Article ID: CJ-16-1055
Article ID: CJ-16-1055
The radiofrequency hot balloon (RHB) catheter enables the creation of contiguous lesions and achieves pulmonary vein isolation (PVI), but real-time monitoring of PVI is not technically available, and the timing of PVI and impact of the ablation protocol on PVI remain uncertain. Herein we describe a case of isolation of the right superior pulmonary vein (RSPV) before reaching the target temperature (TT) and an unexpected isolation of the superior vena cava (SVC), emphasizing the need for real-time monitoring of PVI during RHB energy application.
A 55-year-old man with a history of paroxysmal atrial fibrillation underwent PVI using a novel RHB catheter. Individual isolation of the left superior, left inferior, and right inferior PV was performed using a TT of 70℃ and 180 s. Before the RSPV isolation, a circular catheter was placed at the junction of the right atrium (RA) and SVC for possible real-time monitoring of the far-field potentials of the RSPV (Figure 1). Before energy application, an RA potential followed by 2 different electrocardiograms was recorded. The earlier component was recorded both on the circular mapping catheter and on a multi-electrode catheter placed at the SVC-RA junction, and was thus considered to be the near-field-potential of the SVC. The latter component was considered to be the far-field potential of the RSPV. The order of these potentials changed during an extrasystole from the SVC that clearly discriminated the near-field SVC potential from the far-field RSPV potential. Energy application was initiated in the RSPV and automatically adjusted to reach the TT of 70℃. Just before reaching the TT, the far-field RSPV potential became slightly prolonged, and then the RSPV was isolated (Figure 2). Within another 9 s, the SVC potential recorded by the multi-electrode catheter (RA-SVC 5-6) placed at the RA-SVC junction gradually prolonged and then became unexpectedly isolated (approximately 7 s after reaching the TT). The energy application was continued at 70℃ for a total time of 120 s. After the single energy application, dissociated activity was observed in both the SVC and RSPV, and bi-directional block was confirmed. No complications were observed.

(A) A circular mapping catheter was placed in the superior vena cava (SVC) and a multi-electrode catheter was placed at the junction of the right atrium (RA) and SVC. Before delivering the energy at the right superior pulmonary vein (RSPV), a near-field SVC potential (blue arrowhead) was recorded both on the circular mapping catheter and on the multi-electrode catheter followed by a far-field RSPV potential (asterisk). During an extrasystole from the SVC (third beat), an impulse propagated from the SVC in the caudal direction followed by an RA (black arrow) and SVC potential. (B) Fluoroscopy: anterior-posterior (AP) and left anterior oblique (LAO) views. CMAP, compound motor action potential; CS, coronary sinus; dis, distal; prox, proximal; Stim, stimulation.
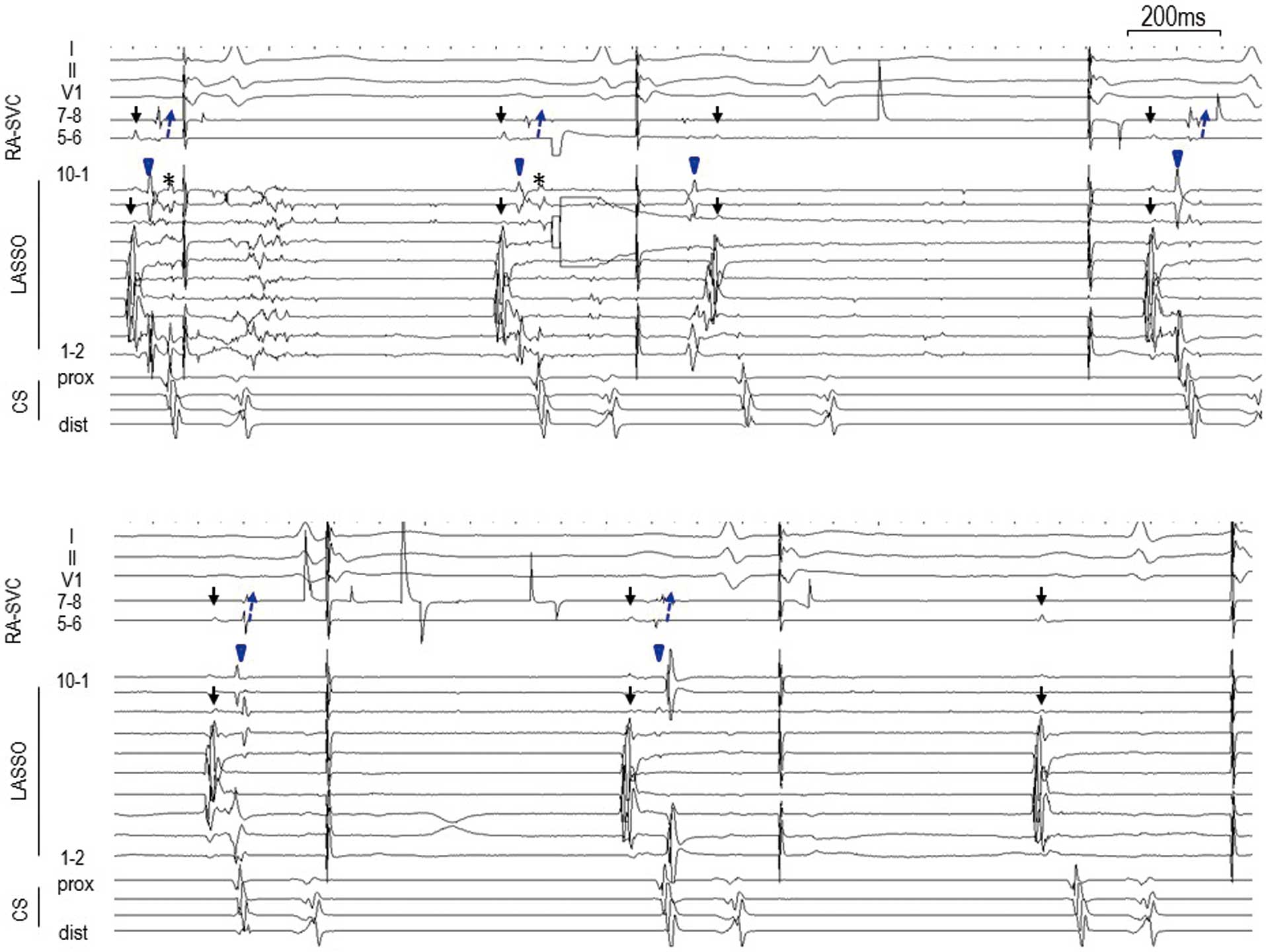
(A) After beginning the energy application at the RSPV, a slight prolongation of the far-field RSPV potential was observed. Then, the RSVP potential disappeared during an extrasystole (third beat) from the SVC. Notably, the near-field SVC potential was also prolonged (fourth beat). (B) Isolation of the SVC was achieved soon after the isolation of the RSPV (third beat). Abbreviations as in Figure 1.
The RHB consists of a compliant balloon that can achieve better contact with irregular shaped PV ostia, enabling the creation of contiguous and transmural lesions.1 The feasibility of PVI using the RHB system has been described,2 but the combination of the central balloon temperature and radiofrequency energy delivery time has been determined empirically and a fixed ablation protocol has not been confirmed. Although real-time monitoring can discriminate between effective and ineffective ablation during energy application when using the cryoballoon, real-time monitoring of the PV potentials is not technically available with the RHB.3 Therefore, this technical issue is critically important in order to establish an optimal ablation protocol and improve the success rate of RHB-PVI. A previous in vivo study had shown that the lesion depth of the RHB depended on the ablation time, and that an energy delivery of 5 min was required to create a lesion depth of 3.8 mm.2 The present case, however, has shown that RSPV isolation could be achieved before reaching TT. Moreover, the SVC was unexpectedly isolated within 9 s after the RSPV isolation. Electrical breakthroughs from the RA to the SVC have been previously reported as being 1.4±0.5 per patient,4 and an inadvertent electrical SVC isolation at the anterior or septal aspect of the RSPV has been described in case reports.5,6 Moreover, the impact of PVI using balloon-based technology on the SVC potentials has recently been described.7 In that report, a conduction delay of the SVC potentials occurred in 57.1% of patients within a median of 6.0 ms during RSPV isolation using a second-generation cryoballoon, and the distance between the RSPV ostium and SVC was the only parameter to correlate with the produced delay. No SVC isolation, however, was detected during the cryoballoon ablation. Although we did not perform computed tomography prior to the ablation, the lesion depth of the RHB may have developed sooner and to a greater degree than in the in vivo study, and a longer energy application at the PV antrum may create collateral damage such as PV stenosis, phrenic nerve injury, or esophageal lesions.8 The development of an inner circular mapping catheter for the recording of the PV potentials is mandatory to establish the optimal ablation protocol and avoid complications in PVI using the RHB.
We thank John Martin for assistance with this article.
None to declare.