Article ID: CJ-18-0483
Article ID: CJ-18-0483
Background: The aim of this single-center study was to report the midterm clinical outcomes and hemodynamic performance of the St Jude Medical Epic porcine bioprosthesis, a tricomposite glutaraldehyde-preserved porcine bioprosthesis, in mitral position.
Methods and Results: Between January 2011 and March 2017, 117 patients (62 men, 55 women; mean age, 66.7±12.8 years) who underwent mitral valve replacement (MVR) with the Epic valve were retrospectively analyzed for early and mid-term morbidity and mortality. The mean follow-up period was 2.6±1.7 years. Three operative deaths occurred, and the operative mortality rate was 2.6%. Sixteen patients died during the follow-up period. On Kaplan-Meier analysis, freedom from all-cause death and major adverse cardiovascular and cerebrovascular events at 5-year follow-up were 80.9% and 54.8%, respectively. There were 6 reoperations: 2 for structural valve deterioration (SVD), 2 for prosthetic valve endocarditis, and 2 for thrombosis. Freedom from valve-related reoperation and SVD at 5 years were 89.0% and 93.1%, respectively. On multivariate analysis, age ≥71 years (HR, 6.78; 95% CI: 2.12–25.2, P<0.01), and NYHA functional class ≥III (HR, 3.20; 95% CI: 1.03–10.4, P=0.04) were independent predictors for all-cause death. Mean mitral pressure gradient at 1 year and 2 years were 5.1±1.9 mmHg and 4.5±1.4 mmHg, respectively.
Conclusions: Mid-term clinical results and durability of the Epic valve in the mitral position are satisfactory.
The biological valve was developed in 1967, when aortic valve replacement (AVR) with a bioprosthesis was first performed. The clinical outcomes and the durability of various biological valves have been described as satisfactory.1–8
The number of elderly people in Japan has been increasing, and in contrast to that in the past, more elderly patients have been undergoing valve replacement with a biological valve in recent years. In Japan, the number of valve replacements with a biological valve increases every year. Recently, >12,000 AVR were performed, and in approximately 80% of cases a bioprosthesis was implanted.9 Moreover, 4,000 mitral valve replacements (MVR) were performed, and >60% of MVR involved a biological valve.9
In 2017, the American Heart Association/American College of Cardiology (AHA/ACC) released a revised guideline for the management of valvular disease,10 and there is an increasing trend toward greater use of biological valve prosthesis in relatively younger patients in their 50 s. In Japan, the use of transcatheter aortic valve replacement has become widespread.11–13 In the near future, transcatheter MVR and valve-in-valve implantation for structural valve deterioration (SVD) in the aortic and mitral positions will be available in Japan, and the number of valve replacements with biological valves will increase. Therefore, continuation of investigation into the performance of biological valves, especially the third-generation bioprostheses, which are mainly used at present, is of great importance.
The St Jude Medical Epic porcine bioprosthesis (St Jude Medical, St Paul, MN, USA) is a third-generation biological valve. It is structurally identical to the St Jude Medical Biocor porcine bioprosthesis, which has a record of very long-term durability.2–5 Moreover, the Epic valve is treated with the Linx anti-calcification process, and a pericardial shield on the outflow edge is designed to prevent abrasion. This valve is expected to perform better than the Biocor valve. In 2007, the Epic valve was approved by the US Food and Drug Administration, and it has been used since May 2011 in Japan. After the Epic valve was released, short-term clinical results were reported in Japan,14 but no studies have been conducted to report on the midterm performance of the Epic valve. In this study, we analyzed the midterm clinical outcomes and hemodynamic performance of the Epic valve in the mitral position.
Between January 2011 and March 2017, 155 patients underwent MVR, of whom 117 patients (female, 47%) received the Epic valve at the present institution. The decision whether to choose a mechanical or biological valve was based on Japanese Circulation Society guidelines.15 The bioprosthesis was selected based on surgeon preference. The Epic valve was implanted in the intra-annular position using pledgeted everting mattress sutures in almost all cases. Postoperative anticoagulation therapy dose was in accordance with the Japanese treatment guidelines.15 After MVR with the bioprosthesis the patients took warfarin for ≥3 months and the international normalized ratio of prothrombin time was controlled between 2.0 and 3.0. Follow-up transthoracic echocardiography (TTE) was performed in an outpatient clinic annually. Mean mitral pressure gradient (mMPG) was calculated using a continuous wave Doppler method.
The mean follow-up duration was 2.6±1.7 years, and the follow-up rates were 100%. Table 1 lists the patient characteristics. Mean patient age was 66.7±12.8 years (range, 11–90 years). The indications for MVR were mitral regurgitation (MR; n=71, 61%), mitral stenosis (MS; n=29, 25%), and acute infective endocarditis (IE; n=17, 15%). Thirty-nine patients had previous cardiac surgery (33%), of whom 31 had undergone mitral valve surgery. The causes of redo MVR were recurrent MR after mitral valve repair (n=12, 10%), MS after open mitral commissurotomy (n=6, 5%), perivalvular leakage after MVR (n=4, 3%), SVD (n=3, 2.6%), MS after mitral valve repair (n=2, 1.7%), IE after mitral valve repair (n=2, 1.7%), valve thrombosis (n=1, 0.9%), and prosthetic valve endocarditis (PVE; n=1, 0.9%). Thirty-three patients (28%) had low cardiac function (left ventricular ejection fraction <40%). Two patients (1.7%) were receiving hemodialysis.
| Characteristics | |
|---|---|
| No. patients | 117 |
| Age (years) | 66.7±12.8 |
| Range (years) | 11–90 |
| Female | 55 (47) |
| BMI (kg/m2) | 20.8±3.3 |
| BSA (m2) | 1.54±0.17 |
| Comorbidity | |
| Hypertension | 20 (17) |
| Diabetes mellitus | 18 (15) |
| Atrial fibrillation | 48 (41) |
| Peripheral artery disease | 2 (1.7) |
| CAD | 14 (12) |
| LVEF <40% | 33 (28) |
| Hemodialysis | 2 (1.7) |
| NYHA functional class | |
| I/II | 70 (60) |
| III/IV | 47 (40) |
| Mitral valve etiology | |
| Mitral regurgitation | 71 (61) |
| Mitral stenosis | 29 (25) |
| IE | 17 (15) |
| Previous cardiac operation | 39 (33) |
| Previous mitral valve surgery | 31 (26) |
| LVEF (%) | 55.2±19.3 |
| LVDd (mm) | 56.0±13.3 |
| LVDs (mm) | 40.6±17.2 |
| LAD (mm) | 51.7±11.5 |
Data given as n (%) or mean±SD. BMI, body mass index; BSA, body surface area; CAD, coronary artery disease; IE, infective endocarditis; LAD, left atrial dimension; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
All the data were retrospectively obtained from the medical records in Osaka University Hospital. The Institutional Review Board approved this study and patient consent was waived due to its retrospective nature.
Definitions of EventsThis study was conducted in accordance with the Guidelines for Reporting Morbidity and Mortality after Cardiac Valve Interventions.16 Operative death and mortality were defined as all-cause death and mortality ≤30 days after the procedure. Valve-related death included not only death caused by valve-related events but also sudden, unexplained death. Cardiac death included valve-related death; sudden, unexplained death; and death caused by non-valve-related cardiac events. Major adverse cardiovascular and cerebrovascular events (MACCE) were defined as cardiovascular death, stroke, reintervention and the postoperative readmission for cardiac events such as heart failure or arrhythmia.
Statistical AnalysisStatistical analysis was performed using SAS version 12.2.0 (SAS Institute, Cary, NC, USA). Normally distributed continuous data are expressed as mean±SD. Kaplan-Meier analysis was performed to determine all-cause or event-free survivals. The risk factors for all-cause death and MACCE were assessed using Cox proportional hazards analysis.
Table 2 lists the operative results. Urgent operation was required in 18 cases (15%). Regarding concomitant procedures, tricuspid annuloplasty had been the most frequently performed (39%), and double valve replacement was performed in 20 patients (17%). As for the size of the Epic valve, 25 mm or 27 mm was used in >80% of cases.
| Variables | |
|---|---|
| No. patients | 117 |
| Urgent operation | 18 (15) |
| Valve size (mm) | |
| 25 | 39 (33) |
| 27 | 56 (48) |
| 29 | 18 (15) |
| 31 | 4 (3) |
| Concomitant procedures | |
| Any | 84 (72) |
| Aortic valve replacement | 20 (17) |
| CABG | 14 (12) |
| Tricuspid annuloplasty | 46 (39) |
| Maze procedure/PVI | 15 (13) |
| Others | 10 (9) |
| Operative time (min) | 364±133 |
| CPB time (min) | 220±78 |
| Aortic cross-clamping time (min) | 76±87 |
Data given as n (%) or mean±SD. CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; PVI, pulmonary vein isolation.
Table 3 presents the clinical outcomes. The early mortality rate was 2.6% (n=3). The all-cause mortality rate was 14% (n=16) during the follow-up period. Cardiovascular and valve-related deaths during the follow-up period occurred in 7 (6%) and in 4 cases (3%), respectively. All valve-related deaths and 4 cases of cardiovascular death occurred due to sudden, unexplained death. Freedom from all-cause death at 5 years was 80.9% (Figure 1A). Twenty-seven patients (23%) had MACCE. Freedom from MACCE at 5 years was 54.8% (Figure 1B).
| Variables | |
|---|---|
| No. patients | 117 |
| Operative death | 3 (2.6) |
| Causes of operative death | |
| Sepsis | 2 (1.7) |
| Aspiration pneumonia | 1 (0.9) |
| All-cause death | 16 (14) |
| Cardiovascular death | 7 (6) |
| Valve-related death | 4 (3) |
| MACCE | |
| Any | 27 (23) |
| Cardiovascular death | 7 (6) |
| Stroke | 5 (4) |
| Reintervention | 4 (3) |
| Postoperative readmission for cardiac events | 11 (9) |
| Sternal re-exploration | 15 (13) |
| Mediastinitis | 4 (3) |
| Thromboembolic event | 13 (11) |
| Hemorrhagic event | 9 (8) |
| Valve-related reoperation | |
| Any | 6 (5) |
| Structural valve deterioration | 2 (1.7) |
| Prosthetic valve endocarditis | 2 (1.7) |
| Prosthetic valve thrombosis | 2 (1.7) |
Data given as n (%). MACCE, major adverse cardiovascular and cerebrovascular events.
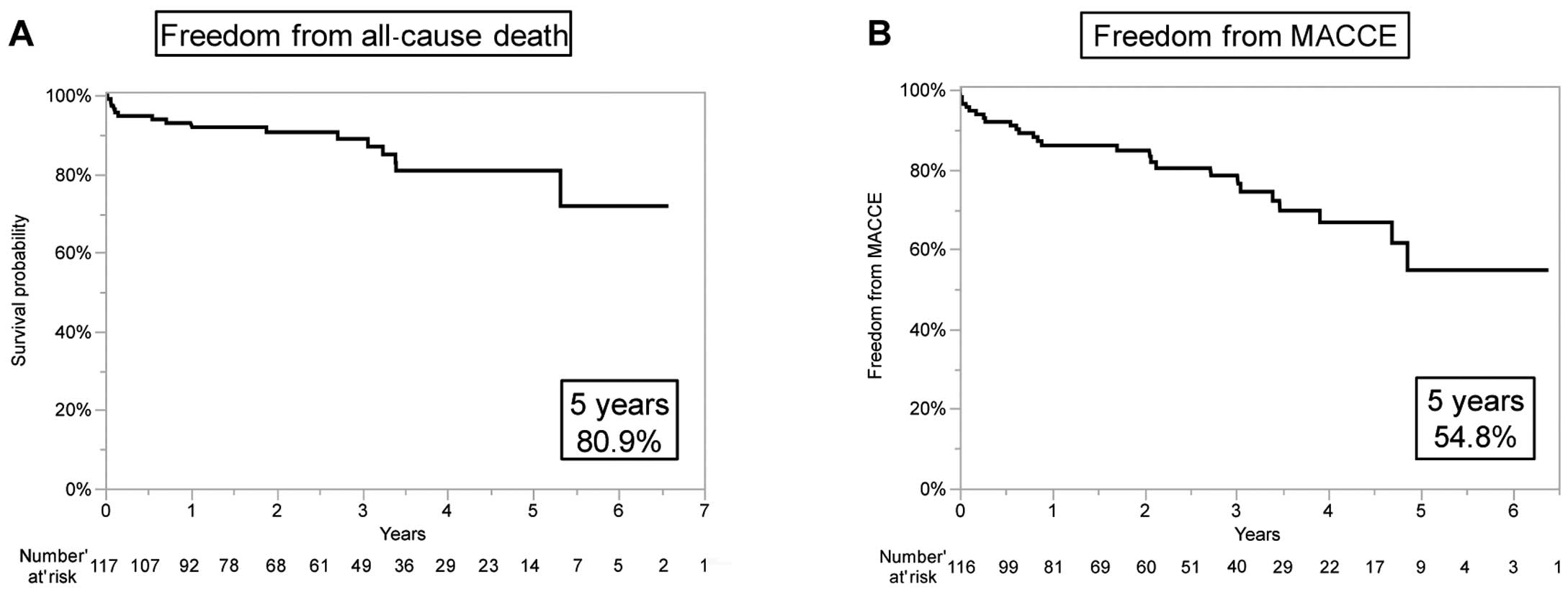
Kaplan-Meier analysis of freedom from (A) all-cause death and (B) major adverse cardiovascular and cerebrovascular events (MACCE).
Valve-related reoperation was performed in 6 patients (5%) during the follow-up period. The indication for reoperation was SVD in 2 patients, prosthetic valve thrombosis in 2 patients and PVE in 2 patients.
Regarding the 2 prosthetic valve thrombosis patients, we performed on-pump beating MVR for functional MR due to dilated phase of hypertrophic cardiomyopathy. During the postoperative period, the patients needed veno-arterial extracorporeal membrane oxygenation (V-A ECMO) because of hemodynamic instability. Although valve thrombosis was suspected on echocardiography, withdrawing V-A ECMO was difficult. The patients then underwent redo MVR and left ventricular assist device implantation on postoperative day 9.
Freedom from valve-related reoperation at 5 years was 89.0% (Figure 2A). In addition, freedom from SVD at 5 years was 93.1% (Figure 2B). Freedom from valve thrombosis at 5 years was 98.3%, and freedom from PVE at 5 years was 97.6%.
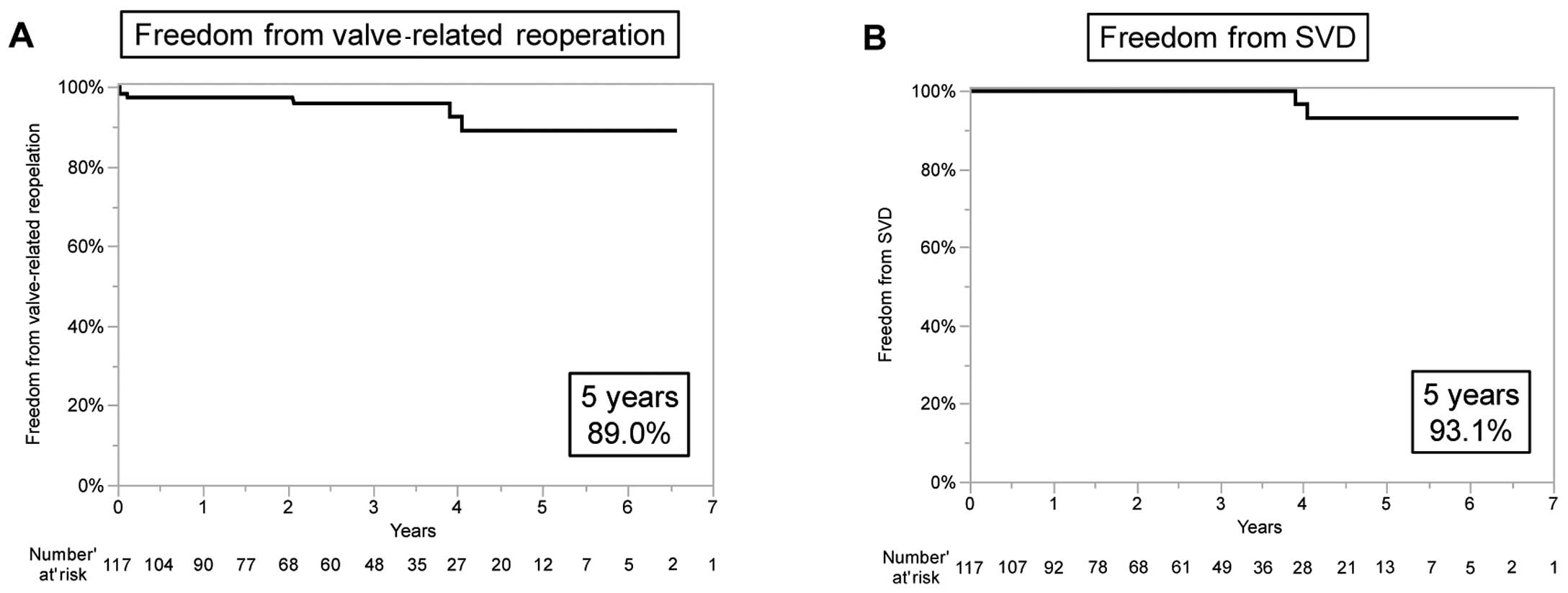
Kaplan-Meier analysis of freedom from (A) valve-related reoperation and (B) structural valve deterioration (SVD).
Regarding all-cause death, the factors with P<0.1 on univariate analysis were as follows: age ≥71 years, hypertension, New York Heart Association (NYHA) functional class ≥III, infective endocarditis, and urgent operation. According to multivariate analysis including these factors, age ≥71 years (HR, 6.78; 95% CI: 2.12–25.2, P<0.01), and NYHA functional class ≥III (HR, 3.20; 95% CI: 1.03–10.4, P=0.04) were independent predictors of all-cause death (Table 4). Conversely, with regard to MACCE, there were no significant risk factors on univariate analysis (Table 5).
| Characteristics | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age ≥71 years | 4.00 | 1.42–13.0 | <0.01 | 6.78 | 2.12–25.2 | <0.01 |
| Female sex | 1.41 | 0.52–3.96 | 0.50 | |||
| Comorbidity | ||||||
| Hypertension | 3.93 | 1.32–10.7 | 0.02 | 1.56 | 0.43–5.32 | 0.49 |
| Diabetes mellitus | 1.69 | 0.38–5.30 | 0.44 | |||
| Atrial fibrillation | 0.91 | 0.31–2.45 | 0.85 | |||
| CAD | 2.41 | 0.54–7.71 | 0.22 | |||
| LVEF <40% | 0.62 | 0.14–1.94 | 0.44 | |||
| NYHA functional class ≥III | 2.37 | 0.88–6.67 | 0.09 | 3.20 | 1.03–10.4 | 0.04 |
| Previous cardiac operation | 1.10 | 0.37–2.97 | 0.85 | |||
| Previous mitral valve surgery | 0.84 | 0.24–2.42 | 0.76 | |||
| Mitral valve etiology | ||||||
| Mitral regurgitation | 0.66 | 0.24–1.80 | 0.41 | |||
| Mitral stenosis | 0.64 | 0.15–2.00 | 0.47 | |||
| IE | 2.94 | 0.93–8.11 | 0.07 | 2.1 | 0.52–7.41 | 0.29 |
| Urgent operation | 3.89 | 1.32–10.5 | 0.02 | 2.55 | 0.59–11.0 | 0.21 |
| Concomitant procedure | ||||||
| Aortic valve replacement | 1.39 | 0.39–4.02 | 0.58 | |||
| CABG | 2.41 | 0.54–7.71 | 0.22 | |||
| Tricuspid annuloplasty | 1.43 | 0.52–4.08 | 0.48 | |||
| Maze procedure/PVI | 0.35 | 0.020–1.75 | 0.24 | |||
Abbreviations as in Tables 1,2.
| Characteristics | Univariable | ||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Age ≥71 years | 0.67 | 0.27–1.48 | 0.33 |
| Female sex | 1.37 | 0.64–3.00 | 0.42 |
| Comorbidity | |||
| Hypertension | 1.10 | 0.32–2.89 | 0.86 |
| Diabetes mellitus | 0.54 | 0.087–1.82 | 0.36 |
| Atrial fibrillation | 0.88 | 0.39–1.90 | 0.76 |
| CAD | 0.74 | 0.12–2.52 | 0.68 |
| LVEF <40% | 1.85 | 0.81–4.01 | 0.14 |
| NYHA functional class ≥III | 1.42 | 0.63–3.10 | 0.38 |
| Previous cardiac operation | 1.72 | 0.79–3.66 | 0.17 |
| Previous mitral valve surgery | 1.75 | 0.77–3.77 | 0.17 |
| Mitral valve etiology | |||
| Mitral regurgitation | 0.98 | 0.46–2.19 | 0.97 |
| Mitral stenosis | 0.74 | 0.27–1.73 | 0.50 |
| Infective endocarditis | 1.59 | 0.53–3.90 | 0.37 |
| Urgent operation | 1.19 | 0.35–3.12 | 0.75 |
| Concomitant procedures | |||
| Aortic valve replacement | 0.89 | 0.30–2.18 | 0.81 |
| CABG | 0.74 | 0.12–2.52 | 0.68 |
| Tricuspid annuloplasty | 0.92 | 0.42–1.98 | 0.83 |
| Maze procedures/PVI | 0.87 | 0.25–2.27 | 0.79 |
Abbreviations as in Tables 1–3.
One- and 2-year follow-up rates after surgery were 83% and 55%, respectively. On postoperative TTE, moderate or severe MR was not observed 1 year after surgery. One patient, however, had transvalvular moderate MR 2 years after operation. mMPG was 5.1±1.9 mmHg and 4.5±1.4 mmHg at 1 year and 2 years after surgery, respectively. The mMPG for each valve size is shown in Figure 3.
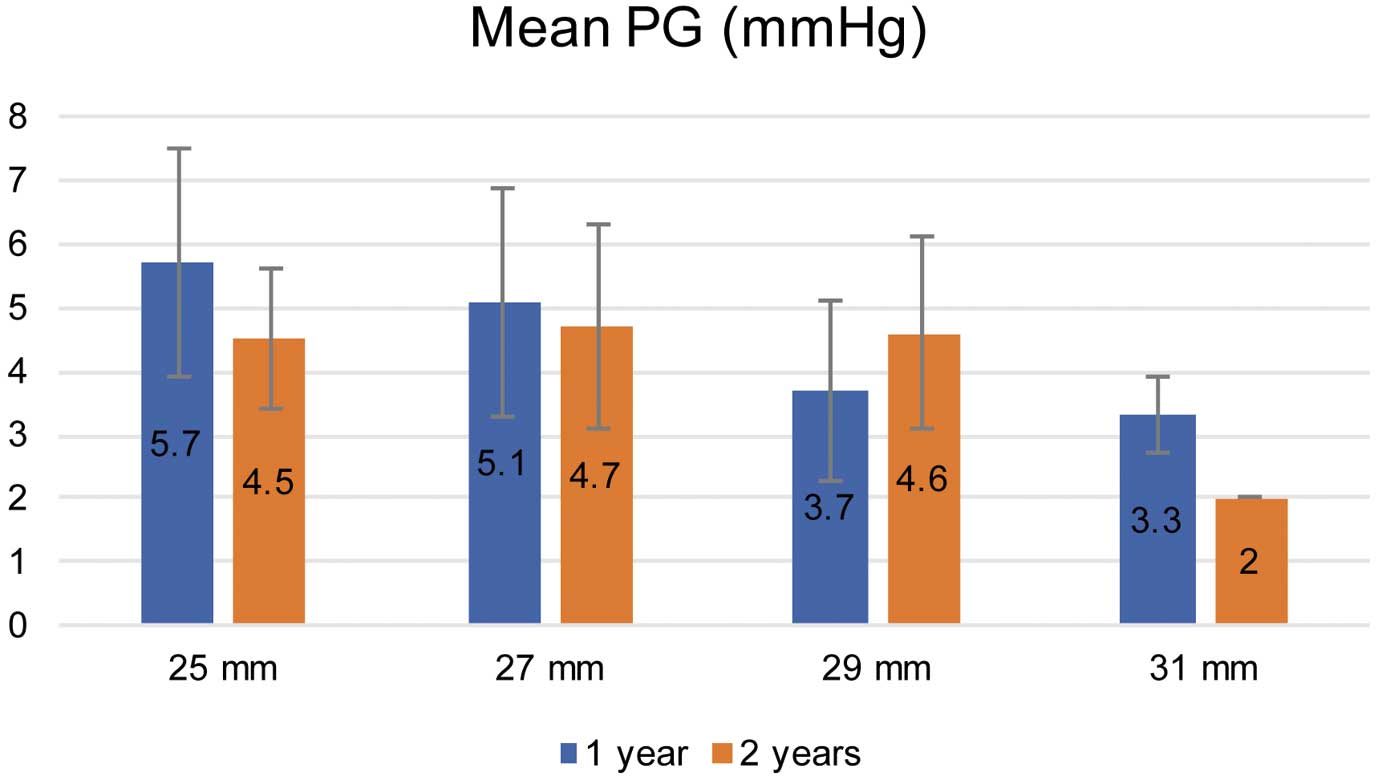
Hemodynamics for mitral valve replacement with the Epic valve at 1 year and 2 years after surgery. PG, pressure gradient.
To the best of our knowledge, this is the first study in Japan to describe the midterm clinical results and hemodynamics of the Epic valve in the mitral position. The mid-term clinical outcomes of the Epic valve were excellent, even though the present cases involved several relatively high-risk patients with low cardiac function or who needed redo surgery.
The St Jude Medical Epic porcine bioprosthesis is a successor model to the St Jude Medical Biocor porcine bioprosthesis. The Biocor valve has been described as having good clinical results and durability.2–5
The Epic valve has a structurally identical design to the Biocor valve and is treated with Linx AC, an ethanol-based anti-calcification therapy. The Epic valve is expected to have better long-term clinical outcomes and durability than the Biocor valve. Studies on the long-term clinical outcomes and durability of the Epic valve, however, are very limited. In 2011, Jamieson et al reported on the midterm performance of the Epic valve.17 There were 204 MVR and the mean age of the population was 73.9 years. The overall survival of the total population was 76% at 4 years. Freedom from reoperation owing to SVD at 4 years was >99%. The actuarial freedom from overall thromboembolism and reoperation for PVE at 4 years were 90% and 96%, respectively.17 In Japan, Kanda et al reported the early clinical outcomes and hemodynamics after 26 cases of MVR with the Epic valve.14 Mean patient age was 71.2±12.1 years. One hospital death occurred, and no reoperation for any valve abnormality was performed in the median follow-up of 24 months. With regard to hemodynamics during follow-up, mMPG was 6.2 mmHg.14 In the present series, mean patient age was slightly younger because of the relatively large number of young patients with cardiomyopathy. Overall survival at 5 years in the present study was 80.9% and freedom from SVD, valve thrombosis, and PVE at 5 years were 93.1%, 98.3%, and 97.6%, respectively. mMPG was <5 mmHg at 2 years after MVR. These data are similar to those of other reports.14,17
Loor et al reported the mid-term efficacy and durability of the Carpentier-Edwards Perimount Magna mitral valve bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) in comparison with other biological valves that are currently used in the mitral position.18 Their mean patient age was 68 years. The 5-year survival rate was 40%, and 90% of the patients were free from SVD. mMPG ranged from 5 to 8 mmHg on postoperative TTE.18 Jamieson et al reported the long-term clinical results and hemodynamics of the Mosaic porcine bioprosthesis (Medtronic, Minneapolis, MN, USA).6 Mean patient age was 67 years. The 10-year survival rate was 58.6%, and freedom from SVD was 95.3% at 10 years, and mMPG was 3.6 mmHg.6 Mean patient age was similar between these reports and the present series, and the present study has demonstrated that the Epic valve is not inferior to other bioprostheses with respect to the early and mid-term clinical outcomes and hemodynamics.
In the present study, age ≥71 years and NYHA functional class ≥III were independent risk factors for all-cause death. This is consistent with that shown in past reports;19,20 these factors are well-known prognostic factors for valve replacement.
There were 2 cases of SVD in the present series. The first patient was an 11-year-old girl who had dilated cardiomyopathy with MR, and SVD with calcification and perforation of the leaflet occurred approximately 4 years later. Some reports are available on bioprosthetic valve replacement in younger patients, indicating the high risk of SVD after valve replacement with bioprosthesis in these patients.21,22 Moreover, Manji et al reported that the calcification and failure of a glutaraldehyde-fixed bioprosthesis were related to humoral and cellular immune system rejection in young patients.23 This mechanism remains controversial.
The second patient had leaflet tear of the implanted valve approximately 4 years after MVR. Grunkemeier et al reported that leaflet tear was responsible for 61% of the SVD explants in the porcine series, and that it occurred more frequently than in the pericardial series (46%).24 Kalejs et al showed that the Epic valve was stiffer and less elastic than the native porcine and human aortic valve.25
Acute mitral bioprosthetic valve thrombosis has been reported after MVR during V-A ECMO support.26–28 The risk has been reported to include reduction of cardiac output from the native heart because the left ventricular afterload increases and the preload decreases on V-A ECMO. Regarding the postoperative anticoagulation therapy in these cases, activated partial thromboplastin time was controlled between 50 and 60 s with the use of heparin. Therefore, we speculated that the reduction of cardiac output from the native heart may have caused valve thrombosis in the present series.
Study LimitationsThis study had several imitations. First, this was a retrospective and single-center study. Therefore, the number of patients was limited. Second, the mean follow-up duration was relatively short. Therefore, further studies with a longer follow-up period are required to clarify the long-term clinical outcomes and durability. Third, the choice of bioprosthesis depended on surgeon preference, and a randomized study should be conducted to compare the Epic valve with other bioprostheses in the future.
The mid-term outcomes of the Epic valve are satisfactory. This is the first study in Japan to report on the midterm clinical outcomes and performance of the St Jude Medical Epic porcine bioprosthesis in the mitral position. Longer follow-up is needed, however, to determine long-term durability.