Article ID: CJ-17-0412
Article ID: CJ-17-0412
Background: Left atrial appendage closure (LAAC) has been developed as an alternative treatment used for the prevention of strokes in high-risk patients with atrial fibrillation. Here, a novel LAAC prosthesis (LACBES® device) is developed, and its translational potential is investigated by performing a pre-clinical study to evaluate its safety and effectiveness.
Methods and Results: LACBES® occluders were implanted in 7 healthy canines percutaneously. Closure effect was evaluated by left atrial angiography. The canines were sacrificed post-procedure on days 45, 80 and 110; gross anatomy was examined subsequently. Endothelialization of device surface was evaluated by HE staining, immunofluorescence staining against CD31 and scanning electron microscope. LACBES® occluders were implanted in all canines successfully; a small residual shunt was observed in 1 canine immediately post procedure. One canine died of groin hematoma within 36 h, which was related to the procedure, but there was no device-related death. A layer of white transparent tissue that failed to cover the nut was formed on the surface of the sealing disc on day 80, but the newborn tissue completely covered the sealing disc on day 110. Immunofluorescence staining against CD31 and scanning electron microscope confirmed complete intima formation and neovascularization within 4 months.
Conclusions: The current research suggested the LACBES® device is feasible for LAAC, with a high success rate, few device-related complications and complete neointima formation in canines.
Atrial fibrillation (AF) is a common arrhythmia, which is one of the main causes of ischemic stroke in the elderly. Left atrial appendage closure (LAAC) can effectively reduce the risk of stroke in patients with non-valvular AF (NVAF), which is non-inferior to oral anticoagulation treatment with warfarin, and it has been an alternative treatment for NVAF patients with a high risk of stroke and contraindication to long-term oral anti-coagulation treatment.1–4 Left atrial appendage (LAA) occluder is a key factor of LAAC, and some LAA occluders have been widely used for LAAC in clinics to date. According to clinical experiences, an ideal LAA occluder should have the following characteristics: occluding LAA completely, being released safely, being retrieved or redeployed easily, less thrombogenicity, being fixed firmly and no impact on adjacent structures. Watchman LAA and ACP devices are the most widely used occluders in clinic, especially in the USA and Europe.1 However, even for them, as emphasized in their instructions, the occluder should be replaced once was completely retrieved. Thus, improvement on current occluders or development for novel occluder should be the future work to focus on. In order to solve these defects, Changhai Hospital and ShangHai PushMed designed and developed a novel LAA occluder, the LACBES® LAA occluder, which can be retrieved or redeployed repeatedly. In this study, the effectiveness and safety of LAAC with the LACBES® occluder were fully evaluated via animal experiments, and this was also the first study to test the feasibility of this occluder in vitro; it would provide an experimental basis for clinical application.
Seven healthy canines (4 males and 3 females) weighing 9–12 kg (10.21±1.04 kg) were utilized in this experiment. After fasting for 8 h, experimental canines were anesthetized with 10% xylazine hydrochloride (0.03 mL/kg) together with atropine (0.02 mg/kg) by intramuscular injection. The anesthesia status was maintained by propofol (i.v.) and ECG was monitored during the whole procedure. All protocols were designed in accordance with China’s National Standard on ‘‘Laboratory animal-requirements of environment and housing facilities’’ (GB 14925–2001).
LACBES® LAA Occluder DescriptionLACBES® occluder (Figure 1) is a novel self-developed LAA occluder, which has just been approved for clinical trials in China. This occluder is made by nitinol wire mesh and mainly consists of 2 parts; an anchor cylinder and a sealing disc, which are filled with polyester fiber membrane. The anchor cylinder will land at the landing zone and deeply anchor to the LAA as a supporting structure for the whole occluder, with the sealing disc sealing the LAA orifice. One important characteristic to note is that the 2 parts are connected by a long and thin waist, which is further constrained and fixed by a short stainless steel ring. This structure can be extended a little and even bend at an angle to make the shape of the occluder more adjustable and flexible after implantation. A short fixing nut and rivet are located at the ends of the sealing disc and the anchor cylinder respectively. The anchor cylinder is made of weaved nitinol wires where localized 9- to 12-integrated micro-barbs are sculptured by using a special technique. The sealing disc is curved inward and 6 mm larger than the anchor cylinder in diameter. The device is available with anchor cylinder sizes from 16 to 34 mm (2-mm size increments) and requires 9- to 14-French sheaths.
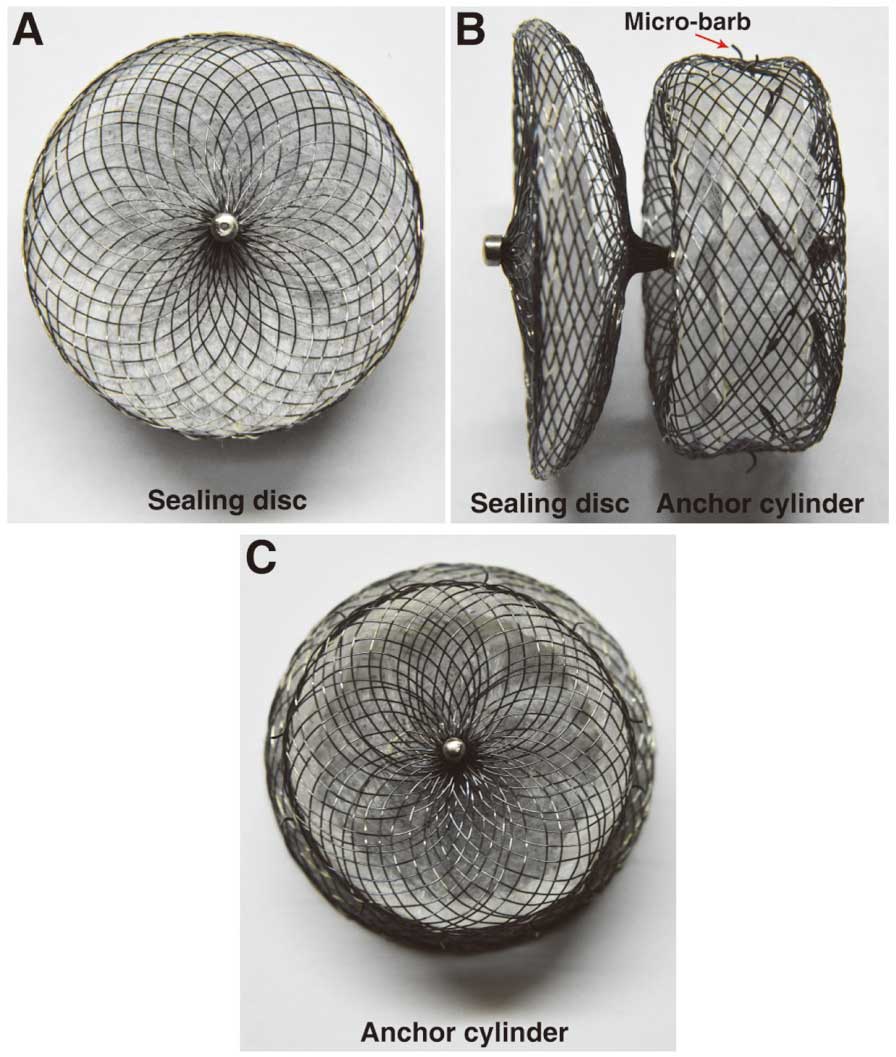
LACBES® occluder. (A) The sealing disc of the occluder. (B) Lateral view of the occluder. (C) The anchor cylinder of the occluder.
The procedure was performed via a femoral vein approach. After the atrial septal was punctured, a spring wire was delivered into the left atrium (LA), and then a 9F dedicated delivery sheath was sent into the LA over a spring wire. Subsequently, a 6F pigtail catheter was placed in the LAA, and an angiography in the 25°right anterior oblique+15°caudal was performed. Diameters of LAA orifice and landing zone were then measured by angiography. The occluder size was generally chosen 1- to 2-mm larger than the LAA landing zone diameter. The anchor cylinder was first partially released and appropriately delivered into the LAA and then anchored in the LAA landing zone, approximately 1–1.5 cm behind the orifice. The sealing disc of the occluder was then unfolded and gently pulled out a little to cover the LAA orifice (Figure 2). The occluder could be released according to the “PAST” principle, which is: (1) P osition: the anchor cylinder is positioned at the landing zone or in a deeper location with a shape of tire; (2) A part: the sealing disc is set apart from the anchor cylinder; (3) S eal: the sealing disc covers the orifice with no or only a small amount of residual shunt; and (4) T ug test: tug the curve sealing disc to cage shape, the anchor cylinder was still fixed in the LAA landing zone without movement.
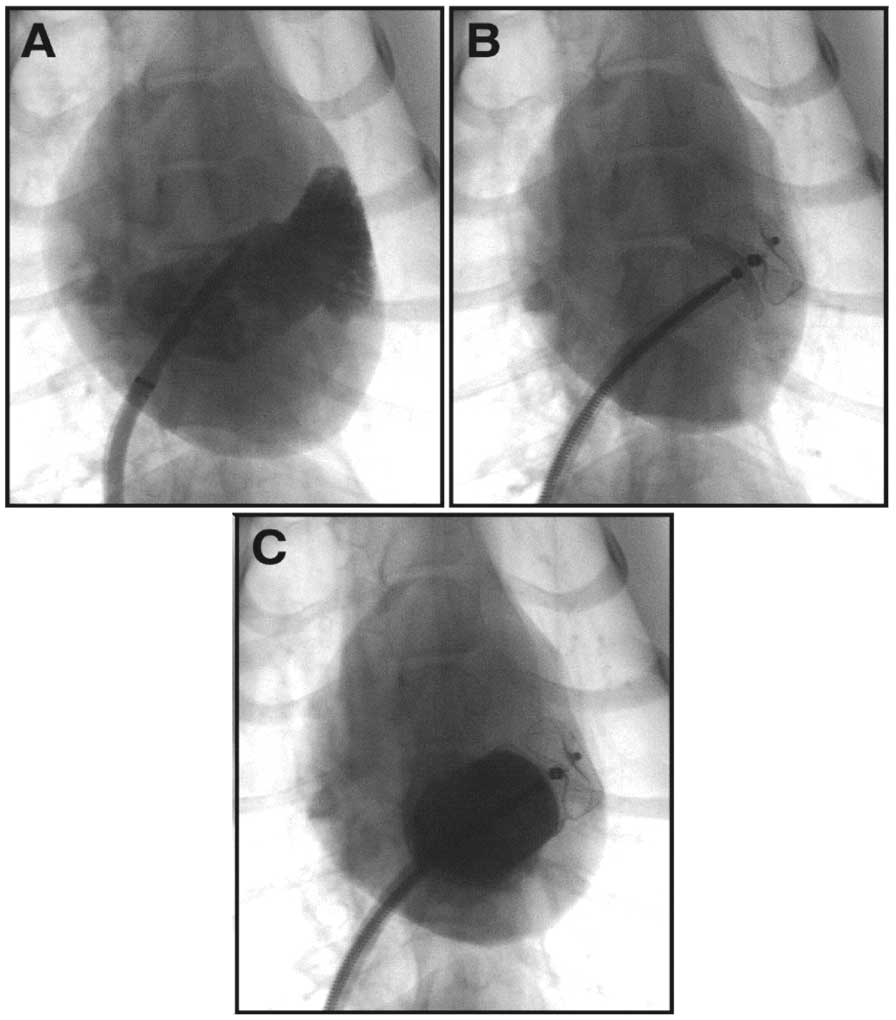
Deployment Procedure of the LACBES® occluder. (A) A left atrial appendage (LAA) angiography in RAO25°+CAU15° was performed to learn about the configuration and size of the LAA. (B) The LACBES® occluder was deployed. (C) a LAA angiography was performed again to evaluate the sealing efficacy.
During the implantation procedure, unfractionated heparin (200 IU/kg) was administered through the sheath. The canines were treated with low molecular weight heparin (1,000 IU/12 h) by subcutaneous injection and penicillin G sodium by muscular injection post-operation for 3 days, and aspirin (3–5 mg/kg, P.O.) for 3 months or until they were sacrificed. The canines were feed separately and their behaviors were observed, especially salivation, hemiplegia and other indications of stroke.
Gross Evaluation and AssessmentCanines were sacrificed after implantation of the LAA occulder on day 45, 80, and 110. Samples from the liver, spleen and kidney were carefully examined to confirm whether infarction existed. The heart was anatomized and the LA was exposed and photographed to check the adjacent relationship of the sealing disc and mitral valve (MV), and left upper pulmonary vein (LUPV). Tissues on the surface of the sealing disc were taken out and fixed for further evaluation by HE staining, immunofluorescence staining against CD31, and scanning electron microscope examination.
Configurations of 7 canines LAA were all single lobe (4 chicken wing and 3 windsock), with an average orifice diameter of 14.40±1.09 mm and a landing zone diameter of 14.11±1.06 mm. The device sizes ranged from 16 to 18 mm (Table). Actually, the occluder was completely replaced with a different sized one in the first two canines and this was because of implantation inexperience at the beginning of the study. For the other 5 canines, an average of 2 times redeployment were performed to get a better occlusion effect during the procedure (Table).
| Occluder size | Redeploy time | |
|---|---|---|
| 1 | 18 | 2 |
| 2 | 16 | 2 |
| 3 | 18 | 2 |
| 4 | 16 | 2 |
| 5 | 16 | 3 |
| 6 | 16 | 1 |
| 7 | 16 | 2 |
A small residual shunt was observed by LA angiography in 1 canine post implantation; there were no residual shunts for the other 6 canines. Unfortunately, 1 canine died 36 h later post implantation due to an undiscovered groin hematoma. An immediate autopsy of the LA showed that the occluder had been perfectly and deeply localized in the LAA and had covered the orifice completely. A layer of thin membrane formed on the surface of the sealing disc and no macroscopic thrombus was observed. In addition, no appendage wall perforation or hemopericardium was observed either. Subsequently, a visualized tugging test was performed by pulling with forceps and confirmed the firmness of anchor cylinder (Figure 3A). Therefore, all animals were implanted with 16- to 18-mm LAA occluders successfully and no device-related death occurred.
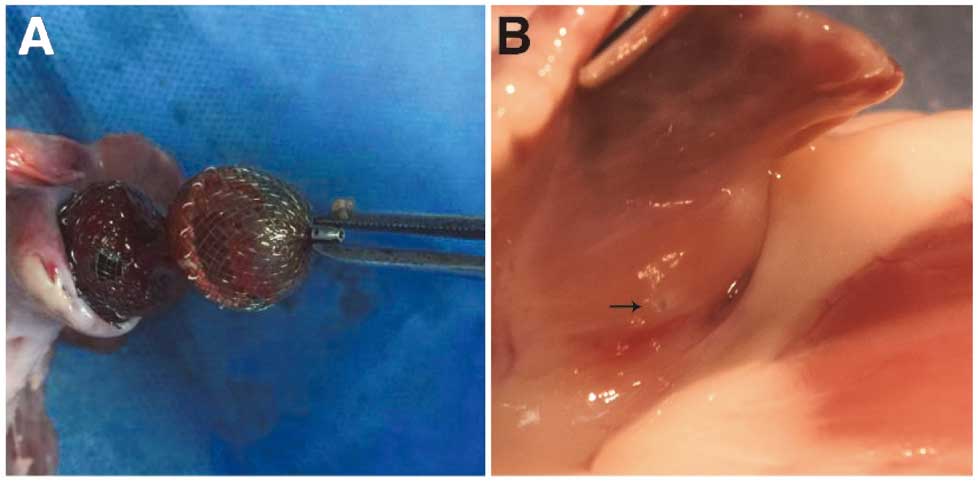
(A) By tugging the sealing disc to cage shape, the anchor cylinder was still fixed in the left atrial appendage (LAA) landing zone. (B) The micro-barb has not penetrated the LAA wall or caused pericardial effusion.
Clinical observation indicated no related behavioral or neurological deficit. Furthermore, no unexpected mortality occurred with the remaining 6 canines.
After being sacrificed, the anatomy of all canines showed no pericardial effusions (Figure 3B) and all hearts were collected carefully for further examination. The atrial aspect of the occluder, including the nut outside, was completely covered by a layer of mature neointima on 110 days post-implantation (Figure 4A). Further HE staining and anti-CD31 immunofluorescent staining both showed neovascularization and confirmed the integrity of the newly formed neointima on the surface of the sealing disc (Figure 4B,C). Furthermore, electron microscope examination also verified that a layer of endothelial cells was formed on the surface (Figure 5). Unfortunately, some endothelial cells in the sample were damaged or rolled up by improper disposal. There was no thrombus formation on the surface of the sealing disc. There was also no interference of the sealing disc on the MV or LUPV for all canines. Moreover, no visible perforation or inflammation was observed on the outside of the appendage, which verified the safety of the integrated micro barbs. Careful anatomy of other organs showed no obvious evidence of infarction on the kidney, liver, or spleen.
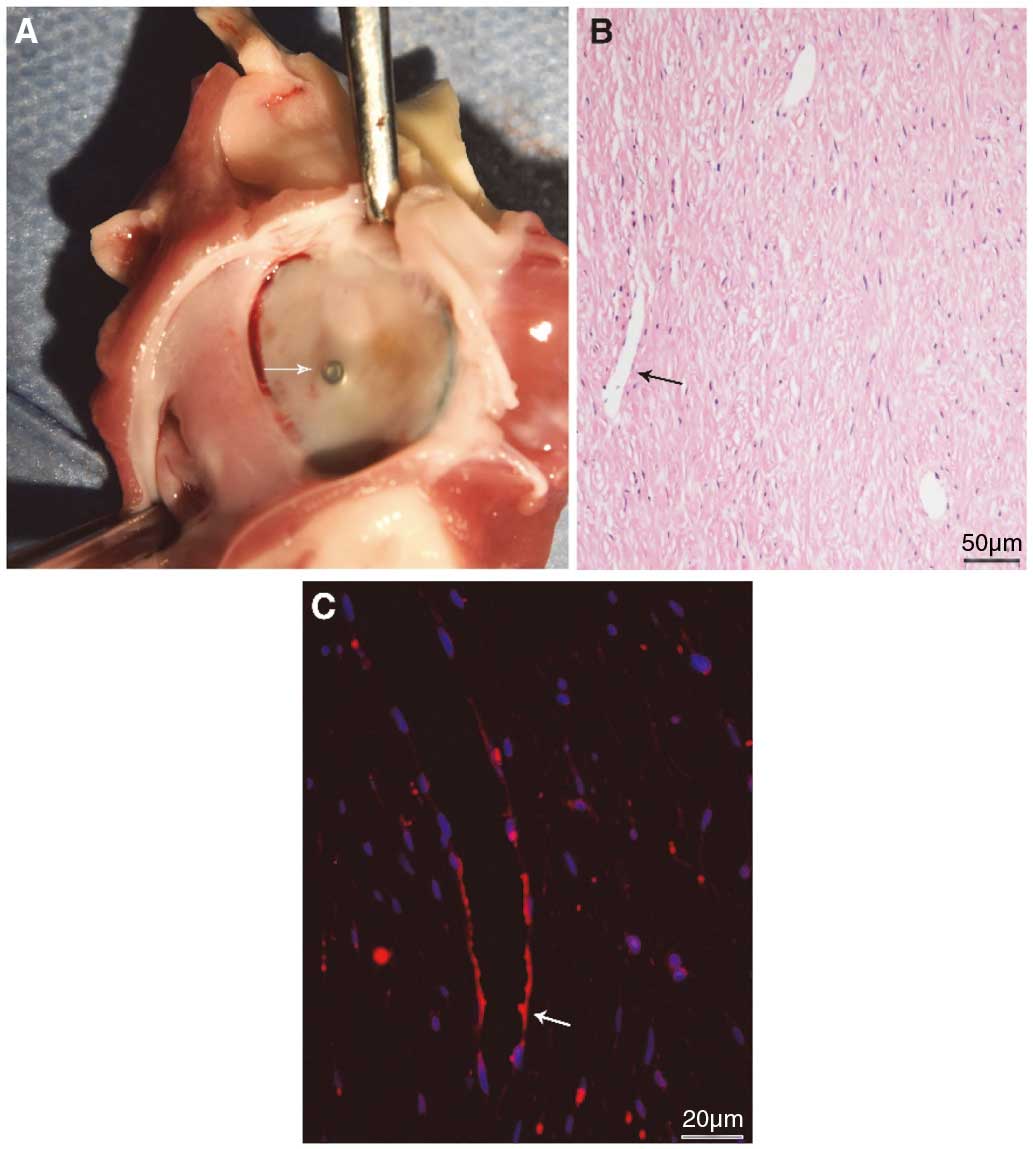
(A) New-born tissue on the surface of the sealing disc on day 110; the tissue has covered the fixing nut completely, and the color of the new-born tissue was nearly the same as that of the left atrium (LA) endocardium. (B) New-born tissue on the sealing disc consisted of endothelial, sub-endothelial and subintima, where neovascularization can be observed on day 110. (C) DAPI (blue) and CD31 (red) immunofluorescence positive-stained cells can be found and new-born vascular can been seen on day 110.

A layer of endothelial cells and the matrix were observed by scanning electron microscope on day 80 and day 110.
AF is the most common type of arrhythmia for older people, with a morbidity of 5.9% among the population aged older than 65 years, which also has an increasing trend with ages up. The Framingham study showed that there was up to a 4- to 5-fold increased incidence of thromboembolic events in AF patients compared with non-AF patients.5 In addition, for NVAF patients, LAA was found to be the primary location in the heart for thrombosis formation, with a ratio 92%.6 Inspirationally, LAAC has been proven to prevent embolism complications effectively, which was non-inferior to oral anticoagulation treatment with warfarin.1,2,4,7,8 LAAC was recommended to be an optional strategy for NVAF patients at a high risk of stroke, but intolerant to oral anticoagulant treatment according to the 2016 ESC Guidelines for the management of AF developed in collaboration with EACTS (recommendIIb, level B).9
Occluder is the most important factor in LAAC. An ideal LAA occluder should seal the LAA completely so that there is no residual shunt, it should anchor inside well, there is no impact on adjacent structures, it is easy deployed and retrieved, and there is also no superficial thrombosis formation. Many types of LAA occluders have finished their pre-clinical experiments since 2001;1 for example, PLAATO, Watchman,10 AMPLATZER Cardiac Plug,11 COHEREX WAVECREST, Occlutech,12 LAmbre occluder,13 et al, some of which have been widely utilized. However, the procedure for the Watchman device was relatively complex; some thrombus formation on the surface of occluder been reported,14,15 and the occurrence of a device-related thrombus on the ACP LAA occluder has been reported to be up to 17.6% in earlier studies,16 and even death has resulted because of pulmonary artery perforation caused by barbs.17 In addition, device embolism during perioperative time and post-procedure was not uncommon.18 Therefore, since these devices have been put on the market, their safety has attracted widespread concern. Thus, one new LAA occluder means one more option for NVAF patients.
The LACBES® occluder is an independently developed LAA occluder that originated from a single disc plug device in 2006. It is a new member of the LAA occluder family, which has just been used to carried out clinical trials in a number of cardiovascular centers in China. Coincidently, it has a similar 2-disc appearance as the Amplatzer Cardiac PlugTM occluder, which has been utilized in clinics. However, they are so different in many aspects. The anchor cylinder and sealing disc of the LACBES® occluder is designed and made independently and uses different nitinol wire hardness. These 2 parts are subsequently connected by a long and thin waist, which is further constrained and fixed with a short stainless steel ring. The hardness of the anchor cylinder is harder than the sealing disc, which allows the anchor cylinder to land at the landing zone more firmly and ensures the sealing disc is more flexible to cover the orifice with the least damage to the LA or LAA wall. In addition, the sealing disc is curved inward slightly, which further reduces the potential damage to the LA or LAA wall caused by the direct incision effect from the rim of the disc. Micro-barbs in the anchor cylinder are integrated sculptured, which render the occluder be retrieved and redeployed completely and repeatedly without influence on its structure.
The most important reason for some other occluders why can’t they retrieve completely repeatedly is that the barbs will be damaged or delivery sheath will be damaged by barbs. However, in the current in-vivo experiment, an average of 2 times complete redeployment of occluder was performed during the procedure. It proved the safety to perform this retrieve and redeployment procedure with LACBES® Occluder. As supplementary evidence, we also performed an in-vitro release and retrieve test of LACBES® in a water bath at 37o C, and recorded it by video. It showed that the tension and orientation of the barbs did not change obviously even after 8 times of release and retrieve cycles (Movie S1), and the delivery sheath did not need to be replaced either. Therefore, the LACBES® occluder could be completely retrieved into delivery sheath, then repositioned and re-released, which simplified the LAAC procedure and reduced the risk of complications. This study showed that the LAA wall was not damaged by barbs of the LACBES® occluder after it was implanted, which can reduce the risk of postoperative pericardial effusion, cardiac perforation and pulmonary artery perforation. However, due to the small sample size, further animal experiments and clinical applications are needed to evaluate its safety. Comparing incidence and the clinical course of thrombus formation following device closure of atrial septal defect significant differences in the incidence of thrombus on closure devices were noted between different devices; the Amplatzer nitinol wire frame was filled with polyester fabric and the Helex nitinol wire was covered by an ultra-thin membrane of expanded polytetrafluoroethylene to be least thrombogenic.19 The sealing disc of the LACBES® occluder consists of 2 layers of self-expanding flexible nitinol mesh filled with a non-woven, biocompatible polyester fiber membrane used for obstructing blood flow. Results of this study showed that a layer of thin endothelial cell and sub-endothelial tissue covered the surface of the sealing disc on day 80 post procedure, and that the tissue has completely covered the disc and its nut. Histological examination, immunofluorescence staining against CD31, and scanning electron microscope confirmed that the new-born tissue consisted of an endothelium, sub-endothelial layer and subintima, where neovascular have been generated. This complete neointima formation cannot only isolate thrombus from a LAA, but also enable thrombosis to be avoided on the surface of the sealing disc, theoretically, no anti-thrombotic therapy is required to prevent device-related thrombosis after neointima has completely formed. Being able to fully complete neointima formation was benefit from this type of inner coating process, even anti thrombus treatment with aspirin alone can also prevent thrombosis post LAAC in this study. But in clinical practice, whether this type of protocol post LAAC with a LACBES® occluder is appropriate, requires further clinical and experimental data.
According to the characteristics of a shallower LAA and the shorter distance between the orifice and MV ring or the LUPV in healthy canines compared to that of AF patient, the length of the anchor cylinder of the LACBES® occluder was adjusted to 5 mm, and the sealing disc of the device was only 3 mm larger than the anchor cylinder in diameter, rendering that the device occluded the LAA in healthy canines well, and also did not affect the MV or LUPV. In this study, all canines had the LACBES® occluder successfully implanted for LAAC, and complete closure was achieved in 6 canines; only 1 canine had a small residual shunt. During the experiment, even in the absence of transesophageal echocardiography guidance, LACBES® occluder does not only obtain satisfactory sealing effect, but it also does not affect the MV or the LUPV, while meeting the PAST releasing standard. In other words, it was relative easy to perform LAAC with the LACBES® occluder, carrying high closure rate.
In this study, there was 1 unexpected canine death, which happened 36 h after the procedure when the canine had completely awoken; this was caused by an undiscovered hematoma at the femoral artery puncture site. An autopsy showed that no hemopericardium existed, the occluder was fixed in the LAA forcefully, the sealing disc completely covered the orifice of the LAA, and a layer of thin membrane formed on the surface of the sealing disc and no macroscopic thrombus was observed. The sealing disc did not affect the MV or the LUPV. Therefore, the cause of death was concluded to be groin hematoma. During the autopsy of this canine, we found that the LACBES® occluder fixed to the LAA well, and that the orifice of the LAA was completely covered. By investigating the cause of death, we believe that the canine death was associated with the procedure and implantation experience, and not related to the device. No unexpected canine deaths occurred during the follow-up period for the remaining 6 canines whose LAA were closed with the LACBES® occluder.
In conclusion, LAAC with the Lacbes® occluder is feasible because the procedure is simple, the closure rate is high, neointima formation is complete, and it is biocompatibile. The LACBES® LAA occluder has a promising future in further clinical application.
Study LimitationsThe main limitations of this study are: (1) the number of experimental canines was very low; (2) the weight of experimental canines was relatively low; (3) the LAA in all the canines was small and similar in size; (4) only 2 specifications of occluder size (16 mm and 18 mm) were used; and (5) there was a lack of neointima forming time and a feasible evaluation of a larger occluder. All the experimental canines in this study were healthy, and their cardiac structure, especially the LAA, had some degree of difference compared with that of canines with AF.
It was relative easy to perform LAAC with the LACBES® occlude; there was a high instant success rate and less device-related complications. The occluder has good biocompatibility, neointima formation can be completed within 4 months post procedure. It was feasible to perform LAAC with the LACBES® occluder.
We thank Tong Kan, Liang Chen and Jiadong Ji, for their assistance with this study.
This work is supported by the National Science Foundation of China (Grand No.81170150) and the 12th Five-year Project (No.BWS11C008).
The authors report no relationships that could be construed as conflicts of interest.
Supplementary File 1
Movie S1. Release and retrieve test of LACBES® was performed in a water bath at 37℃, the tension and orientation of the barbs did not change obviously even after 8 times of release and retrieve cycle.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0412