Article ID: CJ-16-0101
Article ID: CJ-16-0101
Beta-blockers are the cornerstone treatment for congestive heart failure (HF). Current HF guidelines commonly recommend β-blockers for the treatment of HF with reduced left ventricular ejection fraction (LVEF). The effect of β-blockers, however, is less clear for HF patients with preserved LVEF, unstable severe acute HF, or right ventricular failure. This review summarizes the effect of β-blockers in various clinical situations and suggests a strategy for optimal use.
Congestive heart failure (CHF) is a major worldwide public health problem that requires a global response.1 The prevalence of CHF is continuously increasing as a result of enhanced survival after coronary artery disease, congenital or valvular heart disease, and social aging.2–6 Hospitalization for acute heart failure (AHF) is accompanied by substantial post-discharge morbidity, including a significant risk of death, and a high socioeconomic burden.
In HF patients, sympathetic activation compensates for a decline in pump function in the short term. Chronic sympathetic activation followed by hypertrophy and interstitial fibrosis, however, is eventually cardiotoxic and contributes to further deterioration of cardiac function in the later stages.7 Beta-blockers are the cornerstone treatment of CHF (Figure 1).8 Current HF guidelines recommend the use of β-blockers based on many randomized controlled trials (RCT) showing a reduction mortality rate >35%.9–11 In HF with reduced left ventricular (LV) ejection faction (HFrEF), plasma catecholamine concentrations are elevated whereas cardiac β-receptors are downregulated and become less responsive.12 Beta-blockers paradoxically reverse these alterations, reducing sympathetic drive and increasing β-receptor susceptibility. In HF with preserved LVEF (HFpEF),13 plasma catecholamine concentrations are not increased; but, even in the absence of epicardial coronary artery disease HFpEF patients do not normally have an increase in EF in response to exercise or β-adrenergic stimulation,14 suggesting β-adrenergic receptor desensitization.15 Compared with other standard HF medications such as angiotensin-converting enzyme inhibitors (ACEI) and aldosterone antagonists, β-blockers often lead to a more substantial improvement in EF and have an anti-ischemic effect, reducing the risk of sudden cardiac death.10 Here, we review the effect of β-blockers according to the status of HF and the tolerability and ideal dosage of different β-blockers, and suggest an optimal strategy for treatment with β-blockers.
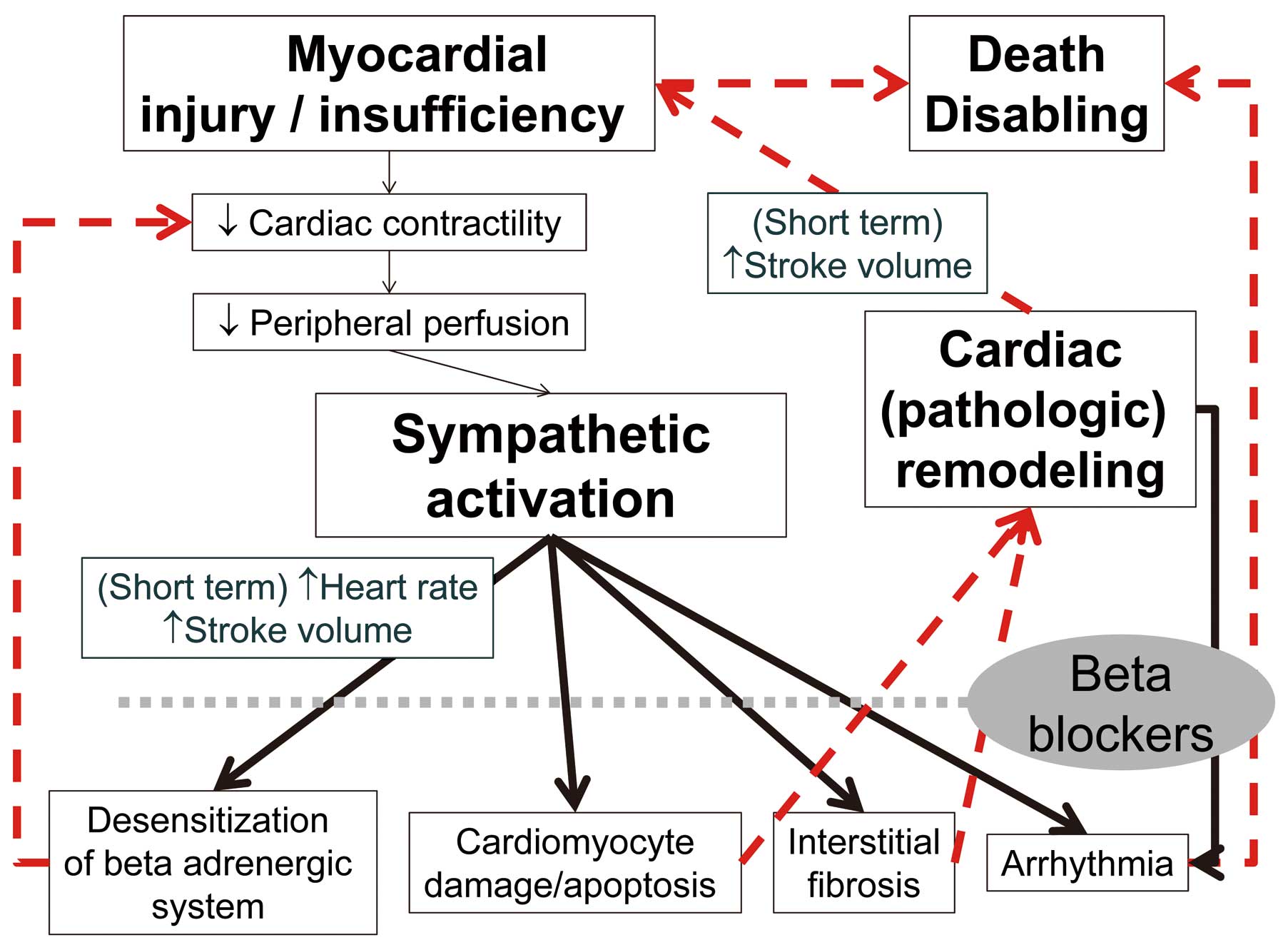
Mechanism of β-blockers in heart failure.
In 3 key trials (Cardiac Insufficiency Bisoprolol Study II [CIBIS II], Carvedilol Prospective Randomized Cumulative Survival [COPERNICUS], and Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure [MERIT-HF]) and 1 other trial (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure [SENIORS]), >11,000 patients with mild-severely symptomatic HF were randomly assigned to treatment with placebo or a β-blocker.16–20
CIBS-II was a European multicenter RCT of 2,647 subjects with HFrEF with New York Heart Association (NYHA) functional class III or IV at baseline using optimal medical therapy including ACEI. Subjects were randomly assigned to receive either up to 10 mg/day bisoprolol or placebo and were followed for an average of 1.3 years. The study was stopped early because interim analysis indicated a robust decrease in all-cause mortality (relative risk [RR], 0.66; P<0.0001). In general, bisoprolol was well tolerated, with an 85% study drug continuation. There were, however, more hospitalizations in the bisoprolol group vs. placebo for bradycardia (14 vs. 2; P<0.004) and stroke (31 vs. 16; P=0.04).16
MERIT-HF evaluated 3,991 subjects with HFrEF with NYHA functional class II–IV. Subjects were randomly assigned to receive either metoprolol succinate or placebo on top of optimal therapy including ACEI and diuretics. Subjects were followed for up to 21 months. Subjects in the metoprolol group were up-titrated to a maximum dose of 200 mg/day. The study was stopped early because interim analysis demonstrated a robust decrease in all-cause mortality (RR, 0.71; P<0.0001) and a decrease in sudden cardiac death (RR, 0.59; P=0.0002).17 Us Carvedilol study21 and COMET study22 also showed the benefits of carvedilol in HFrEF patients.
COPERNICUS evaluated 2,289 subjects with symptomatic HFrEF with LVEF <25% on baseline. Subjects were randomly assigned to receive either carvedilol or placebo on top of optimal medical therapy including ACEI, diuretics, digitalis (65–67%), and spironolactone (19–20%). Subjects were followed up for a mean of 10.4 months. Subjects in the carvedilol group were up-titrated to a maximum dose of 25 mg twice day. The study was stopped early because interim analysis demonstrated a robust decrease in all-cause mortality (RR, 0.65; P=0.0014), and the rate of combined death or HF hospitalization was also lower as a result of treatment with carvedilol (RR, 0.76; P<0.001). Carvedilol was well-tolerated, with more patients stopping the placebo drug (18.5%) than the study drug (14.8%; P=0.02).18
These findings are supported by the SENIORS trial, which evaluated 2,128 elderly (≥70 years) HF patients. Among enrolled patients, 36% had LVEF <35%. Subjects were randomized to receive either nebivolol or placebo on top of optimal medical therapy including ACEI (81.7–82.6%), diuretics (85.5–85.8%), angiotensin receptor antagonists (6.2–7.1%), digitalis (38.9–39.8%), and spironolactone (26.4–28.8%). The rate of combined death or HF hospitalization was significantly lower in the nebivolol group (HR 0.86, P=0.039), but all-cause death was not significantly different (HR 0.88, P=0.21).20
Another large RCT (Beta-blocker Evaluation of Survival Trial [BEST]) with bucindolol, a β-blocker with partial agonist properties, was stopped early at interim analysis because of “totality of evidence regarding the usefulness of β-blocker treatment derived from BEST and other studies”. At the time of study termination (average, 2.0 years), there were a total of 449 deaths in the placebo group (33%) and 411 deaths in the bucindolol group (30%, adjusted P=0.13). Notwithstanding, the findings were generally consistent with those of the aforementioned studies.23
The absolute risk reduction in mortality (after 1 year of treatment) in patients with mild-moderate HF (CIBIS II and MERIT-HF combined) was 4.3%, equating to an number needed to treat (NNT; for 1 year to postpone 1 death) of 23.10 The equivalent figures for severe HF (COPERNICUS) were 7.1% and 14. Similar findings were obtained in network meta-analysis. Beta-blockers credibly reduced mortality compared with placebo or standard treatment after a median of 12 months (OR, 0.69; 95% CI: 0.56–0.80), which seems to be largely due to a “class effect” with no evidence of a clearly superior agent among individual β-blockers.24 Several meta-analyses have suggested that β-blockers improve survival independent of baseline NYHA class25 or geographic region.26
In summary, β-blocker treatment in patients with HFrEF invariably reduced mortality and HF hospitalization within 1 year of starting treatment on top of standard treatment (Table 1).
| Trial | Agent | No. patients |
NYHA class |
Mean follow-up (months) |
Annual placebo mortality rate (%) |
Mortality risk reduction (%) |
Target dose (mg) |
Meandaily dose (mg) |
NNT |
|---|---|---|---|---|---|---|---|---|---|
| CIBIS-II16 | Bisoprolol (highly β1-selective) |
2,647 | III–IV | 15 | 13.2 | ↓34 | 10 once daily |
10† | 23 |
| MERIT-HF17 | Metoprolol succinate (moderately β1-selective) |
3,991 | II–IV | 12 | 11.0 | ↓34 | 200 once daily |
159 | 27 |
| US carvedilol trials21 | Carvedilol (non-β-selective+ α-blocker) |
1,094 | II–IV | 6.5 (median) |
7.8 | ↓65‡ | 25–50 twice daily |
45 | 15 |
| COPERNICUS19 | Carvedilol | 2,289 | III–IV | 10.4 | 19.7 | ↓35 | 25 twice daily |
37 | 15 |
| Carvedilol | 1,511 | II–IV | 58 | NR | ↓27 (carvedilol vs. metoprolol |
25 twice daily |
41.8 | ||
| COMET22 | Carvedilol vs. metoprolol tartrate |
1,518 | II–IV | 58 | NR | NR | 50 twice daily |
85 |
†Most common dose achieved in study. ‡Study design did not constitute a mortality trial. CIBIS-II, Cardiac Insufficiency Bisoprolol Study II; COMET, Carvedilol or Metoprolol European Trial; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival; HFrEF, heart failure with reduced left ventricular ejection fraction; MERIT-HF, Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure; NNT, number needed to treat; NR, not reported; NYHA, New York Heart Association.
HFpEF is a clinical syndrome involving multiple organ systems and is associated with various non-cardiac comorbidities such as renal dysfunction, chronic obstructive pulmonary disease, and obesity.27 Because HFpEF and HFrEF share numerous features such as neurohormonal activation, elevated filling pressure, and clinical signs of HF, β-blockers may be beneficial in HFpEF by lowering blood pressure, reducing LV hypertrophy, reducing diastolic dysfunction, and decreasing heart rate.28 Current evidence supporting the use of β-blocker therapy to treat HFpEF, however, is very limited. The SENIORS trial included a small proportion of HFpEF patients with EF >50%, and the effects of nebivolol were similar between HF patients with EF >35 and ≤35%. The study, however, was underpowered to address the effects of β-blockers in HFpEF.20 The SWEDIC trial reported that carvedilol treatment in HFpEF patients resulted in a significant improvement in echocardiographic parameters such as E/A ratio.29 The Japanese Diastolic Heart Failure (J-DHF) study in Japan randomly assigned 245 HFpEF patients to either the carvedilol group or the control group.30 There was no significant difference in the incidence of the primary endpoint (cardiovascular death or HF hospitalization) between the 2 groups. The average prescribed carvedilol dose, however, was only 7.5 mg daily and the results for patients prescribed carvedilol at >7.5 mg/day were significantly better than for the control.
Currently, there are no other prospective trials or RCT investigating the effects of β-blockers on HFpEF, but the results of several observational studies and meta-analyses have been published. Most recently, a propensity score-matched cohort study using the Swedish Heart Failure Registry reported that during a median follow-up of 709 days, the β-blocker treatment group was associated with a significantly lower rate of overall death (HR, 0.93; 95% CI: 0.86–0.996).28 In the matched HFrEF cohort, mortality reduction with β-blockers was 11% (HR, 0.89; 95% CI: 0.82–0.97). Last, a meta-analysis of 12 previous studies indicated that β-blockers might have beneficial effects on HFpEF.31
In summary, there is still uncertainty regarding the effect of β-blockers in HFpEF. Several observational studies and meta-analyses, however, suggest a significant benefit of β-blockers in HFpEF patients, albeit smaller than for HFrEF. For patients with HFpEF, at least the standard dose of β-blocker should be prescribed as for HFrEF.
Initiation of β-blocker treatment in patients with AHF may transiently worsen the hemodynamic status and increase HF symptoms. Pivotal trials with β-blockers conducted in patients with continuing symptoms and a persistently low EF, however, showed a benefit. Therefore, current guidelines recommend that β-blockers and ACEI should both be started as soon as possible after diagnosis of HFrEF (Table 2).10 Also, the dose should be up-titrated as far as possible before discharge and a plan made to complete up-titration after discharge. It has been shown that β-blocker treatment may be continued in many patients during an episode of decompensation and can be started safely before discharge after an episode of decompensation.
| ESC (2012)10 | ACCF/AHA (2013)40 | NICE (2014)34 | |
|---|---|---|---|
| Continuation during AHF hospitalization |
May be continued in many patients during an episode of decompensation, although dose reduction may be necessary |
Continue in the absence of hemodynamic instability or contraindications |
Continue unless patient has heart rate <50 beats/min, second- or third- degree atrioventricular block, or shock |
| Temporary discontinuation during AHF |
Advised in patients with shock or severe hypoperfusion |
Should be considered only in patients hospitalized after recent initiation or increase in β-blocker therapy or with marked volume overload or marginal/low cardiac output |
No recommendation |
| Initiation after de novo AHF |
Should be started as soon as possible before discharge |
Start or restart during hospital admission | Start or restart during hospital admission |
| Timing of initiation/ restarting |
After stabilization, blood pressure and heart rate permitting |
After optimization of volume status and successful discontinuation of i.v. diuretics, vasodilators, and inotropic agents |
Once patient condition has stabilized, eg, when i.v. diuretics are no longer needed |
| Cautious observation after initiation |
The dose should be up-titrated as far as possible before discharge, and a plan made to complete dose up-titration after discharge |
Should be initiated at a low dose and only in stable patients Caution should be used when initiating in patients who required inotropes during hospital course |
Ensure that the patient’s condition is stable for typically 48 h after starting or restarting and before discharge from hospital |
ACCF, American College of Cardiology Foundation; AHA, American Heart Association; AHF, acute heart failure; ESC, European Society of Cardiology; HF, heart failure; NICR, National Institute for Health and Care Excellence.
In real-world clinical practice, however, β-blockers are still underused for AHF. In the Heart Institute of Japan-Department of Cardiology Registry, only 34.2% of 3,578 consecutive patients hospitalized for CHF were prescribed β-blockers at discharge.32 Similarly, in the Korean Acute Heart Failure Registry (KorAHF), β-blockers were prescribed at discharge for only 51% of patients with AHF.33 The main concern is that if β-blockers are discontinued in patients who are admitted with acute exacerbation of their CHF for fear of additional negative inotropic effects of β-blockers on the myocardium, the patients may be labeled as intolerant to β-blockers and therefore not be restarted on these agents. This worst-case scenario may be preventing patients who would otherwise benefit from this treatment from receiving β-blockers in either the short or long term.34
The most recent AHF guidelines by the National Clinical Guideline Centre, UK, are as follows.34 First, patients with chronic HF presenting with AHF who are already taking β-blockers should continue the treatment unless they have a heart rate <50 beats/min, second- or third-degree atrioventricular block, or shock. Second, AHF patients should start or restart β-blockers during hospital admission once their condition has stabilized. The most likely criterion for AHF stabilization is cessation of i.v. diuretics. Last, the patients should be closely monitored for 48 h after starting or restarting β-blockers before discharge. These statements are very practical although the evidence is based on low-quality data from observational studies or RCT.
RV failure is frequently combined with HFrEF and is a powerful predictor of mortality and morbidity.35 RVEF <20% is an independent predictor of poor outcome in patients with HFrEF.36 There are very limited studies evaluating the effect of β-blockers on RV failure. A study on porto-pulmonary hypertension suggested that β-blockers may be detrimental in patients with RV failure.37 In contrast, a small study demonstrated that β-blockers improve RV systolic function in patients with HFrEF.38 In a subgroup analysis of the BEST, β-blocker treatment was suggested to increase the risk of mortality in HFrEF patients with RVEF <20%.36 BEST participants receiving bucindolol, however, had substantial improvement in both LVEF and RVEF during follow-up,39 which warrants further study.
Although the beneficial effect of β-blocker seems undisputed, whether target heart rate or target dose is more important in β-blocker therapy is the subject of debate. Current guidelines recommend up-titration of β-blockers to the target dose, as established in trials (Table 3).10,40 Many data, however, indicate that the degree of heart rate control is more important than the dose itself. Direct evidence of a relationship between clinical effects and the heart rate was assessed in the CIBIS-II trial.16 The heart rate reduction by bisoprolol was proportionately associated with a better improvement in survival. Also of note, in a meta-regression analysis of 23 β-blocker trials registering >19,000 patients, the mortality benefit was related to the magnitude of heart rate reduction but not to the dose of the β-blocker.41 Another meta-analysis showed that the magnitude of heart rate reduction was more important than achievement of target dose in predicting improved outcome.42
| Beta-blocker | Starting dose | Target dose |
|---|---|---|
| Bisoprolol | 1.25 mg once daily | 10 mg once daily |
| Carvedilol | 3.125 mg twice daily | 25–50 mg twice daily |
| Carvedilol CR | 10 mg once daily | 80 mg once daily |
| Metoprolol succinate (CR/XL) | 12.5/25 mg once daily | 200 mg once daily |
| Nebivolol | 1.25 mg once daily | 10 mg once daily |
These data suggest that heart rate should be considered more important than the actual dose when tailoring β-blocker therapy. In particular, in HF patients the target resting heart rate might be <70 beats/min. The reason why heart rate reduction is more important than β-blocker dose might be related to the large pharmacogenomic heterogeneity of β-blockers.43,44
Clinical response to HF medication varies significantly from patient to patient,45 and pharmacogenomic information has the potential to help tailor HF treatment. Among many single nucleotide polymorphisms in the β-adrenergic receptor genes, Arg389Gly in β-1 adrenergic receptor (ADRB1) has been most widely investigated.46 In vitro, ADRB1 Arg389 shows increased coupling to G proteins compared with Gly389, making the β-1 adrenergic receptor more active.47 In contrast, Gly389 in ADRB1 reduces the receptor sensitivity as if it were partially blocked.48–51 The allele frequency of ADRB1 Gly389 among Asian subjects was reported to be approximately 0.20–0.30, which is lower than that in Caucasian subjects (0.24–0.34) and African-American subjects (0.39–0.46).52–54 HF-ACTION data suggested that CHF patients with the Arg389Arg genotype required a higher dose of β-blockade to achieve a treatment response similar to that of Gly carriers.55 Similarly, in the Association between Beta-adrenergic receptor polymorphism and Bisoprolol therapy in heArt failure (ABBA) study performed in Korean CHF patients with LVEF <45%, the Arg389Arg ADRB1 genotype group required a 30% greater amount of bisoprolol than the Gly389X (Gly389Arg+Gly389Gly) genotype group in order to achieve the same level of heart rate reduction (Figure 2).54 In the β-blocker-naïve state, the Gly389X genotype group was reported to have a better prognosis and greater survival.56,57 In β-blocker-treated patients, the therapeutic impact of the ADRB1 genotype might be different according to the selectivity and dose of the β-blocker.58 The response to metoprolol, which has high β-1 selective blocking activity, was better in patients with the ADRB1 Arg389Arg genotype, resulting in improved LVEF and greater heart rate reduction.59,60 In contrast, the ADRB1 Arg389Arg genotype was reported to be associated with increased mortality in patients treated with carvedilol, a non-specific β-1 and β-2 blocker.61 Similarly, CHF patients with the ADRB1 Arg389Arg genotype receiving low-dose (<25 mg daily) carvedilol had a 2-fold increase in death risk compared with those treated with high-dose (>25 mg daily) carvedilol, which was not observed in the ADRB1 Gly carriers.55
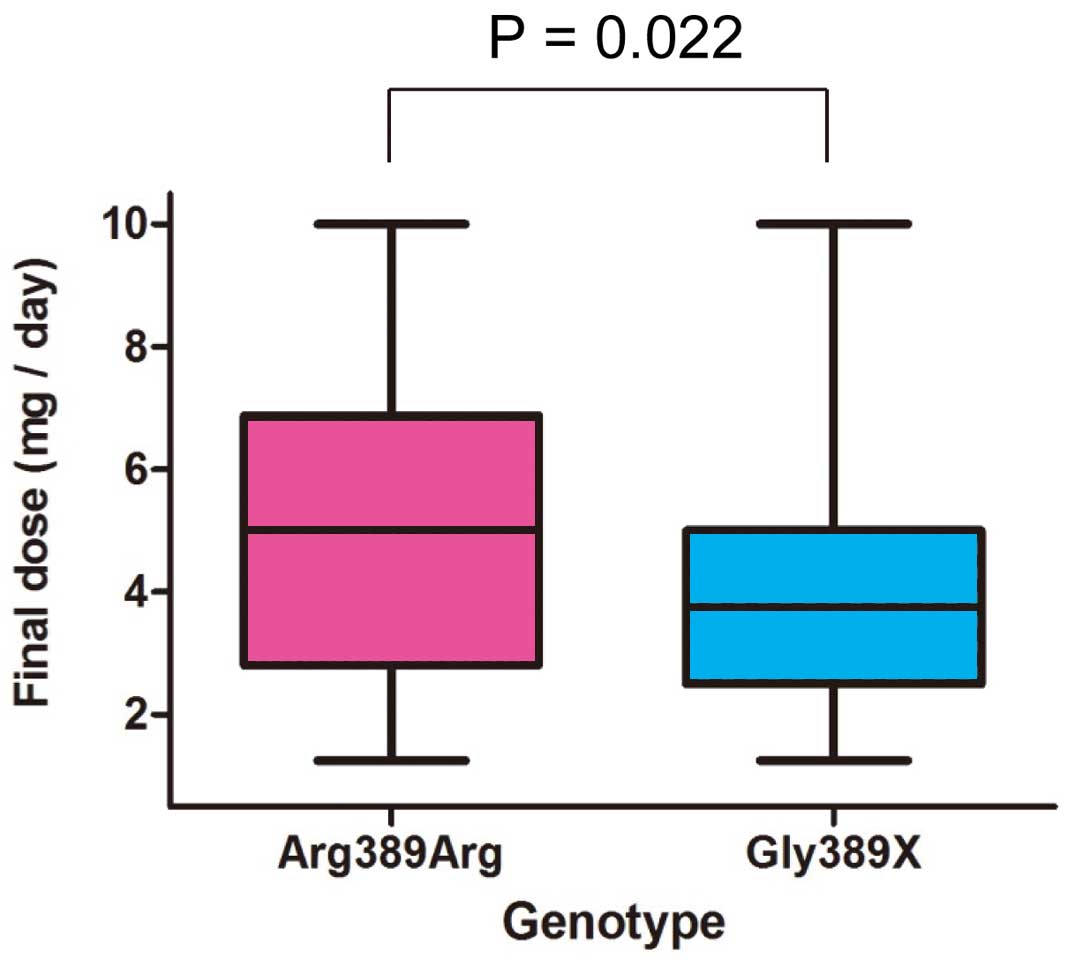
Different dose requirement of β-blockers in order to achieve the same target heart rate according to β-1 adrenergic receptor (ADRB1) genotype. (Adapted with permission from Lee HY, et al.54)
The most important concern of β-blocker therapy in real-world clinical practice is that patients are typically treated with a considerably lower dose than that confirmed to be effective in clinical trials.62 A recent prospective Registry to Improve the Use of Evidence-Based Heart Failure Therapy in the Outpatient Setting (IMPROVE-HF) trial enrolling 7,605 patients with HFrEF reported that only 20.5% of patients were treated with β-blockers at the target dose at enrolment, and after 24 months only 30.3% of eligible patients were taking the target dose.63 In most major HF trials, however, β-blocker therapy was well-tolerated, with study drugs being withdrawn no more frequently than the placebo.
A meta-analysis of 9 RCT of β-blocker use in CHF demonstrated their tolerability in the populations studied.64 Although the patients assigned to β-blocker therapy had higher rates of dizziness (21.5 vs. 16.7%; RR, 1.37) and bradycardia (5.7 vs. 1.8%; RR, 3.61), β-blocker therapy was well-tolerated, with more patients withdrawing from the placebo arm than the β-blocker arm (18 vs. 16%; RR, 0.89). In addition, this meta-analysis confirmed the decrease in all-cause mortality (RR, 0.73; 95% CI: 0.62–0.85) with an absolute risk reduction of 34 deaths per 1,000 patient years (NNT, 29).
Another meta-analysis pooled data from 13 RCT of 15,383 patients with HFrEF randomly assigned to receive either β-blocker (7,836 patients) or placebo on a background of standard treatment.65 Patients on β-blocker therapy had statistically significantly lower rates of tachycardia (RR, 0.51; 95% CI: 0.41–0.65), palpitations (RR, 0.71; 95% CI: 0.55–0.92), depression (RR, 0.72; 95% CI: 0.56–0.93), insomnia (RR, 0.76; 95% CI: 0.61–0.95), CHF aggravation (RR, 0.69; 95% CI: 0.56–0.87), and chest pain (RR, 0.80; 95% CI: 0.69–0.92). Patients on β-blocker therapy, however, were more likely to have hyperglycemia (RR, 1.31; 95% CI: 1.08–1.59), diarrhea (RR, 1.4; 95% CI: 1.18–1.67), dizziness (RR, 1.45; 95% CI: 1.17–1.80), claudication (RR, 2.47; 95% CI: 1.32–4.62), and bradycardia (RR, 3.45; 95% CI: 2.19–5.42). Remarkably, there was no statistically significant difference in the occurrence of commonly perceived β-blocker-related side-effects such as sexual dysfunction, hypotension, fatigue, syncope, or dyspnea.
Current HF guidelines commonly recommend use of β-blockers for HFrEF (Table 4).66,67 Even in situations of AHF, β-blocker treatment may be continued or should at least be started before discharge. Various β-blocker trials in CHF strongly suggest that heart rate reduction is more important than β-blocker dose. Finally, in contrast with safety concerns, β-blocker therapy was reported to be well-tolerated in both large-scale outcome trials and in meta-analyses. Therefore, potentially all patients with stable mild and moderate HF should take a β-blocker unless contraindicated.
| Who should receive β-blocker therapy? |
| - Potentially all patients with stable mild and moderate HF unless contraindicated (symptomatic hypotension or bradycardia, asthma, etc); patients with severe HF should be referred for specialist advice |
| When to start? |
| - In patients with stable NYHA class II–III, start as early as possible in course of disease |
| - In patients with NYHA class IV, as early as physical evidence of improved fluid retention (use diuretics accordingly). It is recommended to start β-blockers in the hospital (ie, before discharge) |
| How to use? |
| - Start with a low dose (Table 2) |
| - Double the dose at no less than 2-week intervals |
| - Aim for target dose or, failing that, the highest tolerated dose |
| - Remember that some β-blocker is better than no β-blocker |
| - Monitor heart rate, blood pressure, clinical status (symptoms and signs, especially signs of congestion, body weight) |
| - Check blood chemistry 1–2 weeks after initiation and 1–2 weeks after final dose titration |
| Problem solving |
| - If symptoms/signs of congestion (eg, increasing dyspnea, fatigue, edema, weight gain) increase, increase dose of diuretic first. If increasing diuretic does not work, consider halving the dose of β-blocker |
| - If marked fatigue (and/or bradycardia) occurs, halve the dose of β-blocker (rarely necessary) |
| - If heart rate is <50 beats/min and symptoms worsen, halve β-blocker dose or, in cases of severe deterioration, stop β-blocker (rarely necessary). First, consider stopping other drugs with heart rate lowering effect (eg, digoxin, amiodarone, diltiazem, verapamil) |
| - In cases of symptomatic hypotension (SBP <80 mmHg), consider reducing other drugs first (eg, nitrates, calcium antagonists, other vasodilators). If no signs/symptoms of congestion, consider reducing diuretic dose or ACEI |
| - Importantly, β-blockers should not be stopped suddenly unless absolutely necessary (there is a risk of a “rebound” increase in myocardial ischemia/infarction and arrhythmias) – ideally specialist advice should be sought before treatment discontinuation |
ACEI, angiotensin-converting enzyme inhibitor; SBP, systolic blood pressure. Other abbreviations as in Tables 1,2.
None declared.