Article ID: CJ-18-0052
Article ID: CJ-18-0052
Vascular endothelial cells (ECs) maintain circulatory system homeostasis by changing their functions in response to changes in hemodynamic forces, including shear stress and stretching. However, it is unclear how ECs sense changes in shear stress and stretching and transduce these changes into intracellular biochemical signals. The plasma membranes of ECs have recently been shown to respond to shear stress and stretching differently by rapidly changing their lipid order, fluidity, and cholesterol content. Such changes in the membranes’ physical properties trigger the activation of membrane receptors and cell responses specific to each type of force. Artificial lipid-bilayer membranes show similar changes in lipid order in response to shear stress and stretching, indicating that they are physical phenomena rather than biological reactions. These findings suggest that the plasma membranes of ECs act as mechanosensors; in response to mechanical forces, they first alter their physical properties, modifying the conformation and function of membrane proteins, which then activates downstream signaling pathways. This new appreciation of plasma membranes as mechanosensors could help to explain the distinctive features of mechanotransduction in ECs involving shear stress and stretching, which activate a variety of membrane proteins and multiple signal transduction pathways almost simultaneously.
The endothelial cells (ECs) that line blood vessels are constantly exposed to hemodynamic forces such as shear stress, which is generated by flowing blood, and stretch-induced tension, which is produced by the blood pressure. The ECs collectively sense changes in shear stress and stretching, transduce them into intracellular biochemical signals, and produce active responses that are accompanied by changes in their own morphology, function, and gene expression.1 Such EC responses are crucial in controlling cardiovascular structure and function. For example, blood vessels increase or decrease their diameters according to similar changes in blood flow. This blood flow-vessel diameter relationship is an EC-mediated adaptive response that ensures the shear stress levels acting on vascular walls remain constant.2 During embryonic development, hemodynamic forces also play critical roles in cardiac and vascular organogenesis.3 The development of devices that can produce controlled levels of shear stress and stretching and that can discharge these forces on cultured cells has made it possible to analyze EC mechanoresponses at the cellular and molecular levels. Studies using such devices have shown that ECs are sensitive to biomechanical forces and modify their functions, including migration, proliferation, and the production of bioactive substances involved in the regulation of blood pressure, coagulation and fibrinolysis, and immune and inflammatory responses, accordingly.4 Impairment of these EC mechanoresponses has been implicated in the onset and progression of vascular diseases, including hypertension, thrombosis, aneurysms, and atherosclerosis.5–7
Although many studies have shown how ECs sense shear stress and stretching and transduce them into biochemical signals, the specific underlying mechanisms remain to be clarified. In particular, the mechanosensors that sense and differentiate between shear stress and stretching, together with their sensing mechanisms, remain to be elucidated. Here, we document the current knowledge of EC mechanotransduction and introduce the novel concept of EC mechanosensing, in which the lipid-bilayer membranes of ECs act as mechanosensors capable of differentiating between shear stress and stretching.
Considerable progress has been made in research conducted to elucidate the pathways underlying EC mechanotransduction. A variety of molecules located at the apical, junctional and basal parts of ECs have been shown to play critical roles in EC mechanotransduction. As for apical mechanosensors, ion channels such as the K+, Cl−, and Ca2+ channels open up in response to shear stress, and the mechanical force alters the cytoplasmic ion concentrations.8 Shear stress also activates G-protein-coupled receptors (GPCRs)9 and tyrosine-kinase-associated receptors,10 which in turn trigger the downstream signaling pathways. Furthermore, membrane microdomain caveolae,11 primary cilia with a rod-like structure that protrude from the apical cell membranes,12 and the membrane-bound glycocalyx are involved in shear stress sensing by ECs.13 Shear stress is also transduced into biochemical signals through the basal mechanosensors, including integrin and cytoskeleton actin filaments,14 which stabilize the cell structure and shape. EC-cell junctional proteins, such as platelet-EC adhesion molecule-115 and vascular endothelium-cadherin,16 are known to act as junctional mechanosensors. Recently, NOTCH1, a single-pass transmembrane receptor that is necessary for the maintenance of junctional integrity, was shown to be responsive to shear stress and to act as a mechanosensor in adult arteries.17
Stretch-induced tension activates multiple signal transduction pathways in ECs via ion channels, receptors, enzymes, adhesion molecules, and the cytoskeleton. The application of stretching to ECs induces extracellular Ca2+ entry through stretch-activated (SA) ion channels, followed by Ca2+ release from intracellular Ca2+ stores.18 An increase in the intracellular Ca2+ concentration phosphorylates focal adhesion proteins such as focal adhesion kinase (pp125FAK) and paxillin via the Src tyrosine kinase.19 The transient receptor potential (TRP) families TRPV4 and TRPC3 have been shown to play a role in the stretch-induced Ca2+ response.20 Stretching also activates a variety of membrane enzymes, including adenylate cyclase, phospholipase C, and phosphoinositide 3-kinase, thereby activating secondary messengers such as cAMP, diacylglycerol, inositol triphosphate, and protein kinase C.21 Vascular smooth muscle cells (VSMCs) are known to respond to stretch-induced tension and to change their functions accordingly. Thus, to understand the mechanoresponse of blood vessels as a whole tissue, VSMC sensing and responses to stretch-induced tension and their interactions with those of ECs must be taken into account.
A distinctive feature of EC mechanotransduction is the rapid and almost concomitant activation of multiple signaling pathways that involve several membrane molecules and microdomains. The underlying mechanisms by which shear stress and stretching activate a variety of membrane molecules almost simultaneously remain unknown. Recent studies, including ours, have shown that lipid-bilayer membranes respond directly to mechanical forces and alter their physical properties, such as lipid order, fluidity, and cholesterol content; these changes, in turn, modify the functions of embedded proteins, thereby activating multiple signaling pathways simultaneously.
Plasma membranes, which are continuous bilayers of phospholipids, are assumed to be in a liquid-crystalline-like state, and 2 distinct lipid phase states coexist in the plasma membrane of each cell: a liquid-ordered state, in which the fatty acyl chains of phospholipids are regularly oriented and their motions are restricted, and a liquid-disordered state, in which the orientation of fatty acyl chains is irregular, and their flexural movement is brisk. Plasma membranes undergo phase transitions between these 2 states in response to various changes. The lipid order of plasma membranes, a physical property parameter, reflects the lipid phase state and increases as it transits from a liquid-disordered state to a liquid-ordered state. Variations in the membrane lipid order can be analyzed in living cells using the environment-sensitive fluorescent probe Laurdan and two-photon microscopy.22 Laurdan shows a 50-nm red shift as the membrane lipid order changes from the liquid-ordered state to the liquid-disordered state depending on the level of water penetration into the lipid bilayer. Generalized polarization (GP) values calculated from Laurdan images reflect changes in the lipid order. GP images show that EC membranes have a non-uniform lipid order in which liquid-disordered phases with low GP values and liquid-ordered phases with high GP values coexist (Figure 1A). When ECs are exposed to laminar shear stress (10 dynes/cm2), the lipid order of the entire plasma membrane rapidly decreases, with reductions in liquid-ordered regions being more apparent than those in liquid-disordered regions (Figure 1A, Left panel).23 The decrease in the lipid order is dependent on the intensity of the shear stress and is reversible. In contrast, uniaxial stretching of ECs (a sustained 10% stretch) increases the lipid order (Figure 1A, Right panel).24 These findings suggest that EC plasma membranes respond differentially to shear stress and stretching by changing their lipid order in opposite directions.
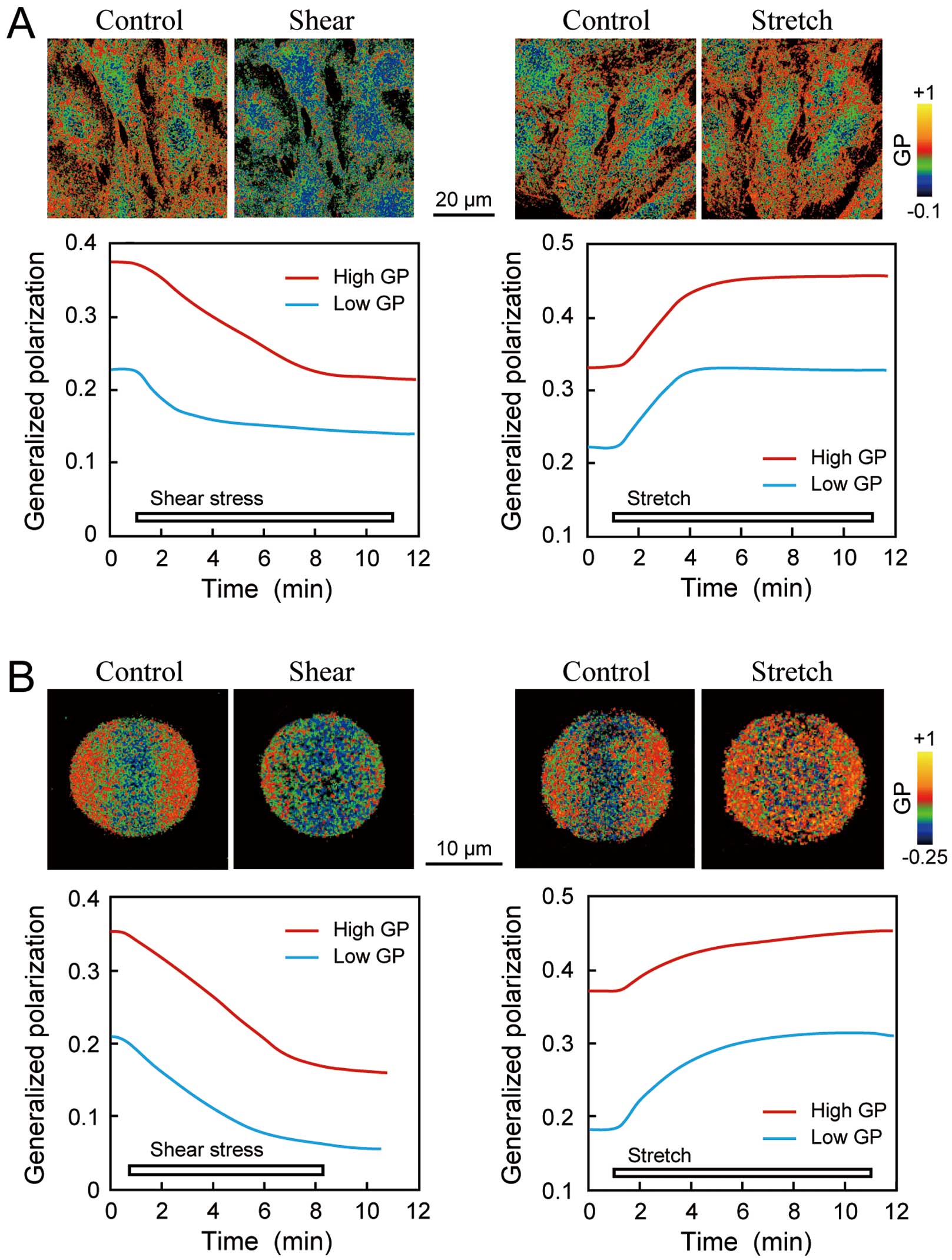
Effects of biomechanical forces on the lipid order of endothelial cells (EC) and model membranes. (A) Biomechanical-force-induced changes in the lipid order of EC plasma membranes. The photomicrographs are Laurdan general polarization (GP) pseudocolor images of human pulmonary artery ECs before and 10 min after biomechanical force loading. GP values were calculated from the Laurdan images using the formula:59 GP=(I400–460−G×I470–530)/(I400–460+G×I470–530), where I is the intensity of the emitted light collected in the 400–460 nm range and 470–530 nm range, and G is a correction factor. GP has been used to estimate changes in the membrane lipid order. EC membranes have a nonuniform lipid order in which liquid-disordered phases with low GP values (blue–green area) and liquid-ordered phases with high GP values (red–orange area) coexist. When the cells were exposed to a shear stress of 10 dynes/cm2, the GP values decreased in both the high- and low-GP regions, indicating that shear stress reduces the membrane lipid order. In contrast, when the cells were exposed to uniaxial stretching (a sustained 10% stretch), the GP values increased in both regions, indicating that stretching increases the membrane lipid order. EC membranes show contrasting responses to stretching and shear stress by changing their membrane lipid order in inverse directions. (B) Biomechanical-force-induced changes in the lipid order of an artificial lipid bilayer. The GP values were calculated from Laurdan-labeled giant unilamellar vesicles (GUVs). GUVs adherent to the cover glass coated with poly-L-lysine were exposed to biomechanical forces. The GP values decreased in response to a shear stress of 15 dynes/cm2 in both the high- and low-GP regions, whereas they increased in response to membrane stretching induced by hypotonic swelling using 50 mmol/L of glucose solution (50 mosmol/L). These findings indicate that the artificial lipid bilayer responded to shear stress and stretching by changing its lipid order in opposite directions. These data were adapted from references 23 and 24.
EC membrane sensitivity to mechanical stimuli has been demonstrated using a variety of techniques other than Laurdan imaging. A study of membrane dynamics using single-molecule spectroscopy showed that plasma membrane lipid domains that can be characterized as liquid-ordered, such as rafts or caveolae, are differentially sensitive to shear stress, compared with domains characterized as liquid-disordered.25 Exposure of bovine aortic ECs (BAECs) to shear stress (10dynes/cm2) induced an early and transient decrease in the viscosity of liquid-disordered domains and a later sustained decrease in that of the liquid-ordered domains. The area of liquid-disordered domains increased as a result of exposure to shear stress, while the area of liquid-ordered domains became smaller and more mobile.
Recent studies have shown that artificial lipid-bilayer membranes change their physical properties in response to mechanical forces. Laurdan-labeled giant unilamellar vesicles (GUVs) composed of the phospholipids dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylcholine (DOPC), and cholesterol have been exposed to laminar shear stress (15 dynes/cm2) and hypotonic swelling (200 mosmol/L), and changes in their lipid order have been analyzed. The Laurdan images of GUVs obtained under static conditions showed a heterogeneous lipid order across the membrane surface, with the liquid-ordered and liquid-disordered states coexisting (Figure 1B). Upon shear stress stimulation, the lipid order rapidly decreased across the entire membrane, and the liquid-ordered regions diminished (Figure 1B, Left panel).23 In contrast, the lipid order rapidly increased across the entire membrane when the GUVs swelled in response to a hypotonic solution, and the liquid-ordered regions expanded (Figure 1B, Right panel).24 This finding indicated that artificial lipid-bilayer membranes are mechanoresponsive and capable of differentiating between shear stress and stretching by changing their lipid order in opposite directions. This also means that the lipid-order changes observed in the EC plasma membranes constitute a physical phenomenon independent of any involvement of membrane proteins, the cytoskeleton, or other biological activities.
Membrane FluidityPlasma membranes are continuous bilayers of phospholipids comprising various lipids and proteins. The lipids and proteins can move about rapidly in the plane of the membrane, and membrane fluidity changes in response to mechanical forces. A study26 used a fluorescent molecular rotor (9-(dicyanovincyl)-julolidine) that integrates into the cell membrane and whose quantum yield varies with membrane viscosity to assess the effect of shear stress on membrane fluidity in human umbilical vein ECs (HUVECs). The results showed that laminar shear stress (≈6–32.7 dynes/cm2) increases membrane fluidity, and the increase occurs within 5 s of being exposed to the shear stress in an intensity-dependent manner. Moreover, fluorescence recovery after photobleaching (FRAP) measurements using DiI-C16 showed that laminar shear stress (10 or 20 dynes/cm2) transiently increased membrane fluidity upstream within 10 s of exposure in BAECs.27 In addition, our own recent FRAP study showed that contrasting changes in membrane fluidity occur in response to shear stress and to stretching (Figure 2). Exposure of ECs to laminar shear stress (15 dynes/cm2) markedly increases their membrane fluidity, whereas exposure to stretching induced by uniaxial cell stretching (a sustained 10% stretch) or hypotonic swelling significantly decreases their membrane fluidity.23,24
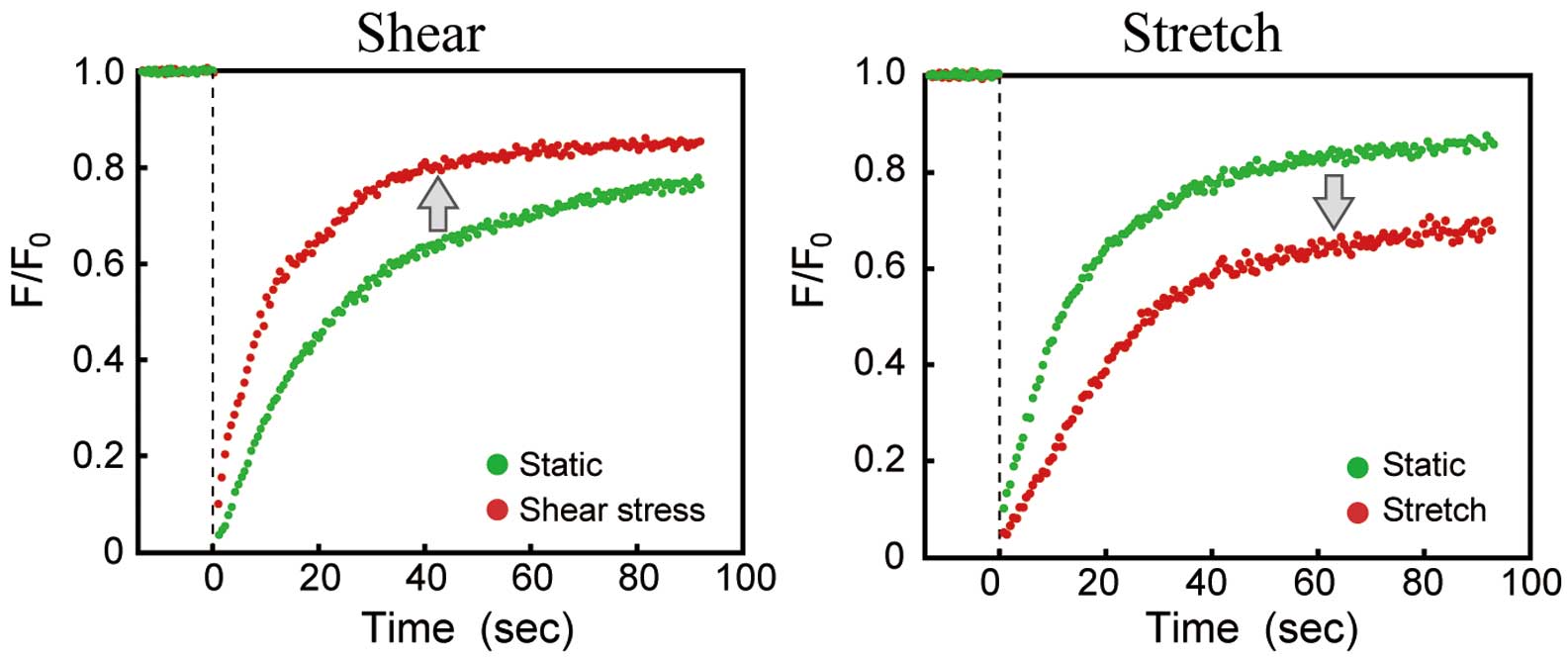
Effects of biomechanical forces on the membrane fluidity of endothelial cells (ECs). Human pulmonary artery ECs were loaded with the fluorescent probe DiIC18 and a predefined region was bleached at full laser power; the recovery of fluorescence was monitored by scanning the bleached region at a low laser power. The curves of fluorescence recovery after photobleaching were drawn by plotting the ratio (F/F0) of fluorescence intensity during recovery from bleaching (F) to the fluorescence intensity immediately after bleaching (F0) against time, and the plots were then curve-fitted. The application of shear stress (15 dynes/cm2, 10 min) accelerated fluorescence recovery, whereas the application of uniaxial stretching (a sustained 10% stretch, 10 min) slowed fluorescence recovery, indicating that opposite changes in membrane fluidity occur in response to shear stress and stretching. These data were adapted from reference 24.
Shear stress and stretching are assumed to alter the mechanical properties of plasma membranes, such as bilayer thickness, bending stiffness, and undulation, and many studies have shown that changes in such properties can affect the function of membrane proteins.28,29 An atomistic molecular dynamics simulation showed that tension in the range of 0–15 mN/m causes a linear reduction in bilayer thickness and induces a significant increase in acyl chain interdigitation.30 Changes in membrane thickness may cause a hydrophobic mismatch, leading to the hydrophobic thickness of a membrane protein becoming larger or smaller than that of the lipid bilayer; such mismatches may modify the conformation and function of a particular protein.31 Using single-channel recording and pipette aspiration techniques in GUVs, Goulian et al examined the effect of membrane tension induced by an externally applied mechanical stress on the dimerization kinetics of a channel-forming peptide, gramicidin A. Their results showed that the lifespan of the gramicidin dimer and its formation rate increased as the membrane tension increased.32 The essential effect of tension likely thinned the membrane and decreased the hydrophobic mismatch between the membrane and gramicidin, thereby promoting its dimerization.33 Moreover, plasma membranes rise and fall like waves, and molecular dynamics simulations of a coarse-grained lipid-bilayer model have shown that shear stress increases the undulation of lipid-bilayer membranes,34 while stretching reduces it.35 Regarding the effect of biomechanical forces on the mechanical properties of the lipid-bilayer membrane, only a few studies have been performed using living ECs; instead, most reports describe results obtained using artificial membranes or computer simulation.
Plasma membrane cholesterol is vital in regulating EC responses to mechanical forces, together with cellular functions such as signaling, adhesion, motility, and remodeling of the cytoskeleton. Laminar shear stress (10 dynes/cm2) activated ERK in BAECs, and pretreatment of the cells with a cholesterol-binding antibiotic, filipin, inhibited the shear-dependent activation of ERK, indicating that plasma membrane cholesterol is a key molecule in ERK activation by shear stress.36 Recent studies have shown that cholesterol levels change in response to mechanical forces. Laminar shear stress (12 dynes/cm2) reduced membrane cholesterol levels in HUVECs, whereas oscillatory shear stress (0.5±4 dynes/cm2, 1 Hz) increased them.37 The decrease in membrane cholesterol in response to laminar shear stress downregulated the mitochondrial-dissociated ATP synthase expression in caveolae, whereas the increase in response to oscillatory shear stress led to its upregulation.38 The plasma membrane cholesterol levels in human pulmonary artery ECs clearly decreased in response to laminar shear stress (10 dynes/cm2), while they increased in response to membrane stretching induced by uniaxial stretching (a sustained 10% stretch) or hypotonic swelling (200 mosmol/L).24 Further studies are necessary to elucidate the dynamics of plasma membrane cholesterol in response to mechanical forces, including changes in its distribution, internalization, efflux, and transport between the cytoplasm and plasma membrane.
Membrane cholesterol regulates the function of many membrane proteins by altering their conformation and properties. Levitan et al list 3 ways through which cholesterol influences plasma membrane proteins: (1) via cholesterol-mediated changes in the properties of the membrane; (2) via specific interactions between cholesterol and proteins that have cholesterol-binding sites; and (3) by maintaining the scaffolds of membrane proteins.39
Cholesterol exerts an effect on the ion channels by modifying the physical properties of the plasma membranes: the membrane-cholesterol levels influence ion-channel gating by changing the bending modulus and pressure profile of the membrane lipid-bilayer.40 Cholesterol also influences the conformation of ion channels by causing a hydrophobic mismatch (i.e., by making the hydrophobic thickness of the lipid bilayer differ from that of the channel, thereby affecting the deformation energy required for the transition between closed and open states).
An example of a cholesterol effect exerted via a direct interaction is the β2-adrenergic receptor, a member of the GPCR superfamily.41 The β2-adrenergic receptor is mainly expressed in muscle tissue and is involved in muscle relaxation following activation. An X-ray crystallography model of the human β2-adrenergic receptor showed cholesterol occupying a shallow surface groove formed by the transmembrane α-helix IV.42 A coarse-grained molecular dynamics simulation showed that the binding of cholesterol to α-helix IV increases the dimerization of the receptor, thereby promoting the interaction between the receptor and G protein.43
An example of a cholesterol effect by maintaining protein scaffolds is that of the cystic fibrosis transmembrane conductance regulator (CFTR). The CFTR is a plasma-membrane anion channel involved in transepithelial fluid secretion and in mucociliary clearance of inhaled bacteria and other particles from the lungs. A CFTR fraction in human bronchial epithelial cells is present in lipid rafts, and the scaffold protein NHERF1, which links the CFTR to the actin cytoskeleton and stabilizes it in the plasma membrane, has a cholesterol-binding motif. Exposure of cells to cholesterol oxidase, which depletes membrane cholesterol, reduces both CFTR confinement and the fraction of CFTR in the confined population, whereas both increase following exposure of the cells to cholesterol esterase, which increases membrane cholesterol.44 These findings show a clear dependence of CFTR expression on cholesterol and suggest that cholesterol influences CFTR expression and channel function by modulating scaffold lipid raft dynamics.
Caveolae are small, flask-shaped invaginations of the plasma membrane that are rich in proteins as well as lipids, including cholesterol and sphingolipids, and their formation and maintenance are attributable to a cholesterol-binding protein, caveolin-1. Caveolae play a key role in signal transduction by forming a platform on which various receptors, ion channels, and signaling factors accumulate. Ample evidence has been presented to show that the caveolae are involved in EC mechanotransduction.11 Indeed, a disrupted caveolar structure caused by treating BAECs with methyl β-cyclodextrin (MβCD), which depletes membrane cholesterol, inhibits flow-induced activation of ERK.45 Similarly, disassembly of caveolae in rat lungs perfused in vivo with filipin, suppressed flow-induced activation of the Ras/Raf/mitogen-activated protein kinase pathway.46 In addition, we have previously shown that laminar shear stress (10 dynes/cm2) stimulated ECs to release ATP at the caveolae, which in turn triggered Ca2+ influx via an ATP-gated cation channel, P2X4, and subsequent propagation of Ca2+ waves throughout the entire cell.47 Knockdown of caveolin-1 with siRNA or disruption of caveolae with MβCD eliminated the shear stress-induced ATP release and Ca2+ signaling.47 Laurdan imaging has showed that caveolar membrane domains respond to laminar shear stress (15 dynes/cm2) by rapidly shifting their lipid order from the liquid-ordered state to the liquid-disordered state, and the addition of cholesterol to the ECs blocks the lipid order responses of the caveolae, together with markedly suppressing the shear stress-induced ATP release.23 These findings suggest that the transition within the caveolar lipid order is involved in the shear stress mechanotransduction that leads to ATP release.
The caveolae are also involved in cellular responses to stretching. A sustained 20% stretching activated the Rho family small GTPases RhoA and Rac1 in rat cardiomyocytes and subsequently dissociated them from the caveolae. Disruption of the caveolae with MβCD blocked the stretch-induced RhoA and Rac1 activation.48 Exposure of mouse mesangial cells to a 1-Hz cyclic strain for 10 min caused epidermal growth factor receptor (EGFR) transactivation and protein kinase B (Akt) activation. Disruption of the caveolae with MβCD or filipin prevented stretch-induced Akt activation, and both EGFR and Akt activation by stretching failed to occur in caveolin-1-knockout mice, indicating that intact caveolae are necessary for the stretch signaling to occur.49
Membrane lipids affect the conformation and function of membrane proteins by altering the membrane structure and physical property. For example, they can change the local membrane thickness and the curvature of the lipid bilayer surrounding proteins, and can modify the physical properties of the membrane, including its cholesterol levels, phospholipid types, acyl chain length and motion, and water content.50 Using a patch-clamp technique and liposomes in which mechanosensitive ion channels, MscS and MscL, were reconstituted, Nomura et al showed that acyl chain length, cholesterol, and lysophosphatidylcholine significantly influenced the mechanosensitivity of these channels.51
Some membrane lipids bind to specific sites on embedded proteins and alter their protein structure and function through chemical interactions.52–54 A study of membrane proteins and their lipid-binding properties revealed that resistance to protein unfolding correlated with specific lipid-binding events, and that while some membrane lipids merely bound to proteins, others modulated membrane protein structures or function.55 Examples of the latter are phosphatidylinositol phosphate, which modulates MscL, cardiolipin, which modulates aquaporin Z, and phosphatidylglycerol, which modulates ammonia channels. β-Secretase, a transmembrane aspartic protease, plays a critical role in the formation of the neurotoxic β-amyloid peptide. To investigate the involvement of membrane lipids in modulating β-secretase activity, purified β-secretase was reconstituted in GUVs, and its specific activity was assessed in vesicles with various lipid compositions.56 The results showed that 3 types of lipids, neutral glycosphingolipids, anionic glycerophospholipid, and cholesterol, could stimulate the proteolytic activity of β-secretase.
EC plasma membranes show differential responses to shear stress and stretching by changing their physical properties, such as the lipid order, fluidity, cholesterol content, and undulations, in opposite directions (Figure 3). Thus, plasma membranes themselves appear to have an intrinsic mechanoresponsive property, which seems to enable them to act as mechanosensors capable of differentiating between shear stress and stretching. Alterations in the membrane lipid order and physical properties modify the functions of the membrane proteins by affecting their conformation, expression, distribution, dimerization, and mutual interactions with other proteins, and they also influence the structure and functions of microdomains, such as caveolae. As a result, downstream signaling pathways are activated via these membrane proteins and microdomains, and information provided by the mechanical stimuli is transmitted to the inside of the cell, leading to the final cellular responses. In fact, changes in the physical properties of the plasma membranes have been shown to contribute to the EC mechanoresponses. For example, ECs exhibit VEGFR phosphorylation in response to shear stress and PDGFR phosphorylation in response to stretching.24 These growth factor receptor responses were markedly suppressed when the biomechanical force-induced changes in the membrane lipid order were prevented by the addition or depletion of cholesterol. Biomechanical force-induced activation of these growth factor receptors has been shown to play an important role in cell differentiation.57,58 Shear-stress induced VEGFR phosphorylation in vascular progenitor cells derived from mouse embryonic stem cells, which was essential for them to differentiate into ECs, whereas stretching induced PDGFR phosphorylation, which was essential for them to differentiate into VSMCs.
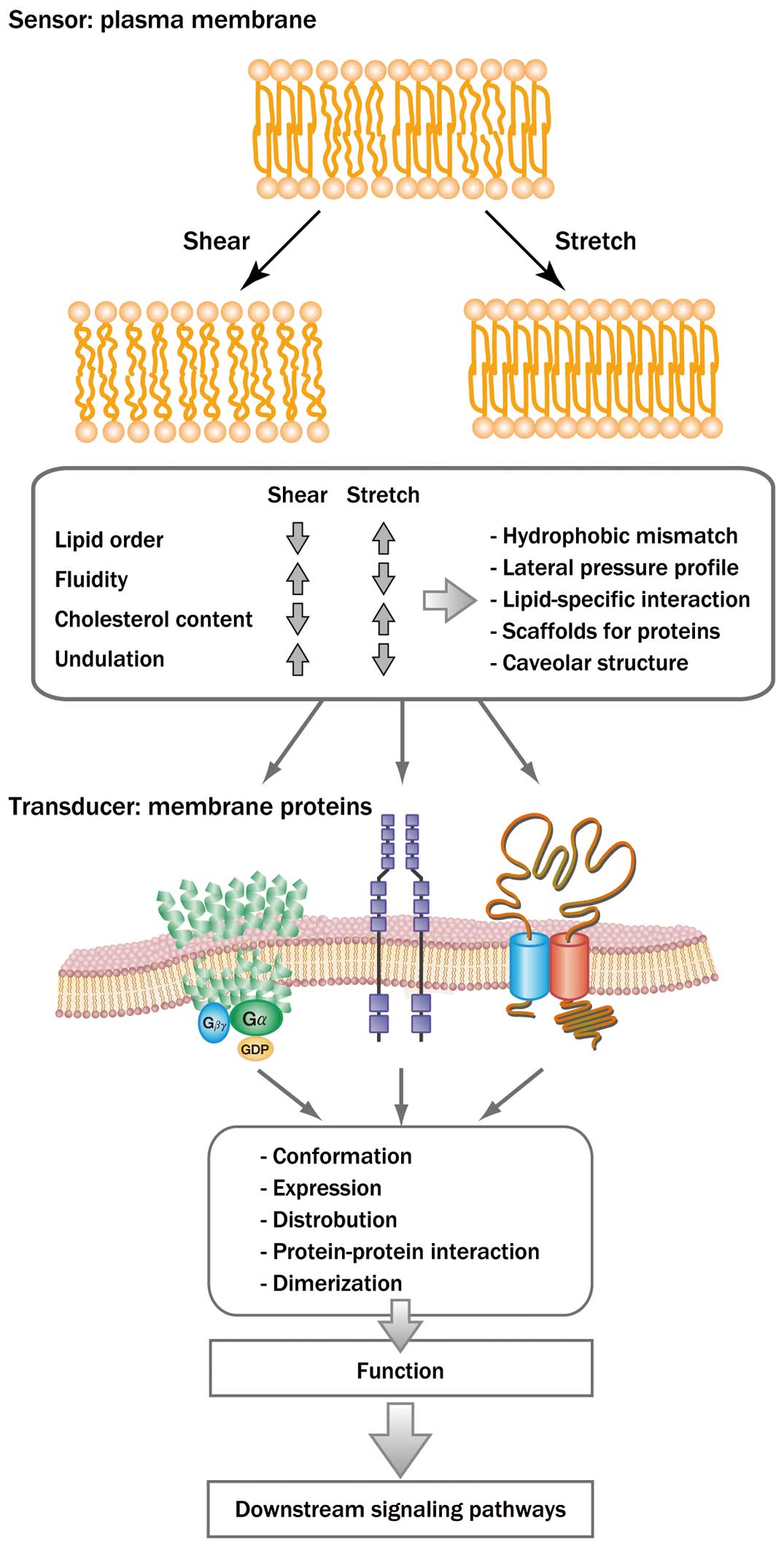
Membrane-lipid-mediated mechanosensing in endothelial cells. Shear stress and stretching differentially change the physical properties of the lipid bilayer, modifying the conformation and function of membrane proteins and microdomains. The changes in the membrane proteins and microdomains trigger a variety of downstream signaling pathways within the cells, leading to the final cellular responses.
The fact that shear stress and stretching differentially change the physical properties of EC plasma membranes, including lipid order, fluidity, and cholesterol content, thereby evoking cellular responses specific to each force, suggests the existence of a novel mechanosensing mechanism via plasma membrane lipids and physical properties. In membrane-lipid-mediated mechanosensing, the mechanosensor is composed of the plasma membrane and membrane proteins: the lipid bilayer, containing cholesterol, acts as the sensor, and the proteins act as transducers. This perspective on mechanosensing could help explain a distinctive feature of EC mechanotransduction, whereby shear stress and stretching activate various membrane proteins and their downstream signal transduction pathways. However, membrane-lipid-mediated mechanosensing does not exclude other types of mechanosensing, which can involve adhesion molecules, the cytoskeleton, the extracellular matrix, and primary cilia. EC mechanosensing seems to be achievable via a variety of mechanisms, and membrane-lipid-mediated mechanosensing appears to be an important component of the entire network of EC mechanotransduction. Further studies are needed to clarify the mechanoresponsive properties of the lipid bilayer and related changes in the conformation and function of membrane proteins. Such studies would provide not only a better understanding of EC mechanosensing, but also help to elucidate the mechanisms by which hemodynamic forces regulate vascular functions and play a role in the pathogenesis of vascular diseases, including hypertension, thrombosis, aneurysms, and atherosclerosis. Furthermore, the development of techniques that modulate EC mechanosensing may aid in the development of novel treatment and prevention of vascular diseases.
We acknowledge Dr. Akira Kamiya’s invaluable support in our work. This work was partly supported by Scientific Research from Japan Agency for Medical Research and Development (AMED) under Grant number JP18 gm0810006 and by Grants-in-Aid for Scientific Research (B) under Grant number 18H03510.