Article ID: CJ-15-0435
Article ID: CJ-15-0435
Angiogenesis, the growth of new capillary from existing blood vessels, is an important natural process that is central to various pathophysiological processes in the body, not only during fetal development but also in postnatal tissue repair and disease development. Angiogenesis is a hallmark of wound healing, cancer development, ischemic and inflammatory diseases.1,2
Article p ????
The formation of new sprouts is dynamic and requires a large number of highly orchestrated processes. Three different types of endothelial cells (ECs), comprising tip, stalk and phalanx cells, have been suggested to be involved in sprouting angiogenesis. Attracted by proangiogenic signals, ECs degrade cell-cell junctions, including VE-cadherin and ZO-1, so that basement membrane and pericytes detach, allowing a tip cell to migrate in response to guidance signals. Following the migration of tip cells, stalk cells proliferate and form a lumen to maintain the integrity and perfusion of the growing vascular bed. Tip cells from neighboring sprouts meet and anastomose to form a perfused branch. Upon the initiation of blood flow, ECs become quiescent phalanx cells. Deposition of basement membrane and recruitment of mural cells stabilize the new connection. A fundamental feature of vessel maturation is the recruitment of the mural cells, pericytes and vascular smooth muscle cells that coat small capillaries and larger vessels, respectively.3 During angiogenesis, bidirectional pericyte-EC signaling is critical for capillary sprout formation. Observations of pericytes leading capillary sprouts also imply their role in EC guidance. As such, pericytes have recently emerged as a therapeutic target to promote or inhibit angiogenesis.4 The prominent signaling pathways that regulate endothelial-mural cell-cell communication are platelet-derived growth factor-β (PDGF-β)/PDGF receptor-β, angiopoietin 1 (Ang1)/Tie2 and transforming growth factor β, which control mural cell recruitment, EC viability and mural cell differentiation, respectively.3–6 However, the mechanisms which regulate their actions in microvascular physiology have been largely underinvestigated.
Nerve injury-induced protein 1, or Ninjurin-1 (Ninj1), is a cell-surface protein and an adhesion molecule. Ninj1 was originally discovered during the identification of molecules related to nerve injury and is known to be upregulated in neuronal and Schwann cells after sciatic nerve injury.7 The Ninj1 gene contains an open reading frame of 152 amino acids (aa), which encodes a predicted 16-kDa polypeptide. Ninj1 has 2 hydrophobic transmembrane domains (72–100 aa, and 118–139 aa) and a putative N-glycosylation site.7 In addition, the 12-residues on the N-terminal ectodomain of Ninj1 are crucial for its hemophilic binding activity (Figure).8 In mammals, there are 2 types of Ninjurin, Ninj1 and Ninj2, which share conserved hydrophobic regions for their transmembrane domains but differ in adhesion motifs and they do not interact with each other.8 Furthermore, the tissue distribution of Ninjurins significantly differs. In addition to its expression in the injured peripheral nervous system, Ninj1 is ubiquitously distributed in all tissues with epithelial origin,7,8 whereas, the expression of Ninj2 is restricted to hematopoietic and lymphatic tissues as well as mature sensory and enteric neurons.9 Ninj1 is responsible for the progression of multiple sclerosis (MS), an autoimmune inflammatory disease of the central nervous system (CNS) characterized by demyelination and axonal damage.10 The expression of Ninj1 is significantly increased in CNS lesions of patients with MS, and functional blockage of Ninj1 decreases neuro-inflammatory responses in an experimental autoimmune encephalomyelitis, an animal model of MS.10 Furthermore, Ninj1 expressed in macrophages is known to contribute to the regression of the hyaloid vascular system, a transiently existing vascular system involved in maturation of the lens during the embryonic period. Systemic neutralization using an anti-Ninj1 antibody delays regression of the hyaloid vascular system.11 Recently, Yin et al reported that inhibition of Ninj1 using neutralizing anti-Ninj1 antibodies promotes penile angiogenesis and neural regeneration through Ang1/Tie2 signaling and restored erectile function in diabetic mice (Figure).12 These findings suggest that Ninj1 has a functional role in the regulation of vascular systems.
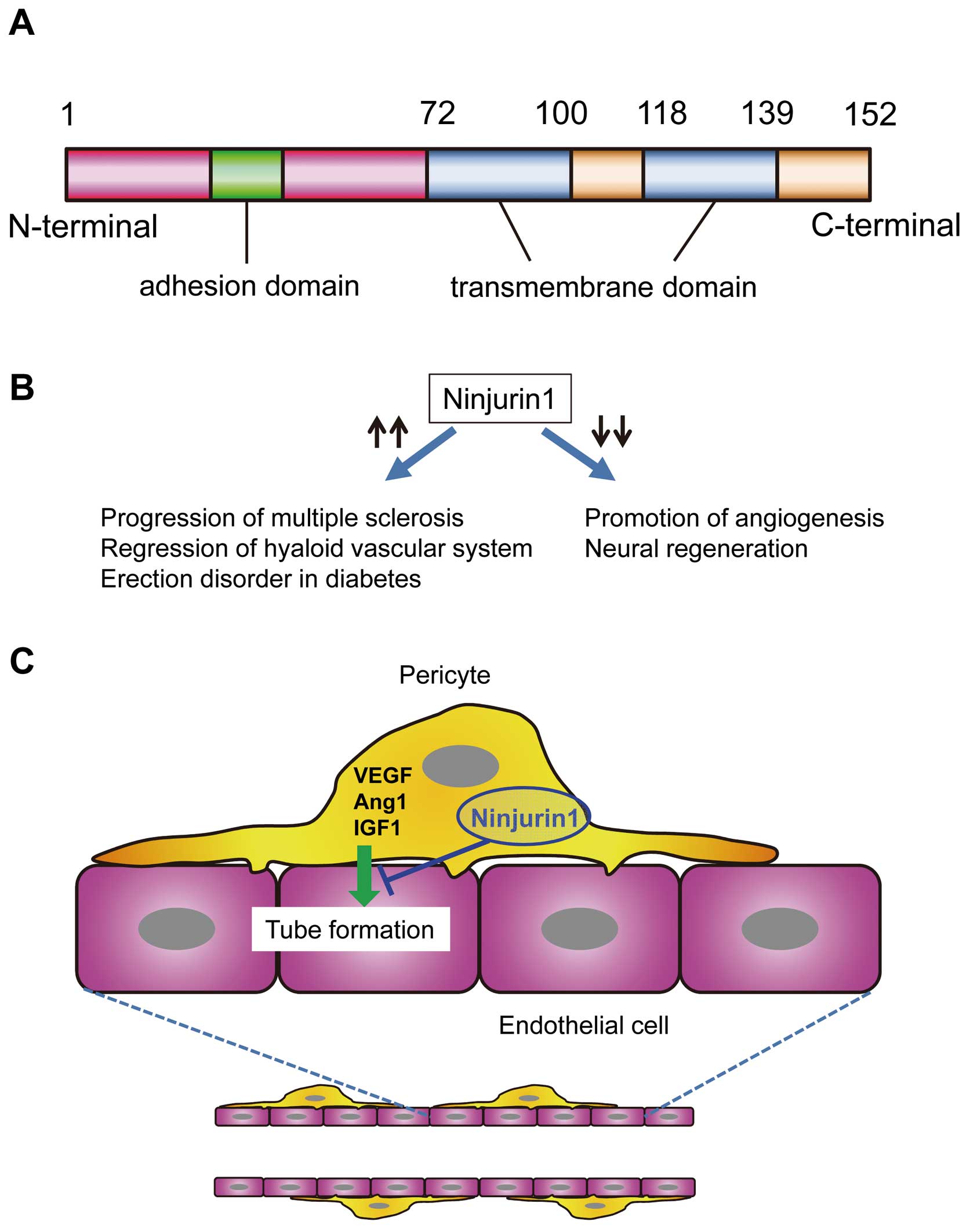
The protein structure (A) and functions (B) of Ninjurin1, which negatively regulates angiogenesis by mediating the interaction between pericytes and endothelial cell tubes (C). Ang1, angiopoietin 1; IGF1, insulin-like growth factor; VEGF, vascular endothelial growth factor.
In this issue of the Journal, Matsuki et al13 clearly demonstrate that Ninj1 is a regulator of function of pericytes and ECs for angiogenesis. They recently established immortalized vascular cell lines, capillary pericytes (cPCs) and endothelial cells (cECs) from microvessels of peripheral tissues.14 When fluorescence-labeled cPCs were co-incubated with a mouse thoracic aorta tissue in a 3D Matrigel system (aortic ring angiogenesis assay), the length of the EC tubes sprouting from the aorta was increased and the expression of angiogenesis-related genes, including vascular endothelial growth factor (VEGF), hepatocyte growth factor, insulin-like growth factor 1, and matrix metalloproteinase was increased in cPCs during neovessel formation (Figure). The expression of Ninj1 was enhanced, together with the production of these angiogenic factors. Hypoxia induced the gene expression of Ninj1 in addition to VEGF in cPCs and they demonstrated in vivo that Ninj1 was predominantly expressed in capillary cells in skeletal muscle tissues in a mouse hindlimb ischemia model. In gain- and loss-of-function experiments, small interfering RNA-mediated downregulation of Ninj1 in cPCs enhanced cPC-mediated angiogenic effects, whereas overexpression of Ninj1 attenuated their effects in the aortic ring angiogenesis assay. The production of angiogenic growth factors, including VEGF and Ang1, in cPCs was enhanced by downregulation of Ninj1, and reduced by overexpression of Ninj1. Ninj1 was also expressed in cECs, but the expression level was low compared with cPCs under normal conditions. Unlike cPCs, downregulation of Ninj1 in cECs did not affect EC tube formation in 3D in-vitro culture; however, overexpression of Ninj1 reduced EC tube formation. These findings provide novel evidence that Ninj1 negatively regulates angiogenesis by mediating the interaction between PCs and EC tubes. Although the physiological or pathological significance of Ninj1 in angiogenesis and the precise mechanism of EC-PC interaction via Ninj1 remain unclear, these findings may open new avenues to treating ischemic diseases and pericyte-associated diseases, including cancer, diabetes and neurodegenerative disorders, by targeting the Ninj1-related pathways in capillary cells.
The authors have no conflict of interest directly relevant to the content of this article.