Abstract
Background:
Circulating pentraxin 3 (PTX3), the main regulator of the inflammatory response, rapidly increases following cardiovascular events, and low PTX3 is associated with high body mass index.
Methods and Results:
We conducted a 12-month longitudinal study, to test the hypothesis that laparoscopic adjustable gastric banding (LAGB)-induced weight loss was associated with changes in platelet activation markers and PTX3. Twelve obese patients, scheduled to undergo LAGB, were enrolled at the University Obesity Center. Urinary 11-dehydro-thromboxane (Tx)B2
excretion rate was measured on radioimmunoassay, and PTX3 and CD40L were determined on immunoassay. Plasma PTX3 increased by 178.8 and 214.9% (P<0.0001), respectively, 6 and 12 months after LAGB. High-sensitivity CRP decreased by 24 and 29.7% (P<0.0001), whereas CD40L decreased by 64.3 and 58.6% (P=0.002), respectively. Urinary 11-dehydro-TxB2
decreased from 1,443 to 715 and 564 pg/mg creatinine, respectively 6 months and 12 months after LAGB (P<0.0001). PTX3 was inversely related to platelet activation markers, 11-dehydro-TxB2
and CD40L. Moreover, multiple regression analysis on pooled data showed that plasma PTX3 was an independent predictor of urinary 11-dehydro-TxB2.
Conclusions:
There is an association between inflammation, platelet activation and metabolic dysfunction in obesity, and PTX3 is a key player within these circuits.
Pentraxin 3 (PTX3) is a member of a superfamily of proteins involved in innate immune response, which includes C-reactive protein (CRP) and serum amyloid P.1
The capability of CRP and PTX3 to modulate inflammation through the complement system and innate immunity suggests their involvement in atherosclerosis, thrombosis, and ischemic heart disease.2
In addition to recognizing pathogens, PTX3 controls the inflammatory response by regulating leukocyte recruitment, thus carrying anti-inflammatory, pro-resolution properties.3
Indeed, PTX3 deficiency is associated with excessive inflammation and tissue damage in pre-clinical models of acute lung and kidney injury,3,4
as well as of cardiac ischemia and reperfusion.5
In clinical settings, circulating PTX3 rapidly increases following cardiovascular events and shortly after revascularization,5,6
and has been shown to participate in contrast-induced acute kidney injury.7
In particular, during acute myocardial infarction (AMI) activated neutrophils, aggregated with platelets, release PTX3, which binds to activated circulating platelets, thereby dampening their prothrombotic action.8
Also, PTX3-bound activated platelets form less aggregates with neutrophils and monocytes, showing reduced capability to stimulate pro-inflammatory functions of these cells. This suggests a PTX3-regulated protective loop that limits polymorphonucleated accumulation and the inflammatory response in AMI.
Editorial p ????
PTX3, however, has been recently implicated in the pathogenesis of vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway9
and as a marker of poor prognosis in patients with stable coronary artery disease after drug-eluting stent implantation,10
or in patients with heart failure,11
suggesting that this molecule may act as fine-tuner of inflammation, dampening an excessive inflammatory reaction, or as an amplifier of the innate immune response according to the cell source and to the surrounding conditions, in terms of ongoing pro- and anti-inflammatory signals.2
In addition to being expressed by leukocytes and endothelial cells,12
PTX3 can be produced by adipose tissue13
and it can be associated with insulin resistance in obese children both at baseline and after completion of a physical activity program.14
Moreover, in a large population study as well as in patients with acute coronary syndrome,15
low PTX3 is associated with higher body mass index (BMI) and waist circumference (WC). Consistent with this, a decrease in BMI and weight after controlled dietary intervention is associated with increased PTX3.16
Thus, it is likely that PTX3 is involved in adiposity-related inflammation and dysmetabolism.
Along these lines, we found that visceral obesity is characterized by persistent platelet activation, partially driven by oxidative stress and inflammatory triggers related to the degree of abdominal adiposity, which is, at least in part, reversible with a successful weight-loss program.17
Moreover, we have recently reported that laparoscopic adjustable gastric banding (LAGB), associated with persistent weight loss, reduced by approximately 60% thromboxane (Tx)-dependent platelet activation.18
In the present study, we tested the hypothesis that LAGB-induced weight loss and reduction in Tx-dependent platelet activation may be associated with changes in circulating PTX3.
Here, we report that the reduction in inflammation, insulin resistance and Tx biosynthesis associated with LAGB, is paralleled by an increase in plasma PTX3, which remains a significant predictor of Tx metabolite excretion during 1-year follow-up, clarifying a novel endogenous, anti-inflammatory mechanism that limits platelet activation and likely its sequelae in this clinical setting.
Methods
Subjects
Between January 2010 and April 2012, 12 consecutive, eligible obese patients (6 male; mean age, 32 years; range, 27–50 years; mean BMI, 43.7±5.02) who were scheduled to undergo LAGB procedure, were enrolled at the University Obesity Center, Chieti, Italy. They completed a 1-year follow-up, which provided sufficient information to assess changes in weight, lipid profile, glucose metabolism, and concomitant comorbidity status. Complete clinical history and physical examination were recorded, including cardiovascular events and comorbidities. The study protocol was approved by the local ethics committee, and patients provided written informed consent.
Inclusion Criteria
Inclusion criteria were: (1) male or female patients aged ≥18 years; (2) BMI >40.0 kg/m2, or >35.0 kg/m2
in the presence of comorbidities;19,20
(3) history of at least 2 previous unsuccessful attempts to lose weight with non-surgical measures; (4) willingness to comply with the substantial lifelong dietary restrictions associated with LAGB implantation; (5) history of obesity ≥5 years; and (6) likelihood to complete all study evaluations and comply with the protocol requirements.
Exclusion Criteria
Exclusion criteria were: (1) any surgical procedure or treatment representing an unreasonable risk to the patient; (2) evidence of cardiovascular disease (by clinical history, physical examination, electrocardiography, and Doppler scanning), cancer, or of altered liver or renal function; (3) history of inflammatory disease of the gastrointestinal tract; (4) obesity secondary to endocrinopathies (Cushing’s disease or syndrome, hypothyroidism); (5) severe cardiopulmonary disease or other serious organic disease; (6) ongoing or planned (within 12 months) pregnancy; (7) alcohol or drug addiction; (8) established infection anywhere in the body at the time of surgery; (9) treatment with lipid-lowering, anti-diabetic, anti-inflammatory, anti-oxidant, anti-coagulant or anti-platelet drugs; and (10) previous or current malignancy. Additional exclusion criteria included the presence of any condition or situation that, in the investigator’s opinion, may have put the subject at significant risk, confounded the study results, or interfered significantly with the subject’s participation.
Surgical Procedure and Diet
All patients received a careful preoperative preparation by the multidisciplinary team. The laparoscopic adjustable band procedure was performed with the standard pars flaccida technique.20
The gastric banding system (REALIZETM, Ethicon Endo-Surgery, Cincinnati, OH, USA) consists of a silicon band with an inflatable inner shell and buckle closure connected by tubing to an accessed port placed subcutaneously under the xiphoid. The inner diameter of the band can be individually adjusted to the patient’s needs by the addition or removal of saline through the access port. We left the band deflated for 1 month and subsequently gradually inflated after a radioscopic control. Restriction was guided according to the patient’s early satiety while there was still sufficient passage of dye as evidenced upon barium swallow.
For the first month after LAGB, a semiliquid diet of 1,000 kcal/day, was prescribed (33% proteins, 19% lipids, 48% carbohydrates). Patients were advised to eat slowly, avoid liquids during meals, use vegetables at each meal and meat or fish at least once a day, and to stop eating when feeling a sense of satiety. One month after LAGB, a solid diet was reintroduced, and by the third month a 1,200-kcal/day diet was prescribed. Diet included 52% carbohydrates, 17% proteins, and 31% lipids. The patients were also asked to do 30-min physical aerobic activity every day to avoid muscular loss, with a gradual increase over a 1-month period.
Measurements
Obese patients were evaluated at baseline and after 3, 6 and 12 months for BMI, WC, waist-to-hip ratio (WHR), arterial blood pressure, fasting glucose and insulin, serum lipids, and CRP. BMI was calculated as body weight divided by the square of height. WHR was defined as the minimum abdominal circumference between the xiphoid process and the iliac crests (waist) divided by the circumference determined over the femoral heads (hip). Homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated from fasting samples of (insulin×glucose)/22.5. Oral glucose tolerance test (OGTT; 75 g) was performed in all patients to identify alterations of glucose metabolism classified according to the WHO definition as normal glucose tolerance (NGT), impaired glucose tolerance (IGT), or type 2 diabetes mellitus (T2DM), respectively, at recruitment and during follow-up evaluations to detect changes in glucose metabolism during weight loss. T2DM was diagnosed according to the American Diabetes Association criteria.21
Serum glucose, cholesterol, triglycerides (TG), and insulin were measured with standard methods. Serum CRP was measured with a highly sensitive immunoassay.22
To convert glucose to mmol (SI units), multiply by 0.055. To convert total and high-density lipoprotein (HDL) cholesterol to mmol, multiply by 0.0259; to convert TG to mmol, multiply by 0.0113. To convert mg/dl CRP to nmol/L, multiply mg/dl by 95.24.
Excess weight was defined as the pre-surgery weight minus the ideal body weight based on a BMI of 25 kg/m2. The % excess weight loss was defined as the pre-surgery weight minus the follow-up weight divided by the excess weight multiplied by 100. Body fat mass and basal metabolic rate were determined using bioelectrical impedance (Tanita Body Fat Analyzer, Sensormedics, Milan, Italy).
Overnight urine collection was performed immediately before blood sampling. The antioxidant 4-hydroxy-Tempo (1 mmol/L; Sigma Chemical, St. Louis, MO, USA) was added to the urine samples, and all samples were frozen and stored at –80℃ until analysis.
Urinary 11-dehydro-TxB2
excretion rate was measured using a previously described radioimmunoassay method.23
This method has been validated using different antisera and by comparison with gas chromatography/mass spectrometry, as detailed elsewhere.23
PTX3 was measured using the quantikine Human Pentraxin 3/TSG-14 Immunoassay (R&D Systems, Minneapolis, MN, USA). Plasma CD40 ligand (CD40L) was determined on enzyme-linked immunosorbent assay (ELISA; R&D Systems), according to recent recommendations.24
Statistical Analysis
Shapiro-Wilk test was used to determine the distribution of each variable. Discrete data are given as counts and percentages, continuous data are presented as median (IQR), unless otherwise specified. The effects of LAGB surgery were assessed using repeated measurements analysis of variance with the post-hoc Bonferroni test for pairwise comparisons (P<0.05) or Friedman’s test, as appropriate. The Pearson or Spearman rank correlation test was used to assess relationships among variables. Stepwise multiple linear regression analysis was performed to assess variables independently associated with urinary 11-dehydro-TxB2
excretion rate, with logarithmically transformed data for this analysis. Covariates included in the multiple regression models were selected on the basis of their significance on univariate analysis and their clinical relevance to the outcome of interest as reported from other studies. They included BMI, excess weight, WC, serum lipids, fasting plasma glucose (FPG), post-prandial glucose (PPG), HOMA-IR, fat mass, CRP, and plasma CD40L.
Only 2-tailed probabilities were used for testing statistical significance, and P<0.05 was considered statistically significant. All calculations were carried out using SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Clinical and Metabolic Outcomes
Table
lists body weight, BMI, WC, glucose variables, arterial blood pressure, and lipid status in the 12 patients followed for 1 year after LAGB. During follow-up, no drug treatment was initiated in any of the study subjects. BMI progressively decreased from a median preoperative value of 43.7 kg/m2
(IQR, 39.8–47.9 kg/m2) to 34.7 kg/m2
(IQR, 32.9–38.4 kg/m2) 12 months after LAGB (P<0.0001), resulting in excess weight and body fat mass reduction of 34.8% (IQR, 23.3–56.2%) and 5.25% (IQR, 2.5–7.5%), respectively (Table;
Figure 1A). A statistically significant improvement in arterial blood pressure, serum total, HDL and low-density lipoprotein cholesterol and TG was also observed during LAGB follow-up. Pre-surgery OGTT identified 2 out of 12 patients (16.6%) as having T2DM and 4 (33.3%) as having IGT. After 6 months only 1 patient still had overt diabetes and 1 patient had IGT, whereas 4 returned to NGT. After 12 months, only 1 patient still had IGT. None of the patients with pre-surgery NGT developed IGT or diabetes during follow-up (Table). In the whole group, HOMA-IR decreased by a median 22.4% (IQR, 9.6–34.2%) and 37.5% (IQR, 23.6–51.2%) after 6 and 12 months, respectively (P<0.0001;
Table;
Figure 1B). Despite a small, but significant, reduction in the basal metabolic rate (–117 kcal/day; IQR, –11.25 to –284 kcal/day) after 12 months, the basal metabolic rate/lean body mass ratio was 31.7 kcal/kg (IQR, 30.9–33.4 kcal/kg). This is not significantly different from that observed before or 6 months after LAGB (31.7 kcal/kg, IQR: 30.9–33.4 kcal/kg; and 31.5 kcal/kg, IQR: 28.2–36.2 kcal/kg; P=0.92;
Table).
Table.
Severe Obese Subject Characteristics After LABG (n=12)
| Variable |
Before LAGB |
3 months
after LAGB |
6 months
after LAGB |
12 months
after LAGB |
P-value† |
| Gender (M) |
6 (50) |
6 (50) |
6 (50) |
6 (50) |
– |
| Body weight (kg) |
116 (102–137) |
111 (92–127) |
100 (89–121) |
93 (85–109) |
<0.0001 |
| BMI (kg/m2) |
43.7 (39.8–47.9) |
41.6 (36.7–42.4) |
36.8 (35.5–41.2) |
34.7 (32.9–38.4) |
<0.0001 |
| Body fat (%) |
45 (41–49) |
– |
43 (37–47) |
40 (38–45) |
0.004 |
| Excess weight (kg) |
50.9 (37.8–65.1) |
45.4 (28.9–51.8) |
33.1 (25.9–47.1) |
25.9 (21.2–39.2) |
<0.0001 |
| Excess weight loss (%) |
|
10.7 (8.9–24.7) |
22.4 (15.1–39.3) |
34.8 (23.3–56.2) |
<0.0001 |
| Waist (cm) |
127 (112–132) |
123 (110–129) |
114 (109–124) |
111 (100–117) |
<0.0001 |
| Systolic BP (mmHg) |
137 (122–150) |
125 (120–139) |
120 (111–137) |
110 (110–120) |
<0.0001 |
| Diastolic BP (mmHg) |
90 (80–95) |
80 (80–84) |
80 (76–80) |
70 (70–80) |
<0.0001 |
| Total cholesterol (mg/dl) |
184 (170–195) |
NA |
174 (166–185) |
160 (147–168) |
<0.0001 |
| HDL-C (mg/dl) |
43 (37–46) |
NA |
46 (40–55) |
49 (46–59) |
0.004 |
| Triglycerides (mg/dl) |
162 (101–224) |
NA |
110 (88–153) |
79 (72–102) |
<0.0001 |
| LDL-C (mg/dl) |
105 (99–112) |
NA |
105 (89–118) |
92 (76–102) |
<0.001 |
| Fasting plasma glucose (mg/dl) |
104 (90–120) |
NA |
99 (88–108) |
94 (80–100) |
0.001 |
Post-prandial plasma glucose
(mg/dl) |
139 (100–197) |
NA |
116 (110–131) |
111 (110–120) |
0.083 |
| Impaired glucose tolerance |
4 (33.3) |
NA |
1 (8.3) |
1 (8.3) |
0.22 |
| Type 2 diabetes mellitus |
2 (16.6) |
NA |
1 (8.3) |
0 (0) |
0.22 |
| HOMA-IR |
5.0 (3.6–11.6) |
NA |
3.9 (3.1–5.9) |
3.4 (2.2–4.5) |
<0.0001 |
| Basal metabolic rate (kcal/day) |
2,064 (1,690–2,392) |
NA |
1,807 (1,646–2,023) |
1,804 (1,742–2,122) |
0.017 |
| CRP (mg/dl) |
2.3 (1.7–2.5) |
1.8 (1.5–2) |
1.8 (1.3–1.9) |
1.6 (1.1–1.8) |
<0.0001 |
| PTX3 (ng/ml) |
1.4 (0.5–2.2) |
3.0 (2.3–3.7) |
3.8 (3.0–5.1) |
3.9 (2.7–5.9) |
<0.0001 |
U-11-dehydro-TxB2
(pg/mg creatinine) |
1,443 (1,048–1,879) |
994 (782–1,260) |
715 (556–1,003) |
564 (418–810) |
<0.0001 |
| CD40L (pg/ml) |
906 (685–1,007) |
484 (416–652) |
290 (193–467) |
306 (149–544) |
|
Data given as n (%) or median (IQR). †Friedman test. BMI, body mass index; BP, blood pressure; CD40L, CD40 ligand; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance index; LAGB, laparoscopic adjustable gastric banding; LDL-C, low-density lipoprotein cholesterol; NA, not available; PTX3, pentraxin 3; Tx, thromboxane; U, urinary.
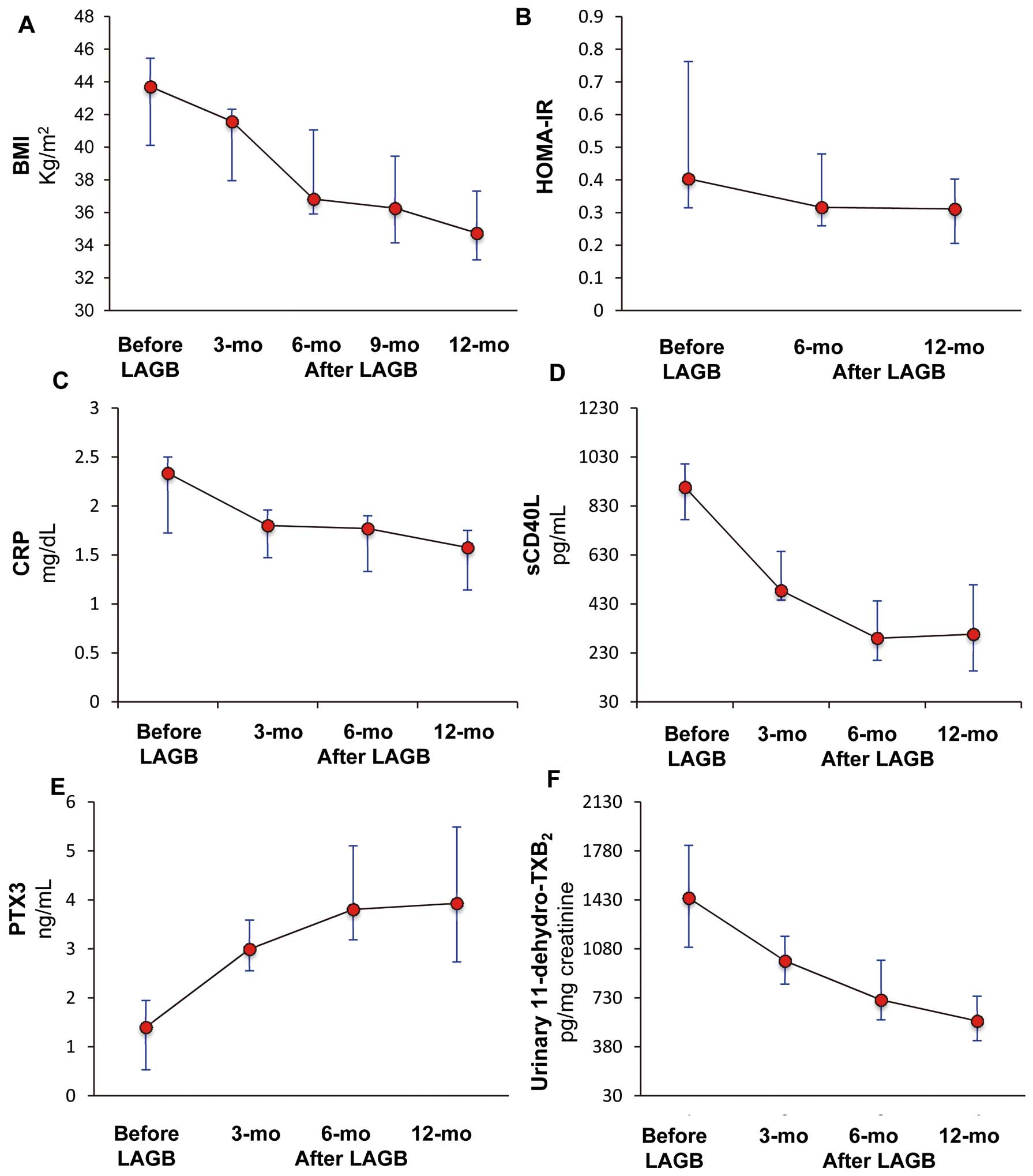
Compared to baseline, high-sensitivity CRP decreased by 24.0% (IQR: 22.0–26.0%) and 29.7% (IQR: 27.7–36.0%; P<0.0001), whereas plasma CD40L decreased by 64.3% (IQR: 44.2–77.3%) and 58.6% (IQR: 40.8–81.4%; P=0.002), respectively (Figures 1C,D). At these time points, plasma PTX3 increased by 178.8% (IQR, 59.2–502.8%) and 214.9% (IQR: 67.8–678.6%; P<0.0001), respectively 6 and 12 months after LAGB (Figure 1E).
Notably, urinary 11-dehydro-TxB2
decreased from 1,443 pg/mg creatinine (IQR: 1,048–1,879 pg/mg creatinine) to 715 pg/mg creatinine (IQR: 556–1,003 pg/mg creatinine) and to 564 pg/mg creatinine (IQR: 418–810 pg/mg creatinine), respectively 6 months and12 months after LAGB (P<0.0001;
Figure 1F).
Correlations
At baseline, urinary 11-dehydro-TxB2
correlated with FPG (rho=0.692, P=0.013) and HOMA-IR (rho=0.755, P=0.005) as well as with HDL and TG (rho=–0.733, P=0.007; rho=0.881, P<0.0001).
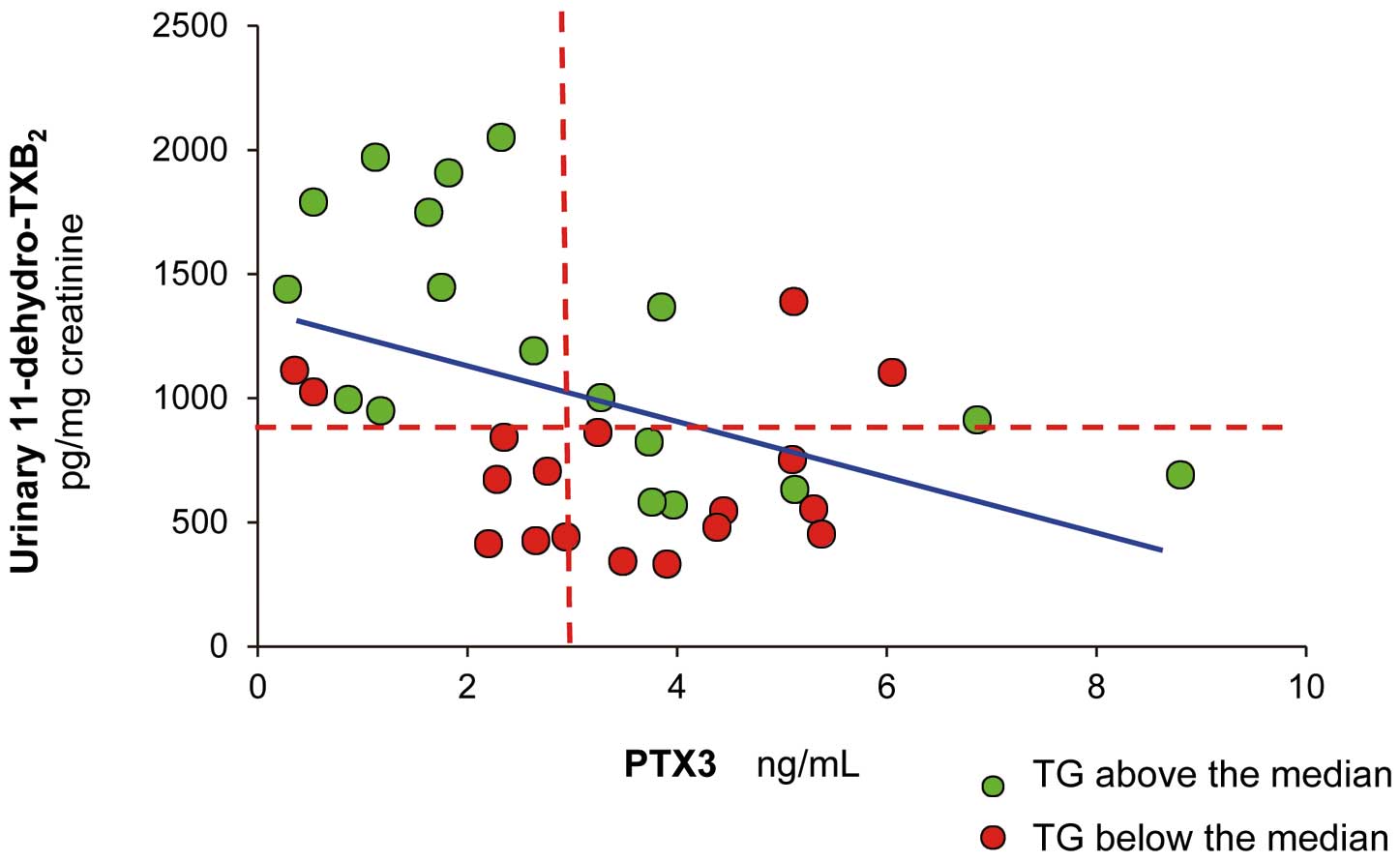
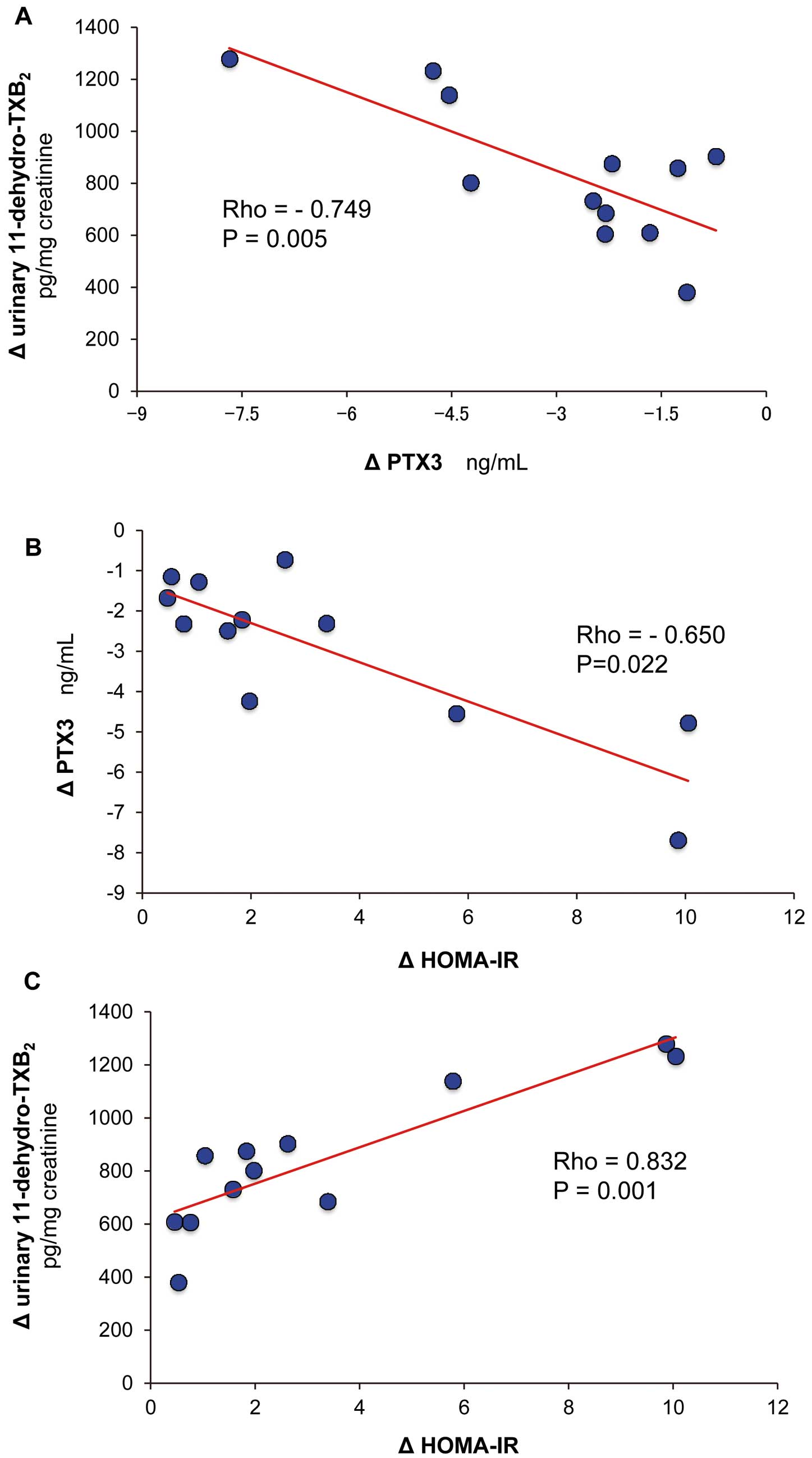
A statistically significant negative correlation between plasma PTX3 and urinary 11-dehydro-TxB2
delta variation from baseline at 12 months after LAGB (r=–0.749, P=0.005;
Figure 2A) was noted. In addition, delta PTX3 negatively correlated with delta HOMA-IR (rho=–0.650, P=0.022,
Figure 2B), and positively correlated with delta 11-dehydro-TxB2
(rho=0.832, P=0.001) and delta serum TG (rho=0.737, P=0.006;
Figure 2C; and data not shown). Serum TG correlated with urinary 11-dehydro-TxB2
delta variation (rho=0.821, P=0.001, data not shown).
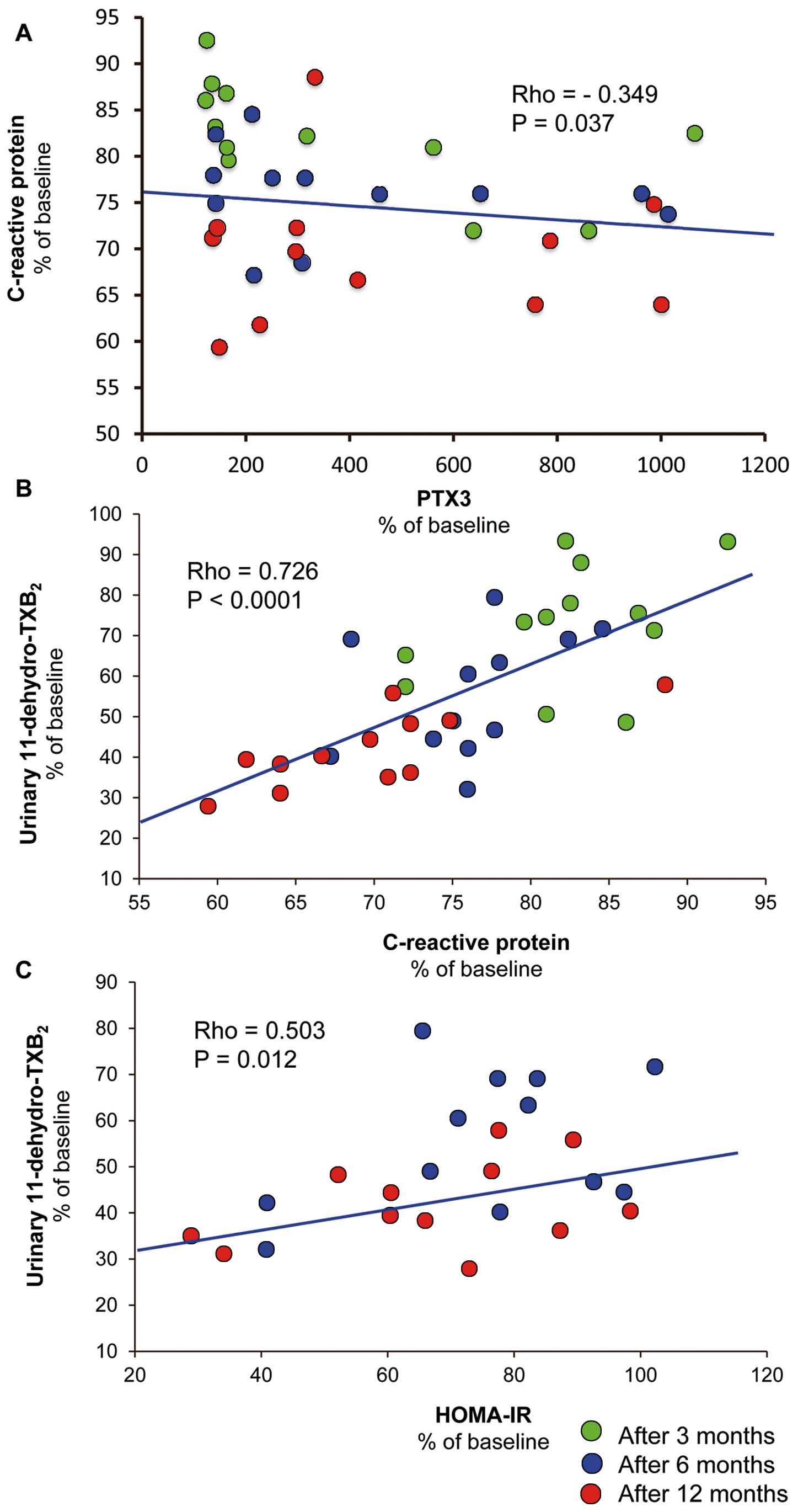
By pooling data from any time point after LAGB, we also observed that plasma PTX3 (% of baseline) negatively correlated with % baseline plasma CRP (rho=–0.349, P=0.037;
Figure 3A), and that urinary 11-dehydro-TxB2
(% of baseline) positively correlated with % baseline plasma CRP and HOMA-IR (rho=0.726, P<0.0001, and rho=0.503, P=0,012, respectively;
Figures 3B,C). It also correlated with % baseline BMI (rho=0.350, P=0.036), WC (rho=0.353, P=0.035), HDL cholesterol (rho=–0.571, P=0.004), and % loss of excess weight (rho=–0.352, P=0.035; data not shown). Plasma CD40L throughout the study was related to both PTX3 and urinary 11-dehydro-TxB2
(rho=–0.487, P<0.0001; rho=–0.500, P<0.0001, respectively).
Multiple regression analysis on pooled data (baseline, 6 and 12 months after LAGB) showed that either PTX3 (β=–0.392; t-value, –2.973; P=0.008), TG concentration (β=0.46; t-value, 3.395; P=0.003) or excess weight (β=0.39; t-value, 2.918; P=0.008) were associated with urinary 11-dehydro-TxB2, independently of HOMA-IR, BMI, WC, total and HDL cholesterol, FPG, PPG, fat mass, CRP and CD40L (adjusted R2=0.611, P=0.008;
Figure 4).
Discussion
Excess adiposity should be considered a chronic disease with serious health consequences. Indeed, obesity is reaching epidemic proportions in many of the industrialized countries.25
Surgery provides the only effective long-term treatment for severely obese patients. Surgical procedures for obesity have evolved in recent years and today they are associated with reduced mortality and nutritional complications, although they produce slower weight loss, compared with malabsorptive procedures.26
In this respect, the analysis of the biochemical consequences of surgical procedures for obesity is of great interest, given that it may identify early predictive biomarkers of the outcome of the procedure before substantial weight loss is achieved. For these reasons, we examined a number of markers of inflammation, glucose and lipid dysmetabolism, and platelet activation at various times after banding surgery.
Previously, we reported that banding surgery is associated with an approximately 60% decrease27
of the excretion rate of 11-dehydro-TxB2, a marker of platelet activation in vivo.28,29
In the present study, we confirmed this finding, showing that significant reduction can already be observed 3 months after the procedure, when the weight loss is approximately 10% (Table). Consistent with early data from cohorts of obese subjects enrolled in dietary weight loss programs, the decrease in 11-dehydro-TxB2
correlated with consistent reduction in systemic inflammation, as reflected by serum CRP,17
and plasma CD40L,28
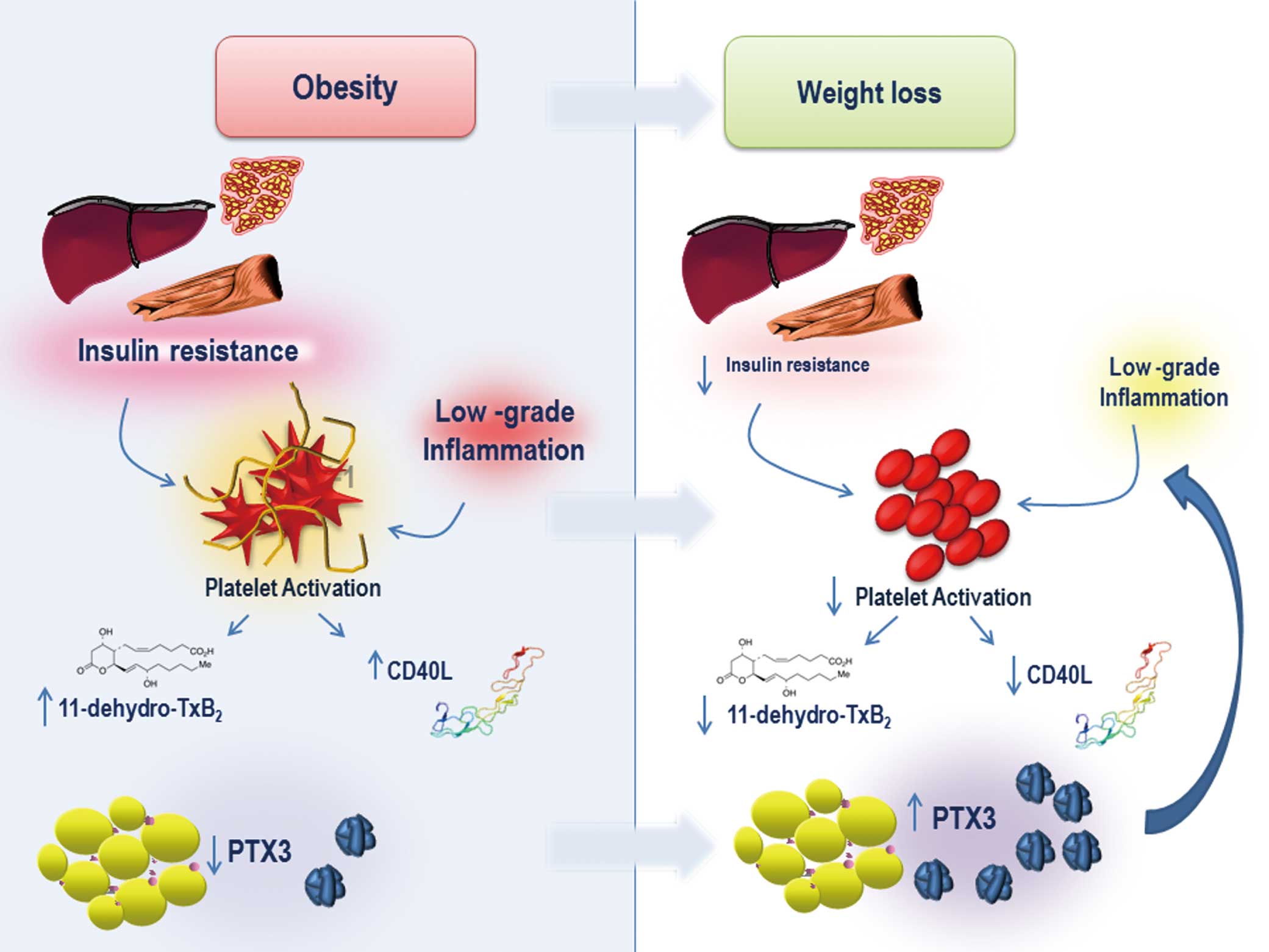
and with an increase in insulin sensitivity. This suggests that amelioration of ongoing low-grade inflammatory, driven by visceral fat and insulin resistance, may favorably downregulate the release of platelet-derived inflammatory molecules that sustain platelet activation, such as CD40L, thus contributing to dampen, at least in part, Tx biosynthesis. The fine-tuning of this complex interplay, in terms of the mechanisms responsible for the waning of inflammation and platelet activation, after successful weight loss, has never been elucidated in this clinical setting.
In the present study, we have shown for the first time that gastric banding is associated with a significant increase in plasma PTX3, which was evident 3 months after banding (+64.4%) to reach approximately +214% at 6 and 12 months (Table;
Figure 1). PTX3 was inversely related to platelet activation markers, that is, 11-dehydro-TxB2
and CD40L (Figure 2). Moreover, plasma PTX3 was an independent predictor of urinary 11-dehydro-TxB2
(Figure 4). Together, these results provide further clinical evidence of the relationship between in vivo platelet activation and inflammation in obesity, pointing to PTX3 as a potential novel and early biomarker of the improvement of endogenous anti-inflammatory circuits following banding. One likely hypothesis is that circulating PTX3 may bind to activated platelets through P-selectin binding, thereby reducing their ability to form aggregates with neutrophils and monocytes, thus limiting their activation.2,8
Along these lines, an effect of bariatric surgery in the modulation of monocyte-platelet aggregates in blood has been recently reported.30
Circulating P-selectin was not assessed in this study, but we have consistently reported a parallel increase and a significant direct correlation between plasma P-selectin and urinary 11-dehydro-TxB2
in several clinical settings, such as diabetes, hypertension and hypercholesterolemia or in sedentary subjects, with reduction of both variables after improvement of the metabolic control with glycemia-reducing agents, statins or with physical exercise.24,31–33
We think that such increase in circulating P-selectin may be rather the consequence of platelet activation, than a primary cause of it. Indeed, P-selectin is a key regulator of platelet-leukocyte interactions more than of platelet activation itself.
In contrast, the present data are consistent with previous studies showing that PTX3 is inversely associated with BMI and WC as well as with insulin resistance16
and with the number of metabolic syndrome components,34
and is significantly reduced in metabolic syndrome.35
Visceral adipose tissue PTX3 gene expression is increased in obesity,36
and has previously been associated with cardiovascular risk factors.37
Moreover, visceral, but not subcutaneous adipose tissue, is associated with circulating PTX3 level.16
Coupled with previous available data, the present findings concur to make the hypothesis of an adipose tissue-specific contribution to circulating PTX3 protein level plausible. It is tempting to hypothesize that in the presence of adipocyte dysfunction associated with visceral obesity and insulin resistance, the adipose-specific PTX3 gene is dysregulated, resulting in reduced plasma PTX3, further contributing to the persistence of low-grade inflammation. In this regard, weight loss, by reducing insulin resistance, would enhance PTX3 secretion by adipose tissue, counteracting inflammation and platelet activation in this clinical setting (Figure 5). The correlation between PTX3 increase and both HOMA-IR and 11-dehydro-TxB2
decrease would support this speculation (Figure 2). In this respect, PTX3 regulation in adipose tissue would be similar to that of the anti-inflammatory adipokine adiponectin, the gene for which is located in the same chromosomal region (3q) of the PTX3 gene,38
and whose protein is homologous to the PTX3 ligand C1q.39
Although PTX3 was not evaluated in our early work,17,28
we can speculate that diet-induced weight loss, by reducing insulin resistance, would, similarly to surgery, enhance PTX3 secretion by the adipose tissue, thus counteracting inflammation and limiting platelet activation.
Although further studies are needed to assess the relative contribution of different cellular sources to circulating PTX3 level in obesity, whatever its origin, plasma PTX3 has emerged as a pro-resolving mediator that counteracts inflammation and platelet activation.
Thus, the present results support a role of PTX3 in obesity, glucose dysmetabolism, platelet activation and cardiovascular risk.
The present findings also underscore the positive impact of bariatric surgery on obesity-associated inflammation and pro-thrombotic state. This is consistent with accumulating evidence showing that, in addition to weight, bariatric surgery reduces several cardiovascular risk factors, such as systemic hypertension, dyslipidemia, sleep apnea, cardiac dysfunction, and glucose metabolism disturbances, with a net survival benefit.25,26
Moreover, bariatric surgery can prevent DM2, particularly in subjects with altered fasting glucose.40
We also observed an 83% reduction in the 1-year incidence of the risk of developing T2DM or impaired fasting glucose. This risk reduction is approximately twice greater than that observed with lifestyle interventions in moderately obese subjects.41
Surgery significantly affected FPG, PPG, and insulin sensitivity (HOMA) in the present obese subjects, with or without IGT, supporting the hypothesis that improvement of insulin resistance induced by means of weight loss may be beneficial not only to diabetic obese individuals, but also to those obese subjects with early disturbances of glucose metabolism.42
The percentage of subjects with overt diabetes was unusually low, as compared with previous reports,39
probably because the Obesity Center, where enrollment took place, preferentially manages non-diabetic patients, unlike the Diabetes Clinic. Nevertheless, we can speculate that the present results would be even more striking for subjects with overt diabetes, given the acknowledged beneficial effects of bariatric surgery in this setting.43
Consistent with previous results, bariatric surgery was associated with a drastic reduction in circulating CD40L (Table;
Figure 1), a pro-inflammatory, pro-thrombotic mediator released by platelets,44
key determinants of increased cardiovascular disease in obesity and metabolic syndrome. Given that adipocytes express CD40 (the CD40L receptor), which triggers MAPK and nuclear factor kB activation, as well as inflammatory adipokine production,44
it is likely that lowering CD40L keeps under control this pro-inflammatory circuit in obesity.
Conclusions
Regardless of some limitations, mainly due to the relatively low number of patients observed, the present study strengthens the concept that interplays between inflammation, platelet activation and metabolic dysfunction do occur in obesity, and that PTX3 represents a key player within these circuits. Thus, the information provided by the gastric banding procedure may facilitate targeted treatment for obesity and its consequences.
Acknowledgments
This study was partially supported by a grant from the Italian Ministry of University and Research (PRIN n. 2010JS3PMZ to F.S.).
Disclosures
Each author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.
Bonacina F, Baragetti A, Catapano AL, Norata GD. Long pentraxin 3: Experimental and clinical relevance in cardiovascular diseases. Mediators Inflamm 2013; 2013: 725102.
- 2.
Vilahur G, Badimon L. Biological actions of pentraxins. Vascul Pharmacol 2015; 73: 38–44.
- 3.
Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol 2010; 11: 328–334.
- 4.
Han B, Ma X, Zhang J, Zhang Y, Bai X, Hwang DM, et al. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest 2012; 92: 1285–1296.
- 5.
Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2008; 117: 1055–1064.
- 6.
Kimura S, Inagaki H, Haraguchi G, Sugiyama T, Miyazaki T, Hatano Y, et al. Relationships of elevated systemic pentraxin-3 levels with high-risk coronary plaque components and impaired myocardial perfusion after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. Circ J 2014; 78: 159–169.
- 7.
Igarashi G, Iino K, Watanabe H, Ito H. Remote ischemic pre-conditioning alleviates contrast-induced acute kidney injury in patients with moderate chronic kidney disease. Circ J 2013; 77: 3037–3044.
- 8.
Maugeri N, Rovere-Querini P, Slavich M, Coppi G, Doni A, Bottazzi B, et al. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J Immunol 2011; 187: 970–979.
- 9.
Carrizzo A, Lenzi P, Procaccini C, Damato A, Biagioni F, Ambrosio M, et al. Pentraxin 3 induces vascular endothelial dysfunction through a P-selectin/matrix metalloproteinase-1 pathway. Circulation 2015; 131: 1495–1505.
- 10.
Haibo L, Xiaofang G, Chunming W, Jie Y, Guozhong C, Limei Z, et al. Prognostic value of plasma pentraxin-3 levels in patients with stable coronary artery disease after drug-eluting stent implantation. Mediators Inflamm 2014; 2014: 963096.
- 11.
Matsubara J, Sugiyama S, Nozaki T, Akiyama E, Matsuzawa Y, Kurokawa H, et al. Incremental prognostic significance of the elevated levels of pentraxin 3 in patients with heart failure with normal left ventricular ejection fraction. J Am Heart Assoc 2014; 3: e000928, doi:10.1161/JAHA.114.000928.
- 12.
Akaike M. Pentraxin-3: A potential novel biomarker detecting vulnerable plaque and predicting prognosis in acute myocardial infarction. Circ J 2014; 78: 65–66.
- 13.
Miyaki A, Choi Y, Maeda S. Pentraxin 3 production in the adipose tissue and the skeletal muscle in diabetic-obese mice. Am J Med Sci 2013; 347: 228–233.
- 14.
Chu SH, Park JH, Lee MK, Jekal Y, Ahn KY, Chung JY, et al. The association between pentraxin 3 and insulin resistance in obese children at baseline and after physical activity intervention. Clin Chim Acta 2012; 413: 1430–1437.
- 15.
Barazzoni R, Aleksova A, Carriere C, Cattin MR, Zanetti M, Vinci P, et al. Obesity and high waist circumference are associated with low circulating pentraxin-3 in acute coronary syndrome. Cardiovasc Diabetol 2013; 12: 167.
- 16.
Witasp A, Carrero JJ, Michaëlsson K, Ahlström H, Kullberg J, Adamsson V, et al. Inflammatory biomarker pentraxin 3 (PTX3) in relation to obesity, body fat depots, and weight loss. Obesity (Silver Spring) 2014; 22: 1373–1379.
- 17.
Davì G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, et al. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002; 288: 2008–2014.
- 18.
Vazzana N, Guagnano MT, Aceto L, Innocenti P, Davì G. Thromboxane-dependent platelet activation after gastric banding in obesity. JAMA Surg 2014; 150: 179–180.
- 19.
Dixon JB, Zimmet P, Alberti KG, Rubino F; International Diabetes Federation Taskforce on Epidemiology and Prevention. On behalf of the International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: An IDF statement for obese type 2 diabetes. Diabet Med 2011; 28: 628–642.
- 20.
Fielding GA, Allen JW. A step-by-step guide to placement of the LAP-BAND adjustable gastric banding system. Am J Surg 2002; 184: 26S–30S.
- 21.
American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care 2013; 1: S11–S66.
- 22.
Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann Clin Biochem 1998; 35: 745–753.
- 23.
Ciabattoni G, Pugliese F, Davì G, Pierucci A, Simonetti BM, Patrono C. Fractional conversion of thromboxane B2 to urinary 11-dehydrothromboxane B2 in man. Biochim Biophys Acta 1989; 992: 66–70.
- 24.
Santilli F, Vazzana N, Iodice P, Lattanzio S, Liani R, Bellomo RG, et al. Effects of high-amount-high-intensity exercise on in vivo platelet activation: Modulation by lipid peroxidation and AGE/RAGE axis. Thromb Haemost 2013; 110: 1232–1240.
- 25.
Poirier P, Cornier MA, Mazzone T, Stiles S, Cummings S, Klein S, et al; American Heart Association Obesity Committee of the Council on Nutrition,
Physical Activity, and Metabolism. Bariatric surgery and cardiovascular risk factors: A scientific statement from the American Heart Association. Circulation 2011; 123: 1683–1701.
- 26.
Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013; 127: 945–959.
- 27.
Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007; 357: 2482–2494.
- 28.
Basili S, Pacini G, Guagnano MT, Manigrasso MR, Santilli F, Pettinella C, et al. Insulin resistance as a determinant of platelet activation in obese women. J Am Coll Cardiol 2006; 48: 2531–2538.
- 29.
DeFilippis AP, Oloyede OS, Andrikopoulou E, Saenger AK, Palachuvattil JM, Fasoro YA, et al. Thromboxane A2 generation, in the absence of platelet COX-1 activity, in patients with and without atherothrombotic myocardial infarction. Circ J 2013; 77: 2786–2792.
- 30.
Periasamy M, Lieb DC, Butcher MJ, Kuhn N, Galkina E, Fontana M, et al. Bariatric surgery decreases monocyte-platelet aggregates in blood: A pilot study. Obes Surg 2014; 24: 1410–1414.
- 31.
Santilli F, Formoso G, Sbraccia P, Averna M, Miccoli R, Di Fulvio P, et al. Postprandial hyperglycemia is a determinant of platelet activation in early type 2 diabetes mellitus. J Thromb Haemost 2010; 8: 828–837.
- 32.
Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: Effects of statin therapy. Circulation 2002; 106: 399–402.
- 33.
Guagnano MT, Ferroni P, Santilli F, Paoletti V, Manigrasso MR, Pescara L, et al. Determinants of platelet activation in hypertensives with microalbuminuria. Free Radic Biol Med 2009; 46: 922–927.
- 34.
Ogawa T, Kawano Y, Imamura T, Kawakita K, Sagara M, Matsuo T, et al. Reciprocal contribution of pentraxin 3 and C-reactive protein to obesity and metabolic syndrome. Obesity 2010; 18: 1871–1874.
- 35.
Osorio-Conles O, Guitart M, Chacón MR, Maymo-Masip E, Moreno-Navarrete JM, Montori-Grau M, et al. Plasma PTX3 protein levels inversely correlate with insulin secretion and obesity, whereas visceral adipose tissue PTX3 gene expression is increased in obesity. Am J Physiol Endocrinol Metab 2011; 301: 1254–1261.
- 36.
Alberti L, Gilardini L, Zulian A, Micheletto G, Peri G, Doni A, et al. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis 2009; 202: 455–460.
- 37.
Breviario F, D’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, et al. Interleukin-1-inducible genes in endothelial cells: Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem 1992; 267: 22190–22197.
- 38.
Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 2002; 13: 84–89.
- 39.
Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012; 367: 695–704.
- 40.
Diabetes Prevention Program Research Group. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374: 1677–1686.
- 41.
Vazzana N, Santilli F, Sestili S, Cuccurullo C, Davi G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr Med Chem 2011; 18: 5267–5280.
- 42.
Santilli F, Davì G, Consoli A, Cipollone F, Mezzetti A, Falco A, et al. Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J Am Coll Cardiol 2006; 47: 391–397.
- 43.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes: 3-year outcomes. N Engl J Med 2014; 370: 2002–2013.
- 44.
Lutgens E, Poggi M, Weber C. CD40L-CD40 fuel ignites obesity. Thromb Haemost 2010; 103: 694–695.