Article ID: CJ-15-0044
Article ID: CJ-15-0044
Pressure-wire technology, most typically fractional flow reserve (FFR), has provided interventional cardiologists with a means of determining the physiological importance of a stenosis during angiography. There has been renewed interest in coronary physiology in the light of guideline recognition, ongoing clinical research and new technologies changing the paradigm of how assessment is performed in the catheter laboratory. We reflect on FFR, with regards the potential effects of changing hemodynamics on FFR and the latest evidence with regards to outcomes. We also review the instantaneous wave-free ratio (iFR), a new pressure-only index, measured at rest, that is under active evaluation in several international randomized controlled trials. We review the accumulated evidence and discuss the important physiological concepts between pressure and flow that underlie the approach to using resting indices. Finally we investigate future developments, including physiological mapping with iFR-Pullback and the potential to predict the hemodynamic effect of stenting.
Patient outcomes are improved and costs are reduced when coronary physiology is used to guide coronary revascularization.1,2 However, the majority of patients are still managed using the angiographic visual appearance of a stenosis, sometimes without formal ischemia assessment.3 Conceptually, revascularization targeted to ischemia-causing stenoses maximizes the benefit while minimizing risk.4,5 Guidelines6 and appropriateness criteria reflect the added value of invasive physiology,7,8 and together with new technologies there has been renewed interest in coronary physiology.9
In this review, we consider the latest advances in coronary physiology. We reflect upon new data for fractional flow reserve (FFR), as well as new technologies, such as the instantaneous wave-free ratio (iFR), which allows assessment of coronary stenosis severity under rest conditions and permits stenosis mapping with anatomical co-registration.
FFR is the standard for clinical physiological assessment in the catheter laboratory and is supported by sound concepts and randomized data.10 FFR as a concept requires stable and minimal microvascular resistance for pressure measurements to equate to flow. In clinical practice this is most commonly achieved using adenosine; if maximal hyperemia is not achieved, there is potential for the pressure ratio calculated not to be the true FFR. Rigorous attention to detail is necessary in the application of physiology in the catheter laboratory and we reflect upon important areas that affect the interpretation of results.
Physiological Effect of Hemodynamic Changes During Vasodilator UseThe AFFECTS study assessed changes in aortic and coronary pressures in response to central infusion of adenosine, during both peak and stable hyperemia.11 Seven distinct different patterns were noted (Figure 1). Infusion of adenosine triggers pulmonary receptors to give an initial peripheral vasoconstriction and a rise in systemic blood pressure (BP).11,12 Shortly after, adenosine increases large artery compliance, causing a fall in peripheral resistance and aortic reservoir pressure. Systemic BP falls, leading to a fall in proximal pressure (Pa); AFFECTS showed a mean fall of 10.2±10.5 mmHg in Pa.11 This fall in Pa accounted for more than half of the change in distal pressure (Pd) in response to centrally administered adenosine.11 That is, half of the change in Pd, which is perceived to be due to a reduction in microcirculatory resistance, was related to a reduction in the aortic proximal driving pressure. This is intuitive, because the purpose of hyperemia is to create conditions in which pressure and flow are linearly related; any situation in which the pressure falls will cause flow to fall also. Therefore when Pa is reduced, then Pd and the ratio (Pd/Pa), which acts as surrogate of flow, will be reduced by the same proportion.

The 7 different hemodynamic responses to adenosine infusion. Both proximal (Pa) and distal (Pd) pressures change during adenosine infusion – with differential behaviors during peak and stable hyperemia. This will affect the ratios of Pd/Pa calculated at peak and stable hyperemia. Reproduced with permission from Tarkin JM, et al.11
Because FFR can be calculated during either initial peak or stable hyperemia, and because Pa and Pd can differ at these 2 time points according to the 7 possible responses to adenosine, it is possible FFR values will differ at peak and stable hyperemia. For example, for a given Pd, if in peak hyperemia the Pa is high, then a low FFR may be calculated; if Pa then falls during stable hyperemia, a higher FFR will result.
This is observed practically: Echavarria-Pinto et al showed the lowest Pd/Pa ratio during hyperemia occurred during the peak phase rather than stable phase in almost one-fifth cases.13 In nearly 3% of cases this produced FFR values that suggested different revascularization decisions at peak and stable hyperemia.13 This result is important because the validation work and clinical outcome data for FFR were based on values calculated in the stable phase, not the lowest number recorded. These findings may be more frequent in an unselected real-world cohort that did not specifically exclude poor quality traces.
The choice of hyperemic agent will have a differential effect on hemodynamics.14 Adenosine (both intravenous and intracoronary) reduced BP significantly more than intracoronary nicorandil (a fall of 12.9±9.1 mmHg vs. 9.2±6.7 mmHg, P<0.001).14 Regadenson reduced BP even more than adenosine, but the changes in the reported subset were smaller, perhaps reflecting methodological differences (adenosine caused a rise in BP 3.2±12.1 vs. regadenoson −2.7±11.1, P<0.001).14 Although no relationship between the difference in FFR values between the 2 hyperemic agents and the change in hemodynamics was found, this is perhaps because FFR was calculated only during “maximal hyperemia” (likely to be the lowest ratio), with no actual comparison of the peak and stable values. Therefore, the true effect of pressure changes on the ratio remains unexplored in that dataset.
When Pa falls considerably, paradoxical vasoconstriction of the microcirculatory bed can occur to preserve tissue perfusion. This is observed as a paradoxical rise in Pd during intravenous adenosine infusion,15,16 particularly if stenoses are severe or infusion time is long. Although 1 min is typical, many centers and studies use 4 min as originally validated (eg, FUTURE (NCT01881555)17).
These findings are consistent with previous theoretical models that suggested FFR is affected by changes in hemodynamics.18 Indeed, the original animal data showing the pressure independence of FFR has been reviewed using a Bland-Altman analysis,19 demonstrating there can be a difference between pressure-based FFR and true flow-based FFR (Figure 2).
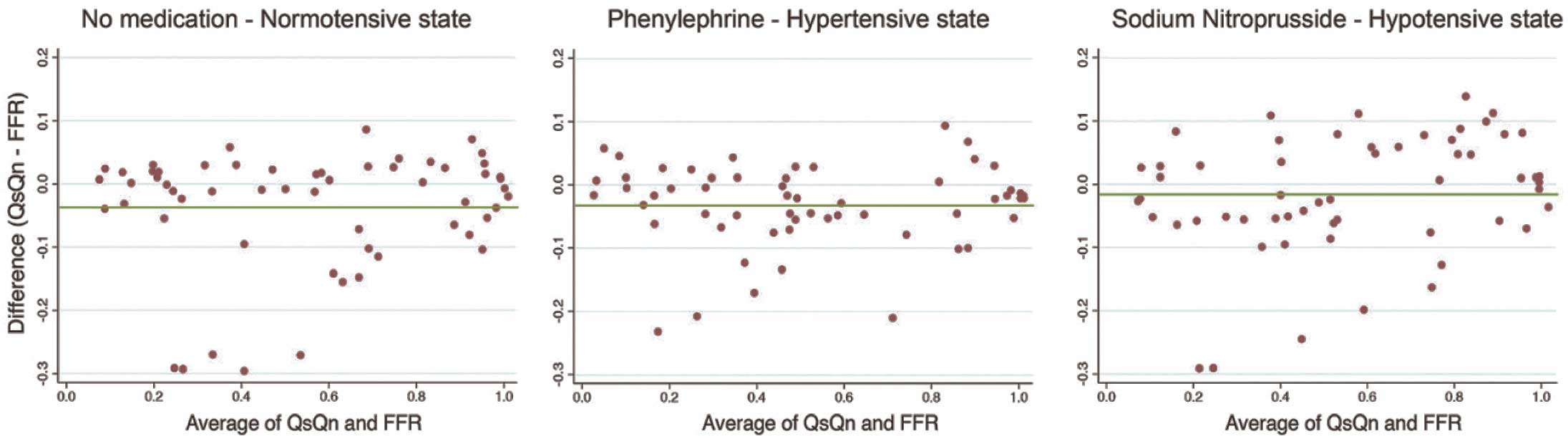
Bland-Altman graphs of Qs/Qn with fractional flow reserve (FFR). The original validation work for FFR measured a true FFR by directly measuring flow velocity in the presence and absence of a stenosis (Qs/Qn). This was compared with FFR, and when plotted with different hemodynamic states had high correlation and R2 values. However, re-plotting the data of the 5 dogs presented in the published paper as a Bland-Altman plot shows that the Qs/Qn values are less tightly associated as may be suggested by correlations.
Additional doses of hyperemic agent are often given when FFR values are close to the threshold, ostensibly to ensure resistance is at its lowest and ensure lower FFR values cannot be achieved.20 However, higher intravenous doses have been shown to adversely affect hemodynamics21 and higher doses have no validation in clinical outcome studies. The current thresholds for treatment (whether 0.80 or 0.75) were derived at much lower doses of adenosine (IC 20–40 μg bolus, IV 140 μg·kg–1·min–1),22 and higher doses may require different thresholds. As such, keeping to recommended doses helps standardize assessment and allows interpretation with the clinical outcome studies.
The route of hyperemic agent administration varies among centers. Although the landmark trials used intravenous infusion, many prefer intracoronary adenosine boluses.23 The reasons include avoiding the need for additional femoral punctures or infusion pumps, increased cost efficiencies and lower incidence of side-effects.24 Intracoronary dosing may avoid hemodynamic confounding because central aortic pressure is less likely to change,21 even when intracoronary infusions are used.25 Although classification agreement is good across a wide spectrum of stenoses,24 in more clinical populations the classification match between intracoronary and intravenous dosing can fall to 78%, near the 0.80 threshold.14 Therefore, revascularization decisions may be affected by using an intracoronary approach in truly intermediate stenoses.
Physiology may have specific traction during diagnostic angiography to guide revascularization decisions earlier in the patient’s clinical pathway. This patient cohort differs from those in whom revascularization by percutaneous coronary intervention (PCI) was already selected, as seen in the DEFER and FAME studies. Two important studies add significantly to our understanding of how such changes will alter future paradigms of the management of coronary disease.
In the RIPCORD study,26 200 patients undergoing coronary angiography had a management plan made based only on the angiographic findings. FFR was then measured in all vessels with ≥2.25 mm diameter and used to guide a second management plan. One-quarter of the patients had a change in management after measurement of FFR; 9 of 72 patients (12.5%) in whom medical therapy was chosen on the basis of angiography were recommended for revascularization when the FFR was known. Similarly, one-quarter of patients first recommended for revascularization required medical therapy once the FFR was known.2 In total, one-third of vessels were considered significantly changed after FFR assessment.
The R3F study27 was a larger study with a similar design, enrolling over 1,075 consecutive patients undergoing coronary angiography in 20 French centers. Unlike RIPCORD, patients were only included if at least 1 ambiguous lesion was present, and FFR was performed only in the vessel of interest. Although the overall proportion of medical therapy, PCI and coronary artery bypass grafting appeared similar, with only a modest but statistically significant change, individual patients had a marked change in plan, with 464 (43%) patients having a different management plan made after FFR disclosure. Importantly, from a safety perspective, the event rate after 1 year of follow-up was identical in the cohort of patients who were reclassified by FFR and those in whom angiographic-guided plans were concordant with the FFR findings (11.2% vs. 11.9% major adverse cardiac event (MACE) rate, P=0.78).
Taken together, RIPCORD and R3F strongly suggest that routine coronary physiology should be considered in all patients referred for coronary angiography.
The key question for the open-label FAME-2 study28,29 was whether patients with ischemia defined by FFR have improved outcomes if treated by PCI compared with optimal medical therapy alone. The study group was 1,600 stable patients scheduled for FFR assessment of any angiographically visible stenoses (>50%). Those with FFR ≤0.80 were randomized to FFR-guided PCI with optimal medical therapy or medical therapy alone, while those with FFR >0.80 were treated medically and followed in a registry. The study was stopped early, after 888 patients had been randomized, because of increased events in the medical therapy arm. The primary endpoint, a composite of death, nonfatal myocardial infarction (MI) or urgent revascularization within 2 years was significantly lower in those undergoing PCI rather than medical therapy (8.1% vs. 19.5%, hazard ratio [HR] 0.39 [0.26–0.56], P<0.001).29 Events were driven by urgent revascularization, with 18 patients (4%) in the PCI arm vs. 72 (16.3%) in the medical arm needing PCI (HR 0.23, [0.14–0.38], P<0.001) with no difference in mortality (HR 0.74, [0.26–2.14], P=0.58) or MI (HR 0.85, [0.50–1.45], P=0.56).29 Those patients who had been deferred with an FFR >0.80 had a low primary endpoint of 3% at 7 months28 and 9% (15 patients) at 2 years.29 There was little difference between the deferred population (FFR >0.80) and those with FFR ≤0.80 who had undergone PCI (HR 0.90, [0.49–1.64], P=0.72).
FAME-2 provides excellent contemporary data and learning points for future trials. It can be seen that blinding patients and operators to the physiological values will have great importance. RIPCORD and R3F demonstrate that physician behavior and management plans are altered when the FFR value is known. Knowledge of the unblinded FFR value may have altered management of patients in FAME-2 and accounted for some of the early revascularization decisions. Furthermore, it is unclear how patients react to the knowledge that they have a stenosis known to be causing ischemia. In those with FFR ≤0.80 who had medical therapy, a dramatic cumulative increase in class IV angina (pain at rest) was observed. The increase was most marked in those reporting symptoms and less so in those with objective markers of instability such as ECG changes or troponin rise. This suggests knowledge of stenosis significance may have influenced patients’ perception of symptoms. Trials such as the DEFINE-FLAIR study (NCT 02053038),30 which is enrolling 2,500 patients into an outcomes-based iFR-FFR comparison, will have patients and those conducting follow-up blinded to the physiological arm or values to minimize such bias.
If revascularization is performed for ischemia, a continuous benefit should be expected related to the severity of ischemia, and FAME-2 demonstrates that the FFR value is related to the number of events. First, decisions were made using the 0.80 threshold; although not specifically validated during the original FFR studies, there is data to suggest there are increased events when stenoses between 0.75–0.80 are deferred,31,32 suggesting that the higher 0.80 cut-point is prudent. An FFR of 0.65 had significant interaction with the primary endpoint at 7 months (when FFR ≤0.65, events in the PCI arm were 7 vs. 35, HR 0.08 [0.03–0.26], when FFR >0.65, events were 12 vs. 21, HR 0.46 [0.21–1.01], P=0.01 for interaction).28 At 2 years, however, the interaction only showed a trend towards significance (P=0.07).29 This suggests that the majority of benefit for revascularization is when stenoses are truly ischemic (FFR ≤0.65), and this value is similar to the mean FFR in FAME-1 in stenoses undergoing PCI (0.60±0.14).1
Further supporting data come from a comprehensive meta-analysis of study (9,173 lesions) and patient-level (6,961 lesions) data from FFR studies reporting events.33 Clinical events increase as the FFR decreases and revascularization demonstrates the greatest benefit the lower the FFR value. Using patient-level data, the optimal FFR threshold to predict benefit from revascularization over medical therapy for a composite of death, MI and revascularization was found to be 0.67.33
Taken together, the real value of the FFR is in the assessment of the severity of all stenoses rather than just the intermediate stenoses. Because anatomical parameters poorly predict the FFR value, limiting FFR to only “intermediate” stenoses will poorly identify potential ischemia; rather, all stenoses should be assessed to maximize gain.
There are limited data for the value of FFR in the ACS setting.34 Value may be added if nonculprit stenoses can be assessed at the time of catheterization to avoid later investigation. Concerns have regarded a possible sub-hyperemic response and underestimation of severity because of microvascular dysfunction leading to a sub-hyperemic response to adenosine, resulting in underestimation of stenosis severity. Repeating FFR measurement 6 weeks after MI (both ST-segment elevation MI [STEMI] and non-STEMI) shows little change in the mean FFR value for the study group, though there can be significant change on a per-patient basis.35 Certainly in STEMI, microvascular responsiveness changes over time, showing significant worsening of FFR in nonculprit vessels when repeated measurements are made 1 day after STEMI and more so at 6 months.36
The FAMOUS-NSTEMI study37 was designed, but not powered, to assess the differences in health outcomes when both culprit and nonculprit vessels undergo FFR-guided treatment vs. angiographic-guidance during NSTEMI. The study group was 350 NSTEMI patients with 706 stenoses (≥30% severity) amenable to revascularization who had 704 FFR measurements and were randomized to FFR-guided or angiographic-guided PCI (FFR was not disclosed); 430 (61.1%) stenoses had FFR ≤0.80 and 80% of each arm had at least 1 physiologically significant vessel. In the FFR-guided arm, 22.7% patients were treated by medical therapy compared with 13.2% in the angiographic arm (P=0.02). Despite this, the number and length of stents was similar in both groups, and there was no difference in the in-hospital costs.
Prospective follow-up noted no difference in clinical events at 1 year,37 with Kaplan-Meier plots showing convergence of a combination of death, MI or heart failure admission. Because both arms had similar stent rates, stent-related complications could be responsible or alternatively, patients with NSTEMI deferred for stenting may have had a catch-up of events (ie, they were under-treated initially). Breakdown of events according to culprit and nonculprit vessel would help interpretation. Larger studies with greater delineation of the drivers of events are required, but FAMOUS clearly demonstrates it is technically feasible to perform pressure-wire assessments during cardiac catheterization for NSTEMI patients.
Coronary stenosis assessment at rest has been available since the dawn of coronary intervention,38 but has been limited by bulky low-fidelity equipment. Exogenous hyperemic agents had to be used to aid discrimination between stenoses by increasing flow across them. However, exogenous induction of hyperemia does not increase flow uniformly across all severities of stenosis. Positron emission tomography (PET)-derived quantification of blood flow shows that adenosine increases blood flow most in unobstructed vessels or those with <50% diameter stenosis.39 In those with >50% stenosis, namely those considered for suitable for revascularization, there was no significant increase in flow.39 Resting blood flow was preserved across all severities of stenosis, and this likely occurs by compensatory microcirculatory vasodilatation in response to the stenosis at the expense of Pd, which falls even at rest (Figure 3). Because pressure falls at rest with stenosis severity, then a resting index should be sufficient to quantify severity, provided there is sufficient flow velocity to distinguish between stenoses.

Schematic representation of the behavior of resting coronary physiology in the face of increasing stenosis severity. Resting flow-velocity is preserved across all severities of stenosis until near occlusion. To maintain flow, the microcirculation dilates at rest at the expense of the distal coronary pressure. Measuring pressure alone at rest gives an indication of how important the stenosis is to the given coronary bed – if there is no transtenotic gradient, then there has been no microcirculatory compensation in response to the stenosis and the stenosis is less likely to be truly flow-limiting.
iFR is a resting index of stenosis severity that provides a physiological quantification of the effect of a stenosis on the coronary circulation. iFR is measured during a specific period of diastole known as the wave-free period, when flow is intrinsically at its highest compared with the whole cycle. By measuring during a higher flow velocity, the capacity to discriminate between stenosis severities at rest is amplified and maximal compared with any other phase of the cardiac cycle.
The wave-free period was originally isolated by application of wave-intensity analysis. Wave intensity demonstrates that the forces propagating from the proximal vessel (the aorta) conflict with those travelling from the distal end (the microcirculation) (Figure 4).40,41 Competing interaction between the waves occurs throughout systole and the largest microcirculatory wave occurs at the beginning of diastole to explain why coronary flow is predominantly diastolic. During diastole the waves are quiescent,42 and during this wave-free period, microcirculatory resistance is at its lowest and most stable compared with the rest of the cardiac cycle. During this time, pressure and flow velocity are linearly related (Figure 4) and pressure ratios can assess the flow limitation imposed by a stenosis.42 The wave-free period exists as a proportion of diastole changing with alterations of the R-R interval. This means that iFR can be calculated during irregular rhythms (eg, atrial fibrillation) and can be calculated for heart rates normally acceptable for physiological assessment (30–130 beats/min).
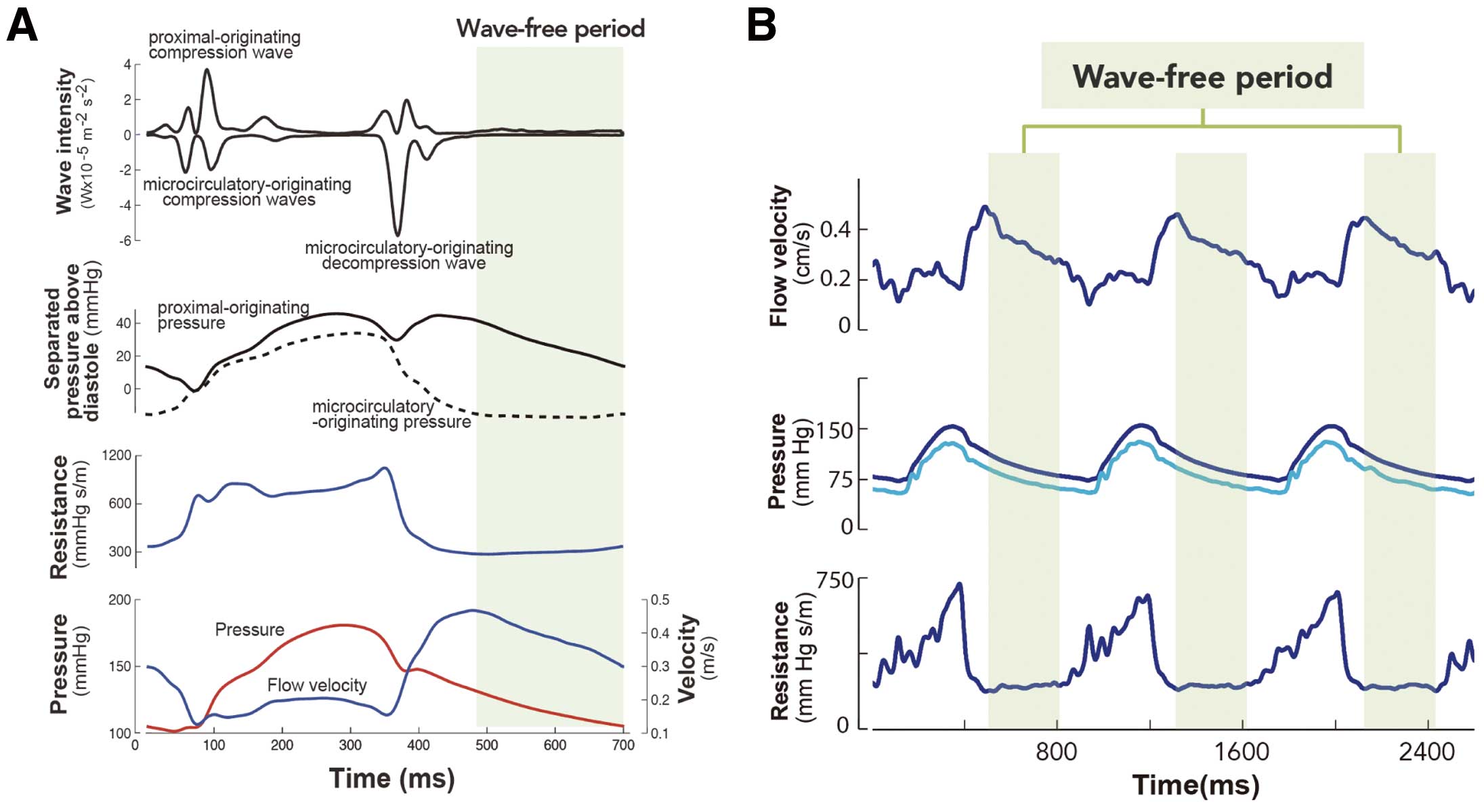
(A) Separated wave intensity plot shows how multiple different waves propagating from the proximal and distal ends of the vessel influence coronary flow. The waves are quiescent during the wave-free period in diastole. Instantaneous resistance plotted throughout the cardiac cycle demonstrates that resistance is lowest and most stable during the wave-free period. Coronary pressure and flow are linearly related during the wave-free period. (B) Flow velocity, proximal and distal pressure traces and instantaneous resistance demonstrate the stability of the wave-free period beat to beat. Flow velocity over the wave-free period is higher than that over the whole cycle, giving greater discrimination between stenosis severities than the whole cycle at rest. Adapted with permission from Sen S, et al.42 Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis.
The ADVISE study42 was the first-in-human assessment of iFR. After describing the wave-free period using combined pressure and flow velocity measurements, computer algorithms were developed to reliably identify the wave-free period using pressure-only technology. The pressure-only iFR algorithm was compared with FFR in 157 stenoses with a good correlation (0.90) and receiver-operating characteristic (ROC) area under the curve (AUC) of 93%.
The ADVISE family of studies demonstrated that in 15–20% cases the iFR, a resting index, was numerically lower than a hyperemic index.42,43 This observation may have been under-reported in clinical practice because many would avoid hyperemia when the resting gradient was already significant. Similar findings have been observed by Seto et al,44 and are explained by the AFFECTS study.11 This phenomenon is more frequent in severe stenoses in which vasodilator reserve is depleted and the trans-stenotic flow is dependent on the proximal driving pressure. Even small hemodynamic perturbations can trigger compensatory microcirculatory vasoconstriction in an effort to preserve perfusion. This is observed as an apparently rising Pd or FFR ratio with continued infusion.
In a small number of cases, a stenosis may have trivial resting gradient but a large hyperemic gradient. Without measurement of flow, this would suggest the resting gradient was “incorrect”. However, coronary flow reserve (CFR) flow studies, whether by PET or invasive measurement, confirm that these events are consistent with a considerable increase in flow across a mild stenosis that gives a false-positive hyperemic pressure gradient (ie, not truly a flow-limiting stenosis). When patients with stenoses such as this are deferred, prognosis appears to be excellent. These concepts are revisited later.
iFR Assessed in Clinical PopulationsThe ADVISE-Registry compared iFR and FFR in a routine clinical population and found a classification match of 80%,45 which is similar to the classification match between repeated measures of FFR in the DEFER study (85% match).45 Therefore, the capacity of iFR (or any other index trying to match FFR) to agree with FFR will be constrained by the intrinsic variability of FFR and its capacity to agree with itself. Adjusting for this, taking maximal agreement as 85% (80/85) gives an agreement of 94%.
DEFER suggested FFR classification could change in 15% of cases when measurements were repeated 10 min apart.46 Lesser change is noted if measurements are 2 min apart;10 greater change when 6 weeks apart.35 Values closest to the cut-point have the greatest propensity to change; datasets made up of data-points away from the cut-point will have less change. Datasets in which values around the cut-point appear to be absent have the least change. Because the ADVISE-Registry included unselected consecutive cases performed as part of routine care, the lesion distribution was unimodal with a mean FFR of 0.81±0.09. With the majority of data-points close to the cut-point, this maximized the chance that small changes in either iFR or FFR would give classification mismatch. Previous FFR validation studies differed: they demonstrated bimodal distributions with a paucity of values at the cut-point.45 This would aid validation against other parameters but not be representative of performance in clinical populations and lead to inadvertent bias.
An independent study from South Korea demonstrated similarity to the ADVISE-Registry.47 In this rigorously collected dataset, resting indices iFR and the whole-cycle resting Pd/Pa had excellent diagnostic accuracy compared with FFR, with iFR demonstrating greater discriminatory power than Pd/Pa. Pd/Pa values are typically clustered around a narrow range, where even a small artifact or drift will affect the diagnostic efficiency. iFR values for the same dataset were more widely spread, with a dispersion range similar to the FFR, meaning that iFR provided greater diagnostic stability over a wider range (Figure 5).

(Left) Sensitivity of the instantaneous wave-free ratio (iFR) and Pd/Pa to detect a stenosis considered significant by fractional flow reserve (FFR). iFR has greater sensitivity over Pd/Pa even in a clinical distribution. (Right) Schematic representation of the spread of values provided by Pd/Pa, iFR and FFR. The greater spread of iFR and FFR afford it greater sensitivity to detect significant stenoses, whereas because all Pd/Pa values are constrained within a very narrow range, the effect of technical variability such as pressure-wire drift or noise on diagnostic accuracy is much higher. Pa, proximal pressure; Pd, distal pressure. Left panel reproduced with permission from Park JJ, et al.47
Given the limitations of iFR-FFR comparisons, where the upper limit of agreement will always be determined by the accuracy of the reference parameter, it is reasonable compare iFR and FFR to third-party parameters of ischemia. The CLARIFY study compared iFR and FFR to the hyperemic stenosis resistance (HSR) index.48 The HSR is a combined pressure and flow-velocity index that essentially calculates the gradient of the pressure-flow curve,48 as originally described by Gould.49 Because it indexes transtenotic gradients by flow velocity, it corrects for false-positive pressure gradients; that is, situations where high flow velocities under hyperemic conditions generate apparently important gradients by turbulence alone without any true flow limitation. In CLARIFY, both iFR and FFR had equal diagnostic efficiency to match an ischemic classification with HSR (both 92%, with no significant difference between the 2 tests).50
The CLARIFY study further addressed key questions brought by the cardiology community. One was whether iFR would be lower in the presence of adenosine, and whether the administration of adenosine over the wave-free period with further improve diagnostic categorization. CLARIFY confirmed that iFR could be numerically lower when measured during adenosine-mediated hyperemia (referred to as iFRa) often far lower than the FFR.50 During iFRa, microvascular resistance was consistently lower than was achievable by adenosine alone over the whole cardiac cycle; that is, resistance was lower than that measured during FFR.50 Therefore, for those who wish to always make measurements at the lowest resistance possible, iFRa is the ideal parameter. However, despite the marked additional reduction in resistance and lower values, iFRa was not diagnostically superior to iFR. There was no statistical differences among iFRa, FFR and iFR in diagnosing ischemia when compared to the third-party hyperemic ischemia parameter, HSR.48 The optimal threshold for ischemia for iFRa was 0.66, corresponding to its much lower values. The implication is that it is certainly possible to elicit lower numbers by combining hyperemia with the wave-free period, but there is no diagnostic advantage in doing so.
CLARIFY confirmed the findings of PET studies showing that adenosine increased flow most in mild stenoses: in CLARIFY, invasive assessment of microvascular resistance showed that mild stenoses had the greatest response to adenosine, with limited effect in significant stenoses.50 In contrast, the wave-free period had a more consistent reduction in microvascular resistance across all severities. In stenoses classed as ischemic by HSR, there was no difference in the resistance reduction offered by adenosine and the wave-free period.50 In nonsignificant stenoses, adenosine hyperemia produced a greater reduction in resistance than offered by the wave-free period, but without any additional diagnostic advantage, as the classification match was identical between parameters.50 This suggests that the wave-free period provides sufficient information for diagnostic classification of stenosis severity and that the addition of hyperemia adds little.
iFR Comparative Studies With Markers of IschemiaDuring the validation of FFR, it was evaluated against noninvasive parameters of ischemia such as exercise testing51 and PET. In many cases, these noninvasive parameters were themselves validated by their capacity to detect anatomical coronary disease. To avoid circular arguments, third-party assessments provide an alternative arbiter of ischemia. iFR has now been compared with other parameters of ischemia in a number of studies. Each demonstrates that a resting index has the capacity to provide as much information as a hyperemic index, even when compared with a reference standard that requires hyperemic stress.
The CLARIFY study has already been discussed, and demonstrated equivalent capacity of iFR and FFR to assess ischemia classified by HSR.50 A second, larger study similarly assessed iFR and FFR against HSR in 120 stenoses. In that study, iFR was found to have a significant higher classification match than FFR (89% vs. 82%, P<0.01).52
A third study assessed iFR, FFR and the basal stenosis resistance (BSR) index53 against a comprehensive combined ischemic reference of myocardial perfusion scintigraphy (MPS) and HSR.54 Stenoses were considered ischemic if both the MPS and HSR were positive, which is pertinent because it confirms both a perfusion maldistribution (MPS) as well as an epicardial stenosis (HSR) as the source of the perfusion defect. No significant difference was found between each index,54 and the results were consistent with other nonselective cohorts using MPS.55
A fourth study compared iFR and FFR against PET, which is recognized as the gold standard for quantifying myocardial blood flow (MBF).56 De Waard et al performed ([15O]H2O) PET imaging in 34 patients with 49 intermediate coronary stenoses followed by invasive pressure-wire assessment. Both iFR and FFR had a 76% classification agreement with PET, and both had similar AUC for ROC analysis (0.85 for FFR and 0.86 for iFR, P=0.71).56 Both iFR and FFR had an identical pattern of agreement and disagreement with PET MBF. This is a remarkable finding and akin to the finding that FFR was related to MBF on PET imaging in 22 patients, which validated FFR as a test for ischemia.57
Finally, iFR and FFR have been compared with invasive CFR.58 Calculated using a Doppler flow-velocity wire and presented as a ratio of hyperemic flow-velocity to resting flow-velocity, CFR is a strongly prognostic index.59 CFR >2 is non-flow-limiting whereas less than this suggests a significant stenosis. When iFR, FFR and CFR were measured in 216 stenoses, iFR had closer agreement with CFR than does FFR, with a statistically significant higher AUROC (iFR 0.82 vs. FFR 0.72, P<0.001).58 Even when constrained to the physiological range of 0.60–0.90, iFR maintained a stronger association with CFR than FFR (AUROC 0.78 vs. 0.59, P<0.001).58 This suggests iFR has a closer association with both hyperemic flow velocity and CFR than FFR. This is understood when it is appreciated that significant FFR values can occur in the presence of high flow velocity across a mild but not flow-limiting stenosis (Figure 6). This mechanism may account for discrepancies between iFR and FFR. In the JUSTIFY-CFR cohort, when iFR was negative and FFR was positive, CFR >2 was noted in 97% of casea (Figure 6). These findings suggest that when iFR and FFR appear to disagree, iFR provides a greater degree of information about the state of coronary flow than does FFR.
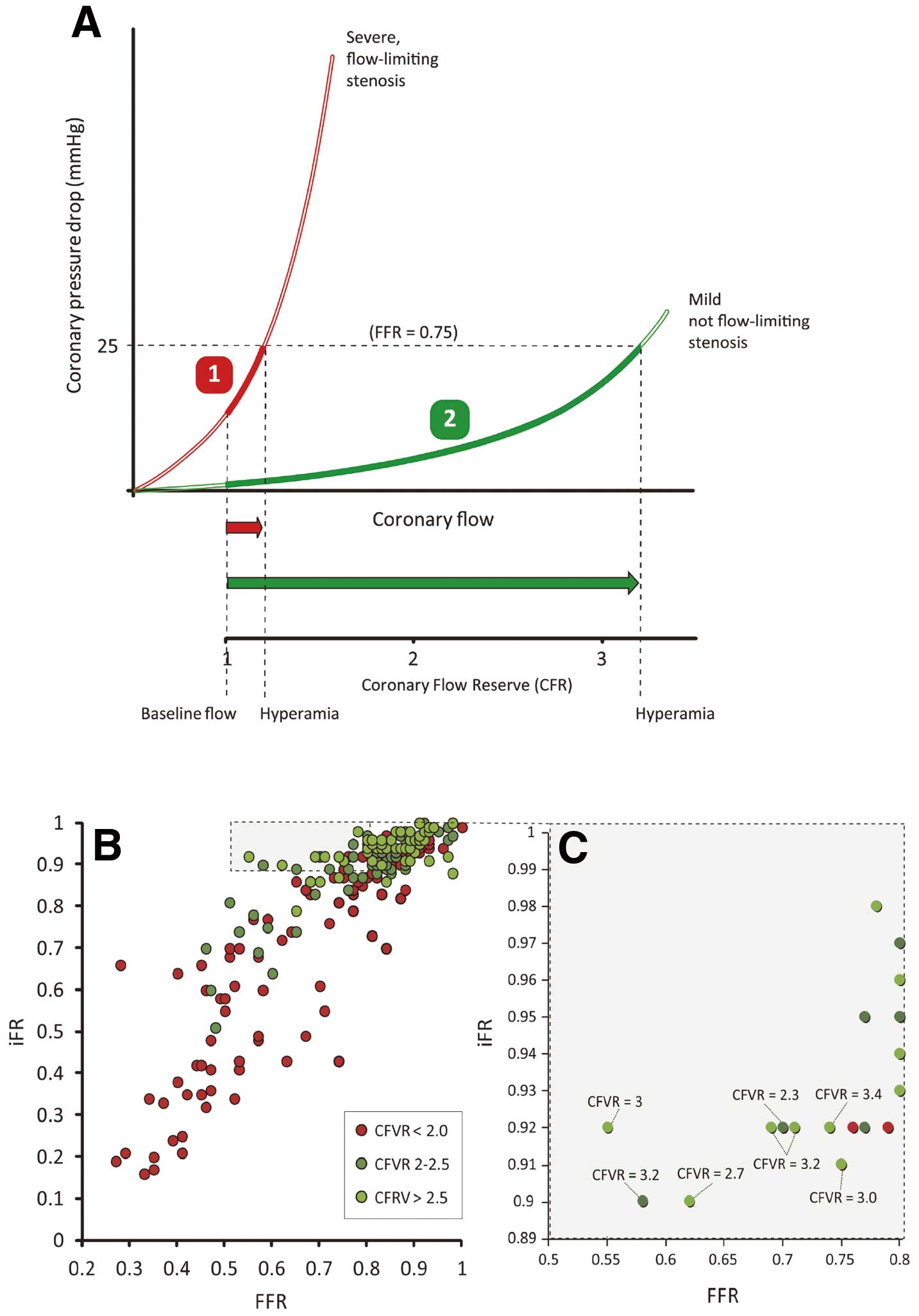
(A) Two stenoses, as described by 2 different pressure-flow velocity curves, can both generate a trans-stenotic gradient of 25 mmHg, equivalent to fractional flow reserve (FFR) value of 0.75. In stenosis (1), the stenosis is truly physiologically significant with a steep curve, such that even a small escalation of hyperemic flow can generate a significant gradient. In stenosis (2), the curve is more shallow, whereby a large increase in flow velocity, equivalent to a coronary flow reserve (CFR) >3, can generate an FFR of 0.75, despite being, by definition, not flow-limiting. (B,C) The relationship between the instantaneous wave-free ratio (iFR) and FFR with color-coding by CFR. In the quadrant where iFR is negative but FFR is positive, the CFR suggests non-flow limiting lesions. Adapted with permission from Petraco R, et al.58
These findings support the concern many physiologists have expressed for some time: that FFR has only modest agreement with CFR.60 Johnson et al studied 438 stenoses and found FFR and CFR matched only in 60%.60 There are 2 areas of discrepancy to consider: when FFR is positive (≤0.80) but the CFR is normal (>2.0), and second, when FFR is negative (>0.80) but the CFR is abnormal (<2.0). The first situation is vexing, as the pressure-based index suggests revascularization while the flow-based index shows the flow can increase sufficiently without intervention.
One possible explanation of the discrepancies between FFR and CFR has been inadequate hyperemia. Because both indices require maximal hyperemia, in the same manner that FFR is affected by inadequate hyperemia, CFR readings may underestimate true flow values. This has been suggested because many studies have used lower doses of intracoronary adenosine than are currently used in practice. However, giving further vasodilator to increase hyperemic flow would mean both indices move even further in opposite directions: FFR would become more positive (FFR <0.80) and CFR would be become more negative (CFR >2.0). Therefore, the discrepancy cannot be resolved by higher doses. Furthermore, higher doses could mean assessments that are congruent could become incongruent: an FFR value close to 0.80 with a CFR >2.0 with higher doses could have an FFR that dips below 0.80 while CFR will only rise.
Although FFR has outcome data, such discrepancies are under-described in FFR trials. Van de Hoef et al reported 10-year follow-up data of patients with discrepant pressure and flow measurements who were deferred for PCI. In those with a positive FFR (≤0.80) but normal CFR (>2.0), MACE rates were no different from those in patients with a negative FFR (>0.80) and a normal CFR (>2.0).61 That is, managing these patients according to their normal flow value achieved the same result regardless of the FFR.61 Those with a negative FFR (>0.80) but abnormal CFR (<2.0) had very high MACE rates, suggesting this pool of patients are not getting appropriate revascularization. This finding may account for the highly variable event rate (2.5–11% at 1 year) reported after deferring stenoses with FFR >0.80.62
By combining iFR and FFR it is possible to more closely attain an estimate of hyperemic flow. If the iFR is negative and FFR is positive, the data suggest these stenoses are likely to represent those with high probability (97%) of CFR >2.0 for which clinical outcomes are identical between PCI and deferral.58,61 Whereas if the iFR is positive and the FFR negative, this suggests a low CFR.58,61 In such cases it may be prudent to revascularize if anatomically feasible; further studies assessing the value of this are underway.
Clinical Application of iFR: Using the iFR-FFR Hybrid ApproachWhile awaiting outcome data, a prudent application of iFR in the clinical setting is to apply the hybrid iFR-FFR strategy.63 The aim is to achieve a high diagnostic agreement with FFR that has outcome data, while reducing the need to administer adenosine. iFR is measured in all patients; if the value found is within a narrow range then adenosine is administered to calculate FFR. Many different hybrid iFR-FFR approaches are possible according to the classification match sought: a match of 95% requires adenosine if iFR values are between 0.86 and 0.93, sparing almost 60–70% of patients from adenosine administration.63–65
External validity of the iFR-FFR hybrid approach has been provided by ADVISE-II and the ADVISE-In-Practice studies. ADVISE-II was a prospective global double-blind multicenter study of 690 truly intermediate stenoses (mean FFR 0.83±0.11).64,65 In nearly 70% of cases, adenosine administration could be avoided by using the hybrid approach while maintaining 94% overall classification match with an FFR-only approach. ADVISE-In-Practice was the first study to use the commercially available iFR console.66 In this international, multicenter study of 392 stenoses in 313 patients, iFR had a high classification match with FFR (80% using the FFR 0.80 cut-point; 88% with ischemic threshold of 0.75 and 92% if the FFR gray-zone was accounted for).66 Using the hybrid approach, 61% of the patients could be spared from adenosine, with a 94% classification match with FFR.66
The hybrid approach has been adopted in routine clinical practice and is being used in SYNTAX-II (NCT 02015832)67 in which 3-vessel disease on angiography will undergo revascularization according to the physiological findings on iFR-FFR assessment. Because physiological assessment can convert angiographic 3-vessel disease to lesser severities,26,68 SYNTAX-II may show that rapid assessment with iFR-FFR can assist in focusing revascularization to functionally important stenoses in patients otherwise treated surgically.
Clinicians experienced in using iFR are increasingly using it alone as a single physiological parameter, with a single threshold of significance (an iFR <0.90 is treated with revascularization, wheareas iFR ≥0.90 is deferred). These thresholds were determined against an FFR threshold of 0.80 in the large and independent RESOLVE study, which combined many different datasets.69 The validity and safety of such an approach is being assessed in 2 international studies: DEFINE-FLAIR30 and the iFR-SWEDEHEART.70
Ongoing Outcome Studies Using iFRClinical outcomes studies are underway to determine the safety of using an iFR-only approach to guide revascularization.
DEFINE-FLAIR is a multicenter international, double-blind outcome study that will be the largest physiology study performed to date (Figure 7): 2,500 patients with intermediate stenoses will be randomized to either an iFR-guided approach (treatment threshold iFR <0.90) or an FFR-guided approach (treatment threshold FFR ≤0.80). Both stable patients and those with ACS (nonculprit vessels) are included. The study is powered to test MACE at 1 year for noninferiority between iFR and FFR. FLAIR is unique because not only will the patients be blinded to the physiological parameter used, but those performing patient follow-up will also be blinded. Other endpoints include the cost efficiencies between iFR and FFR.
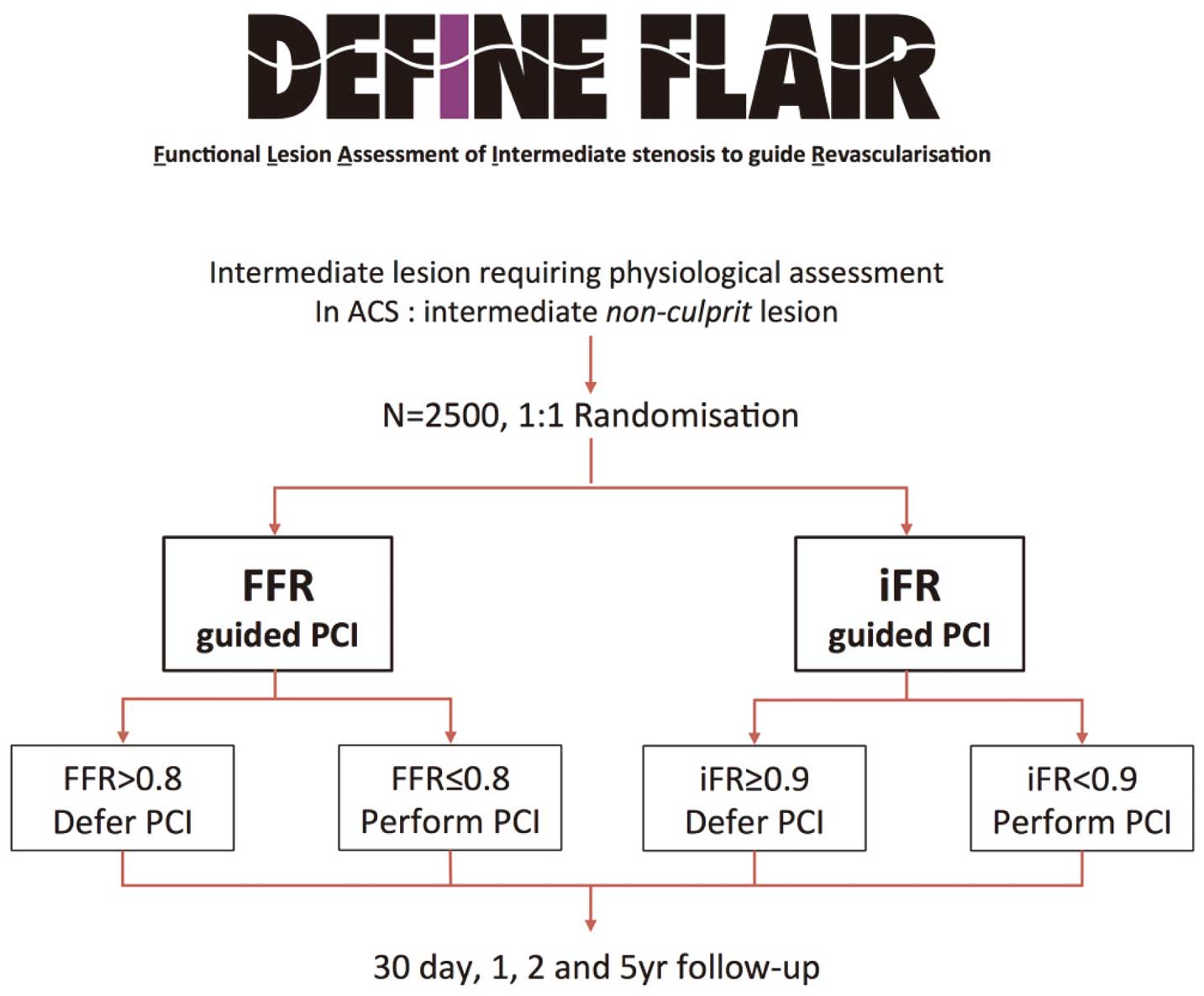
DEFINE-FLAIR study protocol. ACS, acute coronary syndrome; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; PCI, percutaneous coronary intervention. Reproduced with permission from Petraco R, et al.66
The iFR-SWEDEHEART (NCT 02166736) study is simultaneously being performed in Sweden as part of the pioneering Swedeheart registry. In this study, 2000 patients will be randomized to either iFR or FFR and have follow-up within the registry system. Because the clinical outcomes and study design have been harmonized with those of DEFINE-FLAIR, the combined studies will provide data on 4,500 patients and approximately 7,500 coronary stenoses.
iFR in ACSAt present, iFR has very limited data in the setting of ACS. The FORECAST study is an independent study using offline iFR calculation that demonstrated the feasibility of iFR measurements in nonculprit vessels in ACS patients.71 In 123 stenoses in 82 patients with ACS and stable disease, an iFR threshold of 0.92 best matched an FFR of 0.80 with a diagnostic concordance with FFR of 81.3%.71 No difference in diagnostic efficiency between ACS patients and those with stable disease was found and all apparent discrepancies were within the FFR 0.75–0.80 gray-zone.71 Furthermore, when using a hybrid iFR-FFR approach, they found 68% of patients would be spared adenosine. Further data will come from the DEFINE-FLAIR study, which is including patients with ACS.
iFR-Pullback and Virtual-PCIiFR can be calculated beat-to-beat during pressure-wire pullback, which enables the identification of stenoses and their physiological significance at rest without the need for a long infusion of hyperemic agent. The first iFR-Pullback study72 suggested important advantages to performing a resting pullback.
During resting conditions there is less flow interaction between stenoses. Hyperemic flow velocity is readily attenuated by the presence of any stenosis; even minor or diffuse disease can reduce the level of hyperemic flow compared to the flow levels found in normal vessels. Therefore even trivial stenoses or diffuse disease have the capacity to confound hyperemic assessments pressure assessments.73–76 In the resting state, resting coronary flow is preserved despite the presence of even severe stenoses;39 this suggests a transtenotic pressure drop distal to a stenosis will be resultant only from stenoses proximal to the pressure sensor with less confounding from distal disease. If iFR-Pullback is performed, all the gradients in the vessel can be determined to quantify the severity of each lesion in the vessel.
This leads to an exciting new domain. Computer ‘Virtual-PCI’ algorithms can ‘remove’ a stenosis on an iFR-Pullback trace (Figure 8) and then compute the residual gradients to estimate an expected post-PCI iFR,76 under the assumption that any intervention is perfect without any residual gradient in the treated segment.72 In the first iFR-Pullback study, real-world post-PCI iFR values could be predicted with high accuracy and without a significant systematic bias.72

(A) The iFR-Pullback data can be mapped onto the coronary angiogram with pressure loss or iFR intensity plotted as red dots to demonstrate colocalization with the angiographic stenosis. (B) The iFR Intensity can be plotted separately with a measure of physiological lesion length. (C) Virtual-PCI can be performed by selective removal of a stenosis. (D) The expected result of removing a stenosis can be plotted with the prediction of the post-PCI iFR to enable the best stenting option to be selected. iFR, instantaneous wave-free ratio; PCI, percutaneous coronary intervention. Adapted with permission from Nijjer SS, et al.76
The predicted value could provide a target physiological value for the real-world intervention. The process could also permit the evaluation of the potential physiological benefit of multiple different ‘virtual’ stenting strategies. Potentially acceptable physiological results could be achieved by a more limited stenting approach (potentially reducing stented-related complications). Alternatively, iFR-Pullback could reveal more extensive stenting is required to achieve hemodynamic improvement.
This technology is rapidly advancing, thanks to simultaneous developments in pressure-wires, computing power, X-ray imaging and angiographic co-registration systems. Computer tracking of pressure-wire movement could allow manual hand-guided pullback with simultaneous live angiographic co-registration. This would facilitate ease of use and rapid decision making.
All resting measurements should adequate nitrates have been given to ensure epicardial stability, ensure pressure normalization (‘equalization’) has been performed, and avoid wire manipulation during measurement. Patients were not routinely sedated for any of the ADVISE family of studies; all measurements were simply made with a pressure wire without hyperemic agent administration.
Active normalization for iFR is essential because not only must the pressure ratio be 1.0 at the ostium, but any phase- elays between the traces must be removed; measurements with phase delays can invoke significant error.66
Intracoronary nitrates are used not to produce hyperemia, rather to maximize epicardial vessel diameter, and to prevent wire-induced spasm, which can induce a pseudo-stenosis. After any injection of contrast or nitrate, a short delay of 15–20 s should be allowed prior to measurement; because nitrates should be administered soon after guide catheter engagement, there is no practical delay, as this time elapses with wire preparation and passage through the catheter. After PCI, the process of balloon removal, re-administration of nitrates and flushing of the catheter is sufficient to allow a resting state to be restored to allow measurement. If adenosine has been administered, time should be allowed for recovery, which can vary among patients. Finally, manipulation of the wire or pressure manifolds during readings, whether at rest or hyperemia, will alter the values and should be avoided.
There has been an exciting renewal of interest in coronary physiology. Despite the complexity inherent to detailed interrogation of vessel ischemia, patient outcomes are expected to improve. Established technologies such as FFR should be promoted, but with awareness of potential pitfalls. New, potentially disruptive technologies such as iFR may simplify physiological assessment in the catheter laboratory and provide significant added benefit with iFR-Pullback with Virtual-PCI.
The authors thank Dr Rasha Al-Lamee, Dr Jason Tarkin and Dr Christopher Cook.
Dr Justin Davies, Consultant to Volcano Corporation and Medtronic. Dr Justin Davies and Imperial College own intellectual property regarding iFR, which is licensed by the Imperial College to Volcano Corporation. Imperial College London is coordinating the FLAIR study, which is supported by an unrestricted grant from Volcano Corporation. All the authors have had travel expenses to conferences supported by industry, including Volcano Corporation, St Jude Medical, Medtronic, Pfizer and Astra Zeneca.
This review was supported by the Medical Research Council (UK), British Heart Foundation (BHF) and the NIHR Imperial Biomedical Research Centre.