Abstract
Background:
Mutations in
ANK2
have been reported to cause various arrhythmia phenotypes. The prevalence of
ANK2
mutation carriers in inherited primary arrhythmia syndrome (IPAS), however, remains unknown in Japanese. Using a next-generation sequencer, we aimed to identify
ANK2
mutations in our cohort of IPAS patients, in whom conventional Sanger sequencing failed to identify pathogenic mutations in major causative genes, and to assess the clinical characteristics of
ANK2
mutation carriers.
Methods and Results:
We screened 535 probands with IPAS and analyzed 46 genes including whole
ANK2
exons using a bench-top NGS (MiSeq, Illumina) or performed whole-exome-sequencing using HiSeq2000 (Illumina). As a result, 12 of 535 probands (2.2%, aged 0–61 years, 5 males) were found to carry 7 different heterozygous
ANK2
mutations.
ANK2-W1535R was identified in 5 LQTS patients and 1 symptomatic BrS and was predicted as damaging by multiple prediction software. In total, as to phenotype, there were 8 LQTS, 2 BrS, 1 IVF, and 1 SSS/AF. Surprisingly, 4/8 LQTS patients had the acquired type of LQTS (aLQTS) and suffered torsades de pointes. A total of 7 of 12 patients had documented malignant ventricular tachyarrhythmias.
Conclusions:
Various
ANK2
mutations are associated with a wide range of phenotypes, including aLQTS, especially with ventricular fibrillation, representing “ankyrin-B” syndrome.
ANK2
encodes ankyrin-B which links integral membrane proteins to the spectrin-based cytoskeleton.1–3
Ankyrin-B plays critical roles in the anchoring and stabilizing multiple ion channels and exchangers in the cardiomyocyte membrane.4–11
In 1995 a specific form of long QT syndrome (LQTS) with sinus bradycardia was examined using a linkage analysis in a large French family and was found to be associated with locus 4q25–27.12
This was later proved to be mutations in
ANK2
and a cause of LQTS type 4 (LQT4), which is a rare type of LQTS13
and other dysrhythmia phenotypes: ventricular tachyarrhythmia and sino-atrial node (SAN) disturbance.14
Editorial p ????
After these pioneering works, many
ANK2
variants were reported in patients with diverse phenotypes ranging from mild sick sinus syndrome (SSS) without ventricular arrhythmias to sudden cardiac death (SCD) from malignant arrhythmias.12,14–20
Functional assays revealed that these reported
ANK2
variants were associated with various degrees of malfunction in the targeting or stabilization of cardiac ion channels or associated proteins, thereby leading to differences in the in vitro cellular phenotypes.17,18
Clinical and experimental results suggested that
ANK2
variants may exhibit a wide clinical spectrum known as “ankyrin-B syndrome”.14–18
However, only a small number of
ANK2
mutation carriers, mainly in Caucasians, have been reported, with limited clinical features, and to our best knowledge no Japanese patients with
ANK2
mutations have been reported. One reason is that
ANK2
is too big (>45 exons) for screening by the conventional Sanger method. Recent advances in genetic screening using a next-generation sequencer (NGS) have enabled us to screen many target genes in a short time. In this study, using a NGS, we tried to elucidate the prevalence of
ANK2
mutations in inherited primary arrhythmia syndrome (IPAS) patients with no major causative genes in our previous cohort studies, and the clinical characteristic of patients with
ANK2
mutations.
Methods
Subjects and Genetic Analysis
The cohort consisted of 535 probands (male patients=288) with the following IPAS: 341 LQTS, 58 Brugada syndrome (BrS), 40 idiopathic ventricular fibrillation (IVF), 27 catecholaminergic polymorphic ventricular tachycardia (CPVT), 22 SSS/atrial fibrillation (AF), 16 SCD (molecular autopsy), 11 short-coupled variant of torsade des points (SCTdP), 7 ventricular tachycardia (VT), 7 short QT syndrome, and 6 complete atrioventricular block (CAVB) (Table 1). They were suspected to have or diagnosed with IPAS and were referred to the Shiga University of Medical Science (Otsu)/Kyoto University (Kyoto) (n=325) or the National Cerebral and Cardiovascular Center (Suita) (n=210) for genetic evaluation. All subjects gave written informed consent in accordance with the guidelines approved by each institutional review board (SUMS_23-128-2, NCVC_M24-031-4). Their phenotypes were evaluated based on clinical findings and 12-lead ECGs. We defined heart rate ≤60 beats/min as bradycardia for patients aged ≥19 years. For those aged 18 years or younger, we adopted definitions from previous reports.21,22
Table 1.
Prevalence of
ANK2 Mutations in Inherited Primary Arrhythmia Syndrome
| Diagnosis |
n |
No. of ANK2 mutations |
Prevalence (%) |
| Long-QT syndrome |
341 |
8 |
2.3 |
| Brugada syndrome |
58 |
2 |
3.4 |
| Idiopathic VF |
40 |
1 |
2.5 |
| SSS/AF |
22 |
1 |
4.5 |
| VT |
7 |
0 |
0.0 |
| SCD |
16 |
0 |
0.0 |
| CPVT |
27 |
0 |
0.0 |
| SCTdP |
11 |
0 |
0.0 |
| Short-QT syndrome |
7 |
0 |
0.0 |
| CAVB |
6 |
0 |
0.0 |
| Total |
535 |
12 |
2.2 |
AF, atrial fibrillation; CAVB, complete atrioventricular block; CPVT, catecholaminergic polymorphic ventricular tachycardia; SCD, sudden cardiac death; SCTdP, short-couplet variant of torsades des pointes; SSS, sick sinus syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia.
Genomic DNA was isolated from peripheral blood lymphocytes. Genetic screening of all probands was performed by conventional Sanger method, and all were negative for mutations in
KCNQ1,
KCNH2, and
SCN5A.
In 325 probands, we analyzed 46 additional genes including
ANK2
(Table S1) using a bench-top NGS (MiSeq, Illumina, San Diego, CA, USA). These genes have been reported as causative for IPAS or cardiomyopathies. The remaining 210 probands, all LQTS, were analyzed by whole-exome-sequencing (HiSeq, Illumina) as shown previously.23
After analyzing the data obtained by the NGS, we confirmed detected mutations by the Sanger method. The nucleotide number of
ANK2
is based on NM_020977.3, and the amino acid number is based on NP_066187.2. We defined a variants as a mutation of which the minor allele frequency (MAF) was <0.005 in all public databases: the Exome Aggregation Consortium (ExAC: http://exac.broadinstitute.org), NHLBI Exome Sequencing Project Exome Variant Serve (http://evs.gs.washington.edu/EVS/), 1000 Genomes Project (http://browser.1000 genomes.org/index.html), Human Genetic Variation Database (HGVD: http://www.genome.med.kyoto-u.ac.jp/SnpDB/), and the genome cohort study of Tohoku Medical Megabank Organization (ToMMo: https://ijgvd.megabank.tohoku.ac.jp). The severity of each variant was estimated by PolyPhen2, Provean, SIFT and CADD. CADD score >15 was judged as damaging.
Results
Genetic Screening With NGS
We identified 7 different heterozygous
ANK2
mutations in 12 probands (2.2%, age 0–61 years, 5 males) (Table 1): p.Y148H (c.442T>C), p.G761S (c.2281G>A), p.T825I (c.2474C>T), p.R895Q (c.2684G>A), p.L1128V (c.3382C>G), p.W1535R (c.4603T>A), and p.I1855R (c.5564T>G) (Figure 1). p.W1535R was the most frequent and was identified in 6 probands, while the other mutations were identified in a single proband (Table 2). These mutations were scattered in various regions of
ANK2
(Figure 1) and most were predicted as damaging by multiple prediction software (Table 2).

Table 2.
Genetic Characteristics of
ANK2 Mutations in Inherited Primary Arrhythmia Syndrome
Patient
no. |
Mutation |
dbSNP |
Minor allele frequency |
Prediction software |
| Nucleotide |
Amino
acid |
ANK2
domain |
ExAC
Browser |
ESP6500 |
1000
genomes |
HGVD |
ToMMo |
Polyphen2 |
Provean |
SIFT |
CADD |
| 1 |
442T>C |
Y148H |
MBD |
– |
– |
– |
– |
– |
– |
Possibly
damaging
[0.735] |
Deleterious
[−4.131] |
Damaging
[0.04] |
26.6 |
| 2 |
2281G>A |
G761S |
MBD |
rs774769455 |
– |
– |
– |
0.003 |
– |
Probably
damaging
[1] |
Deleterious
[−5.413] |
Tolerated
[0.08] |
19.99 |
| 3 |
2474C>T |
T825I |
MBD |
– |
– |
– |
– |
0.001 |
– |
Possibly
damaging
[0.779] |
Deleterious
[−3.583] |
Tolerated
[0.064] |
26.3 |
| 4 |
2684G>A |
R895Q |
SBD |
rs751513548 |
0.0000248 |
– |
– |
0.001 |
– |
Benign
[0.184] |
Deleterious
[−3.012] |
Tolerated
[0.22] |
18.09 |
| 5 |
3382C>G |
L1128V |
SBD |
– |
– |
– |
– |
– |
– |
Possibly
damaging
[0.987] |
Deleterious
[−2.894] |
Damaging
[0.03] |
11.56 |
| 6–11 |
4603T>A |
W1535R |
DD |
rs199473346 |
0.00003303 |
– |
– |
– |
0.0022 |
Probably
damaging
[1] |
Deleterious
[−3.115] |
Damaging
[0.016] |
31 |
| 12 |
5564T>G |
I1855R |
CTD |
rs201966460 |
0.00000842 |
– |
0.0002 |
0.003 |
– |
Possibly
damaging
[0.547] |
Neutral
[−0.691] |
Tolerated
[0.37] |
16.3 |
CADD, Combined Annotation-Dependent Depletion; ExAC, Exome Aggregation Consortium; ESP6500, NHLBI Exome Sequencing Project; HGVD, HUMAN Genetic Variation Database; SIFT, Sorting Intolerant From Tolerant; ToMMo, Tohoku Medical Megabank Organization. CTD, C-terminal domain; DD, death domain; MBD, membrane-binding domain; SBD, spectrin-binding domain.
Genetic screening by NGS detected multiple variants in various genes in the 12 probands. In an effort to identify disease-causing mutations, we excluded genes not expressed in the heart, variants of which had MAF >0.005 in the public databases, or the severity of each variant estimated by the prediction software was minimal.
Table S2
shows these remaining variants.
Patient 1: ANK2-Y148H
ANK2-Y148H was identified in a female newborn. She was found to have marked bradycardia with high-grade AVB and QT prolongation (QTc: 532 ms, HR: 73 beats/min) at 2 days old, but no syncope. Based on her ECG at 4 months, she no longer had AVB but still had bradycardia for her age (HR: 107 beats/min);21,22
the QTc interval had normalized to 433 ms.
Patient 2: ANK2-G761S
ANK2-G761S was identified in a 61-year-old man who had experienced a transient loss of consciousness after dinner. His resting 12-lead ECG (Figure 2A) showed marked bradycardia (HR: 43 beats/min, QTc: 442 ms). QT prolongation was not detected during exercise stress test, but epinephrine challenge test prolonged his QT interval (from 441 ms to 478 ms).24
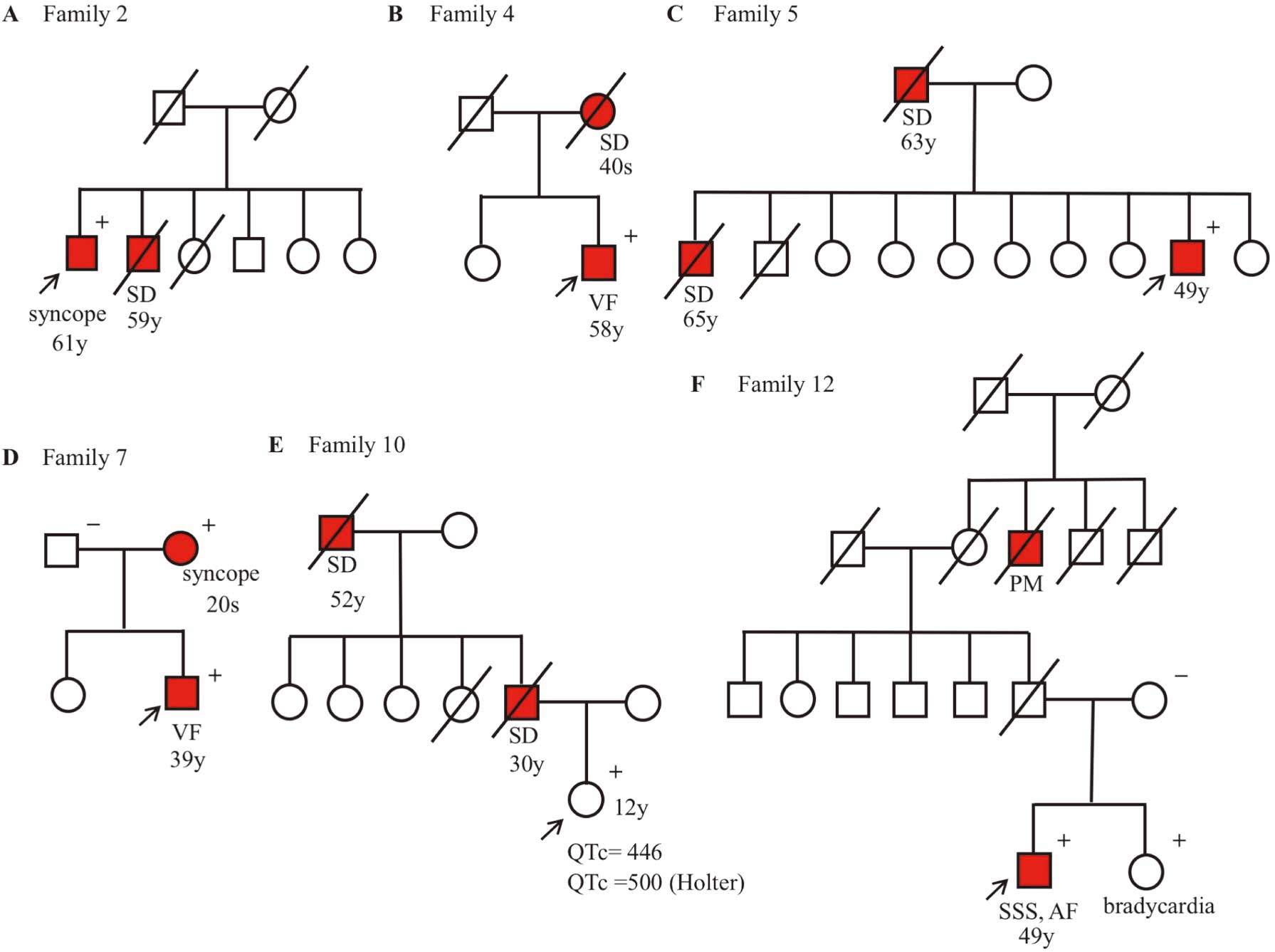
Holter ECG recording revealed 1 episode of nonsustained VT. His syncope was suspected to be caused by LQTS. His brother had died suddenly at age 59, but other family members remained asymptomatic (Figure 3A). The patient carried the S28L variant in
KCNE1, which is known as a causative gene for LQTS type 5 (Table S2), and we could not rule out the possibility that the variant affected his phenotype.
Patient 3: ANK2-T825I
ANK2-T825I was identified in a 30-year-old woman who had experienced repeated episodes of loss of consciousness since she was 12 years old, and the events were mostly exercise-related. Her resting 12-lead ECG showed a normal QTc interval (416 ms), and QT prolongation was not detected during exercise stress test; however, epinephrine challenge test prolonged her QT interval (from 436 ms to 508 ms).24
She had no structural cardiac abnormalities on ultrasound cardiography (UCG) and no notable family history.
Patient 4: ANK2-R895Q
ANK2-R895Q was identified in a 59-year-old man who had experienced syncope because of VF at work and he had been successfully defibrillated by an ambulance crew. There were no abnormal findings on his resting 12-lead ECG, UCG, and other examinations. In the exercise stress test, neither QT prolongation nor polymorphic VT was observed. He was diagnosed as having IVF, and an implantable cardioverter defibrillator (ICD) was implanted. His mother had died suddenly in her 40 s (family tree in
Figure 3B).
Patient 5: ANK2-L1128V
ANK2-L1128V was identified in a 49-year-old man who, although asymptomatic, showed a saddleback-type ST elevation in V1–2 on ECG recorded at a health checkup. Pilsicainide challenge test unmasked a coved-type ST elevation in V1 at the 3rd intercostal position (BrS type 1), and he was suspected of having BrS. His father and brother had died suddenly at ages 63 and 65, respectively (family tree in
Figure 3C); however, no ECGs of his family members were available.
Patients 6–11: ANK2-W1535R
ANK2-W1535R in the death domain (Figure 1) was identified in 6 patients (1 male); 4 had LQTS with documented TdP or VF (patients 6, 8, 9 and 11 in
Tables 2
and
3), 1 was asymptomatic but suspected to have LQTS from the family history (patient 10), and the last patient had symptomatic BrS (patient 7).
Table 3.
Clinical Characteristics of Patients With Inherited Primary Arrhythmia Syndrome With
ANK2 Mutations
Patient
no. |
Age |
Sex |
Dx |
ANK2
mutation |
Symptoms |
TdP/VF |
ECG |
Family
history |
QTc (ms)
at events,
Diagnosis of
Acquired LQTS |
Other ECG
or genetic
abnormalities |
HR
(beats/min) |
Brady |
QTc
(ms) |
| 1 |
0 |
F |
LQTS |
Y148H |
– |
– |
107 |
P |
433 |
– |
QTc 532 ms, HR
73 beats/min |
2:1 AVB, PAT |
| 2 |
61 |
M |
LQTS |
G761S |
Syncope |
– |
43 |
P |
442 |
P |
QTc 478 ms,
epinephrine
load test |
KCNE1-S28L
(rs199473350) |
| 3 |
30 |
F |
LQTS |
T825I |
Syncope |
– |
80 |
– |
436 |
– |
QTc 508 ms,
epinephrine
load test |
|
| 4 |
59 |
M |
IVF |
R895Q |
Syncope |
VF |
58 |
P |
384 |
P |
|
|
| 5 |
49 |
M |
BrS |
L1128V |
– |
– |
60 |
P |
426 |
P |
|
CRBBB,
saddleback-type
ST elevation |
| 6 |
36 |
F |
LQTS |
W1535R |
Syncope |
TdP |
60 |
P |
445 |
– |
QTc 597 ms
with nausea
and vomiting |
|
| 7 |
39 |
M |
BrS |
W1535R |
Syncope |
– |
55 |
P |
353 |
P |
|
Coved ST
elevation,
EPS-induced VF |
| 8 |
45 |
F |
LQTS |
W1535R |
Syncope |
TdP |
67 |
– |
450 |
P |
QTc 550 ms,
hypokalemia and
drug-induced TdP |
|
| 9 |
44 |
F |
LQTS |
W1535R |
Syncope |
VF |
94 |
– |
|
– |
QTc 622 ms after
hemodialysis |
|
| 10 |
12 |
F |
LQTS |
W1535R |
– |
– |
50 |
P |
446 |
P |
|
|
| 11 |
40 |
F |
LQTS |
W1535R |
Syncope |
TdP |
70 |
– |
448 |
– |
QTc 537 ms,
drug-induced TdP |
|
| 12 |
49 |
M |
SSS, AF |
I1855R |
– |
– |
49 |
P |
507 |
P |
|
EPS-induced VF |
P, positive; –, negative. AVB, atrioventricular block; BrS, Brugada syndrome; CRBBB, complete right bundle branch block; EPS, electrophysiological study; IVF, idiopathic VF; LP, late potential; LQTS, long QT syndrome; MRI, magnetic resonance imaging; PAT, paroxysmal atrial tachycardia; PM, pace maker; tdP, torsades des pointes; UCG, ultrasound cardiography; VPC, ventricular premature contraction. Other abbreviations as in Table 1.
Patient 6, a 36-year-old woman, was admitted to an emergency hospital with nausea and vomiting. In the emergency room, she repeatedly lost consciousness because of TdPs. Although her 12-lead ECG displayed a marked QT prolongation (QTc: 597 ms) on admission (Figure 2B-1), her QTc had shortened to 445 ms on the following day (Figure 2B-2). She had experienced no syncope except for this episode and had no notable family history.
Patient 7 was a 39-year-old man who experienced loss of consciousness with seizures after dinner. He had a similar history at age 30 when he had a fever. His 12-lead ECG (Figure 2C) showed a coved-type ST elevation in V1–2 at the 3rd intercostal position and he was diagnosed as having BrS. In the electrophysiological study (EPS), VF was induced by a single extrastimuli (600/240 ms) from the right ventricular outflow tract, and an ICD was then implanted. His mother also had a history of syncope at around 20 years old, and the same mutation was identified (Figure 3D). Her 12-lead ECG showed no Brugada-type ST changes or QT prolongation, though we could not perform a provocation test with sodium-channel blocker or an exercise stress test.
Patient 8, a 45-year-old woman, had experienced repeated episodes of loss of consciousness since taking tolazoline hydrochloride for insomnia. She was hospitalized for pharyngitis with a high fever and had started on ceftriaxone treatment. A few days after admission, she suffered syncope at rest, and TdP was detected on her monitoring ECG. Her QTc was 550 ms with notched T waves, and her serum potassium level was low (3.3 mEq/L) when TdP was recorded. After withdrawal of the culprit drugs, her QT interval remained prolonged (QTc: 450 ms, HR: 67 beats/min). Her mother had also experienced syncope, although the details were uncertain. We could not obtain her consent for genetic analysis.
Patient 9, a 44-year-old woman under artificial hemodialysis for chronic kidney disease, lost consciousness while eating after hemodialysis, and TdP was documented. Her 12-lead ECG after syncope showed marked QT prolongation (QTc: 622 ms, HR: 94 beats/min) and notched T wave. She had never experienced syncope other than this episode. We could not obtain her electrolyte data when she lost consciousness.
Patient 10 was an asymptomatic 12-year-old girl who had a family history of SCD. Her 12-lead ECG showed a borderline QTc interval (QTc: 446 ms) and no sign of Brugada-type ST-T change, but marked bradycardia for her age (HR: 50 beats/min). Her father had died suddenly at 30 while at work, and her grandfather had died suddenly at age 52 while on the way home from work (Figure 3E). Neither had diagnosed cardiac diseases but further clinical information was not available. Her family was suspected to have IPAS, and she was referred for genetic analysis.
Patient 11, a 40-year-old woman, experienced a transient loss of consciousness with seizures at night. In the emergency room, she lost consciousness again, and TdP was documented. Her 12-lead ECG showed marked QT prolongation (QTc: 537 ms). She was suspected of having drug-induced LQTS because she took various medicines, including etizolam, famotidine, NSAIDs, and others. She had never shown QT prolongation at past health checkups. After withdrawal of all the drugs, however, her QTc remained prolonged (448 ms). In addition, she suffered multiple PVCs. Bisoprolol was started, but the PVCs were not suppressed, and marked bradycardia appeared (HR: 38 beats/min). Therefore, β-blocker treatment was stopped, and an ICD was implanted.
Patient 12: ANK2-I1855R
ANK2-I1855R was identified in a 49-year-old man who had been diagnosed with SSS at age 44. Although he was asymptomatic, his bradycardia had progressed gradually. Atrial standstill was detected during EPS, but H-V conduction was normal. In addition, VF was induced by triple extrastimuli from the right ventricle (600/300/280/240 ms). Therefore, an ICD was implanted. His sister carrying the same mutation had bradycardia, but his mother, who was not a carrier, showed a normal heart rate of ECG. His grand-uncle on his father’s side had been implanted with a pacemaker (Figure 3F). Both his grand-uncle and father were already deceased, so their genetic data were not available.
Summary of
ANK2
Mutation Carriers (Table 3)
ANK2
mutations were identified in probands manifesting LQTS (n=8), BrS (n=2), IVF (n=1), and SSS/AF (n=1); 8 of 12 genotyped probands (67%) were symptomatic with syncope, and malignant ventricular tachyarrhythmias were documented in 5 of the 8 symptomatic probands (63%). Bradycardia was present in 8 of the 12 probands (67%; patients 1, 2, 4–7, 10, 12), and patient 1 had CAVB. None of the probands had any structural cardiac abnormalities. All 5 male probands had bradycardia. A patient with BrS and 1 with SSS/AF underwent an EPS, and VF was induced in both cases. In 7 of the 8 LQTS patients carrying
ANK2
mutations, the baseline QT interval was nearly within the normal range, but was markedly prolonged by epinephrine load test or other QT prolongation factors. In particular, 4 of the LQTS patients showed prominent QT prolongation around episodes of TdP; in other words, they were diagnosed as having an acquired type of LQTS (aLQTS).
In 7 families (58%), other members showed clinical phenotypes: SCD, syncope or bradycardia (Table 3). Among the phenotype-positive family members, 5/6 males and 1/4 females had died suddenly. Except for patient 1 and 10, most of the patients were diagnosed in adulthood.
Discussion
This comprehensive analysis using NGS firstly demonstrated that the prevalence of
ANK2
mutations was 2.2% (12 probands, 7 mutations) in the study cohort of 535 Japanese IPAS probands with no major IPAS-related genes identified. Their expressivity varied considerably: LQTS, BrS, IVF and SSS/AF as previously reported.12,14–20
Furthermore, an
ANK2-W1535R variant was identified in 4 patients with aLQTS.
Ankyrin is a multifunctional protein involved in the targeting and stabilization of ion channels and transporters in various tissues.1,2
The ankyrin genes,
ANK1
(chromosome 8p11),
ANK2
(4q25–27), and
ANK3
(10q21) encode 3 different ankyrins: canonical ankyrin-R (210 kD), ankyrin-B (220 kD), and ankyrin-G (190 kD), respectively.1–3
The ankyrins have 4 domains: (1) membrane-binding domain (MBD), (2) spectrin-binding domain (SBD), (3) death domain (DD) and (4) C-terminal domain (CTD) (Figure 1). The MBD of 24 ankyrin repeats interacts with various membrane proteins, and the DD and CTD comprise the regulatory domain.25–27
The known
ANK2
variants in abnormal cardiac function are reportedly localized around the CTD and DD regulatory domains.14–18
Figure 1
summarizes the location of
ANK2
mutations identified in the present patients, showing a wide distribution over the gene.
Caucasian patients with
ANK2
variants present with various dysrhythmia phenotypes, including ventricular tachyarrhythmia and SAN disturbance, a syndrome now known as ankyrin-B syndrome.14–18
Le Scouarnec et al demonstrated that a trafficking dysfunction of ankyrin-B caused abnormal SAN electrical activity and sinus node dysfunction, thereby causing bradycardia and heart rate variability.16
Mohler et al reported that
ANK2
variants around the regulatory domain (DD and CTD) contributed to the various degrees of ankyrin-B loss-of-function, and proposed that it may reflect the clinically wide spectrum.18
Ankyrin-B as a membrane “adaptor” binds to various integral membrane proteins,4–11
probably including the sodium channel. Probands carrying
ANK2
mutations presented with phenotypes of BrS in our study as well as in previous reports.18,28
SCN5A, encoding an α-subunit of the cardiac sodium channel, is the most frequent causative gene responsible for BrS, and its loss-of-function type mutations cause BrS. Ankyrin-B knockout mice have been shown to reduce peak Na while increasing late Na currents,29
indicating that loss-of-function type
ANK2
mutations may present as BrS and long QT phenotypes.
Ankyrin-B also binds to the Na/Ca exchanger (NCX), and several
ANK2
mutations are reported to reduce the NCX expression.18
Bögeholz and colleagues demonstrated that heterozygous NCX-knockout mice had significantly shortened action potential duration (APD).30
ANK2
mutations may therefore affect the APD through NCX reduction and cause heterogeneity of repolarization, which is potentially arrhythmogenic.31
To the best of our knowledge, this is the first report on
ANK2
mutations in a large Japanese population. The phenotypes of the mutation carriers were diverse, and 8 of 12 genotyped probands (67%) showed bradycardia. In addition, across the various diagnoses, 7 of 12 probands presented with documented malignant ventricular tachyarrhythmia, and 2 of the remaining 5 patients had a family history of SCD. As shown in the family trees (Figure 3), the genotype appeared to be associated with the phenotype. These findings suggest that
ANK2
mutations may be associated with malignant ventricular arrhythmias. Although 7 of 12 probands carried several variants in genes expressed in the heart (Table S2), most of the variants were of genes reported as the cause of structural cardiac diseases, and our probands showed no cardiac structural abnormalities that were explained by these variants. Furthermore, the genes reported had no association with IPAS except for
KCNE1
and
ANK2.
Surprisingly, 7 of 8 probands showing LQTS phenotype (87.5%) had the concealed type of LQTS. Additional factors such as epinephrine, hypokalemia, sympathetic nerve stimulator, and bradycardia, aggravated their QT prolongation32
and after removal of these modifiers, the QTc interval nearly normalized (443±37 ms,
Table 3). The mean age at first diagnosis (39±17 years) was older than for typical congenital LQTS, which is also consistent with aLQTS.
In particular, W1535R was identified in 6 of 12 probands (50%), and 4 of those 6 (67%) had aLQTS. TdP/VF was documented in these 4 aLQTS probands. Though functional assay of
ANK2-W1535R was not available, according to prediction software this variant is thought to be damaging, and may be associated with the occurrence of aLQTS. More recently, we demonstrated in 188 clinically diagnosed aLQTS patients that the prevalence of “concealed” congenital LQTS was higher than expected.33
We screened for the 5 major LQTS genes: KCNQ1,
KCNH2,
SCN5A,
KCNE1, and
KCNE2, and identified mutations in these LQTS-related genes in 53 patients (28%).
ANK2
may therefore be a candidate gene for aLQTS.
Study Limitations
We were not able to perform functional analysis of the
ANK2
mutations identified in the probands because ankyrin-B can combine with or affect multiple ion channels and exchangers. We were also unable to further analyze family members to show co-segregation. Therefore, this study may have a limitation to elucidating the pathogenicity of the diseases and diverse phenotypes caused by
ANK2
mutations. In addition, NGS could detect multiple variants of different genes, which were not completely excluded as responsible for the phenotypes.
Conclusions
We identified multiple
ANK2
mutations in IPAS patients with diverse phenotypes using NGS. Ankirin-B syndrome has primary importance in the differential diagnosis of IPAS, especially in those with malignant ventricular tachyarrhythmias and/or the aLQTS phenotype.
Acknowledgments
We thank all the patients and their family members for their willingness to participate in this study. We are also grateful to Dr Hiroko Goto, Dr Tetsuya Haruna, Dr Atsushi Kyodo, Dr Takayuki Maki, Dr Mikiko Mikuri, Dr Akira Nakazawa, Dr Tomohiko Sakatani, Dr Satoshi Shizuta, Dr Yukako Yoshikane, and Dr Tomohide Yoshino for referral and care of the study patients.
Grants
This work was supported by research grants from the Ministry of Education, Culture, Science, and Technology of Japan (T.A., S.O., Y.M., T. Tanaka, W.S., M.H.), Health Science Research grants from the Ministry of Health, Labour and Welfare of Japan for Clinical Research on Measures for Intractable Diseases (T.A., Y.M., T. Tanaka, W.S., M.H.) and Translational Research Funds from the Japanese Circulation Society (T.A., W.S., M.H.).
Supplementary Files
Supplementary File 1
Table S1.
Target genes of the bench-top type of next-generation sequencer
Table S2.
Rare variants in other causative genes
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-0486
References
- 1.
Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev 2001; 81: 1353–1392.
- 2.
Mohler PJ, Gramolini AO, Bennett V. Ankyrins. J Cell Sci 2002; 115: 1565–1566.
- 3.
Otto E, Kunimoto M, McLaughlin T, Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol 1991; 114: 241–253.
- 4.
Cunha SR, Mohler PJ. Cardiac ankyrins: Essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res 2006; 71: 22–29.
- 5.
Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem 1993; 268: 11489–11491.
- 6.
Mohler PJ, Gramolini AO, Bennett V. The ankyrin-B C-terminal domain determines activity of ankyrin-B/G chimeras in rescue of abnormal inositol 1,4,5-trisphosphate and ryanodine receptor distribution in ankyrin-B (–/–) neonatal cardiomyocytes. J Biol Chem 2002; 277: 10599–10607.
- 7.
Bourguignon LY, Jin H. Identification of the ankyrin-binding domain of the mouse T-lymphoma cell inositol 1,4,5-trisphosphate (IP3) receptor and its role in the regulation of IP3-mediated internal Ca2+ release. J Biol Chem 1995; 270: 7257–7260.
- 8.
Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem 2004; 279: 12980–12987.
- 9.
Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 2005; 3: e423, doi:10.1371/journal.pbio.0030423.
- 10.
Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56 alpha targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol 2007; 293: H109–H119.
- 11.
Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem 2007; 282: 4875–4883.
- 12.
Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, et al. Mapping of a gene for long QT syndrome to chromosome 4q25 – 27. Am J Hum Genet 1995; 57: 1114–1122.
- 13.
Mizusawa Y, Horie M, Wilde AA. Genetic and clinical advances in congenital long QT syndrome. Circ J 2014; 78: 2827–2833.
- 14.
Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 2003; 421: 634–639.
- 15.
Sherman J, Tester DJ, Ackerman MJ. Targeted mutational analysis of ankyrin-B in 541 consecutive, unrelated patients referred for long QT syndrome genetic testing and 200 healthy subjects. Heart Rhythm 2005; 2: 1218–1223.
- 16.
Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 2008; 105: 15617–15622.
- 17.
Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA 2004; 101: 9137–9142.
- 18.
Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: Human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation 2007; 115: 432–441.
- 19.
Sedlacek K, Stark K, Cunha SR, Pfeufer A, Weber S, Berger I, et al. Common genetic variants in ANK2 modulate QT interval: Results from the KORA study. Circ Cardiovasc Genet 2008; 1: 93–99.
- 20.
Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol 2009; 47: 203–209.
- 21.
Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011; 377: 1011–1018.
- 22.
Baruteau AE, Perry JC, Sanatani S, Horie M, Dubin AM. Evaluation and management of bradycardia in neonates and children. Eur J Pediatr 2016; 175: 151–161.
- 23.
Shigemizu D, Aiba T, Nakagawa H, Ozaki K, Miya F, Satake W, et al. Exome analyses of long QT syndrome reveal candidate pathogenic mutations in calmodulin-interacting genes. PLoS One 2015; 10: e1030329, doi:10.1371/journal.pone.0130329.
- 24.
Shimizu W, Noda T, Takaki H, Nagaya N, Satomi K, Kurita T, et al. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm 2004; 3: 276–283.
- 25.
Davis LH, Davis JQ, Bennett V. Ankyrin regulation: An alternatively spliced segment of the regulatory domain functions as an intramolecular modulator. J Biol Chem 1992; 267: 18966–18972.
- 26.
Hall TG, Bennett V. Regulatory domains of erythrocyte ankyrin. J Biol Chem 1987; 262: 10537–10545.
- 27.
Mohler PJ, Hoffman JA, Davis JQ, Abdi KM, Kim CR, Jones SK, et al. Isoform specificity among ankyrins: An amphipathic alpha-helix in the divergent regulatory domain of ankyrin-B interacts with the molecular co-chaperone Hdj1/Hsp40. J Biol Chem 2004; 279: 25798–25804.
- 28.
Allegue C, Coll M, Mates J, Campuzano O, Iglesias A, Sobrino B, et al. Genetic analysis of arrhythmogenic diseases in the era of NGS: The complexity of clinical decision-making in Brugada syndrome. PLoS One 2015; 10: e0133037, doi:10.1371/journal.pone.0133037.
- 29.
Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Abnormal cardiac Na(+) channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circ Res 2000; 86: 441–447.
- 30.
Bögeholz N, Pauls P, Bauer BK, Schulte JS, Dechering DG, Frommeyer G, et al. Suppression of early and late afterdepolarizations by heterozygous knockout of the Na+/Ca2+ exchanger in a murine model. Circ Arrhythm Electrophysiol 2015; 8: 1210–1218.
- 31.
Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol 2001; 12: 268–272.
- 32.
Nakajima T, Kaneko Y, Kurabayashi M. Unveiling specific triggers and precipitating factors for fatal cardiac events in inherited arrhythmia syndromes. Circ J 2015; 79: 1185–1192.
- 33.
Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, et al. The genetics underlying acquired long QT syndrome: Impact for genetic screening. Eur Heart J 2016; 37: 1456–1464.