Abstract
Background:
The incidence of hematoma formation following implantation of a cardiovascular implantable electronic device (CIED) is estimated to be 5% even if a pressure dressing is applied. It is unclear whether a pressure dressing can really compress the pocket in different positions. Furthermore, the adhesive tape for fixing pressure dressings can tear the skin. We developed a new compression tool for preventing hematomas and skin erosions.
Methods and Results:
We divided 46 consecutive patients receiving anticoagulation therapy who underwent CIED implantation into 2 groups (Group I: conventional pressure dressing, Group II: new compression tool). The pressure on the pocket was measured in both the supine and standing positions. The incidence of hematomas was compared between the 2 groups. The pressure differed between the supine and standing positions in Group I, but not in Group II (Group I: 14.8±7.1 mmHg vs. 11.3±9.9 mmHg, P=0.013; Group II: 13.5±2.8 mmHg vs. 13.5±3.5 mmHg, P=0.99). The incidence of hematomas and skin erosions was documented in 2 (8.7%) and 3 (13%) Group I patients, respectively. No complications were documented in Group II.
Conclusions:
The new compression tool can provide adequate continuous pressure on the pocket, regardless of body position. This device may reduce the incidence of hematomas and skin erosions after CIED implantation.
The incidence of hematoma following implantation of a cardiovascular implantable electronic device (CIED) is considered to be 2–5%, making it a common complication.1–3
Patients with cardiovascular disease are commonly being administered anticoagulation therapy using warfarin or the new oral anticoagulation drugs (NOACs), and antiplatelet therapy using aspirin and clopidogrel.4–7
In particular, the data specific to CIED implantation significantly favor continuation of warfarin therapy through the procedure over bridging with heparin.8,9
In fact, continuing anticoagulation with warfarin does not increase the risk of hematoma compared with no anticoagulation. Therefore, CIED implantations are performed without interruption of anticoagulation therapy. However, the incidence of hematomas is significantly higher in patients with anticoagulation therapy than in those without. Hematomas are associated with a prolonged length of hospital stay and may require surgical intervention. Furthermore, they increase the risk of infection. To prevent hematomas, pressure dressings are commonly used, but the results of applying pressure dressings have not been consistent among the reports. When a pressure dressing is applied, the center of the pressure should be over the pocket itself rather than the incision. Furthermore, the adhesive tapes designed for pressure dressings can cause skin erosion.10
We hypothesized that a conventional pressure dressing with adhesive tape would not provide adequate pressure between the dressing and pocket. In this study, the first goal was to assess the difference in pressure by using pressure dressings with adhesive tape in both the spine and standing positions. The second goal was to assess whether a new compression tool could improve the pressure in all body positions. The third goal was to compare the incidence of hematomas and skin erosions between conventional pressure dressings and the new compression tool.
Methods
Patient Selection
A total of 46 consecutive patients after a CIED implantation without interruption of anticoagulation therapy were enrolled and divided into 2 groups (Group I: conventional pressure dressing, Group II: new compression tool). Written informed consent was given by all patients. Patients with a body mass index (BMI) >35 kg/m2
were excluded from this study, because at that time the compression tool was designed for patients with a normal BMI. Patients were randomly assigned, in a 1:1 ratio, to receive a conventional pressure dressing or the new compression tool. To avoid dilution of any effect of the compression tools, the randomization was carried out before the CIED implantations.
Periprocedural Management of Anticoagulation and Antiplatelet Therapy
In the patients receiving warfarin, the international normalized ratio (INR) on the day of the implantation was targeted between 1.6 and 2.6.11
Heparin bridging was not performed in any of the patients.9,12
Both anticoagulation therapy including warfarin or NOAC and antiplatelet therapy, comprising clopidogrel and/or aspirin, was also continued even in patients receiving triple therapy.13,14
Patients were instructed to avoid extending the ipsilateral shoulder during physical activity,10
but otherwise were allowed to walk around the ward immediately after CIED implantation. Actually, most of the patients stayed in bed on the day of operation, because of anxiety.
Conventional Pressure Dressing and the New Compression Tool
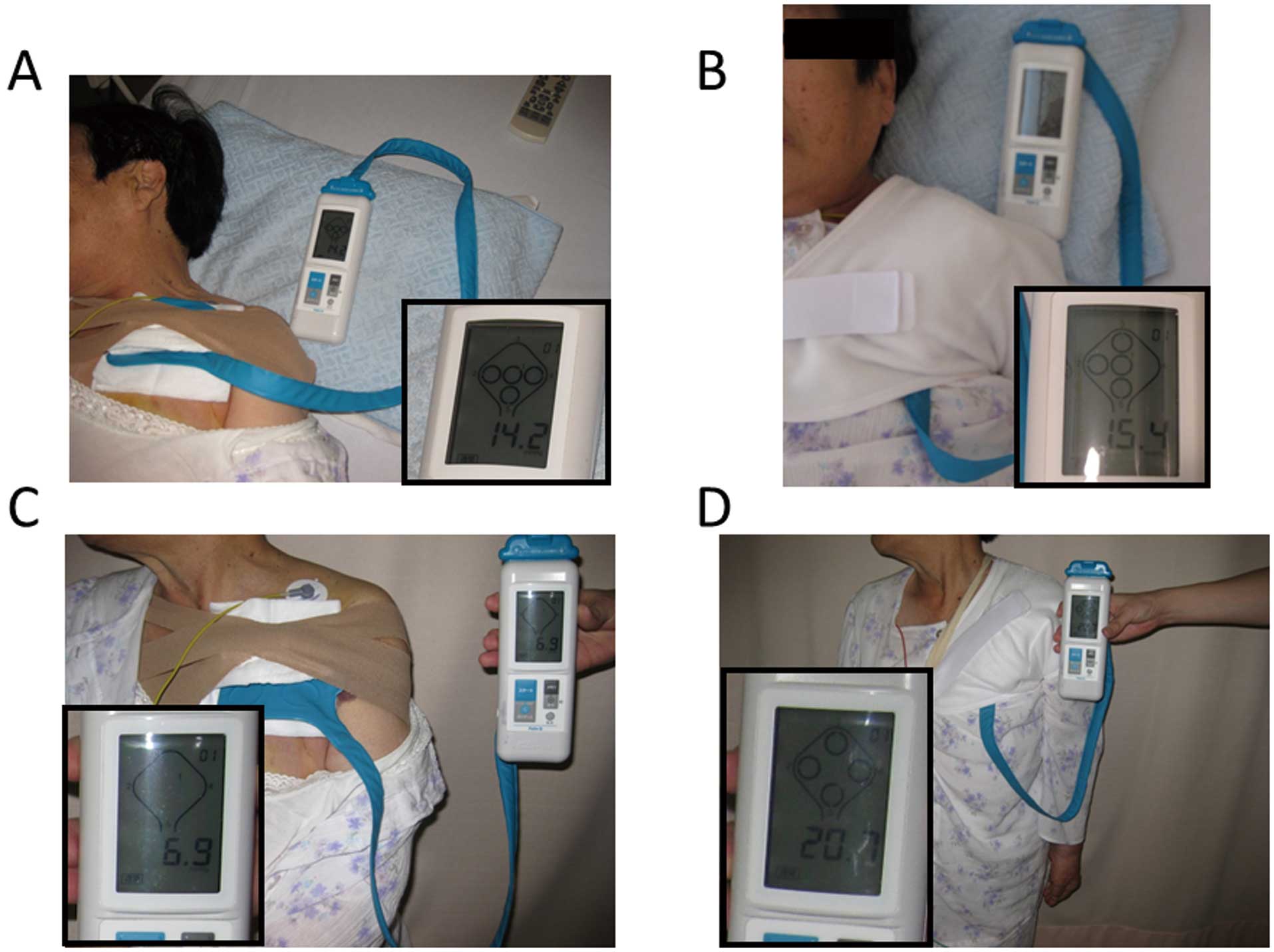
A conventional pressure dressing or the new compression tool was applied immediately after CIED implantation. If a pressure dressing was applied, an attempt was made using adhesive tape to place the center of the pressure over the pocket itself rather than over the incision (Figure 1A). In contrast to the conventional pressure dressing, the new compression tool did not require adhesive tape, which can cause the skin to tear. The compression tool consisted of a pouch filled with gel to compress the pocket, and a shoulder band designed to fix the pouch over the pocket. In detail, the specially designed shoulder band had 2 parts: a shoulder section and an elastic band. The elastic band could be attached to the shoulder section with Velcro tape, and could control the tightness (Figures 1B,C). Of note, the new compression tool could be applied over the clothes. Considering the potential tearing of the skin by the adhesive tape, the conventional pressure dressing and the new compression tool were removed 3 and 7 days, respectively, after CIED implantation.

A portable interface pressure sensor (Palm Q, CAPE, Kanagawa, Japan), used to accurately measure the pressure between the body and contact surfaces to prevent bed sores, was used to measure the pressure over the pocket. In the current study, the measurement was performed with the patients in different positions (supine and standing) on the day after CIED implantation (Figure 2).
Incidence of Hematomas and Skin Erosions
Clinically significant device-pocket hematomas were defined as hematomas requiring further surgery or interruption of OAC therapy during the hospitalization. The decision for further surgery or interruption of OAC agents was made by 2 experienced cardiologists who independently assessed the wound without any information about the compression tools. Skin erosions were defined as skin damage during dressing removal.
Statistical Analysis
The data were tested with the Kolmogorov-Smirnov test, and are presented as the mean±standard deviation for normally distributed variables. The median and quartiles are given for non-normally distributed variables. Categorical variables are expressed as the number and percentage of patients. Continuous and categorical variables were analyzed with the Wilcoxon signed-rank test and Fisher’s exact test, respectively. A value of P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS, version 11.0 software (SPSS Inc, Chicago, IL, USA).
Results
Patients Characteristics
The patients’ characteristics are displayed in
Table. The mean age of the 46 patients was 77±6 years, 22 (48%) were male, the mean BMI was 22±3, and the mean left ventricular ejection fraction (LVEF) was 51±13%. Hypertension was present in 20 (43%) and structural heart disease in 28 patients (61%). The patients’ characteristics, including age, BMI, PT-INR, and LVEF, did not differ between Groups I and II (Table).
Table.
Baseline Characteristics of the Patients According to Compression Device (Group I: Conventional Pressure Dressing; Group II: New Compression Tool)
| |
Group I |
Group II |
P value |
| Sex (male), n (%) |
12 (52) |
10 (43) |
0.77 |
| Age (years) |
78.5±7.5 |
76.0±5.1 |
0.20 |
| Diabetes mellitus, n (%) |
5 (21) |
2 (8) |
0.41 |
| BMI (per 1 kg/m2) |
22.3±3.6 |
22.6±3.2 |
0.73 |
| Hypertension, n (%) |
11 (48) |
9 (39) |
0.76 |
| CKD, n (%) |
10 (43) |
8 (35) |
0.76 |
| Structural heart disease, n (%) |
14 (61) |
14 (61) |
1.00 |
| Warfarin, n (%) |
23 (100) |
22 (96) |
1.00 |
| NOAC, n (%) |
0 (0) |
1 (4) |
1.00 |
| Aspirin, n (%) |
1 (4) |
6 (26) |
0.10 |
| Clopidogrel, n (%) |
2 (9) |
2 (9) |
1.00 |
| DAPT, n (%) |
1 (4) |
1 (4) |
1.00 |
| PT-INR |
1.8±0.6 |
2.2±0.8 |
0.08 |
| Indication of anticoagulation |
|
|
0.59 |
| Atrial fibrillation, n (%) |
19 (83) |
18 (78) |
|
| Post-valvular surgery, n (%) |
2 (8) |
1 (4) |
|
| Other, n (%) |
2 (8) |
4 (17) |
|
| LVEF (%) |
52±13 |
50±13 |
0.61 |
| Device |
|
|
0.11 |
| Pacemaker, n (%) |
19 (83) |
14 (61) |
|
| ICD, n (%) |
3 (13) |
7 (30) |
|
| CRT and CRT-D, n (%) |
0 (0) |
2 (8) |
|
BMI, body mass index; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy with defibrillator; DAPT, dual-antiplatelet therapy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NOAC, novel oral anticoagulants; PT-INR, prothrombin time-international normalized ratio.
Figure 3
shows the compression pressure in both the supine and standing positions between the groups. In Group I, the compression pressure was significantly lower in the standing position than in the supine position (14.8±7.1 mmHg vs. 11.3±9.9 mmHg, P=0.013). The minimum and maximum compression pressures in the supine position were 2.6 mmHg and 35.9 mmHg, respectively, and 1.3 mmHg and 44.9 mmHg in the standing position. In Group II, the compression pressure was comparable between both positions (13.5±2.8 mmHg vs. 13.5±3.5 mmHg, P=0.99). The minimum and maximum compression pressures in the supine position were 9.8 mmHg and 20.4 mmHg, respectively, and 7.0 mmHg and 20.0 mmHg in the standing position.
Incidence of Hematomas and Skin Erosions
Of the 23 patients in Group I, hematomas and skin erosions were observed in 2 (9%) and 3 (13%) patients, respectively. The hematomas in the 2 patients were found 2 and 3 days, respectively, after CIED implantation. In contrast, no hematomas or skin erosions were observed in any patients in Group II.
Discussion
Main Findings
Compression pressure obtained with conventional pressure dressings and adhesive tape was significantly lower in the standing position than in the supine position, which may result in the occurrence of a hematoma. The new compression tool maintained a constant compression pressure in both the supine and standing positions, which may prevent the occurrence of hematomas, even without interruption of anticoagulation and antiplatelet therapy.
Risk Factors for the Development of Hematomas
A previous large, randomized trial reported the safety of performing pacemaker or ICD implantation without interruption of warfarin therapy in patients requiring OAC therapy.1
Furthermore, this strategy was reported to be associated with a significantly lower rate of hematomas, as compared with heparin bridging. However, hematomas still occurred in 3.5% of patients in the group without interruption of warfarin therapy.1,8
Three factors (continued warfarin, diabetes mellitus, and the use of aspirin) were reported to be the independent predictors for the development of hematomas. In the current study, patients with diabetes mellitus and/or using aspirin were less likely to develop hematomas than in the previous study, but the incidence of hematoma was obviously higher in the current study, as compared with the previous study. This discrepancy may be caused by differences in the age of the patients.15
In the current study, the mean age was higher than in the previous study. Considering these issues, the strategy of a compression tool without interruption of anticoagulation/antiplatelet therapy may be optimal for preventing the incidence of hematoma.
Concept of the New Compression Tool
The concept of the new compression tool was as follows: to provide continuous compression over the pocket during hospitalization after CIED implantation. The benefits of conventional pressure dressing application are contradictory16,17
and the pitfalls are as follows: (1) the center of pressure should be over the pocket itself rather than over the incision; (2) the adhesive tape designed for pressure dressings can cause tearing of the skin; and (3) considering the incidence of skin erosions, the maximal duration of the pressure dressing application is only a few days after CIED implantation. We believe that our new compression tool can overcome the deficiencies of conventional pressure dressings. The gel-filled pouch can adjust to the shape and size of all implanted CIEDs and the specially designed shoulder band can completely fix the pouch over the pocket without tearing the skin, which enables continuous compression and adjustment during the hospitalization. In addition, we can perform daily contact-force-guided adjustments and attempt to maintain a contact force of 10 mmHg, because the shoulder band is applied with Velcro tape, not adhesive tape. Unfortunately, during the removal of the pressure dressings, we found that skin damage did occur in the older patients or those with a smaller BMI.
Clinical Implications
The current study demonstrated that the new compression tool could provide continuous compression over the CIED pocket during the hospitalization. This novel tool may reduce the incidence of hematomas and skin erosions after CEID implantation, even with continued anticoagulation therapy.
Study Limitations
Our study had 3 major limitations. First, the sample size was small, but the study was randomized and designed to clearly compare compression pressure between 2 methods methods. Second, the statistical power was not adequate because of the small incidence of hematomas and skin erosions. Third, the difference in the duration of the compression (3 vs. 7 days) may have influenced the incidence of hematoma. Our findings should therefore be verified in a large cohort.
Conclusions
The new compression tool provided adequate compression pressure over the pocket in any body position, which may reduce the incidence of hematomas and skin erosions after CIED implantation without any interruption to anticoagulation therapy. A large, multicenter randomized trial is warranted to clarify this issue.
Acknowledgments
We thank Mr John Martin for his linguistic assistance and all the staff at station 4S of Himeji Cardiovascular Center for their tireless work and professionalism.
Conflict of Interest
None declared.
References
- 1.
Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368: 2084–2093.
- 2.
Rowley CP, Bernard ML, Brabham WW, Netzler PC, Sidney DS, Cuoco F, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111: 1165–1168.
- 3.
Bernard ML, Shotwell M, Nietert PJ, Gold MR. Meta-analysis of bleeding complications associated with cardiac rhythm device implantation. Circ Arrhythm Electrophysiol 2012; 5: 468–474.
- 4.
Korantzopoulos P, Letsas KP, Liu T, Fragakis N, Efremidis M, Goudevenos JA. Anticoagulation and antiplatelet therapy in implantation of electrophysiological devices. Europace 2011; 13: 1669–1680.
- 5.
Kutinsky IB, Jarandilla R, Jewett M, Haines DE. Risk of hematoma complications after device implant in the clopidogrel era. Circ Arrhythm Electrophysiol 2010; 3: 312–318.
- 6.
Kosiuk J, Koutalas E, Doering M, Sommer P, Rolf S, Breithardt OA, et al. Treatment with novel oral anticoagulants in a real-world cohort of patients undergoing cardiac rhythm device implantations. Europace 2014; 16: 1028–1032.
- 7.
Ozcan KS, Osmonov D, Yildirim E, Altay S, Turkkan C, Ekmekci A, et al. Hematoma complicating permanent pacemaker implantation: The role of periprocedural antiplatelet or anticoagulant therapy. J Cardiol 2013; 62: 127–130.
- 8.
Tompkins C, Cheng A, Dalal D, Brinker JA, Leng CT, Marine JE, et al. Dual antiplatelet therapy and heparin “bridging” significantly increase the risk of bleeding complications after pacemaker or implantable cardioverter-defibrillator device implantation. J Am Coll Cardiol 2010; 55: 2376–2382.
- 9.
Yokoshiki H, Mitsuyama H, Watanabe M, Mizukami K, Matsui Y, Tsutsui H. Anticoagulation management in the perioperative phase of implantable cardioverter defibrillator implantation. Circ J 2013; 77: 2003–2008.
- 10.
Harding ME, Chinitz LA. Clinical considerations for allied professionals: Optimizing outcomes: Surgical incision techniques and wound care in device implantation. Heart Rhythm 2014; 11: 737–741.
- 11.
Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T. Target intensity of anticoagulation with warfarin in Japanese patients with valvular atrial fibrillation. Circ J 2015; 79: 325–330.
- 12.
Fujiwara R, Yoshida A, Takei A, Fukuzawa K, Takami K, Takami M, et al. ‘Heparin bridging’ increases the risk of bleeding complications in patients undergoing anticoagulation therapy and device implantation. J Arrhythmia 2012; 28: 96–99.
- 13.
Kosiuk J, Koutalas E, Doering M, Nedios S, Sommer P, Rolf S, et al. Comparison of dabigatran and uninterrupted warfarin in patients with atrial fibrillation undergoing cardiac rhythm device implantations. Circ J 2014; 78: 2402–2407.
- 14.
Senoo K, Lau YC, Dzeshka M, Lane D, Okumura K, Lip GY. Efficacy and safety of non-vitamin K antagonist oral anticoagulants vs. warfarin in Japanese patients with atrial fibrillation. Circ J 2015; 79: 339–345.
- 15.
Nammas W, Raatikainen MJ, Korkeila P, Lund J, Ylitalo A, Karjalainen P, et al. Predictors of pocket hematoma in patients on antithrombotic therapy undergoing cardiac rhythm device implantation: Insights from the FinPAC trial. Ann Med 2014; 46: 177–181.
- 16.
Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract 2013; 2013: 279289.
- 17.
Piromchai P, Vatanasapt P, Reechaipichitkul W, Phuttharak W, Thanaviratananich S. Is the routine pressure dressing after thyroidectomy necessary? A prospective randomized controlled study. BMC Ear Nose Throat Disord 2008; 8: 1.