Article ID: CJ-18-0222
Article ID: CJ-18-0222
Background: The PROTECT AF and PREVAIL trials demonstrated that the WATCHMAN left atrial appendage (LAA) closure device is a reasonable alternative to warfarin therapy for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the USA and Europe. We conducted the SALUTE trial to confirm the safety and efficacy of the LAA closure therapy for patients with NVAF in Japan.
Methods and Results: A total of 54 subjects (including 12 Roll-in) with NVAF who had a CHA2DS2-VASc score ≥2 were enrolled. All subjects were successfully implanted with the LAA closure device. No serious adverse events related to the primary procedure-safety endpoint occurred. The 2nd co-primary endpoint was a composite of all stroke, systemic embolism and cardiovascular/unexplained death. One ischemic stroke (1/42) occurred during the 6-month follow-up. The effective LAA closure rate defined as the 3rd co-primary endpoint was 100% (42/42) at both 45-day and 6-month follow-up.
Conclusions: The procedural safety and 6-month results from the SALUTE trial demonstrated that the LAA closure device was safe and effective, similar to the results of large-scale randomized clinical trials, and provides a novel perspective of LAA closure for Japanese patients with NVAF in need of an alternative to long-term oral-anticoagulation. (Trial Registration: clinicaltrials.gov Identifier NCT 03033134)
Atrial fibrillation (AF) is one of the most common abnormal rhythm disturbances affecting approximately 5.5 million people worldwide, including 10% of people older than 75 years.1 The prevalence of AF in Japan in 2005 was 0.56% (0.72 million people), and with an rapidly aging population, it is expected to increase to >1% in 2050.2 The most debilitating consequence of nonvalvular AF (NVAF) is thrombus formation from stagnant blood flow in the heart, leading to thromboembolism and cardioembolic stroke or systemic embolization. The rate of ischemic stroke attributed to NVAF is estimated to average 5% per year, which is 2–7-fold the rate in those without AF.3
Anticoagulation therapy with either warfarin or direct oral anticoagulants (DOACs) is recommended in the Japanese Circulation Society guidelines of AF treatment,4 as well as in the European and USA guidelines.3,5 Although it is an effective treatment, warfarin administration is complicated by the risk of bleeding, multiple food and drug interactions, and a narrow therapeutic window requiring frequent blood monitoring [international normalized ratio (INR)]. East Asians are also more prone to hemorrhagic stroke compared with Westerners.6 Unlike other guidelines, the Japanese guidelines recommend a reduced target INR of 1.6–2.6 in patients aged ≥70 years to avoid bleeding complications, based on the findings in the NCVC NVAF Secondary Prevention Study7 and J-RHYTHEM Registry.8 However, in the Fushimi AF Registry of Japanese clinical practice, only 53.1% of AF patients were given warfarin or DOACs for stroke prevention and only 54.4% of them reached the therapeutic range of the INR.9,10 In the subanalysis of the RE-LY trial, Japanese patients had lower INRs than those in the overall trial population.11 These results suggest that in Japanese clinical practice warfarin tends to be given at low doses to avoid bleeding complications.
The origin of >90% of thrombus identified in the heart of NVAF patients is the left atrial appendage (LAA).12 The WATCHMANTM LAA Closure Device was developed as a permanent implantable device to seal off the LAA to prevent cardioembolism. Safety and efficacy of the device compared with long-term warfarin therapy were evaluated in 2 randomized trials with warfarin as a control: the Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy (PROTECT AF) trial and the Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial. As a result, non-inferiority to warfarin in reduction of post-implant thromboembolism was demonstrated, and the risk of hemorrhagic stroke, disabling stroke and cardiovascular (CV) death (including unexplained cause) was found to be lower than with warfarin,13 and the device received U.S. Food and Drug Administration approval in March 2015.
To evaluate the safety and efficacy of the device for Japanese NVAF patients, we conducted the SALUTE (A Study to evaluate the safety and effectiveness of the Left atrial appendage closure therapy Using WATCHMAN for patients with NVAF at increased risk of ThromboEmbolism in Japanese medical environment) trial.
SALUTE (NCT 03033134) is a prospective, multicenter, single-arm clinical trial to assess the safety and efficacy of the WATCHMAN LAA closure device (Boston Scientific, St. Paul, MN, USA) in patients with NVAF (paroxysmal, persistent, or permanent).
The trial was conducted at 10 investigational sites in Japan. The protocol was approved by each site’s institutional review board and written informed consent was obtained from each subject prior to enrollment. The sites enrolled their first subject into a roll-in cohort and if necessary, per the discretion of physicians and/or proctor, one more roll-in subject could be enrolled at the site. Roll-in subjects allowed physicians to gain experience with the device implant procedure. After the roll-in subjects were enrolled at each site, subsequent subjects were enrolled in the primary cohort for endpoint analysis.
Subject PopulationThe inclusion criteria for the subjects were: >20 years old; Japanese; documented paroxysmal, persistent or permanent NVAF; CHA2DS2-VASc score ≥2 and recommended for long-term oral anticoagulant therapy; deemed by physicians to be suitable for anticoagulant therapy and had an appropriate rationale to seek a nonpharmacologic alternative to anticoagulation; and eligible to discontinue anticoagulant therapy following LAA closure (i.e., the subject had no other conditions that would require anticoagulant therapy). The major exclusion criteria were: contraindication to aspirin or thienopyridine; previous stroke/transient ischemic attack (TIA) or myocardial infarction within 90 days of enrollment; and either a patent foramen ovale or atrial septal defect requiring treatment.
Study EndpointsThere were 3 co-primary endpoints in this trial. The 1st was periprocedural safety, a composite of all-cause death, ischemic stroke, systemic embolism, or device-/procedure-related events requiring open CV surgery or major endovascular intervention such as pseudoaneurysm repair, AV fistula repair, or other major endovascular repair during the 7 days post-implant or the index hospitalization. Percutaneous catheter drainage of pericardial effusions, snaring of the embolized device, and nonsurgical treatment of access complications were excluded from this safety endpoint. The 2nd endpoint was a composite of ischemic stroke, hemorrhagic stroke, systemic embolism, and CV death (including unexplained cause) during 6-month follow-up. The 3rd endpoint was the effective LAA closure rate at 45-day and at 6-month time points. Both the 1st and 3rd endpoints had prespecified performance targets to assess the trial’s success.
Other endpoints included major bleeding defined as per Bleeding Academic Research Consortium (BARC) definition type 3 or 5,14 clinically overt non-fatal bleeding defined as per BARC bleeding definition type 2, technical success rate, warfarin discontinuation rate, severity of stroke (with modified Rankin Scale, NIH stroke score and Barthel Index), and quality of life assessment with EQ-5D.
Primary endpoint events, as well as other important endpoints such as BARC bleeding definitions (type 2, 3 or 5), were independently adjudicated by a clinical events committee (CEC). The effective LAA closure rate was also independently reviewed by a transesophageal echocardiography (TEE) core laboratory.
ProceduresThe WATCHMAN LAA closure device is a self-expanding, nitinol-framed structure ranging in diameter from 21 to 33 mm to accommodate varying LAA anatomy and size. The device has fixation anchors to minimize embolization and a permeable polyester fabric cover. The study procedure of the device was only to be performed by physicians who had completed the specific training program with the support of an experienced proctor and the implant procedure was percutaneously performed via a transseptal puncture approach and was guided by fluoroscopy and TEE to verify proper positioning and stability. During the study procedure, sterile technique, and anticoagulation therapy, such as heparin administration, were decided at the physician’s discretion. After implantation, subjects were treated with an antithrombotic regimen to allow time for device endothelialization: (1) warfarin with a therapeutic INR of 2.0–3.0 or a therapeutic INR of 2.0–2.6 for subjects aged ≥70 years and aspirin; (2) after approximately 45 days following TEE demonstrating adequate LAA closure (no residual peri-device flow >5 mm in width), warfarin was discontinued, and thienopyridine and aspirin were administered; (3) at the 6-month visit following TEE again demonstrating adequate LAA closure, thienopyridine was discontinued and aspirin alone was continued indefinitely. The dosages of aspirin, ticlopidine and/or clopidogrel followed recommended dosages of Japanese labeling for antiplatelet drugs (i.e., 100 mg/day, 200–300 mg/day, and 75–300 mg/day, respectively) unless otherwise required for any specific reason.
Follow-up visits were scheduled at 45 days, 6 months and up to 24 months with neurologic assessments on a planned and as-needed basis for any neurologic events.
Statistical AnalysisDescriptive statistics were generated for the data collected at baseline, during the procedure, and at follow-up. For continuous variables, the mean, standard deviation (SD), number of subjects, median, range, 95% confidence interval (CI), and discrete values (frequency table and percentage) were calculated and analyzed.
All statistical analyses were done by intention-to-treat (ITT) for the primary cohort with subjects enrolled in the trial regardless of the study device being implanted or not. ITT subjects were included in the primary analysis for the co-primary endpoints as well as the secondary and other endpoints. Roll-in subjects’ data were evaluated separately from the data of the primary cohort.
For the 3 co-primary endpoints, the rates and 95% CIs of each endpoint were calculated and evaluated individually using all subjects’ data available by 6-month follow-up.
The performance target of the 1st co-primary endpoint was 10.0%, derived from the safety event rate observed in the PREVAIL trial with the 95% upper confidence bound as a margin.15 In order to maintain ≥95% probability that the observed estimate from the trial is lower than the performance target of 10.0%, a minimum of 40 subjects were required.
The performance target of the 3rd co-primary endpoint was 94.0%, derived from the effective LAA closure rate observed in the PROTECT AF and PREVAIL trials with 95% lower confidence bound as a margin. Accounting for 10% attrition to 6-month TEE follow-up, 40 subjects were required to maintain ≥95% probability that the observed estimate from the trial was higher than the performance target of 94.0%.
Because of the expected low occurrence of events, the 2nd co-primary endpoint, a composite of any stroke, systemic embolism and CV or unexplained death, had no performance target and the rate was to be compared with the results of the pivotal trials.
A total of 54 subjects were enrolled between February and July 2017 at 10 sites; of those 12 were allocated to the roll-in and 42 to the ITT groups (primary cohort) (Figure 1). The device was successfully implanted in 100% (54/54) of the subjects enrolled and in whom the implant procedure was attempted.

SALUTE study flowchart. There were no subjects in whom implantation of the closure device was attempted but the device was not implanted. Screening failure reasons (17) were: cardiac thrombus (6), consent withdrawn (5), LAA anatomy (3) and others (3: renal function [1], LVEF [1] and safety concern [1]). LAA, left atrial appendage; LVEF, left ventricular ejection fraction.
Baseline characteristics were collected for the ITT subjects (primary cohort) and are presented in Table 1. The mean age was 72.5 years; 16.7% were female; the mean CHADS2 score was 2.5, the mean CHA2DS2-VASc score was 3.6, and the mean HAS-BLED score was 2.9.
| Characteristics | ITT cohort (n=42) |
|---|---|
| Age (years) | 72.5±8.8 (42) (50, 91) |
| Sex | |
| Female | 16.7% (7/42) |
| Male | 83.3% (35/42) |
| Height (cm) | 164.2±8.2 (42) (145.0, 179.0) |
| Weight (kg) | 68.0±13.9 (42) (40.0, 118.2) |
| BMI (kg/m2) | 25.2±4.8 (42) (18.0, 41.4) |
| AF pattern | |
| Paroxysmal | 35.7% (15/42) |
| Persistent | 21.4% (9/42) |
| Permanent | 42.9% (18/42) |
| Paced | 0.0% (0/42) |
| Previous AF ablation | 40.5% (17/42) |
| CHADS2 score (continuous) | 2.5±1.3 (42) (0.0, 6.0) |
| CHA2DS2-VASc score (continuous) | 3.6±1.6 (42) (1.0, 8.0) |
| HAS-BLED score (continuous) | 2.9±1.1 (42) (1.0, 5.0) |
| CHF | 52.4% (22/42) |
| Hypertension | 81.0% (34/42) |
| Age ≥75 years | 47.6% (20/42) |
| Diabetes | 31.0% (13/42) |
| Stroke/TIA/TE | 16.7% (7/42) |
| Vascular disease | 11.9% (5/42) |
| Age 65–74 years | 38.1% (16/42) |
| Abnormal renal function | 54.8% (23/42) |
| Abnormal liver function | 4.8% (2/42) |
| Stroke | 16.7% (7/42) |
| Bleeding | 9.5% (4/42) |
| Labile INR | 7.1% (3/42) |
| Elderly >65 years | 85.7% (36/42) |
| Drugs | 33.3% (14/42) |
| Alcohol | 0.0% (0/42) |
| LVEF (%) | 60.5±9.7 (42) (41.0, 79.0) |
Data are mean±SD (n) (minimum, maximum) or % (n/N). AF, atrial fibrillation; CHF, congestive heart failure; INR, international normalized ratio; ITT, intention-to-treat; LVEF, left ventricular ejection fraction; TE, thromboembolic event; TIA, transient ischemic attack.
Reasons for seeking a non-pharmacologic alternative to warfarin are presented in Figure 2. Approximately 90% of the subjects had an increased risk of bleeding or a bleeding tendency, and 23.8% had a history of overt bleeding related or unrelated to oral anticoagulants.
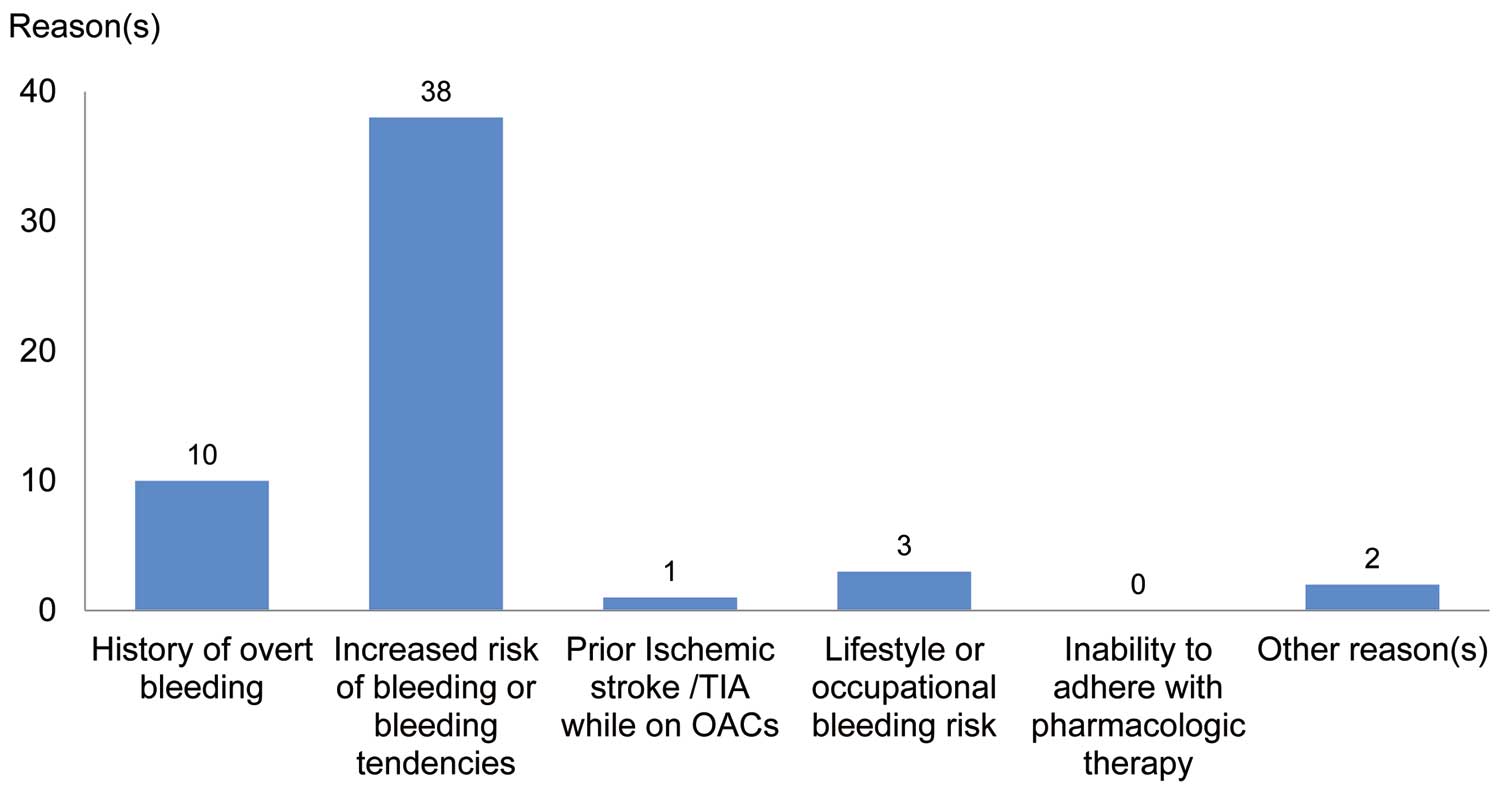
Reasons for seeking a non-pharmacologic alternative to warfarin (ITT cohort). Data are the total number of subjects with each reason (multiple answers possible). There were no subjects who enrolled with other reason(s) only. Other reason(s): flow in the left atrial appendage was markedly reduced and there was a filling defect on contrast computed tomography (1) and taking antithrombotic medication (1). ITT, intention to treat; OAC, oral anticoagulant; TIA, transient ischemic attack.
Baseline characteristics of the LAA in the ITT cohort are presented in Table 2 (LAA characteristics of the roll-in cohort are shown in Table S1). The mean LAA ostial diameter (pre-implant) was 23.6 mm, mean LAA length (pre-implant) was 30.1 mm, and 52.4% of the subjects had >1 LAA lobe.
| ITT cohort (n=42) |
|
|---|---|
| LAA characteristics by site | |
| No. of LAA lobes – screening | |
| 1 | 47.6% (20/42) |
| >1 | 52.4% (22/42) |
| LAA ostium diameter – pre-Implant | 23.6±2.6 (42) (19.0, 30.7) |
| LAA length – pre-Implant | 30.1±4.8 (42) (19.6, 40.9) |
| Diameter of deployed implants | 25.0±2.4 (42) (20.6, 30.3) |
| Closure device size, implanted, final device (mm) | |
| 21 | 0.0% (0/42) |
| 24 | 0.0% (0/42) |
| 27 | 33.3% (14/42) |
| 30 | 42.9% (18/42) |
| 33 | 23.8% (10/42) |
| Total | 100.0% (42/42) |
| Average no. of closure devices used per subject | 1.4 |
| Access system, final access system used | |
| Single curve | 21.4% (9/42) |
| Double curve | 76.2% (32/42) |
| Anterior curve | 2.4% (1/42) |
| Total | 100.0% (42/42) |
| Average no. of access systems used per subject | 1.2 |
Data are mean±SD (n) (minimum, maximum) or % (n/N). LAA, left atrial appendage; ITT, intention-to-treat. Note: the average number of closure devices used per successful implantation calculated as the total number of study devices implanted+total number of study device attempts divided by the total number of study devices implanted. Average number of access systems used per successful implant calculated as the total number of study access systems used+total number of study access system attempts divided by total number of study access system used.
The LAA closure device was implanted under general anesthesia. Mean procedure time, defined as the time from venous access insertion to sheath removal, was 57.7±14.8 (33.0, 93.0) min. Table 2 shows the implanted device sizes and access system used in the ITT cohort. A 30-mm device was implanted most frequently (42.9%, 18/42) and the average number of closure devices used per subject was 1.4. In the ITT cohort, the 21-mm and 24-mm devices were not implanted; 2 of the 21-mm and 1 of the 24-mm devices were implanted in the roll-in cohort (Table S1). All 5 sizes of the LAA closure device were implanted successfully in the trial. The double-curve access system was used most frequently (76.2%, 32/42) and the average number of access systems used per subject was 1.2. All 3 types of access system were used successfully in the trial.
Post-implant, all subjects had at least 6 months’ follow-up without death or study withdrawal.
First Co-Primary EndpointThe 1st co-primary endpoint was periprocedural safety, a composite of all-cause death, ischemic stroke, systemic embolism, or device-/procedure-related events requiring open CV surgery or major endovascular intervention such as pseudoaneurysm repair, AV fistula repair, or other major endovascular repair within 7 days of the procedure or during the index hospitalization. There were no events meeting this definition in the ITT cohort (42 subjects) (Table 3); therefore, this endpoint was met as the event rate was lower than the performance goal of 10% (Figure 3).
| % (n/N) | 95% CI | Performance target |
|
|---|---|---|---|
| 1st co-primary endpoint (periprocedural safety) during the 7 days post-implantation |
0.0% (0/42) | [0.0%, 8.4%] | 10.0% |
| Components | |||
| All-cause death | 0.0% (0/42) | [0.0%, 8.4%] | |
| Ischemic stroke | 0.0% (0/42) | [0.0%, 8.4%] | |
| Systemic embolism | 0.0% (0/42) | [0.0%, 8.4%] | |
| Device- or procedure- related event | 0.0% (0/42) | [0.0%, 8.4%] | |
| 2nd co-primary endpoint (composite endpoint) during the 180 days post-implantation |
2.4% (1/42) | [0.1%, 12.6%] | |
| Components | |||
| All stroke | 2.4% (1/42) | [0.1%, 12.6%] | |
| Ischemic stroke | 2.4% (1/42) | [0.1%, 12.6%] | |
| Hemorrhagic stroke | 0.0% (0/42) | [0.0%, 8.4%] | |
| Systemic embolism | 0.0% (0/42) | [0.0%, 8.4%] | |
| CV death (including unexplained cause) | 0.0% (0/42) | [0.0%, 8.4%] | |
| 45 days | 6 months | Performance target |
|
| 3rd co-primary endpoint (effective LAA closure rate assessed by core laboratory) |
100% (42/42) | 100% (42/42) | 94.0% |
| Complete seal | 50.0% (21/42) | 57.1% (24/42) | |
| Jet size 0 ≤5 mm | 50.0% (21/42) | 42.9% (18/42) | |
| Jet size >5 mm | 0.0% (0/42) | 0.0% (0/42) | |
| Size of largest residual jet around device | 2.8±0.8 (21) (1.0, 4.0) |
2.5±0.6 (18) (2.0, 4.0) |
|
| LAA seal assessed by investigational site | |||
| Complete seal | 59.5% (25/42) | 69.0% (29/42) | |
| Jet size 0 ≤5 mm | 40.5% (17/42) | 31.0% (13/42) | |
| Jet size >5 mm | 0.0% (0/42) | 0.0% (0/42) | |
| Size of largest residual jet around device | 2.3±1.0 (17) (1.1, 4.4) |
1.8±0.7 (13) (1.0, 3.0) |
|
Data are mean±SD (n) (minimum, maximum) or % (n/N). Note: effective LAA closure rate defined as peri-device flow ≤5 mm as demonstrated by TEE and assessed by the core laboratory. The performance target of the 1st co-primary endpoint was 10.0%, derived from the safety event rate observed in the PREVAIL trial. The performance target of the 3rd co-primary endpoint was 94.0%, derived from the effective LAA closure rate observed in the PROTECT AF and PREVAIL trials. CV, cardiovascular; TEE, transesophageal echocardiography. Other abbreviations as in Table 2.

Primary endpoints results (ITT cohort). (A) 1st co-primary endpoint result (0%) met the predefined performance target (<10.0%). (B) 3rd co-primary endpoint result (100%) met the predefined performance target (>94.0%). ITT, intention to treat; TEE, transesophageal echocardiography.
The 2nd co-primary endpoint was the occurrence of composite events including stroke, systemic embolism and CV death (including unexplained cause) during the 6-month follow-up period. One ischemic stroke occurred 118 days post-implant (Table 3) but the modified Rankin Scale score (1) of the subject did not change afterwards.
Third Co-Primary EndpointEffective LAA closure was defined as peri-device flow ≤5 mm demonstrated by TEE and independently assessed by a core laboratory. The 3rd endpoint was observed in 100% (42/42) of the subjects at both the 45-day and 6-month follow-up and the largest residual peri-device jet was 4.0 mm at 45 days and 4.0 mm at 6 months (Table 3). The observed rates were higher than the prespecified performance goal of 94.0% and the performance target was achieved (Figure 3). No subjects with residual peri-device flow >5 mm at 45-day and 6-month time points were observed at the investigational sites.
Other Endpoints/ResultsThe rate of major bleeding defined as per BARC bleeding definition type 3 or 5 was 2.4% (1/42). One case of major bleeding occurred 175 days post procedure and was adjudicated by the CEC to be unrelated to either the device or study procedure. The rate of clinically overt non-fatal bleeding defined as per BARC bleeding definition type 2 was 7.1% (3/42). Of the 3 BARC type 2 events, groin puncture bleeding only, which occurred 4 days post procedure, was adjudicated by the CEC to be related to the study procedure. Warfarin was to be discontinued when the TEE indicated complete LAA seal or a residual jet flow ≤5 mm around the device. The warfarin discontinuation rate was 100% (42/42) during the trial. One subject continued warfarin despite the LAA seal at 45 days because of deep vein thrombosis that was unrelated to the study procedure or device and warfarin was ceased at 6-month follow-up. Two subjects restarted warfarin at 6 months because of a device thrombus observed post-implant. Spontaneous echocardiographic contrast by screening TEE was seen in both of these subjects. Although these findings could be clinically important, associated adverse events were not reported. The size of the thrombus in each case was 0.7 cm2 and 0.5 cm2, respectively, by the core laboratory’s evaluation. Time in therapeutic range (TTR) is the standard benchmark for assessment of effective anticoagulation. It was calculated using Rosendaal method, which incorporates the frequency and values of INR measurements at least 45-day post procedure following TEE demonstrating adequate LAA closure. The observed TTR in accordance with the JCS guideline (therapeutic INR: 2.0–3.0, age ≥70: 1.6–2.6) was 66.8%. EQ-5D scores increased 2.2 from 79.0 at screening to 81.2 at 6 months. There were no deaths through the 180 days post-implant in the trial.
The procedural safety and efficacy results in the roll-in cohort were similar to those in the ITT cohort in the trial.
Adverse Events Related to the Study Procedure and/or Study DeviceIn the ITT cohort, one case of groin puncture bleed and another case of thrombus on the left atrial aspect of the atrial septal puncture site, both related to the study procedure by the site, were reported. Two cases of device thrombi related to the study device by the site were also reported through the 180 days post-implant. Ischemic stroke was reported for a subject but considered to be unlikely related to the study device by the site.
SALUTE is the first trial to confirm the safety and efficacy of a percutaneous LAA closure device for Japanese patients with NVAF. Although randomized trials conducted primarily in the USA (PROTECT AF and PREVAIL) demonstrated similar rates of all stroke or systemic embolism between the LAA closure device and warfarin, there was a large and statistically significant decrease in hemorrhagic stroke (hazard ratio [HR]: 0.20, P=0.0022), substantially fewer disabling/fatal strokes (HR: 0.45, P=0.03) and lower rates of CV death including unexplained cause (HR: 0.59, P=0.03), with the LAA closure device compared with warfarin. Consistent with the results for CV death, the HR for all-cause death also significantly favored the device (HR: 0.73, P=0.04).13
In NVAF patients, >90% of intracardiac thrombi originate in the LAA.12 Because the WATCHMAN mechanism of action is LAA closure, the effective closure rate was evaluated as a co-primary endpoint in this trial. The effective LAA closure rate evaluated by the core laboratory, as well as by the sites themselves, indicated 100% closure at both 45-day and 6-month time points and achieved the prespecified performance goal, indicating the efficacy of the WATCHMAN LAA closure device in Japanese patients. The 30-mm device was implanted most frequently in the SALUTE study, and the 24-mm and 27-mm devices were the most commonly implanted sizes in the PREVAIL study. However, all 5 sizes of the LAA closure device were implanted successfully and were necessary for adequate sealing of the LAA in both studies because the LAA shape, ostium size, depth, number of lobes and anatomic position, as well as that of the left atrium and pulmonary veins, vary from patient to patient. The single case of ischemic stroke reported in this trial was adjudicated by the CEC to be unrelated to either the device or implant procedure because no TEE evidence of device-related thrombus was observed and lacunar infarction was suggested by MRI. Also, the ischemic stroke was considered non-disabling because there was no change in the modified Rankin Scale score. No cases of hemorrhagic stroke, systemic embolism or CV death (including unexplained cause) were reported during the 6-month follow-up. Further evaluation is necessary to determine if the rates of stroke, systemic embolism and CV death post-implant in the Japanese population were consistent with those of the device groups in PROTECT AF and PREVAIL over the long-term follow-up.
Rate of procedural success, defined as device deployment and release, increased from 90.9% in PROTECT AF, to 95.1% in PREVAIL15 to 98.5% in EWOLUTION16 and to 100% in SALUTE. To evaluate the complications in SALUTE by using the identical definitions used in PREVAIL, we assessed periprocedural complications, defined as a composite of all-cause death, ischemic stroke, systemic embolism, or device/procedure-related events requiring open cardiac surgery or major endovascular intervention occurring in the first 7 days after implantation. The 1st co-primary endpoint met the prespecified performance goal for trial success, confirming the safety of the device implant procedure by Japanese operators without previous experience of implanting the device. The rate of periprocedural complications decreased from 2.2% in PREVAIL15 to 0.0% in SALUTE. Furthermore, the potential complications related to the device implant procedure, such as pericardial effusion requiring surgical repair, pericardial effusion requiring pericardiocentesis, procedural and device-related strokes, and device embolization, were not observed in SALUTE and every device implantation requiring the presence of an experienced proctor may have contributed to this result. Therefore, the results from this trial may not be generalizable for a truly new implanter in Japanese clinical practice.
Currently available alternatives to warfarin are DOACs, which include dabigatran, rivaroxaban, apixaban, and edoxaban. The safety and efficacy of DOACs have been established in randomized clinical trials with warfarin as control.17–20 The rates of bleeding with approved dosages of DOACs are either similar to warfarin or, in the case of apixaban, lower, but rivaroxaban and dabigatran have shown an increased risk of gastrointestinal bleeding. In the J-ROCKET trial, the observed rate of major bleeding events was 3.00% per year in the rivaroxaban group compared with 3.59% per year in the warfarin group (HR 0.85), and non-major clinically relevant bleeding event rates were 15.42% per year in the rivaroxaban group compared with 12.99% per year in the warfarin group (HR 1.20).21 In the subanalysis of the Japanese population in the RE-LY trial, the major bleeding rate in the dabigatran group was 5.53% and 3.33% per year compared with 3.59% per year in the warfarin group, and the minor bleeding rate was 24.19%, 33.26%, and 33.14%, respectively.11 In addition, as the bleeding risk of DOACs increases with older age, lower body weight and impaired renal function, the weight loss and/or abnormal renal function that are prominent in the aging Japanese population might increase the bleeding risk of lifelong DOAC therapy. Therefore, the bleeding risk of DOACs, as well as of warfarin, represents a unique challenge for physicians treating NVAF patients in Japan. Postprocedural long-term major bleeding in LAA closure therapy available from the PROTECT AF and PREVAIL studies demonstrated a 52% relative risk reduction (P=0.0003) over warfarin, with consistent results across age subgroups stratified by age (<75 vs. ≥75; interaction P-value=0.98).13
The rates of major bleeding (2.4%; 1/42), defined as BARC bleeding definition type 3 or 5, and clinically overt non-fatal bleeding (7.1%; 3/42), defined as BARC bleeding definition type 2, for the 6-month follow-up tended to be lower than the bleeding rates of warfarin and DOACs observed in the trials, though study designs, subject demographics, endpoint definitions, and follow-up periods differed from those in SALUTE. The results may be even more impressive considering that the subjects enrolled in SALUTE had increased bleeding risk evidenced by their requirement of a nonpharmacologic alternative to long-term anticoagulant therapy.
More importantly, as compared with warfarin, the LAA closure therapy in the PROTECT AF trial was able to reduce CV death by 56%, whereas DOAC reduced it by 12%,22 and this difference was primarily driven by the significant reduction in hemorrhagic strokes without anticoagulants by LAA closure. As Japanese patients have higher rates of hemorrhagic stroke, the CV death reduction may be even more beneficial.
Study LimitationsAs SALUTE is a single-arm study without a control group, this trial was not intended to compare the safety and efficacy of LAA closure with long-term warfarin therapy in Japanese patients with NVAF. Although the results in this trial were comparable to those from large-scale randomized clinical trials, results must still be interpreted with caution in the absence of a matched control arm.
This first report of the SALUTE trial contains only up to 6-months of follow-up results of the ITT cohort and longer follow-up is necessary to further evaluate the efficacy of the device in this population. The numbers of each event related to the primary endpoints or other endpoints were very limited, and the subgroup and multivariable analyses were not tested at this first analysis.
In the trial, the device implanters were proctored by very experienced operators and this could have contributed to the low complication rate.
The procedural safety and 6-month results from the SALUTE trial demonstrated the safety and efficacy of the LAA closure device, similar to the results from previously conducted large-scale randomized clinical trials, and provides a novel perspective of LAA closure for Japanese patients with NVAF in need of an alternative to long-term oral-anticoagulation.
The authors thank Thomas Christen, MD, Nicole Gordon and Yutaka Gomi (Boston Scientific Inc.) for their assistance in the preparation of the manuscript.
This trial was sponsored by Boston Scientific.
Supplementary File 1
Table S1. Left atrial appendage characteristics and summary of device size (roll-in cohort)
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-18-0222