Article ID: CJ-15-0952
Article ID: CJ-15-0952
Background: Although there are several known prognostic determinants in heart failure (HF), individual risk profiles can vary, in particular between ischemic and non-ischemic HF background. This study investigated the difference in prognostic efficacy of cardiac 123I-meta-iodobenzylguanidine (MIBG) imaging between the 2 etiologies.
Methods and Results: All 1,322 patients with HF were enrolled and followed up at most after 10 years. The HF patients were divided into 2 groups: an ischemic group (n=362) and non-ischemic group (n=960), and Cox proportional hazards model was used for data analysis. During 10 years of follow-up, 296 (22.4%) of 1,322 patients died; the mortality rates were 21.8% and 22.6% for the ischemic and non-ischemic groups, respectively. The ischemic group had greater prevalence of sudden death and lethal acute myocardial infarction, and the non-ischemic group had a higher rate of pump failure death. On multivariate Cox proportional hazards analysis using categorized variables, in the ischemic group, delayed heart-to-mediastinum ratio (HMR; P<0.0001), age (P=0.0002) and LVEF (P=0.03) were the independent significant predictors of lethal events. In the non-ischemic group, delayed HMR (P<0.0001), NYHA class (P<0.0001) and age (P<0.0001) were significant determinants of lethal outcome.
Conclusions: Cardiac MIBG imaging has nearly identical prognostic value in both ischemic and non-ischemic HF, independent of cause of cardiac death.
Among the various cardiac imaging modalities, 123I-meta-iodobenzylguanidine (MIBG) imaging has the unique feature of visualizing sympathetic nervous function. MIBG has a similar mechanism to norepinephrine uptake, storage and release in the nerve endings.1 Increased tone of cardiac sympathetic nerve activity impaires MIBG uptake, corresponding to increased spill-over and deficiency of norepinephrine. This cardiac sympathetic nerve dysfunction is associated with increased occurrence of unfavorable cardiac events, including pump failure and lethal cardiac arrhythmia. Recent propective and meta-analytic studies using MIBG scintigraphy in North America, Europe, and Japan have shown that it can predict cardiac death and fatal arrhythmia.2–5 In ischemic heart failure (HF), the prognostic value of myocardial perfusion imaging has been well established.6–8 Although there are several known prognostic determinants of HF, individual risk profiles can vary, in particular between ischemic and non-ischemic HF background. These differences between ischemic profiles have not been investigated as yet in long-term prognostic studies.9–14
This study focused on cardiac death and, in particular, the prediction of fatal cardiac events in both ischemic and in non-ischemic subjects using a long-term pooled database of MIBG imaging.4 The purpose of the present study was to examine the prognostic value of MIBG imaging and to examine the contributing factors in each ischemic and non-ischemic subject with HF.
Individual datasets from 6 prospective MIBG cohort studies performed in Japan between 1990 and 2009 were combined to make a pooled database of 1,322 chronic HF patients, as previously reported.4 Patients with HF from 6 Japanese medical institutions provided data for this study. All patients were enrolled in prospective observational studies, in which MIBG was approved for clinical use in Japan.15,16 All the original cohort studies were approved by the ethics committee or institutional review board in each hospital, and informed consent was obtained from the patients.
To create a single pooled database, cardiology specialists and/or nuclear medicine specialists in 6 hospitals created databases of common clinical parameters and updated the outcome in some institutions. This study includes the patients who were regularly followed up at the outpatient clinic of each facility for 10 years at most. The demographics of this population are summarized in Table 1. The subjects were divided into 2 groups: an ischemic group (n=362) and a non-ischemic group (n=960). The underlying diseases in the non-ischemic group were dilated cardiomyopathy (58%), valvular disease (13%), hypertensive heart disease (8%) and miscellaneous causes of HF (21%) including arrhythmia, secondary cardiomyopathies (diabetes, collagen diseases, etc), myocarditis and cardiac sarcoidosis. Eleven patients had implantable cardioverter defibrillators (ICD) and 7 had cardiac resynchronization therapy (CRT).
| Ischemic group | Non-ischemic group | P-value | |
|---|---|---|---|
| Age (years) | 66.5±11.0 | 59.1±13.5 | <0.0001 |
| Male | 278 (76.8) | 664 (69.2) | 0.0063 |
| DM | 35.2 | 19.2 | <0.0001 |
| Hypertension | 33.8 | 29.9 | 0.41 |
| BNP (pg/ml) (n=512) | 400.4±344.8 | 364±441 | 0.41 |
| Time to event (years) | 5.8±2.8 | 5.8±3.2 | 0.83 |
| LVEF (%) | 36.4±13.4 | 36.9±13.9 | 0.54 |
| HMR | 1.75±0.37 | 1.74±0.34 | 0.30 |
| Drug treatment | |||
| ACEI | 40.1 | 36.6 | 0.25 |
| ACEI or ARB | 66.3 | 64.7 | 0.60 |
| β-blocker | 51.8 | 54.2 | 0.46 |
| Loop diuretics | 69.3 | 69.8 | 0.90 |
| Aldosterone antagonist | 45.9 | 48.0 | 0.71 |
Data given as mean±SD, n (%) or %. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; DM, diabetes mellitus; HF, heart failure; HMR, heart-to-mediastinum ratio; ICD, implantable cardioverter-defibrillators; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The subjects with HF underwent MIBG imaging when they had become stabilized after HF treatment. Anterior planar images using scinticameras equipped with low-energy-type collimators were obtained at 15–30 min (early phase) and 3–4 h (late phase) after injection of 111 MBq 123I-MIBG (FUJIFILM RI Pharma, Tokyo, Japan) in all institutions.4 To calculate heart-to-mediastinum ratio (HMR), a whole heart region and a mediastinal rectangular region in the upper mediastinum were drawn manually.16 Both early and delayed HMR, as well as washout rate (WR) of MIBG, were calculated in each institution.
Left Ventricular (LV) FunctionLV ejection fraction (LVEF) was determined using gated blood-pool study (n=317, 34%), 2-D echocardiography (n=498, 54%), gated single-photon emission computed tomography (SPECT; n=98, 11%) and either gated blood-pool study or echocardiography (n=18, 2%).
OutcomeAll causes of death including HF death, sudden cardiac death (witnessed cardiac arrest and death within 1 h after onset of acute symptoms or unexpected death in patients known to have been well within the previous 24 h), acute myocardial infarction (AMI) and non-cardiac death were determined by the original study investigators. Patient medical records or telephone interviews in each institution confirmed clinical cutcome. All patients were regularly followed up for the mean follow-up interval of 77.6 months. The final outcome and cause of death were evaluated after 10 years.
Statistical AnalysisAll the clinical information and MIBG results were sent to a central institution (Kanazawa University), and were independently analyzed. Data are expressed as mean±SD. Contingency table analysis was examined using likelihood ratio and Pearson statistics. Univariate and multivariate Cox proportional hazard analysis were performed with the categorized variables, for example age (≤65 years, >65 years), New York Heart Association (NYHA) functional class (I/II vs. III/IV), late HMR (HMR <1.4, 1.4≤HMR<1.7, 1.7≤HMR<2.0, HMR ≥2.0) and LVEF (≤35%, >35%).4,5 P<0.05 was considered significant. Survival curves for patient subgroups were created using the Kaplan-Meier method and compared using log-rank test. B-type natriuretic peptide (BNP) was not used in the multivariate Cox analysis because of the limited number of subjects (n=512) available for analysis. Statistical analysis was performed using JMP 10.0.2 (SAS Institute, Cary, NC, USA).
Table 1 lists clinical background according to presence of ischemic background. LVEF was 36.4±13.4% in the ischemic group and 36.9±13.9% in the non-ischemic group (Table 1). The ischemic HF subjects were older and more frequently diabetic and had a prevalence of male sex compared with the non-ischemic group. Representative planar MIBG imaging in patients with and without cardiac events is shown in Figure 1.

Representative planar 123I-meta-iodobenzylguanidine (MIBG) imaging in patients with non-ischemic heart failure and (A) no cardiac event, and (B) cardiac event. (A) A 73-year old man with dilated cardiomyopathy (DCM) in New York Heart Association (NYHA) class II. Left ventricular ejection fraction (LVEF) was 40%, and MIBG heart-to-mediastinum ratio (HMR) was calculated as 1.83. He had been free from cardiac event since the MIBG study. (B) A 72-year-old woman with DCM in NYHA class III. MIBG HMR was 1.34 and LVEF was 36%. She experienced an episode of ventricular tachycardia after MIBG study and lethal cardiac event occurred.
Nineteen variables, including clinical factors and LVEF, were analyzed using univariate Cox hazard analysis in the ischemic group (n=362) and in the non-ischemic group (n=960; Table 2). In the ischemic group, age, LVEF, WR, early HMR, delayed HMR, dyslipidemia and NYHA functional class were the significant determinants of cardiac death. In the non-ischemic group, age, LVEF, WR, early HMR, delayed HMR, dyslipidemia, ventricular tachycardia (VT) and NYHA were the significant determinants of cardiac death.
| Ischemic group | Non-ischemic group | |||||
|---|---|---|---|---|---|---|
| χ2 | HR (95% CI) | P-value | χ2 | HR (95% CI) | P-value | |
| Age | 3.74 | 1.02 (1.00–1.04) | 0.053 | 1.91 | 1.02 (1.01–1.04) | <0.0001 |
| Male | 0.27 | 1.14 (0.71–1.92) | 0.60 | 3.7 | 1.32 (1.00–1.87) | 0.053 |
| LVEF | 21.7 | 0.96 (0.94–0.98) | <0.0001 | 21.0 | 0.98 (0.97–0.99) | <0.0001 |
| WR | 23.5 | 1.04 (1.02–1.06) | <0.0001 | 55.6 | 1.03 (1.02–1.04) | <0.0001 |
| Early HMR | 10.6 | 0.32 (0.15–0.65) | 0.0011 | 7.56 | 0.65 (0.48–0.89) | 0.0059 |
| Delayed HMR | 22.9 | 0.20 (0.10–0.40) | <0.0001 | 70.6 | 0.16 (0.10–0.25) | <0.0001 |
| DM | 2.26 | 1.38 (0.90–2.10) | 0.13 | 0.49 | 1.12 (0.81–1.53) | 0.49 |
| Hypertension | 2.76 | 1.23 (0.96–1.53) | 0.090 | 3.74 | 1.18 (1.00–1.39) | 0.050 |
| Dyslipidemia | 3.98 | 0.63 (0.38–1.00) | 0.046 | 9.59 | 0.55 (0.36–0.81) | 0.0020 |
| VT | 1.34 | 1.38 (0.85–2.17) | 0.19 | 7.72 | 1.46 (1.12–1.90) | 0.0055 |
| NYHA (I/II vs. III/VI) | 10.8 | 2.06 (1.34–3.12) | 0.0010 | 72.2 | 3.08 (2.39–4.00) | <0.0001 |
| Log10BNP† | 10.7 | 4.18 (1.74–10.6) | 0.0010 | 48.6 | 1.50 (1.07–1.95) | <0.0001 |
†n=512. AF, atrial fibrillation; CI, confidence interval; HR, hazard raio; VT, ventricular tachycardia; WR, washout rate. Other abbreviations as in Table 1.
Multivariate Cox proportional harzards analysis using categorized variables demonstrated that in the ischemic group, delayed HMR (HR, 0.58; P<0.0001), age (HR, 2.23; P=0.0002) and LVEF (HR, 0.62; P=0.03) were the independent significant predictors of lethal events (Table 3). On multivariate Cox proportional harzards analysis using categorized variables, in the non-ischemic group, delayed HMR (HR=0.61, P<0.0001) and HYHA (HR=2.43, P<0.0001) and age (HR=1.76, P<0.0001) were significant determinants of lethal outcome (Table 4).
| HR (95% CI) | P-value | |
|---|---|---|
| Late HMR | 0.58 (0.46–0.73) | <0.0001 |
| Age (<65 years, ≥65 years) | 2.36 (1.49–3.85) | 0.0002 |
| LVEF (≤35%, >35%) | 0.62 (0.40–0.95) | 0.03 |
HMR <1.4, 1.4≤HMR<1.7, 1.7≤HMR<2.0, HMR ≥2.0. Abbreviations as in Tables 1,2.
| HR (95% CI) | P-value | |
|---|---|---|
| Late HMR | 0.61 (0.53–0.70) | <0.0001 |
| NYHA (I/II,III/IV) | 2.43 (1.87–3.18) | <0.0001 |
| Age (<65 years, ≥65 years) | 1.76 (1.36–2.28) | <0.0001 |
HMR <1.4, 1.4≤HMR<1.7, 1.7≤HMR<2.0, HMR ≥2.0. Abbreviations as in Tables 1,2.
Kaplan-Meier event-free curves according to HMR and LVEF in the ischemic group and in the non-ischemic group are shown in Figure 2. The optimal cut-off threshold for HMR, which represents maximum log-rank chi-squared on the Kaplan-Meier event-free curve, was 1.7 for both the ischemic and the non-ischemic groups.
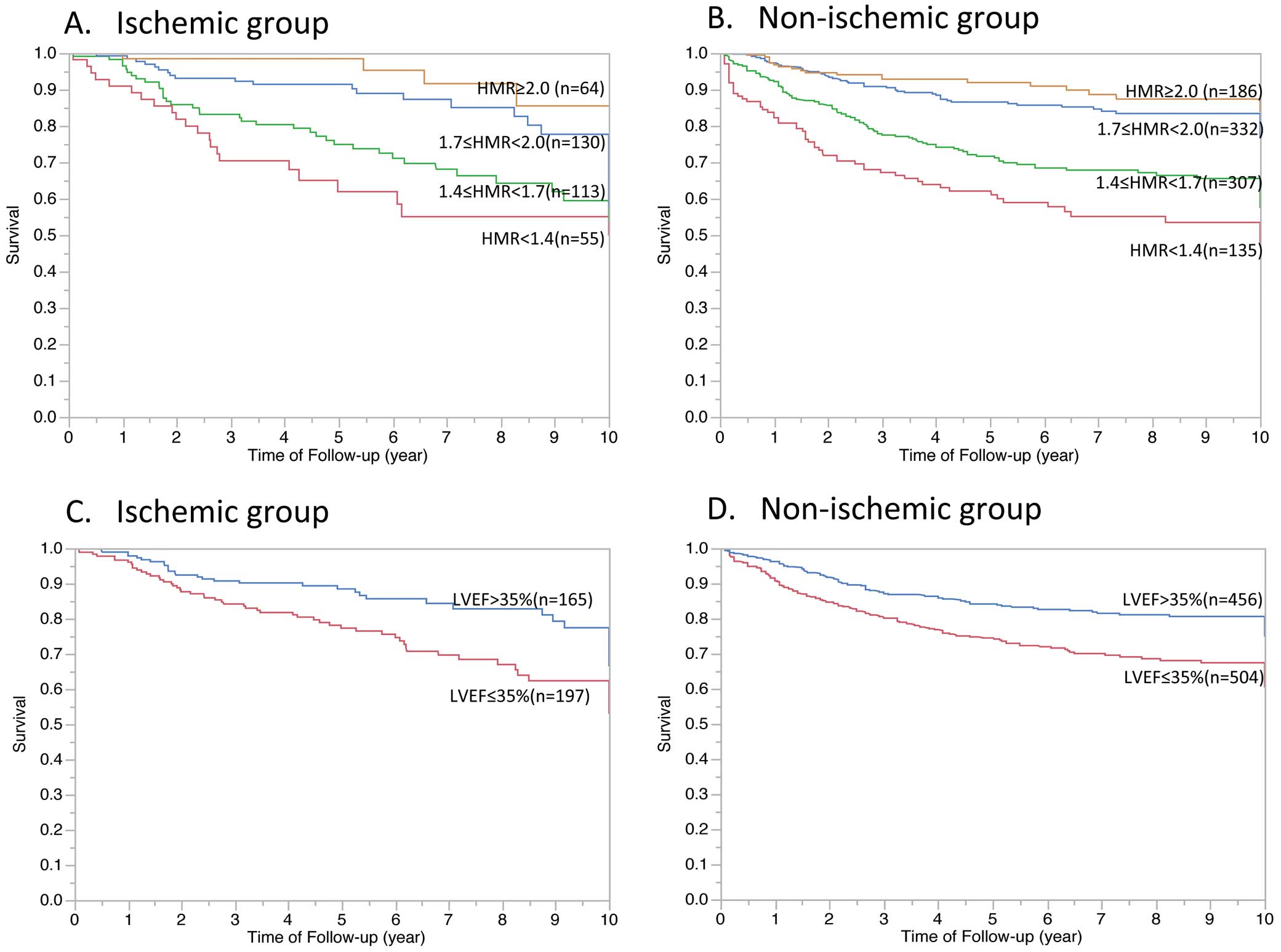
Kaplan-Meier event-free curves according to (A,B) heart-to-mediastinum ratio (HMR) and (C,D) left ventricular ejection fraction (LVEF) in (A,C) ischemic and (B,D) non-ischemic heart failure patients.
In the ischemic group, 85 patients had died during the follow-up period: 29 (34%) due to sudden cardiac death, 34 (40%) due to HF, 9 (11%) due to AMI and 13 (15%) due to non-cardiac causes. Among 217 subjects who died in the non-ischemic group, death was due to sudden cardiac death in 46 (21%), HF in 123 (57%), AMI in 4 (2%), and non-cardiac causes in 44 (20%). Table 5 lists the causes of death in patients with delayed HMR <2.0, given that HMR ≥2.0 has a low risk of mortality.5 In the ischemic group, sudden cardiac death and death due to AMI were significantly more common (P=0.018, P=0.0005, respectively). Death due to pump failure occurred much more frequently in the non-ischemic group compared with the ischemic group (P=0.0023).
| Ischemic group, n (%) | Non-ischemic group, n (%) | P-value | |
|---|---|---|---|
| n | 75 | 199 | |
| Sudden cardiac death | 27 (36) | 36 (18) | 0.018 |
| Pump failure | 28 (37) | 118 (59) | 0.0023 |
| AMI | 9 (12) | 3 (2) | 0.00050 |
| Non-cardiac death | 11 (15) | 42 (21) | 0.17 |
Abbreviations as in Table 1.
The present study of HF patients using large-scale multicenter data showed that delayed HMR on 123I-MIBG imaging was a strong predictor of cardiac mortality, either for ischemic or non-ischemic status. Cardiac sympathetic nerve study plays an important role in predicting death in HF patients. The present study clearly demonstrated the MIBG delayed HMR can determine both high-risk and low-risk probability for patient survival, with only minor differences in the cardiac death contributing factors. Thus the measurement of cardiac sympathetic nerve function on 123I-MIBG imaging might be a better approach for assessing the severity of the disease and for better risk stratification in patients with HF in accordance with previous studies.13,14 The present long term follow-up study showed that this usefulness of 123I-MIBG imaging could apply to both ischemic and non-ischemic patients with HF. This study also demonstrated that there were more deaths due to AMI or sudden cardiac death in ischemic HF patients, and that there was a higher possibility of death due to pump failure in the non-ischemic HF group.
Recent Multicenter MIBG StudiesRecent multicenter and meta-analytical studies have demonstrated the clinical value of 123I-MIBG imaging in patients with HF.2–4 Use of cardiac 123I-MIBG activity thresholds such as for HMR can identify patients at increased risk of fatal outcome. Therefore, decreased cardiac MIBG activity predicts decreased patient survival. Furthermore, cardiac 123I-MIBG improves prognostic value when it is used together with conventional prognostic markers such as NYHA functional class, age and LVEF.4 Increased prognostic value with regard to sympathetic nerve imaging is also supported by recent clinical studies using nuclear imaging techniques.16,17 A prediction model for stratifying risk in HF patients has been constructed using 5-year follow-up data,5 but multi-center studies did not elaborate on the ischemic profile of HF patients, and could not establish the usefulness of MIBG scintigraphy with regard to ischemia status.5
Mechanism of Decreased MIBG Uptake in Ischemic HFSeveral mechanisms in the ischemic group could be responsible for lethal clinical outcome related to impaired sympathetic activity in HF.18 The sympathetic nerve is sensitive to ischemia. The repeated episodes of ischemia in the myocardium due to coronary spasm or stenotic lesion may cause functional changes in the nerve fiber.19 123I-MIBG imaging defects were always more extensive than those for 201Tl, thus indicating that the area of sympathetic degeneration was not limited to the necrotic zone but also extended to the non-infarcted area.20 Sympathetic nerves may be more susceptible than cardiac muscle to permanent ischemic damage.21 In ischemic HF, 123I-MIBG indices indicated that long-term mortality rate was dependent on HMR. The present study showed that LVEF was one of the prognostic determinants in the ischemic group. Ischemic cardiomyopathy including ischemia and infarction causes reduced systolic function in accordance with disease progression.21,22 Ischemic profile is related closely to abnormal sympathetic nerve function, and can be a therapeutic target through coronary intervention. Although the role of myocardial perfusion imaging has been established in ischemic HF,6–8 MIBG imaging may have additional or similar prognostic value over myocardial perfusion imaging.14,17,23–25
Mechanism of Cardiac Sympathetic Abnormality in Non-Ischemic HFSeveral mechanisms in non-ischemic cardiomyopathy are involved in impaired cardiac sympathetic activity. In hypertrophic cardiomyopathy, decreased MIBG uptake is closely related to myocardial damage including myocardial hypertrophy, disarray or fibrosis.26,27 Cardiac neuron deficits may occur in conjunction with myocyte injuries due to inflammation or a degenerative process such as necrosis in advanced HF.28,29 Non-ischemic HF was observed in a relatively large area of Japan, although ischemic HF is the most common etiology, and as much as 50% lower than in many Western countries.30 A total reduction in the number of sympathetic nerve terminals is related to the amount of damage to the affected neurons, and this myocardial damage incudes insufficiency of energy production with mitochondrial dysfunction.31,32 Increased plasma norepinephrine itself may reduce MIBG uptake in non-ischemic conditions, including HF and pheochromocytoma, and stressful situations can trigger cardiac sympathetic abnormalities, which can be a causative mechanism of HF.33 In dilated cardiomyopathy, delayed HMR predicted mortality more accurately, and the prognostic value of LVEF was limited.34,35 MIBG HMR was superior to LVEF in the non-ischemic group in the present study, and NYHA functional class was one of the significant determinants of prognosis in patients with non-ischemic HF. Previous studies reported that patient symptoms were relevant to diastolic function. Sympathetic nervous dysfunction is closely related to diastolic function or mechanical dyssynchrony in phase analysis in non-ischemic cardiomyopathy.36,37 Low HMR (<1.6) was shown to predict response to CRT in HF patients.38,39
Clinical Value of MIBG in HF Irrespective of Ischemia StatusRegardless of HF etiology, myocardial ischemia may be involved in the HF process because of coronary microcirculation abnormalities due to elevated LV end-diastolic pressure and mitochondrial dysfunction.31
Evaluation of the sympathetic nerve provides prognostic information and contributes to better risk stratification in these patients. In the present study, the powerful prognostic value of HMR was irrelevant to ischemic status in patients with HF. Therefore, MIBG imaging can give cardiologists much better information than LV functional test with regard to risk stratification, selection of therapeutic strategy, and prediction of long-term survival in HF patients. MIBG HMR could be used to predict therapeutic outcome before pharmacologic intervention. Drugs such as β-blocker or renin-angiotensin-aldosterone system inhibitors can prolong survival and improve quality of life. MIBG study can provide critical information on drug efficacy and non-pharmacological therapy. In a recent study, the efficacy of device treatment, including ICD and CRT therapy, seemed to be better related to MIBG HMR.39 Arrhythmic death or appropriate ICD discharge for fatal ventricular arrhythmias are associated with denervated myocardium.17,18 Further investigation is needed to establish the cost-effectiveness and decision-making for these clnical treatments.
MIBG and Collimator ChoiceAll MIBG examinations in each institution were performed using low-energy-type collimators. The choice of collimator substantially influences estimation of HMR, given that the presence of high-energy photons leads to a significant amount of septal penetration, and multiple complex scattering due to background activity. The low-medium energy collimator has characteristics to cover the higher energy scatter portion of the 123I energy spectrum, in accordance with the widely used 123I-labeled radiopharmaceutical in Japan. Quantitative MIBG imaging might be best performed using ME collimators. Standardized methods are required to be used with various gamma camera systems.40 Furthermore, the cross-calibration method could be used to apply previous data to one’s own institution using a cardiac phantom.40 The present study successfully demonstrated the prognostic value of MIBG HMR derived from a similar low-energy-type collimator.
Study LimitationsFirst, BNP measurement was not available for all patients in the 1990 s and it could not be included in the hazard model. In the prognosis evaluation, the utility of BNP is limited because BNP increases depending on blood sampling time points.41 Relatively high BNP was observed after starting β-blocker treatment.41 MIBG HMR is more appropriate as a baseline assessment for long-term risk. Second, SPECT could be used to assess regional innervation, which indicates denervated but viable myocardium.42 Patients with severe HF tend to have markedly reduced sympathetic nerve function, such that SPECT images may be unable to be reconstructed, thereby making it difficult to achieve regional assessment.43 Therefore, further study of regional myocardial sympathetic nerve is needed using novel collimators, to facilitate treatment of localized denervation. Third, perfusion data were not available in the present study. Although the combined use of MIBG and perfusion agents had prognostic value in previous studies,39,44 the added value of MIBG for perfusion agent was not demonstrated in this study. Finally, recent reports showed that use of β-blocker has increased, in association with improved HF prognosis. Therefore, the treatment strategy for HF is very different between the early 1990 s and 2000 s.45 Therapeutic outcome should be evaluated with regard to recent optimal medical therapy.
MIBG imaging, as determined in the present large-cohort multicenter study, has prognostic value in both ischemic and non-ischemic heart disease, irrespective of ischemia status.
The present multicenter pooled dataset was created through the collaboration of the following 6 Japanese medical centers: Sapporo Medical University School of Medicine (Akiyoshi Hashimoto); Tokyo Women’s Medical University (Mitsuru Momose); Toho University Medical Center Ohmori Hospital (Junichi Yamazaki, Shohei Yamashina); Shiga University of Medical Science (Shintaro Yoshida, Toshiki Matsui); Cardiovascular Hospital of Central Japan (Shu Kasama); and Osaka Prefectural General Medical Center (Takahisa Yamada).
The authors declare no conflicts of interest.