Abstract
Background:
The long-term prognosis of cardiac ryanodine receptor (RyR2) positive catecholaminergic polymorphic ventricular tachycardia (CPVT) patients after initiation of medical therapy has not been well investigated. This study aimed to assess the recurrence of fatal cardiac event after initiation of medical therapy in
RyR2-positive CPVT patients.
Methods and Results:
Thirty-four
RyR2-positive CPVT patients with a history of cardiac events were enrolled. All patients had medical treatment initiated after the first symptom or diagnosis. Exercise stress tests (ESTs) were performed to evaluate the efficacy of the medical therapy. Even after the initiation of medical therapy, high-risk ventricular arrhythmias (VAs), including premature ventricular contraction couplets, bigeminy, and ventricular tachycardia, were still induced in the majority of patients (80.6%). During 7.4 years of follow-up after the diagnosis, 7 of the 34 (20.6%) patients developed fatal cardiac events. Among those 7 patients, 6 (85.7%) were not compliant with either exercise restriction or medication therapy at the time of the events.
Conclusions:
Even after initiation of medical treatment, high-risk VAs were induced during EST in most
RyR2-positive CPVT patients. Most fatal recurrent cardiac events occurred in patients who were noncompliant with exercise restriction and/or medical therapy. Medical management including strict exercise restriction should be emphasized to prevent recurrent cardiac event in most
RyR2-positive CPVT patients.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare arrhythmogenic inherited disorder characterized by physical activity- or emotional stress-induced ventricular arrhythmias (VAs) in the absence of cardiac structural abnormalities. Mutations in genes encoding the cardiac ryanodine type 2 receptor (RyR2)1,2
and calsequestrin 2 (CASQ2)3,4
have been identified and are recognized as causing the autosomal-dominant and -recessive forms of CPVT, respectively.3,5
Inward rectifier potassium channel 2 (KCNJ2) mutations are also reported to cause CPVT.6–8
At the molecular level, gain-of-function mutations in the cardiac ryanodine receptor encoded by
RyR2
account for at least 50% of CPVT cases and are annotated as type 1 CPVT (CPVT1).9
Editorial p ????
CPVT is one of the most malignant cardiac channelopathies expressed predominately in the young and has been recognized as a significant cause of sudden cardiac death in children and young adults. Because CPVT is a rare disease, there have been limited reports regarding the management and prognosis of these patients.9–14
In addition, medical management of CPVT has changed over the past decades and the prognosis after contemporary medical therapy has not been well investigated either.
Exercise stress tests (ESTs) are one of the most important tests for the diagnosis of CPVT and should be done for suspected patients.9,12,13
The reproducibility of VAs during ESTs in suspected patients is considered as strong evidence of CPVT and ESTs are also useful for the assessment of the efficacy of medical therapy. The main purpose of this study was to investigate the result of ESTs and the recurrence cardiac events in
RyR2-positive CPVT patients receiving medical therapy.
Methods
Patients Characteristics
The study population consisted of 34 CPVT patients with
RyR2
mutations (32 probands and 2 family members), in whom a genetic analysis was conducted between 2006 and 2014 at the National Cerebral and Cardiovascular Center and Shiga University of Medical Science. All patients had a history of cardiac events before initiation of medical treatment. All probands were referred to the 2 centers for the diagnosis and treatment of suspected CPVT because of a bidirectional ventricular tachycardia (VT), polymorphic VT and catecholaminergic idiopathic VT, syncope, or aborted cardiac arrest occurring during physical exercise or emotional stress. Evaluation of the patients included a 12-lead ECG, EST, echocardiography, 24-h Holter ECGs, and genetic analyses for
RyR2
mutations. CPVT was diagnosed clinically in probands with a history of sudden cardiac arrest or syncopal episodes during physical activity or acute emotional stress in conjunction with exercise- or catecholamine-induced polymorphic or bidirectional VT. In all patients, ECG was normal at rest and echocardiogram showed no structural abnormalities.13
All probands underwent genetic testing for
RyR2
mutations and family screening was recommended when a
RyR2
mutation was identified in a proband. First-degree relatives of affected individuals were also evaluated, and CPVT was diagnosed if polymorphic or bidirectional VT was observed on Holter monitoring during exercise or catecholamine challenge, or if genetic testing was positive for the disease-causing mutation in the family.15
All patients were registered on our database at the time of genetic diagnosis and followed prospectively. Clinical prognosis and additional clinical information, including cardiac events and results of EST, were obtained before the final analysis.
Genetic Analysis
All probands and family members provided informed consent for the clinical and genetic evaluations. The institutional ethics committee approved the protocols for the genetic analysis. Genomic DNA was isolated from whole blood using a DNA analyzer (QIAGEN GmbH, Hilden, Germany). In all clinically suspected CPVT patients, we screened the
RyR2
gene.
RyR2
mutations were screened in all 105 exons of the gene. A
RyR2
mutation was defined when the mutation was not identified in any of the 100 control subjects.
Therapy and Follow-up
Medical management, including β-blockers, calcium-channel blockers, and flecainide, was initiated immediately after the first symptom or clinical diagnosis. After the diagnosis was confirmed, all patients (and their parents when appropriate) were strongly advised to avoid any strenuous physical activity and emotional stress. The β-blocker was the first line of therapy, and titrated up until the maximum tolerable dose, based on the patients’ age and body weight. Propranolol was used most commonly and titrated up to 2–4 mg·kg–1·day–1, as much as we could, until the patient developed side effects including hypotension, dizziness, and bradycardia. When the patients could not tolerate one type of β-blocker, we switched to another. When there was evidence of incomplete suppression of VAs, such as recurrent syncope or a high-risk VA during the EST, we titrated the β-blocker up as much as we could. If β-blocker could not suppress the VA sufficiently, then we added flecainide or a calcium-channel blocker. Flecainide was started as low as 50 mg/day and increased up to 200 mg/day. Therapy such as medications and implantable defibrillators (ICDs) was decided according to contemporary guidelines,16,17
depending on each individual physician and patient. ICD was strongly recommended for patients with previous cardiac arrest, or with recurrent syncope or documented VT despite medical management. In patients with an ICD, accurate information on recurrence of VAs and aborted cardiac arrest was confirmed using the device. The rest of the patients were determined to have a cardiac event if they experienced syncope likely caused by VA or cardiac arrest under physical or emotional stress during the follow-up. For all cardiac events, detailed investigations were performed to identify the activity during the event. Light, moderate, and vigorous intensity activity was defined as 1.1–2.9 metabolic equivalents (METs), 3.0–5.9 METs, and ≥6.0 METs, respectively, according to the Physical Activity Guidelines for Americans (http://www.health.gov/). We defined non-vigorous activity as activity <3.0 Mets.
The subjects were routinely seen at the outpatient clinic every 3–6 months for a clinical review including a device interrogation or whenever the patient experienced symptoms possibly related to VA or a device discharge. The follow-up period was counted from the date of the clinical or genetic diagnosis (whichever came first) to the date of the last visit. Patients who had not been followed up for 1 year were excluded.
EST (Exercise Stress Test)
An EST was performed using a treadmill according to the Bruce or Ramp protocol. The EST was continued until maximum exercise capacity or bigeminy premature ventricular contraction (PVC), bidirectional VT, polymorphic VT, or VF was induced.
Statistical Analysis
Continuous variables are expressed using interquartile ranges (IQR). Comparison between groups was performed with a Student’s t-test for paired data. Categorical variables were compared with Fisher’s exact test. A value of P<0.05 was regarded as significant. Survival curves were plotted using Kaplan-Meier methods and analyzed by a log-rank test. Data were analyzed with JMP Version 11 software (SAS Institute Inc, Cary, NC, USA).
Results
Characteristics of the Study Subjects
The demographic characteristics of all patients are shown in
Table 1. A total of 32 probands and 2 family members of
RyR2-positive probands were included; 56% of patients (19/34) were females. All 34 patients had a history of cardiac events before initiation of medical treatment and the average age at the time of the first symptom was 8.6±4.8 (IQRs 5.0) years. Among the 32 probands, 7 (21.9%) had a family history of a cardiac event, including sudden cardiac death or multiple syncopal episodes, during exercise or emotional stress. Regarding the other comorbidities, 8 of 34 (23.5%) patients had a history of sustained atrial arrhythmias.
Table 1.
Clinical Characteristics of CVPT Patient Cohort
| No. of patients |
34 |
| No. of probands |
32 |
| Age at clinical or genetic diagnosis (years) |
13.9±9.8 |
| Age at first symptom (years) |
8.6±4.8 |
| Female |
19 (55.9%) |
| Family history |
7 (21.9%) |
| CPA before diagnosis |
13 (38.2%) |
| Trigger of cardiac episodes before diagnosis |
| Physical activity |
26 (76.5%) |
| Non-physical activity |
20 (58.8%) |
| Emotional stress |
12 (35.3%) |
| Normal activity |
12 (35.3%) |
| Probands with ≥3 cardiac events before diagnosis |
20 (62.5%) |
| Parameters on ECG |
| Resting HR (beats/min) |
65.3±14.6 |
| QTc (ms) |
407±33 |
| Documented atrial arrhythmia |
8 (23.5%) |
| Documented ventricular arrhythmia* |
| Bigeminy |
21 (61.8%) |
| Bidirectional VT |
23 (67.7%) |
| Polymorphic VT |
22 (64.7%) |
| Medication at last follow-up (34 patients) |
| β-blocker |
32 (94.1%) |
| Calcium-channel blocker |
5 (14.7%) |
| Flecainide |
23 (67.7%) |
| ICD |
9 (26.5%) |
| Pacemaker |
2 (5.9%) |
*Includes ventricular arrhythmias during exercise stress test. CPA, cardiopulmonary arrest; CPVT, catecholaminergic polymorphic ventricular tachycardia; HR, heart rate; VT, ventricular tachycardia.
Before the clinical or genetic diagnosis, 13 patients had a cardiac arrest, while the 21 other patients had syncopal episodes during exercise or emotional stress (Tables 1,2).
Table 2.
Results of EST After Initiation of Medical Therapy, Medication During EST* and Situation of Cardiac Event During Follow-up
| |
Proband
or family |
Mutation |
Age at
EST |
Most
severe
episodes |
Medication
dose/day |
Achieved
METs |
Reason for
termination |
Induced
ventricular
arrhythmia |
Recurrence
after
initiation of
treatment |
Situation |
| 1 |
Proband |
g/a
12066_12067ins
CAA g5656a |
25 |
Syncope |
Pindolol
5 mg |
7.2 |
pVT |
pVT |
Syncope† |
Vigorous
activity |
| 2 |
Proband |
c.6643 T>C |
7 |
CPA |
Propranolol
140 mg,
flecainide
100 mg |
11.4 |
Dyspnea |
Bigeminy |
No recurrence |
|
| 3 |
Proband |
c.11836 G>A |
9 |
CPA |
Atenolol
100 mg |
9.5 |
pVT |
pVT |
Syncope |
Quarrel |
| 4 |
Proband |
c. 11812 A>G |
4 |
Syncope |
Bisoprolol
10 mg,
verapamil
180 mg |
7.2 |
pVT |
pVT |
No
recurrence |
|
| 5 |
Proband |
c.12513 G>T |
8 |
Syncope/VT |
Bisoprolol
10 mg,
verapamil
180 mg,
flecainide
150 mg |
7.2 |
pVT |
pVT |
No
recurrence |
|
| 6 |
Proband |
c.5170 G>A |
39 |
Syncope |
Carvediolol
2.5 mg,
flecainide
100 mg |
15.2 |
Fatigue |
Bigeminy |
No
recurrence |
|
| 7 |
Daughter of
Patient 6 |
c.5170 G>A |
8 |
CPA |
Flecainide
100 mg |
11.4 |
Fatigue |
Bigeminy |
CPA |
Vigorous
activity |
| 8 |
Proband |
c.7204 T>C |
28 |
CPA |
Atenolol
50 mg |
14.8 |
Fatigue |
Single PVC |
No
recurrence |
|
| 9 |
Proband |
c.14461 G>A |
10 |
Syncope/VT |
Atenolol
25 mg,
verapamil
75 mg |
14.8 |
pVT |
pVT |
Syncope |
Playing with
other kids |
| 10 |
Proband |
c.13489 C>T |
14 |
CPA |
Propranolol
60 mg |
14.0 |
Bigeminy,
fatigue |
Bigeminy |
No
recurrence |
|
| 11 |
Proband |
c.1258 C>T |
14 |
CPA |
Propranolol
60 mg |
16.8 |
Bigeminy,
fatigue |
Bigeminy |
Syncope |
Vigorous
activity |
| 12 |
Proband |
11997 G>A |
17 |
Syncope |
Propranolol
60 mg |
4.6 |
pVT |
pVT |
CPA, VF |
Unknown |
| 13 |
Proband |
1244 C>G |
42 |
Syncope |
Propranolol
60 mg,
mexiletine
100 mg |
7.8 |
Fatigue |
Single PVC |
No
recurrence |
|
| 14 |
Proband |
11200 C>T |
14 |
CPA VF |
Propranolol
60 mg |
12.1 |
Fatigue |
Single PVC |
VF and
ICD shock |
Vigorous
activity |
| 15 |
Proband |
6737 C>T |
– |
CPA VF |
– |
– |
– |
– |
No
recurrence |
|
| 16 |
Proband |
11590 A>G |
12 |
CPA VF |
Propranolol
90 mg |
12.0 |
Bigeminy,
fatigue |
Bigeminy |
No
recurrence |
|
| 17 |
Proband |
IVS40–2 a/g
splicing error |
20 |
CPA VF |
Propranolol
60 mg |
12.0 |
pVT |
pVT |
VF |
Vigorous
activity |
| 18 |
Proband |
13175 A>G |
– |
Syncope |
– |
– |
– |
– |
VT and
syncope |
Sleeping |
| 19 |
Proband |
535 G>A |
– |
Syncope |
– |
– |
– |
– |
No
recurrence |
|
| 20 |
Proband |
1259 G>A |
15 |
CPA VF |
No
medication |
14.4 |
Bigeminy,
fatigue |
Bigeminy |
No
recurrence |
|
| 21 |
Proband |
11812 A>G,
12331 A>C |
19 |
CPA VF |
Atenolol
200 mg,
verapamil
120 mg,
mexiletine
300 mg |
11.4 |
pVT |
pVT |
VF and
ICD shock |
Vigorous
activity |
| 22 |
Proband |
c.1221A>T |
18 |
Syncope |
Atenolol
50 mg,
flecainide
150 mg |
9.5 |
pVT |
pVT |
VF and
ICD shock |
Vigorous
activity |
| 23 |
Proband |
6507 G>T |
7 |
Syncope |
Bisoplolol
7.5 mg |
15.2 |
Fatigue |
Single PVC |
No
recurrence |
|
| 24 |
Proband |
12559 G>A |
7 |
Syncope |
Propranolol
15 mg |
10.2 |
Fatigue |
Single PVC |
No
recurrence |
|
| 25 |
Proband |
6649 C>T |
8 |
Syncope |
Flecainide
150 mg |
14.4 |
Unknown |
Couplet PVCs |
No
recurrence |
|
| 26 |
Mother of
Patient 25 |
6649 C>T |
36 |
Syncope |
Carvedilol
20 mg,
flecainide
150 mg |
4.7 |
Bigeminy,
fatigue |
Bigeminy |
No
recurrence |
|
| 27 |
Proband |
718 T>C |
26 |
Syncope |
Metoprolol
120 mg |
10.5 |
pVT |
pVT |
No
recurrence |
|
| 28 |
Proband |
11836G>A |
7 |
Syncope |
Propranolol
30 mg,
flecainide
120 mg |
17.6 |
Dyspnea |
Couplet PVCs |
No
recurrence |
|
| 29 |
Proband |
12301 C>T |
16 |
Syncope |
Propranplol
110 mg,
flecainide
150 mg |
2.3 |
pVT |
pVT |
No
recurrence |
|
| 30 |
Proband |
c.13759 g>a |
37 |
Syncope |
Metoprolol
60 mg,
flecainide
100 mg |
12.1 |
Bigeminy,
fatigue |
Bigeminy |
No
recurrence |
|
| 31 |
Proband |
c.4652 a>g,
12475c>a |
10 |
Syncope |
Propranolol
120 mg,
verapamil
120 mg,
flecainide
100 mg |
14.2 |
Bigeminy,
fatigue |
Bigeminy |
CPA |
Noncompliant‡,
details
unknown |
| 32 |
Proband |
c.6737c>t |
19 |
Syncope |
Atenolol
25 mg,
flecainide
100 mg |
10.2 |
Bigeminy,
fatigue |
Bigeminy |
Syncope† |
Walking |
| 33 |
Proband |
c.14593 c>g |
15 |
CPA |
Propranolol
60 mg,
flecainide
100 mg |
Unknown |
Bigeminy |
Bigeminy |
Syncope |
Quarrel |
| 34 |
Proband |
c.14427 g>t |
10 |
CPA |
Bisoprolol
5 mg,
flecainide
200 mg |
14.2 |
Fatigue |
Single PVC |
No
recurrence |
|
Most severe episode: CPA 14, syncope 20. *No. of patients: β-blocker 12, β-blocker+flecainide 10, β-blocker+verapamil 2, β-blocker+mexiletine 1, β-blocker+verapamil+flecainide 2, β-blocker+verapamil+mexiletine 1, flecainide 2, no medication 1. Single PVC 6, couplet PVCs 2, bigeminy 12, polymorphic VT 11. †Patients 1 & 32: recurrence occurred after diagnosis, before EST. ‡Patient 31 was noncompliant with medication at time of recurrence. EST, exercise stress test; METs, metabolic equivalents; PVC, premature ventricular contraction; pVT, polymorphic VT; THR, target heart rate; VF, ventricular fibrillation. Other abbreviations as in Table 1.
Figure 1
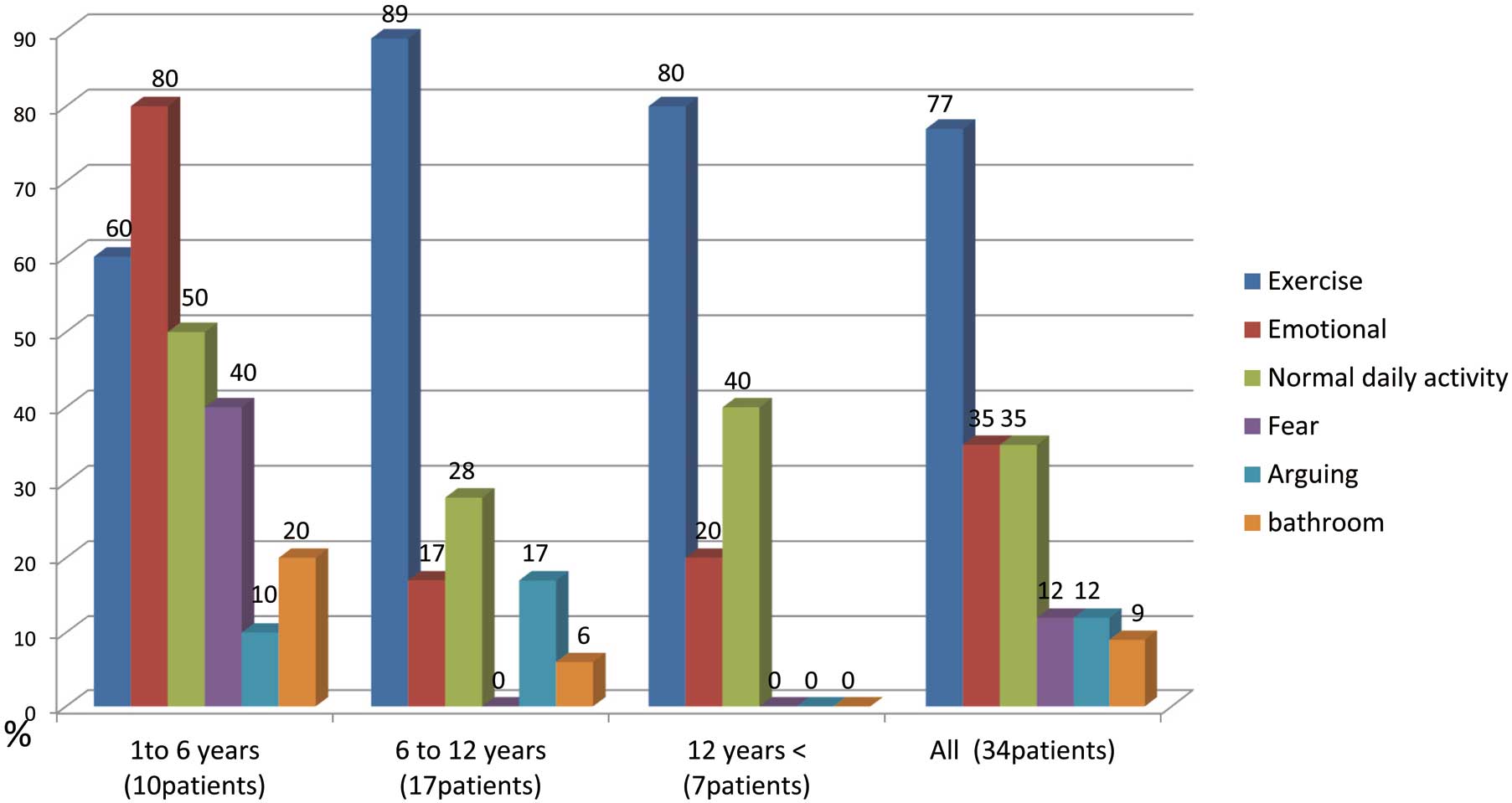
shows the cardiac events before the diagnosis according to the age of onset. Of the 34 patients, 26 (76.5%) had exercise-induced cardiac events before the diagnosis: 12 patients (35.3%) developed cardiac events while swimming; 20 patients (58.8%) developed cardiac events at rest or during light vigorous activities, including emotional stress (12 patients, 35.3%), normal daily activity (12 patients, 35.3%), fear (4 patients, 11.8%), arguing (4 patients, 11.8%), and in the bathroom (3 patients, 8.8%). Among the 10 patients who developed the first event before the age of 6 years, 8 (80%) had a history of an emotion-induced cardiac event. All 4 patients with fear-induced cardiac events were younger than 5 years old.
Diagnosis
The average age at the time of the clinical or genetic diagnosis was 13.9±9.8 (IQR 6.4) years. Among the 32 probands, 12 (37.5%) were diagnosed with CPVT at the initial evaluation; 9 patients (28.1%) were diagnosed with epilepsy before the age of 8 years and all of them had recurrent cardiac events after initiation of antiepileptic medications. Three patients with aborted cardiac arrests were diagnosed with idiopathic VF and discharged without initiation of any medical treatment. A total of 20 (62.5%) of the 32 probands had experienced 3 or more episodes before the diagnosis. Among those 20 patients, 7 developed catastrophic cardiac events (syncope with a documented VA or cardiopulmonary arrest [CPA]) after a missed diagnosis at the initial evaluation.
EST (Exercise Stress Test)
The results of the ESTs are shown in
Table 2. Three probands were unable to perform an EST because of young age, mental status, or hypoxic brain injury after the initial cardiac event. The remaining 31 patients underwent an EST after initiation of medical therapy. The medications at the time of the EST were as follows: β-blockers 12, β-blocker+flecainide 10, β-blocker+verapamil 2, β-blocker+mexiletine 1, β-blocker+verapamil+flecainide 2, β-blocker+verapamil+mexiletine 1, flecainide 2, and no medication 1. One proband had not had an EST under medication. At the time of the EST, 28 of 31 (90.3%) patients were taking β-blockers and 14 (45.2%) patients were on flecainide. During the EST, 25 patients (80.6%) developed high-risk VAs (PVC couplets, bigeminy, or VT) and 11 (35.5%) patients had polymorphic VT.
Therapy and Recurrence After Initial Diagnosis
The average follow-up period after diagnosis was 7.4±6.2 (IQR 7.3) years. At the last follow-up, 32, 5, and 23 of 34 patients took β-blockers, calcium-channel blockers, and flecainide, respectively. Of the 34 patients, 14 (41.2%) experienced at least 1 cardiac event (CPA, VF/VT or syncope) and the 5-year event-free survival rate was 58.9% (Figure 2). CPA, VF, or sustained VT occurred in 7 of 34 patients (20.6%) and the 5-year event-free survival rate was 81.0% (Figure 2). No difference was observed in the occurrence of all cardiac events (estimated 5-year event-free rates were 61.7% vs. 58.4%, P=0.3623) and lethal cardiac events (VF or CPA; estimated 5-year event-free rates were 92.3% vs. 75.4%, P=0.7210,
Figure 3) between the patients with and without a history of a CPA or VF.

Among 7 patients who developed recurrent CPA or VF/VT, 5 developed those events during vigorous exercise; 2 patients (nos.12 and 31) had CPA but the trigger was not clear. One of those patients (no. 31) was noncompliant with medication at that time.
Follow-up Result According to EST
After EST under optimal medical treatment, 11 (35.5%) of 31 patients developed recurrent cardiac events during an average follow-up period of 4.2±2.6 years. We compared the event-free survival rate between 25 high-risk patients and 6 low-risk patients. The average age at the time of the EST was similar between the 2 groups (18.5±10.9 vs. 18.7±14.2 years, P=0.9755) and there were no significant differences in the baseline characteristics of the 2 groups.
Figure 4
shows the Kaplan-Meier curves of freedom from all cardiac events and lethal cardiac events (VF or CPA) after the EST. Among the 25 high-risk patients, 10 (40.0%) developed cardiac events (estimated 5-year event-free rate 49.1%), and 6 (24.0%) developed lethal cardiac events (VT, VF, and CPA; estimated 5-year event-free rate 69.3%). On the other hand, only 1 of the 6 low-risk patients developed recurrent cardiac events. This patient initially had a cardiac arrest during swimming and underwent ICD implantation. During the EST, he achieved 12 METs and only single PVCs were induced. However, he would not follow the exercise restrictions and multiple episodes of VT were recorded during vigorous exercise. None of the low-risk patients developed cardiac events (including syncope) during non-vigorous activity, while 6 of the 25 (24.0%) high-risk patients did during non-vigorous activity (estimated 5-year event-free rate: 100% vs. 73.6%, P=0.2016,
Figure 5). All 6 patients with cardiac events during non-vigorous activity had a history of non-vigorous activity-induced cardiac events before the diagnosis, and they were younger than 18 years old (9.9–17.9, 14.1±3.0 years) at the time of the recurrence.

Discussion
Main Findings
Our data demonstrated that (1) approximately 21% of
RyR2-positive CPVT patients developed recurrent fatal cardiac events (CPA or VF) no matter whether they had a history of cardiac arrest or not; (2) high-risk VAs were induced during EST in most patients even after initiation of medical therapy (80.6%); and (3) most of the recurrent cardiac arrest or VF episodes occurred because of noncompliance with either exercise restriction or medication therapy (85.7%).
Efficacy of Flecainide
Beta-blockers and calcium-channel blockers have been used widely to suppress VAs in CPVT patients. Since the first report by Watanabe et al,18
the efficacy of flecainide in CPVT patients with
RyR2
mutations has been reported.19
In our study, 60% of the CPVT patients were on flecainide during the follow-up. Flecainide is reported to cause partial or complete suppression of high-risk VAs.19
In our cohort, high-risk VAs (PVC couplets, bigeminy or VT) were induced during the EST in 13 of 14 patients (92.9%) with flecainide, and 3 of 14 patients (21.4%) developed cardiac arrest or VF. It is true that flecainide can increase the arrhythmia threshold during exercise,20
but high-risk VAs were still induced in most patients. Most of the study patients could not increase flecainide to 200 mg/day because of small body weight or side effects. A high recurrence rate after initiation of flecainide might be related to insufficient dose.
Importance of Exercise Restrictions and Compliance With Medications
Restriction of vigorous exercise and competitive sports is recommended for all patients with CPVT, but it is more difficult for school-age children to comply with exercise restrictions than adults. Among 7 patients who developed CPA or VF after the diagnosis, 5 developed it during vigorous physical exercise. One patient stopped taking medication and had a cardiac arrest at home. The other patient was found in a state of CPA outside of her house and the trigger was not identified. Overall, noncompliance with exercise restriction or medication therapy was associated with recurrent cardiac events in most patients. After reaching 15 years of age, none of these patients with optimal medical therapy developed emotion-induced CPA or VF, suggesting that optimal medical therapy is effective for suppressing emotion-triggered cardiac arrests or VF in adult patients.
In the present study, the incidence of all cardiac events and lethal cardiac events was not statistically significant between the high- and low-risk groups of patients according to the EST. However, it is of particular importance to identify high-risk patients who may develop cardiac events during non-vigorous activity, because such cardiac events cannot be prevented by exercise restriction and can happen unexpectedly. Of the high-risk patients, 6 of 25 (24.0%) developed syncope, VT, VF, or cardiac arrest during the EST and they were younger than 18 years old at the time of the recurrence during non-vigorous activity. In contrast, none of the low-risk patients developed cardiac events during non-vigorous activity. These facts may suggest that avoidance of emotional stress should be more emphasized in the high-risk group especially while young.
A Challenging Diagnosis in Young Patients
Less than 40% of patients were diagnosed correctly after the initial evaluation at the hospital. Among 32 probands who had cardiac events before the diagnosis, 11 developed a first syncopal episode during non-exercise activity, including emotional stress, crying, micturition, and defecation. Especially in patients with their first symptoms occurring when they were ≤6 years old, 8 of 10 (80%) had a history of emotion-induced cardiac events. In these young patients, the triggers for cardiac events were more heterogeneous and the diagnosis is challenging for physicians. Young patients have a faster heart rate at rest and are probably more vulnerable to emotional stress, including fear. Epilepsy is the most important differential diagnosis for arrhythmia-related syncope, and all patients who were misdiagnosed with epilepsy were younger than 8 years old. Physicians, especially pediatricians, should keep in mind that CPVT patients may develop cardiac events during normal activities (moderate or light intensity activity). Unfortunately, most preschool children cannot undergo an EST. Although epinephrine stress tests can be used as an alternative, their efficacy and accuracy are not the same as an EST.21,22
If CPVT is suspected in young pediatric patients who cannot undergo adequate EST, a genetic analysis should be strongly considered.
Study Limitations
First of all, the number of patients was relatively small because CPVT is a rare inherited disease. However, the 34 patients were all genetically confirmed as
RyR2-positive CPVT patients from 2 representative Japanese centers that cover most genetic tests for CPVT in Japan. In addition, the average follow-up of 7 years was long enough to provide clinically important data. Second, the definition of compliance with medication and exercise restriction was vague. Physicians inquire further into compliance with medications and exercise restriction when their patients develop a cardiac event. Thus, we might overestimate a compliance with medical therapy and exercise restriction in patients without cardiac events. Third, the antiarrhythmic effect of dantrolene was recently reported in patients with CPVT, but we did not have a chance to use this novel treatment. Lastly, none of the patients underwent sympathetic cardiac nerve denervation, partly because few centers in Japan performed this procedure during the study period.
Conclusions
Even after medical treatment, high-risk VAs (PVC couplets, bigeminy, or VT) were still induced in most (80.7%)
RyR2-
positive CPVT patients. During 7.4 years of follow-up after the diagnosis, 7 of 34 (20.6%) patients had fatal cardiac events (VF or CPA). Among those 7 patients, 6 (85.7%) was noncompliant with either exercise restriction or medication therapy. Noncompliance with exercise restriction and medical therapy is common in patients with recurrence of cardiac arrest or VF and medical management including strict exercise restriction should be emphasized to prevent recurrent cardiac event.
Grants
T.A., N.S., S.K., Y.M., I.S., M.H., and W.S. were supported in part by Grants from the Ministry of Health, Labor and Welfare of Japan for Clinical Research on Intractable Diseases (H24-033, H26-040). W.S. was supported in part by a Grant from the Nippon Medical School Grant-in-Aid for Medical Research.
Acknowledgments
The following investigators and institutions participated in this study. We would like to acknowledge their kind assistance with collecting the current patients’ information.
Tamotsu Matsunaga, Department of Pediatric Cardiology, International Medical Center, Saitama Medical University, Hidaka; Hiroshi Morita, Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama; Michiko Motoki, Tomoki Kosho, Department of Pediatrics, Shinshu University School of Medicine, Matsumoto; Junichi Takagi, Department of Pediatrics, Miyazaki University Hospital, Miyazaki; Yoshiaki Kanaya, Takuya Hara, Department of Pediatrics, Oita Prefectural Hospital, Oita; Yoshiaki, Kato, Department of Child Health, Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba; Keiko Toyohara, Daiji Takeuchi, Department of Pediatric Cardiology, Tokyo Women’s Medical University, Tokyo; Naoki Toyota, Department of Pediatrics, Otsu Red Cross Hospital, Otsu; Takahiro Shindo, Department of Pediatrics, Graduate School of Medicine, University of Tokyo, Tokyo; Masanobu Ikoma, Tameo Hatano, Department of Pediatrics, Japanese Red Cross Nagoya Daiichi Hospital, Nagoya; Ken Miyazaki, Department of Obstetrics and Gynecology, Japanese Red Cross Nagoya Daiichi Hospital, Nagoya; Hiroko Goto, Department of Pediatrics, Gifu Prefectural General Medical Center, Gifu; Masaru Miura, Division of Cardiology, Tokyo Metropolitan Children’s Medical Center, Fuchu; Makoto Takenaga, Yayoi Nishimoto, Internal Medicine, Fujimoto Central Hospital, Miyazaki; Toshiyuki Itoi, Department of Pediatric Cardiology and Nephrology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto; Masato Yokozawa, Department of Pediatrics, Hokkaido Medical Center for Child Health and Rehabilitation, Sapporo; Yoshihide Nakamura, Yoko Yoshida, Department of Pediatric Cardiology, Pediatric Medical Care Center, Osaka City General Hospital, Osaka; Nakao Konishi, Department of Pediatrics, Chugoku Rosai Hospital, Kure.
The authors also thank Naotaka Ohta, Toshiko Shibata, Hiromi Fujiyama, Miyuki Hozan and Akihiro Fujiwara for their excellent technical support with the gene analysis.
Lastly, we thank John Martin for his linguistic assistance.
References
- 1.
Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001; 103: 485–490.
- 2.
Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 2001; 103: 196–200.
- 3.
Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 2001; 69: 1378–1384.
- 4.
Laitinen PJ, Swan H, Kontula K. Molecular genetics of exercise-induced polymorphic ventricular tachycardia: Identification of three novel cardiac ryanodine receptor mutations and two common calsequestrin 2 amino-acid polymorphisms. Eur J Hum Genet 2003; 11: 888–891.
- 5.
Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 2002; 91: e21–e26, doi:10.1161/01.RES.0000038886.18992.6B.
- 6.
Itoh H, Dochi K, Shimizu W, Denjoy I, Ohno S, Aiba T, et al. A common mutation of long QT syndrome type 1 in Japan. Circ J 2015; 79: 2026–2030.
- 7.
Kimura H, Zhou J, Kawamura M, Itoh H, Mizusawa Y, Ding WG, et al. Phenotype variability in patients carrying KCNJ2 mutations. Circ Cardiovasc Genet 2012; 5: 344–353.
- 8.
Nakajima T, Kaneko Y, Kurabayashi M. Unveiling specific triggers and precipitating factors for fatal cardiac events in inherited arrhythmia syndromes. Circ J 2015; 79: 1185–1192.
- 9.
Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002; 106: 69–74.
- 10.
Sy RW, Gollob MH, Klein GJ, Yee R, Skanes AC, Gula LJ, et al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2011; 8: 864–871.
- 11.
Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009; 119: 2426–2434.
- 12.
Hayashi M, Denjoy I, Hayashi M, Extramiana F, Maltret A, Roux-Buisson N, et al. The role of stress test for predicting genetic mutations and future cardiac events in asymptomatic relatives of catecholaminergic polymorphic ventricular tachycardia probands. Europace 2012; 14: 1344–1351.
- 13.
Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: A 7-year follow-up of 21 patients. Circulation 1995; 91: 1512–1519.
- 14.
Roston TM, Vinocur JM, Maginot KR, Mohammed S, Salerno JC, Etheridge SP, et al. Catecholaminergic polymorphic ventricular tachycardia in children: An analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015; 8: 633–642.
- 15.
Bauce B, Rampazzo A, Basso C, Bagattin A, Daliento L, Tiso N, et al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: Early diagnosis of asymptomatic carriers. J Am Coll Cardiol 2002; 40: 341–349.
- 16.
Crosson JE, Callans DJ, Bradley DJ, Dubin A, Epstein M, Etheridge S, et al. PACES/HRS expert consensus statement on the evaluation and management of ventricular arrhythmias in the child with a structurally normal heart. Heart Rhythm 2014; 11: e55–e78, doi:10.1016/j.hrthm.2014.05.010.
- 17.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in Collaboration With the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006; 114: e385–e484, doi:10.1161/CIRCULATIONAHA.106.178233.
- 18.
Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med 2009; 15: 380–383.
- 19.
van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol 2011; 57: 2244–2254.
- 20.
Dochi K, Watanabe H, Kawamura M, Miyamoto A, Ozawa T, Nakazawa Y, et al. Flecainide reduces ventricular arrhythmias via a mechanism that differs from that of β-blockers in catecholaminergic polymorphic ventricular tachycardia. J Arrhythm 2013; 29: 255–260.
- 21.
Marjamaa A, Hiippala A, Arrhenius B, Lahtinen AM, Kontula K, Toivonen L, et al. Intravenous epinephrine infusion test in diagnosis of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol 2012; 23: 194–199.
- 22.
Lieve KV, van der Werf C, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia. Circ J 2016; 80: 1285–1291.