Article ID: CJ-17-0112
Article ID: CJ-17-0112
Background: Transcatheter aortic valve implantation (TAVI) is a viable alternative to surgical aortic valve replacement in high-risk or inoperable patients with aortic stenosis (AS). Here we report the midterm outcomes of high-risk Japanese patients with severe AS who underwent TAVI with a self-expandable TAV.
Methods and Results: The CoreValve Japan Trial was a prospective, multicenter trial of the CoreValve System. A group of 55 patients (mean age 82.5±5.5 years, 30.9% male, 100% NYHA class III/IV, STS 8.0±4.2%) were enrolled in the 26-mm/29-mm CoreValve study, and 20 patients (mean age 81.0±6.6 years, 5.0% male, 100% NYHA class III/IV, STS 7.0±3.3%) were enrolled in the 23-mm CoreValve study, which started 1 year later. For the 26-mm/29-mm cohort, the 3-year all-cause mortality rate was 32.6%; major stroke was 15.4%. Mean pressure gradient (MPG), effective orifice area (EOA), and NYHA class showed sustained improvement. Paravalvular regurgitation (PVR) at 3 years was 28.6% (none), 25.7% (trace), 40.0% (mild), 5.7% (moderate), and 0.0% (severe). For the 23-mm cohort, the 2-year all-cause mortality rate was 5.0%; major stroke was 5.0%. MPG, EOA, and NYHA class showed sustained improvement. PVR at 2 years was 16.7% (none), 33.3% (trace), 44.4% (mild), 5.6% (moderate), and 0.0% (severe).
Conclusions: TAVI with the CoreValve System was associated with sustained clinical and functional cardiac improvement in high surgical risk Japanese patients with severe AS. (Clinicaltrials.gov Identifiers: NCT01437098 and NCT01634269.)
Transcatheter aortic valve implantation (TAVI) has been well-studied in the United States, Canada, and Europe, where it has been shown to be an acceptable therapeutic alternative to surgical AV replacement in high-risk or otherwise inoperable patients with severe, symptomatic aortic stenosis (AS).1–3 We recently reported the early outcomes of TAVI with the 26-mm and 29-mm CoreValve TAV (Medtronic, Minneapolis, MN, USA) in Japanese patients with severe, symptomatic AS who were deemed to be at high risk for surgical AV replacement.4 The CoreValve is a trileaflet, bioprosthetic, porcine pericardial tissue valve mounted and sutured in a self-expanding nitinol stent frame. The bioprosthesis is housed in a collapsed position for percutaneous delivery via an 18Fr catheter-based technique and then implanted within the diseased AV. Data obtained at 6 months showed the CoreValve System to be safe and effective for the treatment of Japanese patients with severe AS.4 Here we report the 3-year clinical outcomes and performance of the CoreValve bioprosthesis in the same patient cohort. Additionally, we report the 2-year outcomes of a separate cohort of Japanese patients with severe AS who had a small aortic annulus and received the 23-mm CoreValve bioprosthesis, which became available 1 year after the start of the 26-mm/29-mm CoreValve study.
The CoreValve Japan Trial was a prospective, multicenter, single-arm trial to evaluate the efficacy and safety of the 23-, 26-, and 29-mm CoreValve TAV for the treatment of symptomatic, severe AS in Japanese patients who were high-risk surgical AV replacement candidates. The study’s design has been previously reported.4 Briefly, key patient inclusion criteria were severe symptomatic AS with a mean gradient >40 mmHg and an aortic valve area ≤0.8 cm2. Primary anatomical exclusion criteria were: a native aortic annulus size <20 mm or >27 mm for the 26-mm/29-mm cohort and <18 mm or >20 mm for the 23-mm cohort; a pre-existing prosthetic heart valve in any position; mixed AV disease with predominant aortic regurgitation (AR); moderate to severe mitral or tricuspid valve regurgitation; an ascending aorta diameter >40 mm for the 26-mm/29-mm cohort and >34 mm for the 23-mm cohort; bicuspid or unicuspid valve; or femoral or iliac arterial diameter <6 mm (unable to accommodate an 18Fr sheath). Native aortic annuli were measured using computed tomography (CT) and transthoracic or transesophageal echocardiography; however, CT was the primary method used. Major-minor aortic annulus diameter and aortic annulus perimeter assessments were performed. Preprocedural CT was mandatory in the CoreValve Japan Studies, and all patients met this study requirement.
Antiplatelet and anticoagulation therapies including daily aspirin 81–325 mg and ticlopidine (according to the manufacturer’s instructions for use) were recommended for at least 3 months following the procedure. Continuation beyond 3 months was left to the treating physician’s discretion. The primary endpoint for the study was a composite of improvement of at least 1 New York Heart Association (NYHA) class and an effective orifice area (EOA) of >1.2 cm2 for the 26-mm/29-mm valve cohort or ≥1.0 cm2 for the 23-mm valve cohort at 6 months post-procedure in patients who underwent TAVI with the CoreValve via iliofemoral access. The primary endpoint result for the 26-mm/29-mm valve cohort has been previously reported;4 the primary endpoint for the 23-mm valve cohort is presented in this report. Additionally, for both cohorts, key endpoints analyzed were major adverse cardiovascular and cerebrovascular events (MACCE), NYHA class, echocardiographic valve assessment, valve-related death, adverse events, and quality of life. Except for the primary endpoint for the 23-mm cohort, results are reported for each study arm’s cohort in aggregate regardless of the TAVI access approach.
DefinitionsMyocardial infarction (MI), stroke, vascular complications, life-threatening or disabling bleeding events, and paravalvular regurgitation (PVR) were defined using Valve Academic Research Consortium (VARC) I definitions, which were the current VARC definitions in use when this study was conducted.5 MACCE was defined as a composite rate of all-cause death, MI, all stroke and reintervention. Reintervention was defined as any cardiac surgery or percutaneous catheter intervention (PCI) procedure that repaired, otherwise altered or adjusted, or replaced a previously implanted valve. Valve-related death was defined as any death caused by prosthetic valve dysfunction, valve thrombosis, embolism, bleeding event, or implanted valve endocarditis or related to reintervention on the operated valve. Quality of life was evaluated using the Short Form (SF)-36 health survey.
Study OversightAn independent Echocardiographic Core Laboratory (Mayo Clinic Echocardiographic Core Laboratory, Rochester, MN, USA) was responsible for the review and analysis of echocardiographic images. A Clinical Events Committee (CEC), also independent from the study investigators, evaluated all clinical endpoints. The study was conducted in accordance with the ethical principles originating from the Declaration of Helsinki, pharmaceutical affairs law, and good clinical practice. The institutional review board at each center approved the study protocol, and a signed informed consent form was received from all patients.
Statistical AnalysisDescriptive statistics were used to report baseline demographics and clinical variables. For the 23-mm cohort, the comparator for the primary study objective was conventional therapy, which included medical management and balloon aortic valvuloplasty. Categorical variables are summarized as frequencies and percentages. Continuous variables are summarized as mean±standard deviation. For NYHA class, echocardiographic assessment of valve performance, and the SF-36, Wilcoxon signed-rank tests were performed to evaluate the median change from baseline at discharge, then 1, 6, 12, and 24 months (23-mm CoreValve cohort), and discharge, and 1, 6, 12, 24, and 36 months (26-mm/29-mm CoreValve cohort) for implanted patients. Kaplan-Meier analyses were performed for safety endpoints.
From October 2011 to October 2012, 55 patients with severe symptomatic AS were enrolled at 4 investigational centers in Japan, of whom 44 underwent attempted TAVI with the 26- or 29-mm CoreValve via the iliofemoral approach, 5 by the subclavian route, and 6 by the direct aortic approach; 38 (70.4%) patients received the 26-mm valve and 16 (29.6%) received the 29-mm valve. For all patients, mean age was 82.5±5.5 years, 30.9% were male, mean Society of Thoracic Surgeons (STS) score was 8.0±4.2%, mean logistic EuroSCORE was 21.5±9.9%, and all patients had NYHA class III (89.1%) or IV (10.9%) symptoms.
From July 2012 to August 2013, 20 patients with severe, symptomatic AS and a small aortic annulus were enrolled at the same 4 investigational centers in Japan, of whom 16 underwent attempted TAVI with the 23-mm CoreValve via the iliofemoral approach and 4 by the direct aortic approach. For all patients, mean age was 81.0±6.6 years, 5.0% were male, mean STS score was 7.0±3.3%, mean logistic EuroSCORE was 22.3±10.1%, and all patients had NYHA class III (95.0%) or IV (5.0%) symptoms. Baseline characteristics for both cohorts are summarized in Table 1. Patient disposition for both cohorts is presented in Figure 1.
| Characteristic | 26/29 mm | 23 mm |
|---|---|---|
| All patients (n=55) | All patients (n=20) | |
| Age (years) | 82.5±5.5 | 81.0±6.6 |
| Male | 17 (30.9) | 1 (5.0) |
| NYHA classification | ||
| II | 0 (0.0) | 0 (0.0) |
| III | 49 (89.1) | 19 (95.0) |
| IV | 6 (10.9) | 1 (5.0) |
| STS score (%) | 8.0±4.2 | 7.0±3.3 |
| <4 | 7 (12.7) | 1 (5.0) |
| 4–10 | 34 (61.8) | 16 (80.0) |
| >10 | 14 (25.5) | 3 (15.0) |
| Logistic EuroSCORE (%) | 21.5±9.9 | 22.3±10.1 |
| Diabetes mellitus | 19 (34.5) | 3 (15.0) |
| Creatinine level >2 mg/dL | 0 (0.0) | 0 (0.0) |
| History of hypertension | 50 (90.9) | 16 (80.0) |
| Peripheral vascular disease | 18/53 (34.0) | 8 (40.0) |
| Prior stroke | 15/54 (27.8) | 0 (0.0) |
| Prior TIA | 3/54 (5.6) | 0 (0.0) |
| Cardiac history | ||
| CAD | 27 (49.1) | 10 (50.0) |
| Prior CABG | 11 (20.0) | 3 (15.0) |
| Prior PCI | 17 (30.9) | 6 (30.0) |
| Prior balloon valvuloplasty | 3 (5.5) | 1 (5.0) |
| Previous MI | 5 (9.1) | 1 (5.0) |
| Pre-existing pacemaker | 3 (5.5) | 0 (0.0) |
| Congestive heart failure | 49 (89.1) | 16 (80.0) |
| Prior atrial fibrillation/atrial flutter | 7/54 (13.0) | 5 (25.0) |
| Chronic lung disease/chronic obstructive pulmonary disease | 24 (43.6) | 14 (70.0) |
| Echocardiographic parameters | ||
| MPG (mmHg) | 57.8±16.1 | 63.4±18.0 |
| Mean aortic valve area (cm2) | 0.6±0.1 | 0.5±0.1 |
| Moderate or severe AR | 3 (5.5) | 1 (5.0) |
| Frailty | ||
| Anemia with transfusion | 1 (1.8) | 1 (5.0) |
| BMI <21 kg/m2 | 16 (29.1) | 8 (40.0) |
| Albumin <3.3 g/dL | 3/54 (5.6) | 1 (5.0) |
| Unplanned weight loss | 7 (12.7) | 3 (15.0) |
| Fall in past 6 months | 2 (3.6) | 2 (10.0) |
| 5-m gait speed (s) | 8.5±4.2 (n=39) | 8.1±4.2 (n=19) |
| 5-m gait speed >6 s | 30/39 (76.9) | 12/19 (63.2) |
| Grip strength below threshold | 50 (90.9) | 17 (85.0) |
| Disability | ||
| Wheelchair bound | 16 (29.1) | 5 (25.0) |
| Does not live independently | 17 (30.9) | 8 (40.0) |
| Does not bathe independently | 16 (29.1) | 8 (40.0) |
| Does not dress independently | 3 (5.5) | 2 (10.0) |
| Does not toilet independently | 4 (7.3) | 1 (5.0) |
| Does not transfer independently | 10 (18.2) | 3 (15.0) |
Values are mean±SD or N (percentages) or n/N (percentages). AR, aortic regurgitation; BMI, body mass index; CABG, coronary artery bypass surgery; CAD, coronary artery disease; MI, myocardial infarction; MPG, mean pressure gradient; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack.

Patient disposition for the 26-mm/29-mm and 23-mm CoreValve cohorts.
26-mm/29-mm Cohort Kaplan-Meier rates for adverse events for patients in the 26-mm/29-mm CoreValve cohort are presented in Table 2. The Kaplan-Meier rate for all-cause death was 13.0% at 1 year, 18.7% at 2 years, and 32.6% at 3 years (Figure 2). Two deaths were adjudicated by the CEC as valve-related. One patient underwent successful implantation of a 26-mm CoreValve bioprosthesis but became unstable after transfer to the intensive care unit and subsequently underwent pericardiocentesis. The next evening emergency surgery revealed a left retroperitoneal hemorrhage from the left external iliac artery, the patient remained unstable and died on postoperative day 2. The other patient died from an intracranial bleed 3 years post-procedure. The definition of a valve-related death includes any death caused by prosthetic valve dysfunction, valve thrombosis, embolism, bleeding event, or implanted valve endocarditis or related to reintervention on the operated valve. No patients required reintervention through 3 years’ follow-up. Two cases of MI occurred within 1 month of the procedure. The first patient had a history of prior coronary artery bypass grafting and PCI. Following valve implantation, a post-TAVR balloon valvuloplasty was performed after moderate AR was noted. The patient became hypotensive and coronary angiography revealed absence of flow in the left circumflex coronary artery. Creatinine kinase (CK) and troponin levels the following day confirmed the MI. On the 2nd postoperative day PCI of the affected vessel was performed. The second patient also had a history of prior PCI. During the TAVR procedure cardiac perforation and tamponade occurred after pre-TAVR balloon valvuloplasty. Transient myocardial damage caused by hypotension was suspected, which was treated with percutaneous cardiopulmonary support. CK and CK MB elevation was noted on the 1st postoperative day, but no ischemic-event related ECGs were provided. The CEC noted that the periprocedural MI was related to the CoreValve device. There were no subsequent cases of MI reported through 3 years; thus, the rate for MI remained 3.6%. The rate of major stroke was 5.8% at 1 year, 10.1% at 2 years, and 15.4% at 3 years; however, only 3 of the 7 major strokes that occurred over the 3-year follow-up were adjudicated by the CEC as related to the device or procedure. The CEC adjudicated stroke events by a few criteria, one of which was proximity to the implant procedure. For 2 of the patients, the stroke event occurred within 30 days of the implant procedure, so was adjudicated as related to the device or procedure. In the 3rd patient, the stroke occurred 605 days after the implant procedure, but the patient also had endocarditis, attributed to the valve, and the CEC considered the stroke likely device-related because of the valve-associated endocarditis. There were no signs of valve deterioration or thrombus observed during follow-up.
| Assessment | 1 month | 6 months | 1 year | 2 years | 3 years |
|---|---|---|---|---|---|
| MACCE | 6 (10.9) | 10 (18.4) | 13 (23.9) | 17 (31.4) | 23 (42.7) |
| All-cause death | 1 (1.8) | 5 (9.2) | 7 (13.0) | 10 (18.7) | 17 (32.6) |
| Cardiovascular death | 1 (1.8) | 3 (5.5) | 3 (5.5) | 5 (9.6) | 8 (17.2) |
| Stroke | 4 (7.4) | 5 (9.3) | 6 (11.4) | 8 (15.7) | 10 (21.0) |
| Major stroke | 2 (3.7) | 2 (3.7) | 3 (5.8) | 5 (10.1) | 7 (15.4) |
| Ischemic | 1 (1.9) | 1 (1.9) | 2 (3.9) | 4 (8.3) | 5 (10.9) |
| Hemorrhagic | 1 (1.9) | 1 (1.9) | 1 (1.9) | 1 (1.9) | 2 (4.9) |
| Myocardial infarction | 2 (3.6) | 2 (3.6) | 2 (3.6) | 2 (3.6) | 2 (3.6) |
| Reintervention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Valve thrombosis* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Major vascular complication | 6 (10.9) | 6 (10.9) | 6 (10.9) | 6 (10.9) | 6 (10.9) |
| Life-threatening/disabling bleeding | 7 (12.8) | 8 (14.7) | 9 (16.6) | 10 (18.7) | 11 (21.5) |
| Atrial fibrillation** | 3/51 (5.9) | 5/47 (10.6) | 6/45 (13.3) | 4/42 (9.5) | 3/36 (8.3) |
| Permanent pacemaker | 12 (22.2) | 14 (26.3) | 14 (26.3) | 15 (28.6) | 15 (28.6) |
Data reported as no. of patients (Kaplan-Meier rates as percentages). *Doppler ultrasound echocardiography used for valve thrombosis evaluation. **Atrial fibrillation is reported as incidence [n/N, (percentage)] and not as a Kaplan-Meier rate. MACCE, major adverse cardiovascular and cerebrovascular events.
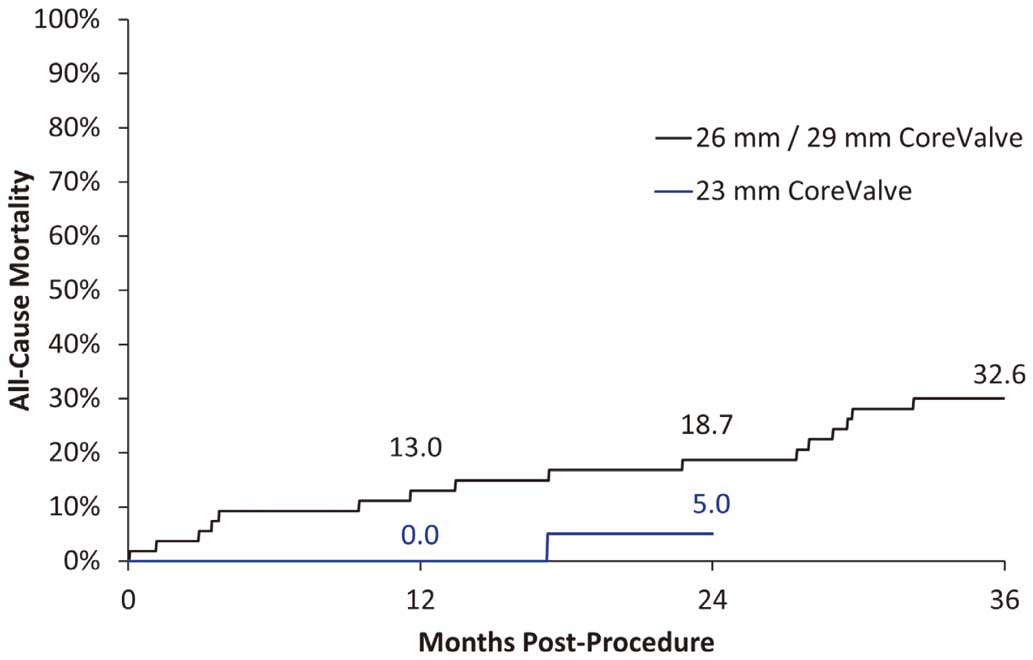
Kaplan-Meier estimates of all-cause mortality to the longest follow-up for each cohort.
The rate of MACCE was 23.9%, 31.4%, and 42.7% at 1, 2, and 3 years, respectively. Using VARC I definitions, the rate of life-threatening or disabling bleeding was 16.6% at 1 year, 18.7% at 2 years, and 21.5% at 3 years. The rate of major vascular complications was 10.9% at 1 month and remained unchanged throughout the remainder of the follow-up period. Permanent pacemaker implantation was required in 14 patients in the 1st year of follow-up, with 1 additional patient needing a permanent pacemaker in the 2nd year, and none in the 3rd year. The pacemaker implantation rates are shown in Table 2.
For patients with follow-up data, improvement in NYHA classification was sustained over 3 years (Figure 3A). As shown in Figure 4A, the rate of PVR remained stable over time, with 2 (5.7%) patients having moderate and no patients having severe PVR at 3 years. Improvements in mean pressure gradient (MPG) and EOA were also stable over time (Figure 5A). Quality of life measurements showed improvement in the SF-36 physical component summary score of 10.8 points (P=0.008) at 1 year, 9.3 points (P=0.002) at 2 years, and 4.8 points (P=0.151) at 3 years. However, the SF-36 mental component summary score showed only slight improvement, increasing by 2.8 points (P=0.016) at 1 year, 2.9 points (P=0.220) at 2 years, and 1.5 points (P=0.240) at 3 years.

New York Heart Association (NYHA) class over time for the (A) 26-mm/29-mm CoreValve cohort and (B) 23-mm CoreValve cohort. Note the follow-up time points vary for each panel based on completed follow-up for each cohort.

Paravalvular regurgitation over time for the (A) 26-mm/29-mm CoreValve cohort and (B) 23-mm CoreValve cohort. Note the follow-up time points vary for each panel based on completed follow-up for each cohort.

Mean pressure gradient and effective orifice area over time for the (A) 26-mm/29-mm CoreValve cohort and (B) 23-mm CoreValve cohort. Note the follow-up time points vary for each panel based on completed follow-up for each cohort.
23-mm Cohort A total of 16 iliofemoral patients were enrolled in the 23 mm study. Analysis of the primary endpoint showed that iliofemoral patients achieved a composite NYHA and EOA success rate of 87.5% (14/16). The lower bound of the 2-sided 95% confidence interval was 61.7%, indicating the performance of the 23-mm CoreValve was superior to conventional therapy outcomes of 50% improvement and the primary study objective was met (P=0.002).
Kaplan-Meier rates for adverse events for patients in the 23-mm CoreValve cohort (n=20) are presented in Table 3. The Kaplan-Meier rate for all-cause death was 0.0% at 1 year, and 5.0% at 2 years (Figure 2). No cases of MI, reintervention, or valve-related death occurred through 2 years. The rate of major stroke was 0.0% at 1 year and 5.0% at 2 years. The rate of MACCE was 0.0% at 30 days, 5.0% at 6 months and 1 year, and 10.0% at 2 years. Using VARC I definitions, the rate of life-threatening or disabling bleeding was 10.0% at 30 days, 15% at 1 year, and remained unchanged at 2 years. The rate of major vascular complications was 15% at 30 days and remained unchanged throughout the remainder of the follow-up period. Permanent pacemaker implantation was required in 4 patients in the 1st month of follow-up, with an additional patient needing a permanent pacemaker in the 1st year, and none in the 2nd year. The percentages of patients with a pacemaker are shown in Table 3.
| Assessment | 1 month | 6 months | 1 year | 2 years |
|---|---|---|---|---|
| MACCE | 0 (0.0) | 1 (5.0) | 1 (5.0) | 2 (10.0) |
| All-cause death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Cardiovascular death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stroke | 0 (0.0) | 1 (5.0) | 1 (5.0) | 1 (5.0) |
| Major stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Ischemic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) |
| Hemorrhagic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myocardial infarction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Reintervention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Valve thrombosis* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Major vascular complication | 3 (15.0) | 3 (15.0) | 3 (15.0) | 3 (15.0) |
| Life-threatening/disabling bleeding | 2 (10.0) | 3 (15.0) | 3 (15.0) | 3 (15.0) |
| Atrial fibrillation** | 2/20 (10.0) | 1/20 (5.0) | 2/20 (10.0) | 1/18 (5.6) |
| Permanent pacemaker | 4 (20.0) | 4 (20.0) | 5 (25.0) | 5 (25.0) |
Data reported as no. of patients (Kaplan-Meier rates as percentages). *Doppler ultrasound echo used for valve thrombosis evaluation. **Atrial fibrillation is reported as incidence [n/N, (percentage)] and not as a Kaplan-Meier rate. MACCE, major adverse cardiovascular and cerebrovascular events.
At baseline, all patients were in NYHA class III or IV; all patients followed to 6 months were in NYHA class I or II and remained so through 2 years (Figure 3B). PVR showed sustained improvement over time, with only one (5.6%) patient having moderate and no patients having severe PVR at 2 years (Figure 4B). Improvements in MPG and EOA also remained stable over time (Figure 5B). There was no statistically significant improvement in the SF-36 physical or mental component summary scores through 2 years of follow-up.
This study reports the midterm experience with the CoreValve 23-, 26-, and 29-mm bioprostheses in the Japanese population. Overall, the results demonstrated that TAVI with the CoreValve System was associated with sustained clinical and functional cardiac improvement in high-risk Japanese patients with severe AS.
In the 26-mm/29-mm cohort, MACCE occurred in 23 patients through 3 years of follow-up; 17 patients died, of whom 8 died from cardiac causes; 2 deaths were related to the TAV. The 3-year mortality rate was 32.6%. Although high, this mortality rate is not unexpected given the severity of AS, advanced age, and frailty of this patient cohort. The 3-year mortality rate is also similar to that reported by other larger studies evaluating the CoreValve in high-risk AS patients.6,7
Stroke remains an important problem with any AV intervention, be it surgical or catheter-based.8 In the 26-mm/29-mm CoreValve cohort, 7 patients experienced a major stroke through 3 years of follow-up, resulting in a Kaplan-Meier rate for major stroke of 15.4%, which is higher than that reported by larger, long-term studies of the CoreValve device;8,9 however, the small sample size (n=55) in the present study had a larger effect on calculated event rates compared with other much larger CoreValve studies. Additionally, in review of the 7 major stroke events, only 3 were adjudicated by the CEC as being related to the CoreValve bioprosthesis or procedure. Of these, 1 was a subarachnoid hemorrhage, which occurred within 30 days of the procedure, and 2 were ischemic strokes, one of which occurred within 30 days of the procedure, and 1 which occurred between the 1- and 2-year follow-up visits.
Cardiac conduction disorders requiring permanent pacemaker implantation are one of the most common complications seen after TAVI. In the present study, 15 patients who received either the 26-mm or 29-mm device required a permanent pacemaker through 3 years, resulting in a Kaplan-Meier rate of 28.6%. However, pacemaker implantation was required in the majority of cases (12 patients) within 30 days of the TAVI procedure. The incidence of permanent pacemaker implantation in the present study is similar to that reported for this TAV device, and several recent studies have found no adverse effect of permanent pacemaker implantation after TAVI with this device on stroke or survival.10,11
Echocardiography core laboratory data continued to demonstrate the durability of the CoreValve bioprosthesis. No significant changes in the hemodynamic status of the implanted 26-mm/29-mm bioprostheses were seen, and the MPG and EOA remained stable from discharge to 3 years. There was no evidence of valve thrombosis during the follow-up period. PVR remained stable over time, with only 2 (5.7%) patients having moderate PVR and no patients having severe PVR at 3-year follow-up.
In the 26-mm/29-mm cohort, NYHA functional classification showed sustained improvement over time, with all surviving patients classified as either NYHA class I or II at 3 years compared with class III or IV at baseline. There was also statistically significant improvement in the SF-36 physical component summary score through 2 years; however, this diminished by the 3rd year. The SF-36 mental component summary score showed only modest improvement, achieving statistical significance only at 1-year follow-up. Although the reason for the more limited improvement in the mental component summary results is unclear, other studies of TAVI in high-risk AS patients have documented similarly modest benefits in psychological measures.12
We also evaluated the 2-year outcomes of a separate cohort of Japanese patients with severe AS and a small aortic annulus who received the 23-mm CoreValve bioprosthesis. Overall, this valve performed well in this patient group, and the primary endpoint was met. There were 3 cases of MACCE occurred in 2 patients: 1 patient died from non-cardiac causes, and 1 patient had 2 stroke events over 2 years; 5 patients required a permanent pacemaker through 2 years, resulting in a Kaplan-Meier rate of 25.0%. No significant changes post-procedure in the hemodynamic status of the implanted 23-mm bioprosthesis were seen and PVR was stable over time, with only one patient having moderate and no patients having severe PVR at 2 years. NYHA functional classification also showed sustained improvement over time. We did not observe significant improvement in the SF-36 physical or mental component summary scores at any follow-up time point, which may have been limited by the small study cohort.
Study LimitationsThe primary limitation of both these studies was the small sample size, which also resulted in each event having a larger effect on event rates than is seen in larger trials.
This study demonstrated that TAVI with the self-expanding CoreValve was associated with sustained, multi-year clinical and functional cardiac improvement in high-risk surgical Japanese patients with severe, symptomatic AS.
Angie Zhang is an employee and shareholder of Medtronic, Plc.
The authors thank Janice Hoettels, PA, MBA and Jane Moore, MS, ELS for their assistance in the preparation of this manuscript. Both are employed by Medtronic.
This work was supported by Medtronic Japan Co. Ltd.