Abstract
Background:
The trend of the initial treatment strategy for pulmonary arterial hypertension (PAH) has changed from monotherapies to upfront combination therapies. This study analyzed treatments and outcomes in Japanese patients with PAH, using data from the Japan PH Registry (JAPHR), which is the first organized multicenter registry for PAH in Japan.
Methods and Results:
We studied 189 consecutive patients (108 treatment-naïve and 81 background therapy patients) with PAH in 8 pulmonary hypertension (PH) centers enrolled from April 2008 to March 2013. We performed retrospective survival analyses and analyzed the association between upfront combination and hemodynamic improvement, adjusting for baseline NYHA classification status. Among the 189 patients, 1-, 2-, and 3-year survival rates were 97.0% (95% CI: 92.1–98.4), 92.6% (95% CI: 87.0–95.9), and 88.2% (95% CI: 81.3–92.7), respectively. In the treatment-naïve cohort, 33% of the patients received upfront combination therapy. In this cohort, 1-, 2-, and 3-year survival rates were 97.6% (95% CI: 90.6–99.4), 97.6% (95% CI: 90.6–99.4), and 95.7% (95% CI: 86.9–98.6), respectively. Patients on upfront combination therapy were 5.27-fold more likely to show hemodynamic improvement at the first follow-up compared with monotherapy (95% CI: 2.68–10.36).
Conclusions:
According to JAPHR data, initial upfront combination therapy is associated with improvement in hemodynamic status.
Pulmonary arterial hypertension (PAH) is a progressive disorder. PAH is defined as an elevation in mean pulmonary artery pressure (mPAP) >25 mmHg, as well as pulmonary vascular resistance (PVR) >3 Wood units associated with normal pulmonary artery wedge pressure (PAWP).1
PAH is a complex and multifactorial disorder with a poor prognosis, and leads to right ventricular overload associated with severe right-sided heart failure.2
According to recent reports from several countries, the prevalence of PAH is approximately 12–50 per million people.3–5
The prognosis of PAH has improved since the approval of potent drugs, such as prostacyclin, endothelin receptor antagonists, and phosphodiesterase type 5 inhibitors. The average survival time after diagnosis is now estimated to be 5–7 years.6–8
Data from the US Registry to Evaluate Early and Long-Term PAH Disease Management show that the 1-year survival rate for PAH is approximately 91%.9
Currently, combination therapies in PAH, especially upfront combination therapies, are becoming more important, especially for patients with severe PAH. Evidence for this increase in importance includes a report from a French group, who showed positive efficacy of triple combination therapy in patients with New York Heart Association (NYHA) classes 3–4.10
Additionally, the AMBITION study showed a survival benefit of upfront combination therapies compared with single-sequential therapy.11
People in Japan have had universal health coverage since 1961, with coverage by employee-based and community-based insurance plans.12
The medical services and fees set for physicians and hospitals are uniform across the nation. Moreover, especially for patients with rare diseases, such as PAH, almost 100% of medical fees are covered by insurance. Surprisingly, not only are almost all potent drugs for PAH approved, but also reimbursements to perform combination therapies are permitted. Accordingly, after 2008 when sildenafil was approved in Japan and 3 types of PAH drugs, including epoprostenol, sildenafil, and bosentan, started to be used, physicians treating patients with pulmonary hypertension (PH) in Japan actively performed combination therapies.
Given the progression of knowledge on upfront combination therapy and the absence of data on combination therapies from nationwide multicenter registries, we investigated PAH survival in the combination therapy era using registry data for PAH in Japan.
Methods
The Japan Pulmonary Hypertension Registry (JAPHR) network was established by government grant support in Japan. This network started to collect data from 8 PH centers in Japan. For the purpose of this study, we evaluated all patients with pre-capillary PH who were recruited between April 2008 and March 2013 at each center. The registry was approved by the Ethics Committees both at Keio University (approved No. 2010-227) and at International University of Health and Welfare (approved No. 5-16-23), followed by the Institutional Review Boards of all participating centers, and all participants provided written informed consent to participate in the study (UMIN000026680). All of the centers were monitored to verify the data and to avoid missing data. All of the institutions are members of a research group to establish a national registry for PH, which is supported by the Japanese government and funded by Health and Labor Sciences Research Grants in Japan (No. H24-nanchito-general-020).
Pre-capillary PH was defined as mPAP ≥25 mmHg at rest and PAWP ≤15 mmHg, as measured on right-heart catheterization. Among the patients diagnosed with pre-capillary PH, the participants were also categorized as follows: PAH (Dana Point classification group 1); chronic thromboembolic PH (Dana Point classification group 4); or PH due to lung disease and/or hypoxemia associated with significant reduction in forced expiratory volume in 1 s (FEV1) and total lung capacity (TLC) (Dana Point classification group 3). To avoid misclassification of patients into group 1, we excluded those with FEV1 <60% predicted or with significant parenchymal lung disease on HRCT regardless of spirometry from the PAH cohort. PAH was classified as idiopathic, heritable, or associated with anorexigen exposure, connective tissue disease, portal hypertension, congenital heart disease, and pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis.
For each case, we collected data on patient age, sex, Dana Point classification, date of diagnosis and of initial cardiac catheterization, NYHA functional class, 6-min walk distance, hemodynamics, laboratory data, and detailed information on medications for PH via a Web-based data registration system. Data for all patients visiting the participating centers were entered consecutively into the registry.
Data are collected at the time of the first right heart catheterization as a baseline measurement, and at least in 12-month intervals or whenever the patient had a serious clinical event, such as death or transplantation. Every change in PH drugs regarding whether they were scheduled was noted. For data quality management, out-of-range data or missing values were automatically queried in the system during data entry.
Inclusion criteria for the present analysis were diagnosis of PAH, age ≥18 years with availability of data from right heart catheterization at diagnosis, and mPAP ≥25 mmHg and mean PAWP ≤15 mmHg. The treatment-naïve group was defined as those with a diagnosis of PAH on right heart catheterization between April 2008 and March 2013. Treatment-naïve patients included those who were diagnosed and who started medication during the study period. The background therapy group was defined as those with a diagnosis made prior to starting the study. We divided the patients into 2 groups according to the performance of upfront combination therapy. Upfront combination therapy was defined as receiving multiple types of approved PH-specific drugs including bosentan, ambrisentan, sildenafil tadalafil and epoprostenol within <90 days without any additional evaluation, such as an echocardiogram or right heart catheterization, or with no tolerability issues.
Symptomatic and Hemodynamic Status Improvement
We examined the proportion of NYHA classifications at baseline and at the first follow-up visit according to treatment group (monotherapy vs. upfront combination therapy). We also measured mPAP, cardiac index, and PVR from baseline and at right heart catheterization on first follow-up in the treatment groups. For patients with information on right heart catheterization at follow-up, we assessed the proportion of those with improved hemodynamic status since baseline. We defined improvement using the following 3 criteria: (1) ≥20% reduction in mPAP; (2) ≥20% upregulation of cardiac index; and (3) ≥20% reduction in PVR. To exclude the possibility of confounding effects, we initially excluded the patients treated with only calcium channel blockers because such patients have extremely good response to each drug.
Statistical Analysis
We tabulated the characteristics of the 2 cohorts based on etiology of PAH, demographics, baseline cardiac conditions, and baseline hemodynamic status and laboratory data. We report the type(s) of starting drugs. We plotted the 3-year Kaplan-Meier survival curves for survival from all-cause death or lung transplant according to the 2 cohorts, as well as the subgroups defined by etiology type and baseline NYHA classification. We compared the proportion of patients with improvement in 3 indices (mPAP, cardiac index, and PVR) at the earliest follow-up of right heart catheterization between the groups (upfront combination and monotherapy) using Fisher’s exact test. We created modified Poisson regression models13
for the 3 outcomes to assess the association between the treatment group and the improvement in each of these criteria. We also created a fourth model for the outcome of improvement in all 3 indices to estimate the relative likelihood of the outcome for the upfront therapy group compared with those on regular treatment. In these models, we adjusted for baseline NYHA classification class, dividing them into binary groups of classes I–II vs. III–IV. P<0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Subjects
A total of 189 consecutive adult patients with PAH were enrolled in this study. In this total cohort, the number of patients with idiopathic, heritable, and drug-induced PAH was 108 (Table 1). With regard to the treatment-naïve group, this consisted of 108 patients who received initial treatment at the time of enrollment (Table 1). Hemodynamic parameters at baseline in both of the cohorts are given in
Table 2.
Table 1.
Subject Clinical Characteristics
| |
Treatment-naїve cohort
(n=108) |
Total cohort
(n=189) |
| n |
% |
n |
% |
| Etiology |
| Idiopathic/heritable PAH |
50 |
46.3 |
105 |
55.6 |
| PAH associated with connective tissue disease |
36 |
33.3 |
48 |
25.4 |
| PAH associated with portal hypertension |
10 |
9.3 |
13 |
6.9 |
| PAH associated with CHD |
6 |
5.6 |
16 |
8.5 |
| Pulmonary veno-occlusive disease |
3 |
2.8 |
4 |
2.1 |
| Drug- and toxin-induced PAH |
3 |
2.8 |
3 |
1.6 |
| |
n |
% |
n |
% |
| Sex |
| Female |
86 |
79.6 |
144 |
76.2 |
| |
Mean |
SD |
Mean |
SD |
| Age (years) |
| At diagnosis |
– |
– |
43.9 |
16.9 |
| At treatment initiation |
48.8 |
17.3 |
45.1 |
16.6 |
| |
n |
% |
n |
% |
| NYHA class |
| I |
1 |
0.9 |
4 |
2.1 |
| II |
36 |
33.3 |
64 |
33.9 |
| III |
55 |
50.9 |
96 |
50.8 |
| IV |
16 |
14.8 |
25 |
13.2 |
| Cardiac rhythm |
| Sinus |
105 |
97.2 |
183 |
96.8 |
| Atrial fibrillation |
2 |
1.9 |
4 |
2.1 |
| Other |
1 |
0.9 |
2 |
1.1 |
| Use of anticoagulants |
| Yes |
46 |
42.6 |
78 |
41.3 |
| No |
62 |
57.4 |
111 |
58.7 |
| |
Mean |
SD |
Mean |
SD |
| 6-MWD (m) |
281 |
145 |
306 |
146 |
| Blood sample data |
| Bilirubin (mg/dL) |
1.1 |
0.9 |
1.0 |
0.7 |
| Creatinine (mg/dL) |
0.8 |
0.3 |
0.8 |
0.3 |
| Uric acid (mg/dL) |
6.5 |
2.3 |
6.5 |
2.4 |
| BNP (pg/mL) |
245 |
292 |
213 |
273 |
6-MWD, 6-min walk distance; BNP, B-type natriuretic peptide; CHD, congenital heart disease; NYHA, New York Heart Association; PAH, pulmonary arterial hypertension.
Table 2.
Hemodynamics Parameters at Study Entry
| |
Treatment-naїve cohort
(n=108) |
Total cohort
(n=189) |
| Mean |
SD |
Mean |
SD |
| Mean PAP (mmHg) |
46.9 |
14.4 |
48.2 |
13.8 |
| PAWP (mmHg) |
7.8 |
3.7 |
8.2 |
3.4 |
| Mean right atrial pressure (mmHg) |
6.5 |
4.0 |
6.6 |
4.1 |
| PVR (dyn·s·cm−5) |
1,106 |
733.0 |
1,036 |
653.0 |
| Cardiac index (L/min/m2) |
2.2 |
0.7 |
2.4 |
0.8 |
| Mixed venous oxygen saturation (%) |
65.0 |
8.9 |
66.6 |
9.7 |
PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance.
Among the 108 patients in the treatment-naïve cohort, all patients received PAH-specific therapy of epoprostenol, bosentan, ambrisentan, sildenafil, and/or tadalafil (Figure 1). Patients receiving only calcium channel blockers or beraprost were excluded from this cohort. The characteristics of drug use just after diagnosis are given in
Table 3. Surprisingly, 34 patients (31.5%) had already received upfront combination therapy at diagnosis, including 3 (2.8%) with triple combination therapy, including epoprostenol. A total of 65.7% of the patients had severe PAH symptoms and were categorized in NYHA classes 3–4. We analyzed the dosage of epoprostenol as an initial target. Sixteen patients received epoprostenol as the first-line therapy, including single and upfront combination therapies, and the mean (±SD) dosage was 40.4±19.4 ng/kg/min.
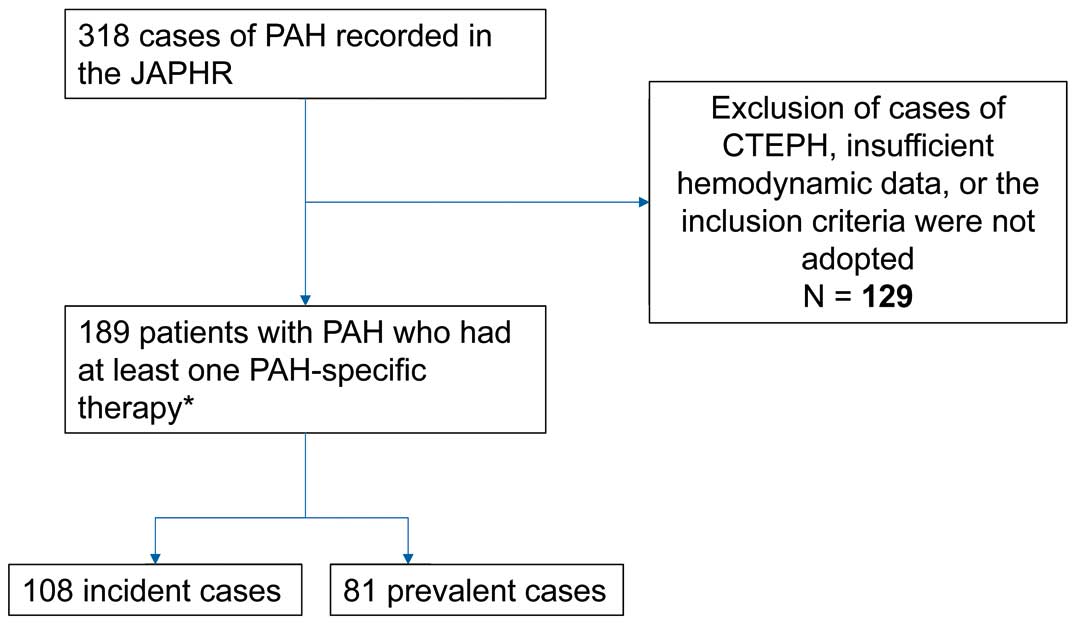
Table 3.
Characteristics of Initial Drug Use
| Drug combination |
n |
% |
| Treatment-naїve cohort (n=108): drug use just after diagnosis |
| Single |
| Sildenafil |
38 |
36.2 |
| Tadalafil |
16 |
14.8 |
| Bosentan |
14 |
13 |
| Ambrisentan |
4 |
3.7 |
| Epoprostenol |
2 |
1.9 |
| Upfront double combination |
| Bosentan and sildenafil |
7 |
6.5 |
| Ambrisentan and sildenafil |
7 |
6.5 |
| Bosentan and epoprostenol |
5 |
4.6 |
| Sildenafil and epoprostenol |
5 |
4.6 |
| Bosentan and tadalafil |
4 |
3.7 |
| Ambrisentan and tadalafil |
2 |
1.9 |
| Tadalafil and epoprostenol |
1 |
0.9 |
| Upfront triple combination |
| Bosentan, sildenafil, and epoprostenol |
2 |
1.9 |
| Ambrisentan, tadalafil, and epoprostenol |
1 |
0.9 |
| Total cohort (n=189): drug use at study entry |
| Single |
| Sildenafil |
46 |
24.3 |
| Bosentan |
46 |
24.3 |
| Tadalafil |
17 |
9 |
| Ambrisentan |
5 |
2.7 |
| Epoprostenol |
29 |
15.3 |
| Double combination |
| Bosentan and sildenafil |
13 |
6.9 |
| Ambrisentan and sildenafil |
8 |
4.2 |
| Bosentan and epoprostenol |
6 |
3.2 |
| Sildenafil and epoprostenol |
6 |
3.2 |
| Bosentan and tadalafil |
5 |
2.7 |
| Ambrisentan and tadalafil |
3 |
1.6 |
| Tadalafil and epoprostenol |
1 |
0.5 |
| Triple combination |
| Bosentan, sildenafil, and epoprostenol |
2 |
1.1 |
| Ambrisentan, tadalafil, and epoprostenol |
1 |
0.5 |
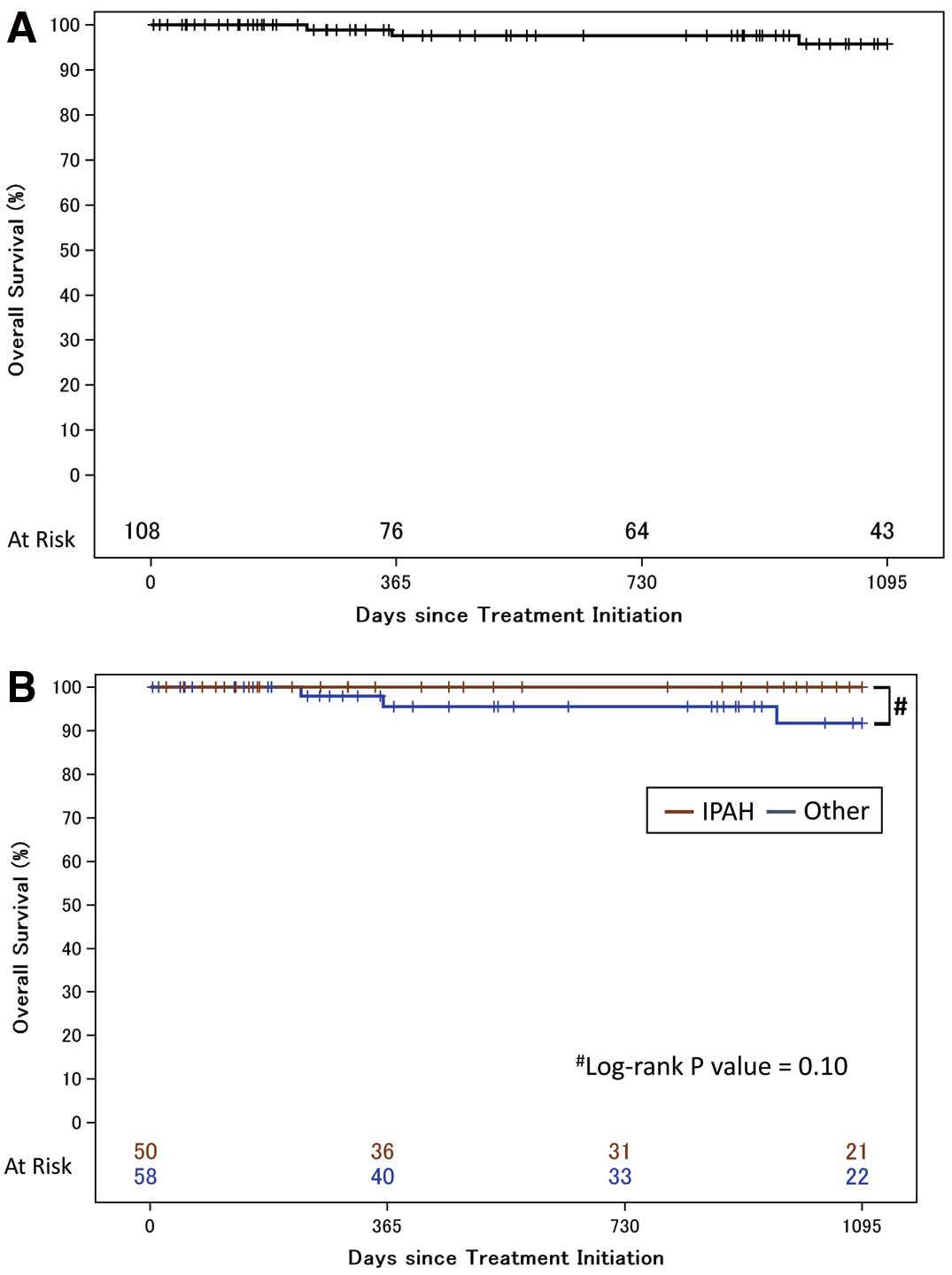
We performed 3-year survival analysis in the treatment-naïve cohort. Among the 108 patients in this cohort, 3 patients died or had lung transplantation performed during the follow-up period (Figure 2A). There was no loss to follow-up in each of the centers during the 3-year follow-up. Kaplan-Meier survival estimates for the 108 treatment-naïve PAH patients at 1, 2, and 3 years were 97.6% (95% CI: 90.6–99.4), 97.6% (95% CI: 90.6–99.4), and 95.7% (95% CI: 86.9–98.6), respectively.
Figure 2B
shows the Kaplan-Meier survival estimates for patients with idiopathic, heritable, and drug-induced PAH (n=50), and for patients with other types of PAH (n=58). The estimated 3-year survival rates were 100% and 91.7% (95% CI: 75.4–97.4), respectively, with no significant difference between the 2 subgroups. In the subgroups of PAH associated with other diseases, the estimated 3-year survival rate for patients with autoimmune disease, congenital heart disease, and portal hypertension was 93.0%, 80.0%, and 100%, respectively (Figure S1).
Among the 189 patients in the combined cohort, all patients also received PAH-specific therapy. Patients receiving only calcium channel blockers or beraprost were excluded from this cohort. The mean (±SD) age at diagnosis was 43.9±16.9 years. Therefore, the mean time from diagnosis to study enrollment was 1.2 years. The characteristics of drug use at study entry are listed in
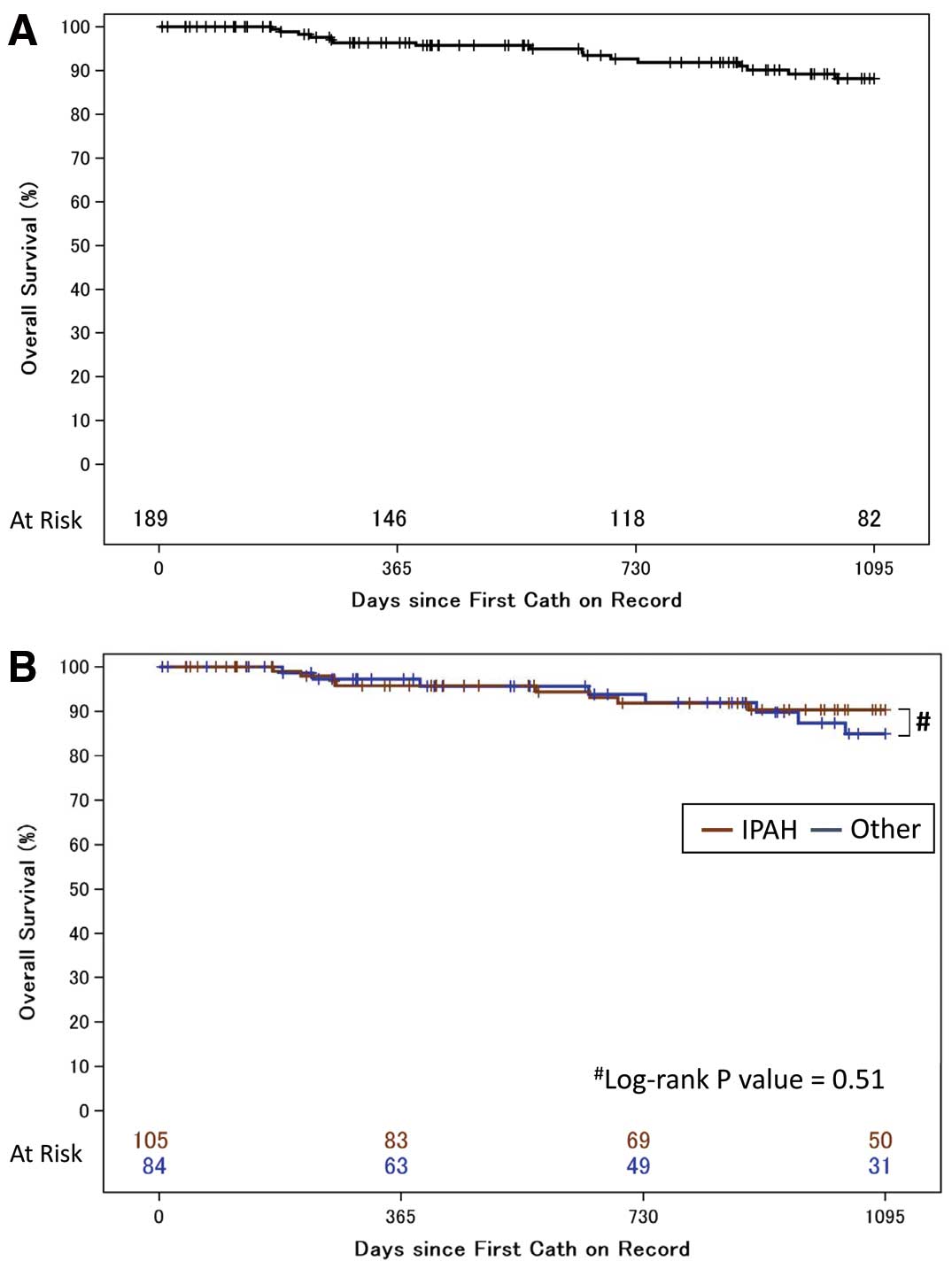
Table 3. A total of 143 patients (75.6%) received monotherapy, including 29 (15.3%) with epoprostenol monotherapy. The number of patients who had double and triple combination therapies, including sequential and upfront combination therapy, was 42 (22.3%) and 4 (2.1%), respectively. On 3-year survival analysis of the 189 patients in this cohort, 16 patients died or had lung transplantation during the follow-up period (Figure 3A). There was no loss to follow-up in each center during the 3-year follow-up. Kaplan-Meier survival estimates for the 189 patients with treatment-naïve PAH at 1, 2, and 3 years were 97.0% (95% CI: 92.1–98.4), 92.6% (95% CI: 87.0–95.9), and 88.2% (95% CI: 81.3–92.7), respectively.
Figure 3B
shows the Kaplan-Meier survival estimates for idiopathic, heritable, and drug-induced PAH (n=105) and for patients with other types of PAH (n=84). The estimated 3-year survival rate was 90.4% (95% CI: 81.6–95.1) and 84.9% (95% CI: 71.5–92.3), respectively, with no significant difference between the 2 subgroups.
Among the 108 patients in the treatment-naïve cohort, we obtained follow-up information on NYHA classification and right heart catheterization measures for 92 patients. Baseline and first follow-up NYHA classification, hemodynamic status and other clinical characteristics are listed in
Tables 4,S1. The median time to first follow-up from baseline was 292.5 days. Among the 66 patients on monotherapy, 36 (54.6%) had NYHA class III or IV, while 23 of 29 (88.4%) had NYHA class III or IV in the upfront combination therapy group. At first follow-up, 34.8% and 53.8% of patients were in NYHA classes III and IV, respectively. And hemodynamic improvements for each treatment strategy were associated with the improvements in exercise function (Tables 4,S2). Among 26 patients on upfront combination therapy, 84.6% had a ≥20% reduction in mPAP at the first right heart catheterization. Improvement in the cardiac index and pulmonary ventricular resistance was observed in 84.0% and 88.5% of the patients, respectively. In contrast, 42.4%, 43.1%, and 64.1% of patients had improvement in mPAP, cardiac index, and PVR in the monotherapy group, respectively. After adjustment for baseline NYHA classification status, those on upfront combination therapy were 1.98-fold more likely to have an improvement in PAP at their first follow-up right heart catheterization (95% CI: 1.37–2.84) compared with those on regular treatment (Table 5). The relative risks for improvement of the cardiac index and PVR were 1.90 (95% CI: 1.32–2.73) and 1.39 (1.06–1.81), respectively. Taken together, patients on upfront combination therapy were 5.27-fold more likely to show improvement in all 3 of these indices (95% CI: 2.68–10.36) compared with those on regular treatment, and the results were not different after adjustment for age and gender (Table S3). Finally, the Kaplan-Meier survival estimates for monotherapy and for upfront combination therapy are given in
Figure S2. Interestingly, there was no significant difference between the 2 subgroups, nevertheless the monotherapy group had less severe PAH at baseline. This result was in line with the hemodynamics results.
Table 4.
Improvement vs. Therapy Type in the Treatment-Naїve Cohort
| |
Monotherapy (n=66) |
Upfront combination therapy (n=26) |
P-value |
| Baseline |
First follow-up |
Baseline |
First follow-up |
| n |
% |
n |
% |
n |
% |
n |
% |
| NYHA class |
| I |
1 |
1.5 |
1 |
1.5 |
0 |
0 |
1 |
3.8 |
|
| II |
29 |
43.9 |
42 |
63.6 |
3 |
11.5 |
11 |
42.3 |
|
| III |
31 |
47.0 |
23 |
34.8 |
14 |
53.8 |
11 |
42.3 |
|
| IV |
5 |
7.6 |
0 |
0.0 |
9 |
34.6 |
3 |
11.5 |
|
| |
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
| Hemodynamic changes |
| Mean PAP (mmHg) |
44.4 |
13.8 |
38.6 |
13.4 |
55.9 |
14.5 |
37.6 |
16.7 |
|
| Cardiac index (L/min/m2) |
2.21 |
0.68 |
2.76 |
1.01 |
1.94 |
0.68 |
2.96 |
1.02 |
|
| PVR (dyn·s·cm−5) |
1,023.2 |
697.4 |
664.8 |
424.6 |
1,405.7 |
852.1 |
564.1 |
430.6 |
|
| |
|
|
n |
% |
|
|
n |
% |
|
| Improved hemodynamic status |
| Mean PAP (≥20% reduction) |
|
|
28/66 |
42.4 |
|
|
22/26 |
84.6 |
<0.001 |
| Cardiac index (≥20% upregulation) |
|
|
28/65 |
43.1 |
|
|
21/25 |
84.0 |
<0.001 |
| PVR (≥20% reduction) |
|
|
41/64 |
64.1 |
|
|
23/26 |
88.5 |
0.02 |
| All 3 indices |
|
|
10/64 |
15.6 |
|
|
19/25 |
76.0 |
<0.001 |
Abbreviations as in Tables 1,2.
Table 5.
Estimated Relative Risk of Improvement at First Follow-up RHC
| Improvement index |
Relative risk |
P-value |
| Mean PAP |
1.98 (1.37–2.84) |
<0.001 |
| Cardiac index |
1.90 (1.32–2.73) |
<0.001 |
| PVR |
1.39 (1.06–1.81) |
<0.02 |
| All 3 indices |
5.27 (2.68–10.36) |
<0.001 |
†In the upfront combination therapy group vs. the regular treatment group. RHC, right heart catheterization. Other abbreviations as in Table 2.
Discussion
This is the first report of a multicenter registry on PAH from Japan, and it strongly supports the benefit of upfront combination therapy. Even in patients with NYHA functional class III or IV, upfront combination therapy resulted in improvement in hemodynamics, as well as exercise capacity. Such improvement might result in a better prognosis of PAH. Despite most patients having NYHA functional class III or IV, 26 patients who received initial upfront combination therapy had remarkable improvement in PVR, cardiac index, and PAP.
The combination therapy paradigm has advanced over the span of several years. Initially, a French network of PAH investigators reported their initial experience with upfront triple combination therapy, including epoprostenol.10
Despite the fact that the investigators retrospectively reviewed only 19 patients with severe PAH who were treated with bosentan, sildenafil, and i.v. epoprostenol simultaneously as initial treatment, there was significant improvement in the hemodynamics of 18 of 19 patients (cardiac index, 3.5±0.7 vs. 1.7±0.4 L/min/m2; PVR, 7.1±3.3 vs. 21.5±7.8 Wood units). They also showed improvement in the 6-min walk distance (463±94 vs. 227±171 m) at the initial follow-up evaluation compared with baseline. These beneficial effects were sustained to the final follow-up evaluation at 32±10 months. Additionally, the AMBITION study compared dual upfront combination therapy with ambrisentan and tadalafil with monotherapy, with the 2 agents as a pooled group in treatment-naïve PAH patients.11
This previous study demonstrated that upfront combination therapy was superior to monotherapy with either single agent and it had long-term benefits.11
The advantage of combination therapy is suggested to be ubiquitous because the French network of PAH investigators also reported their retrospective data of initial dual oral combination therapy.14
All regimens of a combination of sildenafil or tadalafil plus bosentan or ambrisentan showed significant improvement with a better prognosis than the expected survival rates from the French equation. The 2015 European Society of Cardiology/European Respiratory Society PH guidelines recommend performing initial combination therapy, especially in critically ill patients with PAH.1
Data from only a few studies, however, were used to support these guidelines. The present data strongly support the advanced recommendation because improvement in hemodynamics and prognosis were hopeful in severe patients.
Another unique point of the treatment method in Japan is that the use of high-dose epoprostenol is eligible for insurance reimbursement. In the present study, the initial mean target dosage of epoprostenol was 40.4 ng/kg/min, which is similar to previous reports.15,16
We cannot conclude yet that “more is better” for use of epoprostenol because side-effects of long-term epoprostenol use have been reported, such as IgG4-related disease,17
and we also should consider the cost-effectiveness of high-dose epoprostenol. We should reconsider, however, the optimal dosage of epoprostenol, because in the French double combination study, patients who received tadalafil appeared to have greater hemodynamic improvement than those who received sildenafil.14
This could be explained by the fact that the approved dose of sildenafil was 20 mg 3 times daily. Additionally, the change in PVR from baseline was dose dependent from 20 mg 3 times daily to 80 mg 3 times daily in an initial randomized, controlled trial of sildenafil vs. placebo (SUPER-1).18
Such findings suggest that the method “more is better” could have considerable advantages if compliance and side-effects are acceptable.
The present study has a few limitations with regard to the interpretation of findings. This study was retrospectively designed and the initial follow-up period was not uniform between each center. The data, however, were well monitored and validated to exclude inclusion bias. Additionally, because of the limited number of patients, extracting and adjusting all of the conceivable factors to decide whether each patient should be enrolled in upfront combination therapy was difficult. Therefore, residual confounding may exist in the assessment of the association between hemodynamic improvement and treatment choice. Given that patients with severe PAH were treated with combination therapy, the baseline difference was by nature associated with better improvement. Second, the data were not useful with regard to health economics. The higher the number of drugs, the higher the cost of treatment, therefore we should analyze this aspect at the next step. Additionally, we defined “upfront combination therapy” as receiving multiple types of approved PH-specific drugs within <90 days without any additional evaluation. The consensus of the term has not been established as yet, and although we adopted a definition similar to previous reports,10,14
the present findings may not be generalizable to upfront combination therapies based on other definitions. Finally, the centers that were enrolled in this registry were experienced in the treatment of PAH. Therefore, the present data may not reflect the practices and observations at other locations across Japan. This is because there is an imbalance in treatment strategies owing to the difference in relative experience with patient care and drug use.
Conclusions
Initial upfront combination therapy, which is a standard treatment strategy in Japan, appears to have an advantage in the treatment of PAH. This therapy was associated with improvement in hemodynamic status at first follow-up, similar to the AMBITION trial. Because the prognosis of PAH is poor on classical treatment regimens, the present results suggest the use of combination therapies for these patients.
Disclosures
Y.T. is affiliated with Pulmonary Hypertension Center, International University of Health and Welfare Mita Hospital, endowed by Nippon Shinyaku, and received lecture fees from Actelion Pharmaceuticals Japan, and Nippon Shinyaku, and research grants from Actelion Pharmaceuticals Japan and Mochida Seiyaku.
H. Kumamaru, and H. Miyata are affiliated with the Department of Healthcare Quality Assessment at The University of Tokyo, and the department is endowed by Johnson & Johnson, Nipro Corporation, Teijin Pharma, Kaketsuken, St. Jude Medical Japan, Novartis Pharma, Taiho Pharmaceutical, W. L. Gore & Associates, Olympus Corporation, and Chugai Pharmaceutical.
T.S. received lecture fees from Actelion Pharmaceuticals Japan, and a research grant from Actelion Pharmaceuticals Japan.
A.O. has received lecture fees from Actelion Pharmaceuticals Japan, GlaxoSmithKline, Nippon Shinyaku, and Pfizer Japan, and a research grant from GlaxoSmithKline.
N.T. works in a department endowed by Actelion Pharmaceuticals Japan., and received lecture fees from Actelion Pharmaceuticals Japan, Bayer Yakuhin, GlaxoSmithKline, Nippon Shinyaku, and a research grant from Nippon Shinyaku.
M.H. works in a department endowed by Bayer Yakuhin, GlaxoSmithKline, and Nippon Shinyak, and received lecture fees from Actelion Pharmaceuticals Japan and Bayer Yakuhin.
A.Y. received lecture fee from Actelion Pharmaceuticals Japan.
I.T. received a research grant from Actelion Pharmaceuticals Japan.
H. Kimura works in a department endowed by Actelion Pharmaceuticals Japan., and received lecture fees from Actelion Pharmaceuticals Japan.
M.K. received lecture fees from Actelion Pharmaceuticals Japan, Bayer Yakuhin, GlaxoSmithKline, Nippon Shinyaku, and a research grant from Actelion Pharmaceuticals Japan, and Bayer Yakuhin.
H. Matsubara has received lecture fees from Actelion Pharmaceuticals Japan, AOP Orphan Pharmaceuticals, Bayer Yakuhin, GlaxoSmithKline, Nippon Shinyaku, and Pfizer Japan, and a research grant from Nippon Shinyaku.
Author Contributions
Figures: Y.T., H. Kumamaru. Study design: Y.T., T.S., H. Miyata, H. Matsubara, K.T. Data collection: Y.T., A.O., N.T., M.H., A.Y., K.A., I.T., K.F., H. Kimura, M.K. Data analysis: H. Kumamaru, H. Miyata. Data interpretation: Y.T., H. Kumamaru, H. Miyata. Writing: Y.T., H. Kumamaru, N.T., K.A., H. Kimura.
Sources of Funding
This work was supported by Health and Labor Sciences Research Grants in Japan (No. H24-nanchito-general-020 and H28-nanchito-general-022).
Appendix
List of JAPHR Investigators
The JAPHR study included the following principal investigators:
Takahiro Sato, Hiroshi Ohira, Taku Watanabe and Masaharu Nishimura (Hokkaido University, Tokyo, Japan), Yucihiro Shirai (Nippon Medical School, Tokyo, Japan), Hisataka Maki, Toshiro Inaba, Minatsuki Shun and Hironori Muraoka (The University of Tokyo Hospital, Tokyo, Japan), Mai Kimura, Makoto Takei, and Tomohiko Ono (Keio University, Tokyo, Japan), Seiichiro Sakao and Yasunori Kasahara (Chiba University, Chiba, Japan), Hiroki Uyama, Atsuhiro Nakamura and Takefumi Itoh (Nara Medical University, Nara, Japan), and Akiko Ohina (National Hospital Organization Okayama Medical Center, Okayama, Japan).
Supplementary Files
Supplementary File 1
Figure S1.
Three-year survival analysis for patients with (A) autoimmune diseases; (B) congenital heart disease; and (C) portal hypertension.
Figure S2.
Three-year survival analysis for the treatment-naïve cohort by treatment strategy.
Table S1.
Baseline clinical characteristics vs. therapy type in the treatment-naïve cohort
Table S2.
Hemodynamic improvement vs. therapy type in the treatment-naïve cohort
Table S3.
Relative risk for improvement at first follow-up RHC
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0139
References
- 1.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975.
- 2.
Wilkins MR. Pulmonary hypertension: The science behind the disease spectrum. Eur Respir Rev 2012; 21: 19–26.
- 3.
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: Results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030.
- 4.
Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007; 30: 104–109.
- 5.
Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US contemporary registries. Chest 2011; 139: 128–137.
- 6.
Gomberg-Maitland M, Dufton C, Oudiz RJ, Benza RL. Compelling evidence of long-term outcomes in pulmonary arterial hypertension?: A clinical perspective. J Am Coll Cardiol 2011; 57: 1053–1061.
- 7.
Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1285–1293.
- 8.
Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456.
- 9.
Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172.
- 10.
Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA, et al. Upfront triple combination therapy in pulmonary arterial hypertension: A pilot study. Eur Respir J 2014; 43: 1691–1697.
- 11.
Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844.
- 12.
Ikegami N, Yoo BK, Hashimoto H, Matsumoto M, Ogata H, Babazono A, et al. Japanese universal health coverage: Evolution, achievements, and challenges. Lancet 2011; 378: 1106–1115.
- 13.
Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706.
- 14.
Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jaïs X, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016; 47: 1727–1736.
- 15.
Kimura M, Tamura Y, Takei M, Yamamoto T, Ono T, Kuwana M, et al. Rapid initiation of intravenous epoprostenol infusion is the favored option in patients with advanced pulmonary arterial hypertension. PLoS One 2015; 10: e0121894.
- 16.
Tokunaga N, Ogawa A, Ito H, Matsubara H. Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J Cardiol 2016; 68: 542–547.
- 17.
Shirai Y, Tamura Y, Yasuoka H, Satoh T, Kuwana M. IgG4-related disease in pulmonary arterial hypertension on long-term epoprostenol treatment. Eur Respir J 2014; 43: 1516–1519.
- 18.
Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157.




