Article ID: CJ-17-0111
Article ID: CJ-17-0111
Background: Transcatheter aortic valve replacement (TAVR) has been an alternative less invasive therapy for high-surgical risk/inoperable patients with aortic valve stenosis (AS) in Japan. We report 5-year outcomes of the first pivotal clinical trial of TAVR in Japan (PREVAIL JAPAN).
Methods and Results: A total of 64 patients with AS who were considered unsuitable candidates for surgery were enrolled at 3 centers in Japan (mean age: 84.3±6.1 years, female: 65.6%, STS score: 9.0±4.5%). Transfemoral approach (TF) and transapical approach (TA) was performed in 37 patients and 27 patients, respectively. At 5 years, freedom from all-cause death was 52.7% (TF: 51.3%, TA: 56.3%). Risk of all stroke at 5-year was 15.8% (TF: 8.9%, TA: 25.5%) and risk of major adverse cardiac and cerebrovascular events at 5 years was 58.0% (TF: 51.3%, TA: 69.2%). Mild or greater aortic regurgitation (AR) at 1 week was not associated with increased all-cause death at 5 years (69.1%) compared with none or trace AR (48.3%) (P=0.184). Patients with high STS score (>8) had higher mortality rate than those with low STS scores (≤8).
Conclusions: The 5-year data from PREVAIL JAPAN show the clinical benefit of TAVR and suggest that balloon-expandable TAVR is an effective treatment option for Japanese patients with severe AS who are not suitable for surgery. (Funded by Edwards Lifesciences Limited; ClinicalTrials.gov number, NCT01113983.)
Severe symptomatic aortic stenosis (AS) is a common cardiac disease in elderly patients and the natural history is very poor unless aortic valve replacement procedure is performed.1 However, there are a number of patients who are not suitable for surgical aortic valve replacement (AVR). Recently, transcatheter AVR (TAVR) has become the treatment option for patients with severe aortic stenosis2,3 and long-term outcomes of TAVR in high risk and inoperable patients have been reported as favorable.4,5
The first TAVR was performed in Japan in 2009.6 After the Transfemoral and Transapical Placement of Aortic Balloon Expandable Transcatheter Valve Trial (PREVAIL JAPAN), which was the first pivotal clinical trial in Japan,7 the SAPIEN XT (Edwards Lifesciences, CA, USA) became commercially available in 2013. Since then, short-term and mid-term outcomes in Japanese and Asian cohorts have been reported.8–11 However, long-term clinical outcomes in a Japanese cohort are lacking. We herein report the first 5-year follow-up outcomes of Japanese patients after TAVR in the PREVAIL JAPAN trial.
Details of the trial were published in January 2015.7 PREVAIL JAPAN is a prospective, multicenter, non-randomized, and pivotal clinical trial. The trial was conducted in accordance with Japanese Good Clinical Practice at 3 centers in Japan. Inclusion criteria were severe AS (aortic valve area (AVA) <0.8 cm2 or effective orifice area index (EOAI) <0.5 cm2/m2, a mean pressure gradient (PG) ≥40 mmHg, or peak aortic-jet velocity ≥4.0 m/s) and New York Heart Association (NYHA) functional class ≥II. Patients were determined to be high surgical risk by at least 1 surgeon and 1 cardiologist. The trial was approved by the institutional review board at each participating site and all patients provided written informed consent prior to enrollment.
ProcedureWe used the 2nd-generation Edwards SAPIENTM XT transcatheter heart valve (Edwards Lifesciences LLC) in this study. It consisted of a balloon-expandable, discrete cobalt chromium frame with a trileaflet bovine pericardial valve. The procedure was performed in a hybrid operating room under general anesthesia via a transfemoral (TF) or transapical (TA) approach. Only 23-mm and 26-mm valves were used in this trial. The study valve was implanted under fluoroscopic and transesophageal echocardiographic guidance.
EndpointsThe prespecified primary endpoint of this trial was a composite of improvements in AVA (≥1.0 cm2) and NYHA functional classification converted from a specific activity scale (SAS) at 6 months after the procedure. Prespecified secondary endpoints for safety were signs of valve dysfunction and incidence of adverse events at 30 days and 6 months after the procedure. The “signs of valve dysfunction” were defined as “any change in valve function (a decrease of 1 NYHA functional class or more) of an operated valve resulting from an intrinsic abnormality of the valve that causes stenosis or regurgitation in this study”. Prespecified secondary endpoints also included improvement or maintenance of left ventricular ejection fraction, quality of life at 30 days and 6 months after the procedure, procedural success, and improvement in AVA and NYHA functional classification based on conventional physician assessment at 6 months after the procedure. These endpoints have been reported elsewhere.7 In this report, we present the 5-year analyses. In this study, stroke was defined as a neurological deficit lasting 24 h, or lasting <24 h with a brain imaging study using CT scan showing infarction, and a major adverse cardiovascular and cerebrovascular event (MACCE) was defined as a composite of death, myocardial infarction (MI), stroke, and renal failure. Furthermore, cardiac death was defined as all deaths resulting from cardiac causes, which includes valve-related deaths (including sudden unexplained deaths) and non-valve-related cardiac deaths (e.g., congestive heart failure, acute MI, documented fatal arrhythmias), and non-cardiac death was defined as a death not having a cardiac cause.
All patients were followed up during scheduled visits or contacted by telephone if they missed scheduled visits yearly up to 5 years after the procedure.
Statistical AnalysisContinuous variables are described with mean±SD. Categorical variables are described by frequencies and percentages. Group comparisons of time-dependent variables were performed using the log-rank test. Survival curves for time-to-event variables were constructed on the basis of all available follow-up data with the use of Kaplan-Meier estimates. A two-sided test at an α level of 0.05 was used for all superiority testing. A forest plot was used to display the hazard ratio (HR) and respective 95% confidence interval (CI) of 5-year death using a Cox regression model. The variables included as predictors were: sex, cardiovascular risk factors and non-cardiovascular risk factors. All statistical analyses were performed using SAS software, version 9.3 or above (Cary, NC, USA).
Between April 2010 and April 2011 we enrolled 64 patients with severe AS who were not suitable for surgical AVR at 3 centers (mean age 84 years; mean STS score 8.96%; 65.6% female); 37 and 27 patients had TF and TA valve implantation, respectively (Figure S1, Table S1). Surviving patients were followed up for 5 years (follow-up rate: 94%).
At 5 years, the all-cause mortality rate was 52.7% for all patients and 51.3% and 56.3% for the TF and TA groups, respectively (P=0.85, Table, Figure 1A). Likewise, risk of cardiac death, which was adjudicated by trial centers, was much lower in the TF group (9.8%) than in the TA group (25.3%) (Figure 1B). The risk of all stroke, as reported by trial centers, was 15.8% (TF: 8.9%, TA: 25.5%): 30 days through to 5 years after the procedure, only 1 TF and 2 TA patients experienced stroke (Figure 2A); MACCE at 5 years was 58.0% (TF: 51.3%, TA: 69.2%) (Figure 2B). Landmark analysis of all-cause death and MACCE showed no significant difference between each approach at each timeframe assessed: 0–30 days, 30 days to 5 years (Figure 3A,B).
| 1 year | 5 years | ||||||
|---|---|---|---|---|---|---|---|
| Pooled (n=64) | TF (n=37) | TA (n=27) | Pooled (n=64) | TF (n=37) | TA (n=27) | P value | |
| Death | 14.6% | 8.3% | 23.3% | 52.7% | 51.3% | 56.3% | 0.85 |
| Cardiac death | 11.5% | 5.5% | 19.9% | 16.4% | 9.8% | 25.3% | 0.13 |
| Stroke | 11.7% | 5.8% | 19.7% | 15.8% | 8.9% | 25.5% | 0.09 |
| Death or stroke | 25.9% | 13.9% | 42.2% | 58.5% | 51.2% | 71.1% | 0.13 |
| Myocardial infarction | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | NA |
| Renal failure | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | NA |
NA, not applicable; TA, transapical; TF, transfemoral.
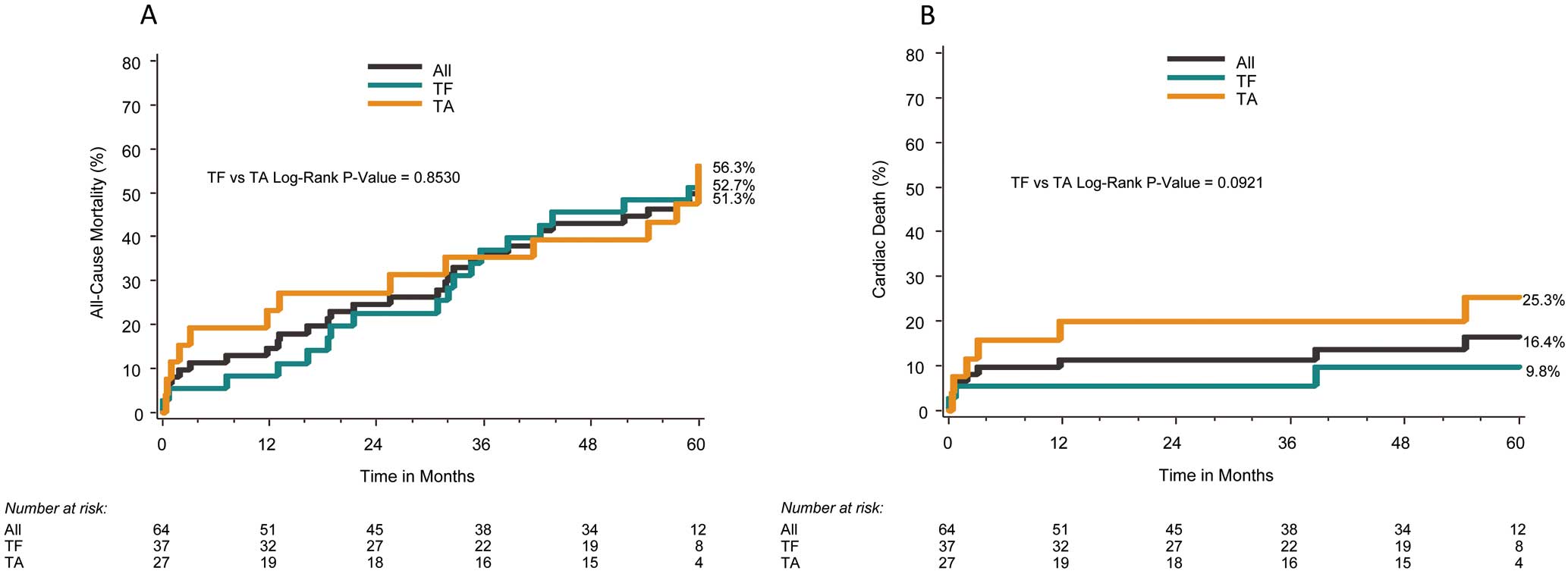
Kaplan-Meier analysis of all-cause death (A) and cardiac death (B) at 5 years. TA, transapical; TF, transfemoral.

Kaplan-Meier analysis of all stroke (A) and major adverse cardiac and cerebrovascular events (MACCE, B) at 5 years. TA, transapical; TF, transfemoral.
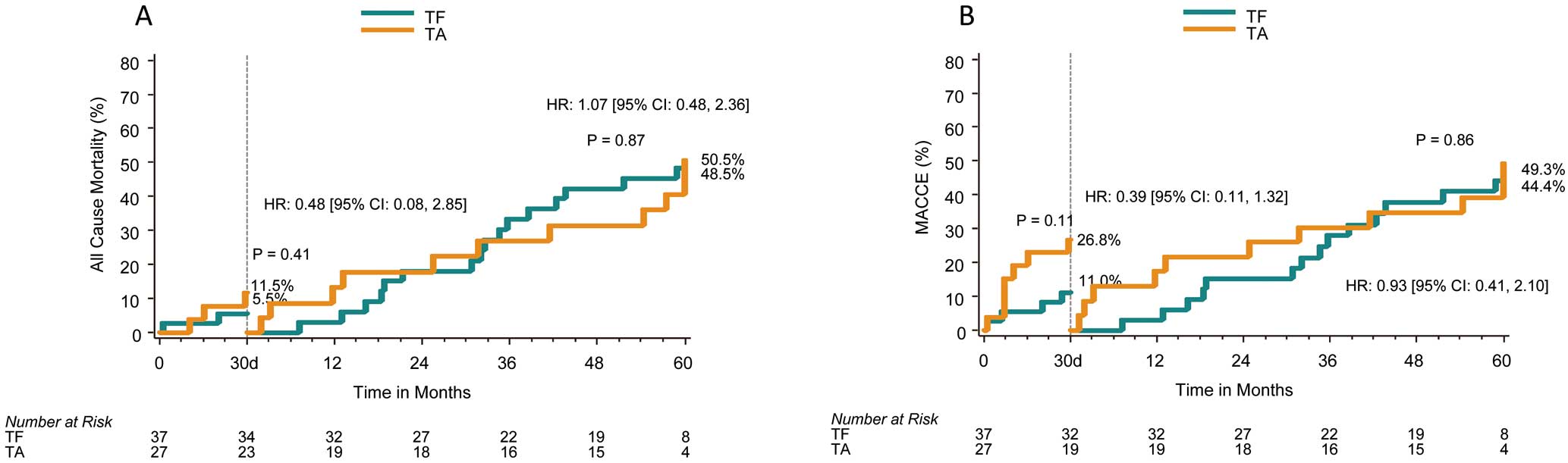
Landmark analysis of all-cause death (A) and major adverse cardiac and cerebrovascular events (MACCE, B). CI, confidence interval; HR, hazard ratio; TA, transapical; TF, transfemoral.
The echocardiographic findings are shown in Figure 4 (Figure S2). After valve implantation, the mean AVA was significantly increased (P<0.001), while the mean PG was significantly decreased (P<0.001), and these improvements were stable throughout the follow-up period. Left ventricular mass regression was also significantly at 5 years (P=0.017). One case of structural valve deterioration (SVD) was reported in this trial. The patient was an 88-year-old woman who underwent TA aortic valve replacement with 23-mm SAPIEN XT 5 years ago. At 7 days after the procedure, mean PG was 16 mmHg and EOA was 1.49 cm2. Postoperative course was uncomplicated until the 5-year follow-up visit (31 August 2015), when echocardiography revealed SVD (mean PG: 41 mmHg, EOA: 0.88 cm2). On CT there was calcification of the valve without malposition or deformation/fracture of stent. Echocardiography revealed peak velocity, mean PG, and EOA were 4.4 m/s, 46 mmHg, and 0.9 cm2, respectively. The patient underwent a valve-in-valve procedure with a 23-mm SAPIEN XT within the failed study valve. Echocardiography 1 month later revealed mean PG and EOA were 12 mmHg and 1.4 cm2, respectively.
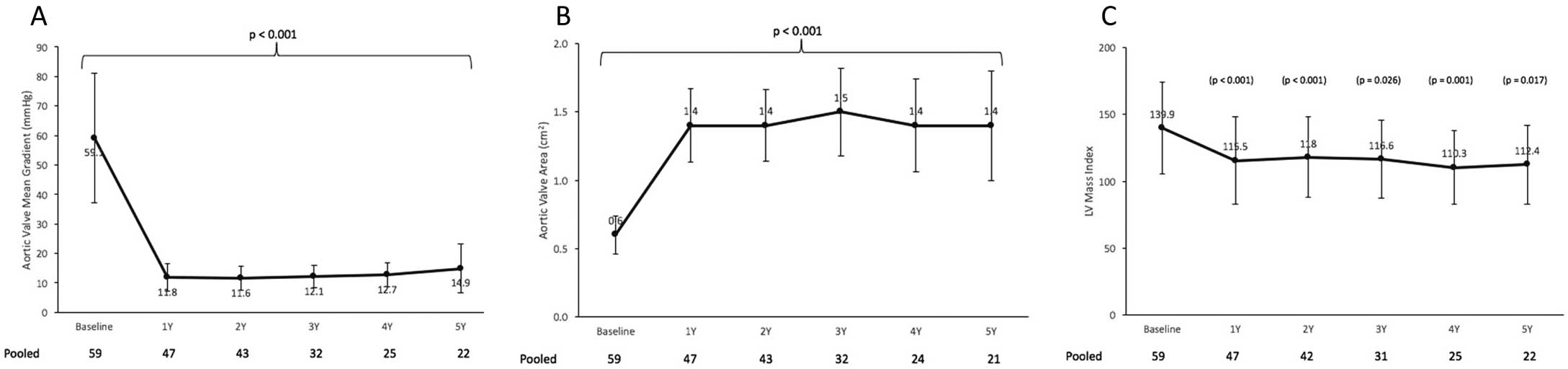
Valve hemodynamics: aortic valve mean gradient (A), aortic valve area (B), and left ventricular mass index (C).
To evaluate the effect of postprocedural aortic regurgitation (AR) on long-term death, we compared the mortality rate with and without postprocedural AR. By echocardiography at 7 days after the procedure, none (0)/trace (1+), mild (2+) or greater AR was found in 80.3% and 19.7%, respectively, and mild AR at 7 days was not associated with increased all-cause death at 5 years (69.1%) compared with none/trace AR (48.3%) (P=0.184) (Figure 5). In terms of the predictors of death at 5 years, the presence of renal dysfunction (HR: 3.62; 95% CI: 1.65–7.94), chronic obstructive pulmonary disease (COPD; HR: 3.04; 95% CI: 1.08–8.57), and peripheral vascular disease (PAD; HR: 3.45; 95% CI: 1.41–8.43) were associated with increased risk of death (Figure 6). Moreover, patients with high STS score (>8) had higher mortality rates than those with low STS scores (≤8) (Figure 7).

All-cause death according to the degree of aortic regurgitation after transcatheter aortic valve replacement.
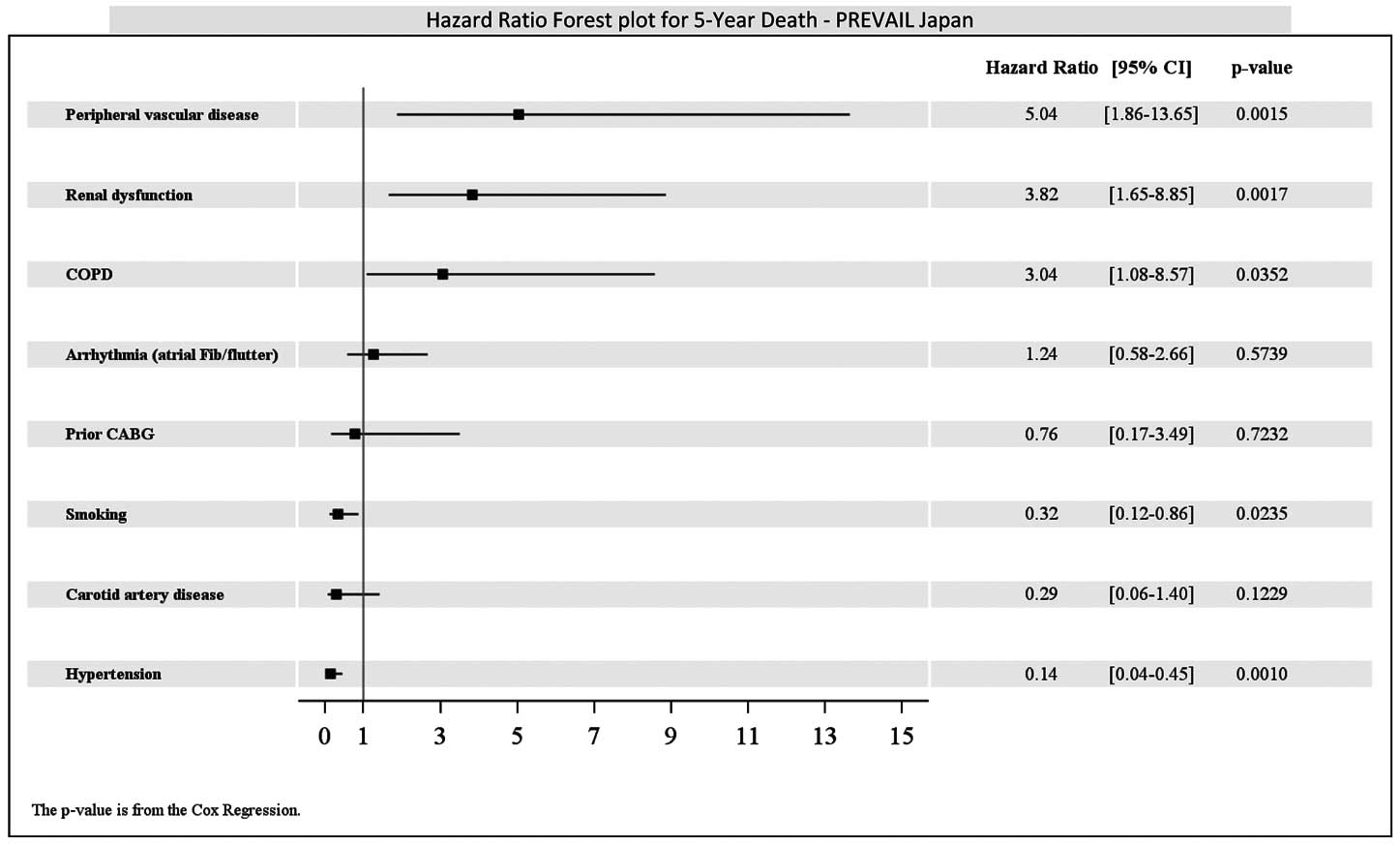
Subgroup analysis. CABG, coronary artery bypass graft; CI, confidence interval; COPD, chronic obstructive pulmonary disease; Fib, fibrillation.

All-cause death stratified by Society of Thoracic Surgeons (STS) score.
PREVAIL JAPAN was the first pivotal clinical trial of TAVR in Japan. Our 5-year follow-up results showed a sustained clinical benefit of TAVR. At 5 years, the all-cause mortality rate was 52.7% in the high surgical risk patients with severe AS, which was comparable to or better than that previously reported.4,5,12–14 Though the all-cause mortality rate for TF at 6 months was lower than that for TA (TF: 5.6%, TA: 19.2%), as previously reported, the 5-year all-cause mortality rates for TF and TA were similar (TF: 51.3%, TA: 56.3%). In contrast, cardiac mortality at 6 months was lower in the TF group (5.6%) than in the TA group (15.7%) and this trend was preserved at 5 years (TF: 9.8%, TA: 25.3%). Though the sample size was limited, landmark analysis of all-cause death and MACCE showed no significant difference between each approach at each assessed timeframe; however, at 0–30days, death and MACCE in the TA group were higher than for TF, but the rates were similar to each other at 30 days to 5 years. Because all stroke included asymptomatic cases in the definition used for this trial, 3 out of 6 strokes in the TA group were asymptomatic whereas all 3 of the strokes in the TF group were symptomatic. This may have caused the higher event rate for stroke with the TA approach. Moreover, we considered that higher event rates for cardiac death, death and MACCE were related to higher learning curve for TA as previously described.7,15 In this study, furthermore, the presence of renal dysfunction, COPD, and PAD were associated with increased risk of death. Although renal dysfunction and COPD were common in previous reports as predictors of long-term mortality, PAD was uncommon. The definition of PAD was left up to each site’s judgement, which may have caused this result. Although the device size was selected according to pre- or intraoperative transesophageal (TEE)/transthoracic echocardiographic (TTE) findings, the rate of paravalvular leakage that was defined as mild or greater was very low (19.7%) in this trial. Moreover, in our findings, postprocedural AR was not associated with long-term mortality, whereas other studies show an adverse effect of periprocedural AR on mortality.16–18 Only the 23-mm and 26-mm SAPIEN XTTM valves were available during the PREVAIL JAPAN trial and the number of patients who completed the 5-year follow-up was limited. Newer valves and a wider range of valve size have become available, so our findings may differ in current clinical settings.
Higher risk of stroke associated with TAVR is a major concern.19 In the present study, we observed a 15.8% rate of stroke at 5 years. Because our results were based on trial center-reported events, including asymptomatic stroke without adjudication by a data core laboratory, and the generation of investigational devices differed between PREVAIL JAPAN and PARTNER I, it is difficult to precisely compare these trials. However, our finding was comparable to the results of the PARTNER I trial (High risk cohort: 15.9%, Inoperable cohort: 16.0%).4,5 Though 18 or 19Fr sheath with TF and 24Fr sheath with TA were used during the PREVAIL JAPAN trial, a 3rd-generation device with a 14Fr sheath for TF has become available in recent years. Lower profile device and the accumulation of operator experience may contribute to a reduction in the stroke rate in the future. The incidence of stroke was higher with TA (6 events) than with TF (3 events), and 3 of the 6 events with TA were asymptomatic stroke while no cases of asymptomatic stroke was reported for TF.
This report is not only the first report of long-term outcomes of TAVR using the SAPIEN XTTM in Japan but also in the world.20 Long-term valve durability was a concern with TAVR when it became the treatment option for the patients with AS.21 In this trial, 5-year follow-up echocardiography revealed 1 case of SVD. The patient had “valve-related dysfunction” according to the VARC 2 definition22 (mean PG 41 mmHg and EOA 0.88 cm2) and was treated by a valve-in-valve procedure as previously reported.23 Although no cases of SVD were reported in the PARTNER trial,4 Toggweiler et al reported 3.4% of 88 patients experienced moderate prosthetic valve failure,12 and Barbanti et al reported 1.4% of 353 patients with significant prosthetic valve degeneration.13 Overall valve performance in this trial was well preserved at 5 years, and the mean PG, AVA and LV mass index showed significant improvements from baseline. A propensity score analysis in the PARTNER trial indicated that TAVR with SAPIEN 3 in intermediate-risk patients with severe AS was associated with low mortality, stroke, and regurgitation rates at 1 year.24 This 3rd-generation balloon-expandable transcatheter device was reimbursed in May 2016 in Japan. Therefore, it is mandatory to investigate systematic long-term echocardiographic follow-up including valve hemodynamics and SVD.
Study LimitationsThe small sample size and low “number at risk” at 5-year follow-up are the primary limitations of this study. Furthermore, TAVR was relatively new in Japan at the time of the trial, so most of the surgeons and interventional cardiologists involved were rather inexperienced with the procedure, which was likely related to the complications. The protocol-mandated selection criteria excluded important patient subgroups, such as those requiring treatment of coronary stenosis and those with severe PAD.
PREVAIL JAPAN was the first pivotal clinical trial of TAVR in Japan and showed the long-term clinical benefit of TAVR. Our findings suggested that balloon-expandable TAVR is an effective treatment option for Japanese patients with severe AS who are not suitable for surgery. (Funded by Edwards Lifesciences; ClinicalTrials.gov number, NCT01113983).
Y. Sawa and S.T. serve as a consultant for Edwards Lifesciences Limited. M.T., T. Komiya and K.M. have no conflicts of interest. T.G., T. Kuratani and T.T. serve as a proctor for Edwards Lifesciences Limited. Y. Sakata received a research grant from Edwards Lifesciences Limited.
Supplementary File 1
Figure S1. Patient disposition in PREVAIL JAPAN.
Figure S2. (A–C) Comparison of echocardiographic parameters between the transfemoral (TF) and transapical (TA) approaches for transcatheter aortic valve replacement.
Table S1. Characteristics of patients in PREVAIL JAPAN
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0111