CD14++CD16+ monocytes in Rx(+) patients (10.8 [0.2–23.5] %) was significantly lower than in Rx(−) patients (23.0 [11.5–38.4] %, P=0.001). After corticosteroid therapy, the COV of the SUV was significantly improved from 0.32 [0.14–0.62] to 0.17 [0.04–0.43] (P=0.017). The proportion of CD14++16+ monocytes showed a significant decrease from 22.2 [8.8–38.4] % to 8.4 [1.8–16.8] % (P=0.001). The decrease in the proportion of CD14++16+ monocytes significantly correlated with the decrease in the COV of the SUV (r=0.495, P=0.027).
Conclusions: CD14++16+ monocytes are a possible surrogate marker of the therapeutic effect of corticosteroid therapy in CS.
Sarcoidosis is characterized by noncaseating granulomas, consisting mainly of epithelioid cells and multinucleated giant cells derived from monocyte-macrophage lineage cells.1 Although it is not commonly fatal, cardiac involvement may be responsible for more than two-thirds of deaths.2 Therefore, clinical diagnosis of cardiac sarcoidosis (CS) is critically important for planning therapeutic strategies. Until now, the serum level of angiotensin-converting enzyme (ACE) has been used for diagnosis and evaluation of disease activity.3,4 However, this marker is influenced by ACE inhibitors, and both the sensitivity and specificity of ACE are relatively low.5 Recently, the findings of several studies have suggested that the number of circulating monocytes, especially the proportion of CD14+CD16+ monocytes, is significantly higher in extra-CS patients than in healthy subjects.6–8 Taking these findings into account, we hypothesized that the CD14+CD16+ monocyte subsets could be a novel biomarker for both disease activity and monitoring the therapeutic effect in CS.
Editorial p ????
Noncaseating granuloma is rarely found by subendomyocardial biopsy in CS, making it difficult to histologically diagnose cardiac involvement.9 Recent studies have shown the promising potential of 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET) in the diagnosis of active sarcoid lesions and their response to corticosteroid treatment of CS.10–13 Accumulation of 18F-FDG is closely associated with an active inflammatory process in patients with CS.14,15
The aim of this study was to evaluate whether specific monocyte subsets could serve as a biomarker of disease activity and surrogate maker of the therapeutic effect of corticosteroid therapy in CS patients evaluated by 18F-FDG PET.
We enrolled 31 patients diagnosed with CS based on guidelines established in 2006 by the Japanese Ministry of Health and Welfare (JMHW) (Table 1)16 between July 2010 and November 2013 at Wakayama Medical University Hospital. We did not include patients with a history of coronary artery disease and to exclude them, we performed stress myocardial perfusion imaging and/or coronary angiography in those with multiple coronary risk factors. Using echocardiography, we also excluded subjects with valvular heart disease and cardiomyopathy. Three patients were excluded for prior myocardial infarction, but none for valvular heart disease or cardiomyopathy. Therefore, the final study population consisted of 28 patients (8 men, 20 women; mean age: 61±9 years).
| Major criteria* |
| a. Advanced AVB. |
| b. Basal thinning of the interventricular septum. |
| c. Positive 67 gallium uptake in the heart. |
| d. Depressed ejection fraction of the left ventricle (50%). |
| Minor criteria** |
| a. Abnormal ECG findings: ventricular arrhythmias (VT, multifocal or frequent PVCs), CRBBB, axis deviation or abnormal Q-wave. |
| b. Abnormal echocardiography: regional abnormal wall motion or morphological abnormality (ventricular aneurysm, wall thickening). |
| c. Nuclear medicine: perfusion defect detected by 201thallium or 99mtechnetium myocardial scintigraphy. |
| d. Gadolinium-enhanced CMR imaging: delayed enhancement of myocardium. |
| e. Endomyocardial biopsy: interstitial fibrosis or monocyte infiltration over moderate grade. |
*Two or more of the 4 major criteria are satisfied. **One in 4 of the major criteria and 2 or more of the 5 minor criteria are satisfied. AVB, atrioventricular block; CMR, cardiac magnetic resonance; CRBBB, complete right bundle branch block; ECG, electrocardiogram; PVC, premature ventricular contraction; VT, ventricular tachycardia.
We divided these patients into 2 groups: known CS receiving corticosteroid therapy (Rx(+)) and new-onset CS (Rx(−)). We then compared the groups in terms of specific monocyte subsets.
The study was approved by the regional ethics committee, and all patients provided written informed consent to participate.
Protocol 2: Correlation Between Monocyte Subsets and Inflammatory Activity in New-Onset CS Patients Initiating Corticosteroid TherapyOf the 15 Rx(−) patients, 10 started corticosteroid therapy and 5 refused this treatment. We analyzed the monocyte subsets and performed 18F-FDG PET computed tomography (CT) before and 12 weeks after starting corticosteroid therapy to assess the response to treatment.
Cytometric AnalysisFor cytometric analysis, monoclonal antibodies against CD14 (fluorescein isothiocyanate [FITC]-conjugated, clone M5E2; BD Bioscience, San Jose, CA, USA) and CD16 (phycoerythrin [PE]-CyTM5-conjugated, clone 3G8; BD Bioscience) were used as described previously.17–20 Matched-isotype antibodies (FITC-conjugated mouse IgG2aκ isotype, clone G155-178, and PECyTM5 mouse IgG1κ isotype, clone MOPC-21; BD Bioscience) were used as negative controls. A total of 100 μl of blood was incubated for 15 min at room temperature in the dark. For erythrocyte lysis and leukocyte fixation, 1 ml of lysis solution was added (BD FACS Lyse, Lysing Solution; Becton Dickinson, Germany). Cytometric analysis was performed in a flow cytometer (BD FACSAriaTM, Becton Dickinson) using BD FACSDiva software (Becton Dickinson). As shown in Figure 1, monocytes were first gated in a forward scatter/sideward scatter dot-plot, and 2-color fluorescence was then measured within the monocyte gate. CD14+CD16– cells were defined as monocytes expressing CD14 but not CD16, and CD14+CD16+ cells as monocytes expressing CD16, which included either a higher level of CD14 (CD14++CD16+) or a lower level of CD14 (CD14+–CD16+). The recently updated classification of monocyte heterogeneity acknowledges the existence of 3 monocyte subsets: classical monocytes (CD14++CD16–), intermediate monocytes (CD14++CD16+), and non-classical monocytes (CD14+CD16+).21 Therefore, we obtained data on the CD14+CD16–, CD14++CD16+, and CD14+–CD16+ subsets in this study. Leukocytes were counted by an automated method (Coulter Counter, Beckman Coulter, Miami, FL, USA).

Fluorescence-activated cell scanner analysis. (A) Monocytes gated in a forward scatter/sideward scatter (FSC/SSC) dot-plot. (B) CD14+CD16− cells defined as monocytes expressing CD14, but not CD16 (lower right quadrant). CD14+CD16+ cells defined as monocytes expressing CD16 and divided into 2 monocyte subsets: high levels of CD14 (upper right quadrant; CD14++CD16+) and low levels of CD14 (upper left quadrant; CD14+−CD16+). FITC, fluorescein isothiocyanate.
Echocardiograms were performed with a Vivid E9 System (GE Healthcare, Horten, Norway) equipped with a 2.5-MHz transducer. Parasternal long- and short-axis views at the basal, mid-ventricular, and apical levels, as well as 3 standard apical views (4-chamber, 2-chamber, and long axis), were acquired from 3 consecutive beats (frame rate: 56–92 frames/s). Left ventricular (LV) ejection fraction was determined by manual tracing of end-systolic and -diastolic endocardial borders using the apical 4- and 2-chamber views, and the biplane Simpson’s method from the average of 3 beats.
ElectrocardiographyA 12-lead surface ECG was recorded. The findings were interpreted by an experienced cardiologist and classified as abnormal if any of the following abnormalities, as set out in the JMHW guidelines, were noted: right or left bundle branch block, axis deviation, atrioventricular nodal conduction disease, ventricular tachycardia, premature ventricular contraction (grade >2 in Lown’s classification), pathological Q-wave, or ST-T abnormality.
18F-FDG PET CTAll patients underwent 18F-FDG PET on the same day as the cytometric analysis. PET imaging was performed using a 16-channel GEMINI TF PET system (Philips Electronics Japan, Tokyo, Japan). All patients were instructed to eat a low-carbohydrate meal and to have fasted for at least 18 h before 18F-FDG injection. They were given unfractionated heparin (50 IU/kg) intravenously to reduce physiological 18F-FDG uptake in the myocardium. Prior to 18F-FDG PET imaging, we checked that none of the patients had contraindications to heparin use such as active bleeding, bleeding risk, or a history of heparin-induced thrombocytopenia. Transmission scanning for PET or a low-dose CT scan for PET/CT was performed for attenuation correction. Scans were performed 50 min after administration of 3.7 MBq/kg of 18F-FDG. Acquired images were resliced into a series of short-axis, horizontal long-axis and vertical long-axis images, and glucose metabolic activity in the LV myocardium was evaluated. There were no complications related to the PET procedure, and all patients tolerated the procedure well.
The 18F-FDG PET images were visually evaluated for the presence of FDG uptake in the heart on the basis of agreement of 2 cardiologists (T.N. and I.T.) blinded to the clinical information and treatment assignment of each subject. Reorientation of the axial images to a standard cardiac orientation was undertaken and the standardized uptake value (SUV) was determined. The intensity of myocardial FDG uptake was quantified by measuring the SUV in all 17 segments.22 Analysis of myocardial FDG uptake was based on recognition of the endomyocardial and epimyocardial borders and by subdividing the LV in each segment. We determined the SUV for each of the 17 segments for each subject and then calculated the mean SD of the SUV for each patient. The coefficient of variation (COV) of the SUV was calculated by dividing the SD by the mean SUV and it was used to quantify inflammatory activity.22
Two nuclear radiologists (S.O. and T.T.) blinded to patient data measured the SUV, and the measurements were averaged. The intraobserver and interobserver variabilities of SUV measurements were 5±2% and 7±3%, respectively.
Corticosteroid TherapyThe 10 patients were treated with oral prednisolone 30 mg/day for the first 4 weeks, followed by a decrease to 10 mg/day for the next 8 weeks. Thereafter, the dose was maintained at 10 mg/day. Other medications were unchanged during the course of corticosteroid treatment. Cytometric analysis, measurement of ACE levels, and 18F-FDG PET were repeated at 12 weeks after starting corticosteroids therapy.
Statistical AnalysisAll statistical analyses were performed using SPSS version 11.0.1 (SPSS Inc, Chicago, IL, USA). Continuous variables are expressed as mean±SD for variables with a normal distribution and median [interquartile range] for skewed variables. Categorical variables are presented as number (%) and compared using the chi-squared test. The nonparametric Mann-Whitney U test was used to test for differences between the Rx(+) and Rx(−) groups as well as to evaluate differences between before and 12 weeks after starting corticosteroid therapy. The correlations between the measurements of the CD14++16+ monocytes, ACE, and COV of the SUV by 18F-FDG PET CT were analyzed by simple linear regression. All tests were 2-sided and values of P<0.05 were considered statistically significant.
The clinical baseline characteristics of the CS patients in the Rx(+) group (n=13) and Rx(−) group (n=15) are shown in Table 2. The incidence of ventricular tachycardia was significantly higher and the ACE level was significantly lower in the Rx(+) patients than in the Rx(−) patients. LV ejection fraction in the Rx(+) patients was significantly lower than in the Rx(−) patients. There were no significant differences in age, sex, coronary risk factors, lung and eye involvement, or levels of B-type natriuretic peptide, and high-sensitivity C-reactive protein between the 2 groups. A total of 13 patients underwent cardiac biopsy, of which 5 were positive for CS. Other medication use is shown in Table 2.
| Rx(+) (n=13) |
Rx(−) (n=15) |
P value | |
|---|---|---|---|
| Age, years | 61±9 | 60±9 | 0.778 |
| Female, n (%) | 7 (54) | 13 (87) | 0.096 |
| Coronary risk factors, n (%) | |||
| Hypertension | 2 (15) | 7 (47) | 0.114 |
| Diabetes mellitus | 1 (8) | 1 (7) | 1.000 |
| Dyslipidemia | 4 (31) | 5 (33) | 1.000 |
| Lung involvement, n (%) | 4 (31) | 7 (47) | 0.390 |
| Eye involvement, n (%) | 3 (23) | 1 (7) | 0.311 |
| Cardiac biopsy performed, n (%) | 9 (69) | 4 (27) | 0.024 |
| Positive, n (%) | 4 (31) | 1 (7) | 0.097 |
| Extracardiac biopsy performed, n (%) | 8 (62) | 8 (53) | 0.662 |
| Positive, n (%) | 3 (23) | 5 (33) | 0.549 |
| Advanced AVB, n (%) | 5 (38) | 11 (73) | 0.063 |
| VT, n (%) | 8 (61) | 2 (13) | 0.016 |
| Heart failure, n (%) | 5 (38) | 4 (27) | 0.689 |
| ACE, IU/L | 10.4±4.6 | 18.7±4.6 | 0.001 |
| BNP, pg/ml | 103±92 | 106±103 | 0.945 |
| hs-CRP, mg/dl | 0.11±0.13 | 0.11±0.18 | 0.939 |
| Medication use, n (%) | |||
| ACEI or ARB | 4 (31) | 3 (20) | 0.670 |
| β-receptor blocker | 7 (54) | 3 (20) | 0.114 |
| LVEF, % | 41±12 | 52±13 | 0.024 |
ACE, angiotensin-converting enzyme; ACEI, ACE inhibitor; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; Rx, corticosteroid. Other abbreviations as in Table 1.
Peripheral blood samples were obtained from patients in both groups at enrollment and analyzed for the 3 distinct monocyte subsets (CD14+CD16−, CD14++CD16+, and CD14+−CD16+). The proportion of CD14++CD16+ monocytes in the Rx(+) patients (10.8 [0.2–23.5] %) was significantly lower than in the Rx(−) patients (23.0 [11.5–38.4] %, P=0.001) (Figure 2).

Comparison of the proportion of CD14++16+ monocytes in patients with known cardiac sarcoidosis receiving corticosteroid therapy (Rx(+)) and new-onset cardiac sarcoidosis (Rx(−)). Data are presented as a box and whisker plot with median and 25–75th percentiles (boxes) and 10–90th percentiles (whiskers).
Table 3 shows baseline clinical characteristics of 10 CS patients started on corticosteroids. At 12 weeks after starting corticosteroid therapy, the COV of the SUV had significantly improved from 0.32 [0.14–0.62] to 0.17 [0.04–0.43] (P=0.017) (Figure 3). In line with the decrease in inflammatory activity, the proportion of CD14++16+ monocytes was significantly decreased from 22.2 [8.8–38.4] % to 8.4 [1.8–16.8] % (P=0.001) (Figure 3). Figure 4 shows a representative 18F-FDG PET and the monocyte subsets analysis of a patient before and 12 weeks after starting corticosteroid therapy. The ACE level was also significantly suppressed from 19.7 [13.1–25.5] IU/L to 12.7 [7.0–18.6] IU/L (P=0.002) after corticosteroid therapy (Figure 5). The decrease in the COV of the SUV significantly correlated with the decrease in the proportion of CD14++16+ monocytes (r=0.495, P=0.027) (Figure 3). However, the correlation between the decrease in the COV of the SUV and the decrease in ACE level did not reach statistical significance (r=0.368, P=0.110) (Figure 5).
| Case no. | Age (years) |
Sex | Extracardiac lesions |
ECG findings |
BNP (pg/ml) |
ACE (IU/L) |
hs-CRP (mg/dl) |
FDG PET COV |
LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | F | LN, lung | IRRBB | 147.7 | 25.5 | 0.03 | 0.20 | 50.4 |
| 2 | 56 | M | None | VT, 1 st AVB | 127.9 | 14.7 | 0.02 | 0.28 | 44.3 |
| 3 | 55 | F | Lung | 3rd AVB (ASVP) |
399.0 | 23.8 | 0.02 | 0.34 | 24.3 |
| 4 | 48 | F | LN, lung | 3rd AVB (ASVP) |
192.5 | 18 | 0.04 | 0.42 | 40.0 |
| 5 | 44 | M | None | 3rd AVB (ASVP) |
79.6 | 22.5 | 0.05 | 0.40 | 31.8 |
| 6 | 61 | F | LN, lung | 3rd AVB (ASVP) |
184.3 | 13.1 | 0.05 | 0.14 | 45.4 |
| 7 | 61 | F | LN, lung | 3rd AVB (ASVP) |
99.4 | 23.4 | 0.11 | 0.46 | 62.7 |
| 8 | 51 | F | LN, skin, eye | 3rd AVB (ASVP) |
19.6 | 20.3 | 0.03 | 0.62 | 63.8 |
| 9 | 77 | F | LN, lung | 3rd AVB (ASVP) |
49.5 | 18.9 | 0.01 | 0.24 | 57.0 |
| 10 | 65 | F | LN | 3rd AVB (ASVP) |
101.7 | 16.6 | 0.02 | 0.16 | 35.8 |
ASVP, atrial sense ventricular pace; COV, coefficient of variation; FDG, 18F-fluorodeoxyglucose; IRBBB, incomplete right bundle branch block; LN, lymph node; PET, positron emission tomography. Other abbreviations as in Tables 1,2.

Correlation between the proportion of CD14++16+ monocytes and the coefficient of variation (COV) of the standardized uptake value (SUV) assessed by 18F-FDG PET before and 12 weeks after starting corticosteroid therapy in patients with new-onset cardiac sarcoidosis. The COV of the SUV positively correlated with the proportion of CD14++16+ monocytes (r=0.495, P=0.027).
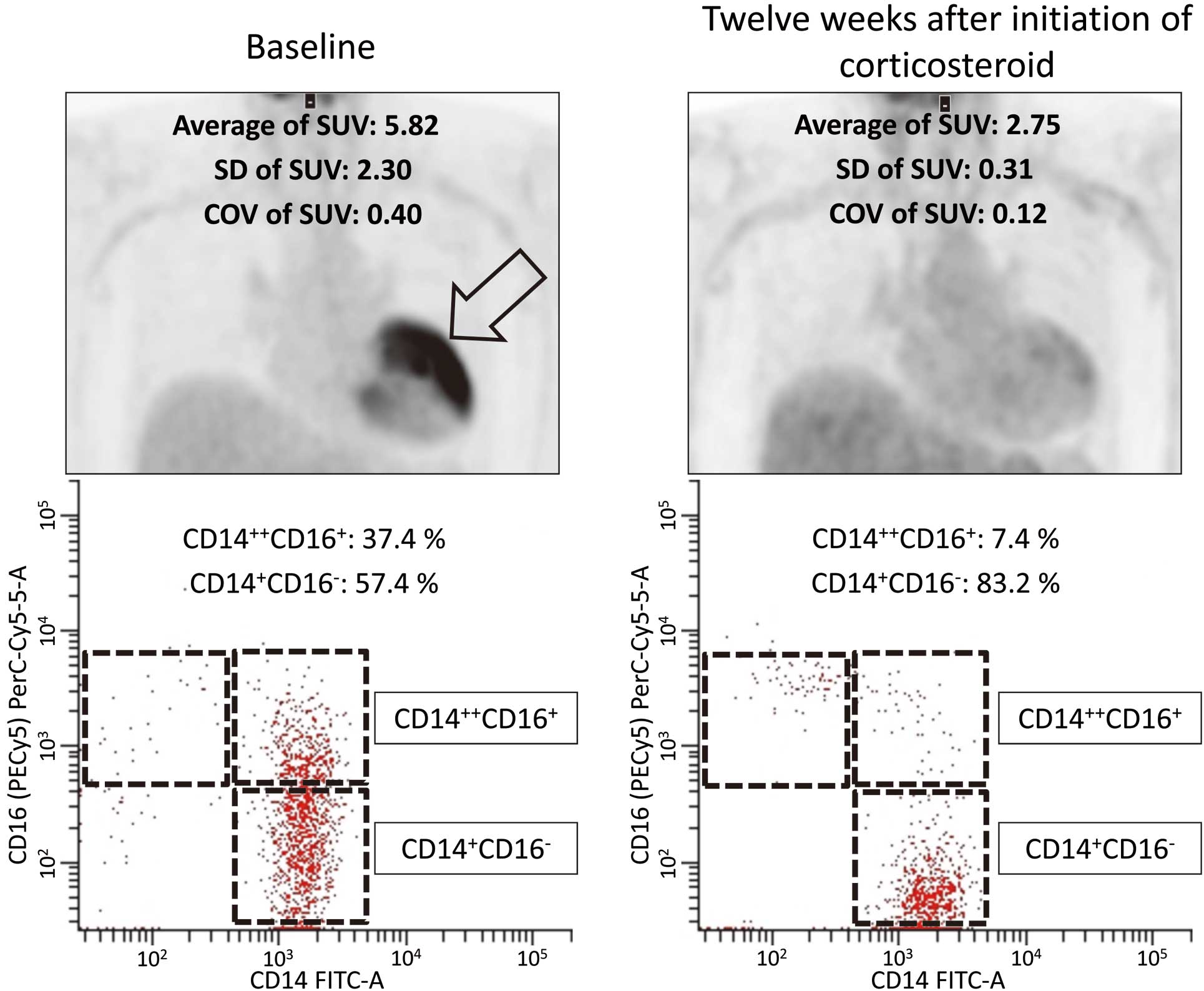
Representative 18F-FDG PET images and fluorescence-activated cell scanner analysis of the 3 monocyte subsets (CD14+CD16–, CD14++CD16+, and CD14+–CD16+) in a patient with new-onset cardiac sarcoidosis. At 12 weeks after starting corticosteroid therapy, 18F-FDG uptake (arrow) has disappeared, accompanied by a decrease in the proportion of CD14++CD16+ monocytes. COV, coefficient of variation; SUV, standardized uptake value.

Correlation between the level of angiotensin-converting enzyme (ACE) and the COV of the SUV assessed by 18F-FDG PET before and 12 weeks after starting corticosteroid therapy in patients with new-onset cardiac sarcoidosis. The ACE level was associated with the COV of the SUV but the correlation did not reach statistical significance (r=0.368, P=0.110). COV, coefficient of variation; SUV, standardized uptake value.
Contrary to expectations, we found no favorable changes in LV systolic function despite corticosteroid treatment for 12 weeks (LV ejection fraction 48±14 vs. 49±12, P=0.852).
In this study, we demonstrated for the first time that the proportion of CD14++CD16+ monocytes was significantly lower in CS patients receiving corticosteroid therapy compared with those without this treatment. More importantly, improvement in inflammation assessed by 18F-FDG PET was significantly associated with the decrease in CD14++16+ monocytes after corticosteroid therapy.
Cardiac PET in CSRecent studies have demonstrated the promising potential of 18F-FDG PET in the diagnosis and assessment of CS. 18F-FDG PET reportedly provides better sensitivity than other radioisotope imaging modalities such as 201Tl, 99mTc, and 67Ga scintigraphy.23–28 18F-FDG PET can also monitor the disease activity of CS quantitatively, demonstrating a reduction in FDG uptake in the heart after corticosteroid therapy.12,22,26–29 However, there are limitations to 18F-FDG PET that should be recognized. Normal myocardial cells use not only free fatty acids but also glucose as their main energy sources and thus, FDG can accumulate in the normal heart.24–27 This physiological FDG uptake by the normal myocardium is problematic because it could lead to blurring of the sarcoid lesions in the heart and/or false-positive results. To correctly detect CS lesions using 18F-FDG PET, a long fasting period (>18 h), a low-carbohydrate diet, and intravenous preadministration of unfractionated heparin have been introduced to reduce the physiological FDG uptake by the normal myocardium.30
CD14++16+ Monocytes in CSCD14+16+ monocytes play a key role in various infectious and inflammatory diseases.31–33 Recent reports of an elevated percentage of CD14+16+ monocytes from pulmonary tuberculosis patients indicate that CD14+16+ monocytes may also be involved in granulomatous disorders.34 We have extended those findings by demonstrating that CD14++16+ monocytes were upregulated in CS patients, followed by a significant decrease after corticosteroid therapy. The migration of mononuclear cells into the myocardial tissue is a key event in inflammatory myocardial disease. Chemokines interacting with their receptors are known to have a crucial role in the recruitment of inflammatory cells in myocardial lesions and are crucial in the initiation, maintenance and resolution of inflammation.35 Several studies have shown that the expression of both fractalkine and monocyte chemoattractant protein (MCP-1) in endomyocardial biopsies was significantly increased in inflammatory myocardial disease.36,37 Receptor-mediated chemokines and MCP-1 secretion were also significantly increased in peripheral blood mononuclear cells.37 Although we have no data on chemokines in cardiac and extracardiac sarcoidosis, the circulating CD14++CD16+ monocyte subsets may reflect the recruitment of inflammatory cells in the myocardial lesions of sarcoidosis.
There are several variables that can be used to monitor the inflammation of sarcoidosis, including serum ACE, lysozyme, and soluble interleukin-2 receptors.38 Although ACE is a clinically useful biochemical maker of systemic sarcoidosis, serum levels soon return to the normal range after corticosteroid therapy, even when disease activity remains high.5 ACE inhibitors are often used to treat heart failure in CS, and therefore serum ACE levels are likely to be influenced by this treatment. In this study, we showed a correlation between the serum level of ACE and the COV of the SUV by 18F-FDG PET; however, the proportion of CD14++16+ monocytes more strongly correlated with the COV of the SUV than with the serum level of ACE.
We also showed that the proportion of CD14++16+ monocytes corresponded well with inflammatory activity in CS. The proportion of CD14++16+ monocytes significantly decreased when inflammatory activity was improved by corticosteroid therapy. In cases 6 and 9, the serum level of ACE was suppressed after corticosteroid therapy, although the proportion of CD14++16+ monocytes and the COV of the SUV did not improve. It is interesting that these patients were readmitted to hospital with congestive heart failure. Taken together, our findings suggest that CD14++16+ monocytes may reflect the inflammatory status of cardiac lesions, which means this monocyte subset can act as a surrogate marker of the therapeutic effect of corticosteroid therapy in patients with CS.
Study LimitationsFirst, the number of cases was small. Because CS is a rare disease, multicenter participation is necessary for a large study. Second, investigation of patients with active sarcoidosis but without cardiac involvement would be needed to clarify the specific pathophysiology in CS. Finally, we did not confirm the immunohistologic findings of the CD14++16+ monocytes using biopsy specimens of the myocardium and extracardiac lesions. It remains unclear whether the elevation in the proportion of circulating CD14++16+ monocytes reflects the extent of monocyte infiltration of the myocardium. Further studies are needed to analyze the chemokines using myocardial biopsies and peripheral blood mononuclear cells in addition to circulating monocyte subsets in CS patients.
We found a significant association between the proportion of CD14++16+ monocytes and inflammatory activity of CS assessed by 18F-FDG PET. In addition, a preferential decrease in peripheral CD14++16+ monocytes was observed after corticosteroid therapy in association with an improvement of the COV of the SUV by 18F-FDG PET. These findings suggest that CD14++16+ monocytes might be a surrogate maker of inflammatory activity in CS.
None.
There are no conflicts of interest in this study.