Article ID: CJ-18-0849
Article ID: CJ-18-0849
Background: The ideal surgical technique for ischemic mitral regurgitation (MR) is controversial. We introduced an extended posterior mitral leaflet (PML) augmentation technique for functional MR with severe tethering, which detached the PML from the annulus almost completely and augmented it with a large 3×6-cm oval pericardial patch.
Methods and Results: A total of 17 mitral repairs using the new technique were performed for ischemic MR with no 30-day mortality and 2 hospital deaths. The NYHA class was III in 47% and IV in 13%. The EuroSCORE II was 9.7±4.9. The ring size was 32±1.4 mm. Concomitant coronary bypass was performed in 67% and left ventricular repair in 28%. The mechanism of leaflet closure was evaluated using transthoracic echocardiography in 15 survivors. MR decreased to none or trivial with a significant increase in coaptation length (Pre: 4.7±0.8 mm vs. Post: 10.0±2.4 mm; P<0.001). The PML flexibly moved forward and tightly contacted as if “snuggling up” to the anterior leaflet. There were no late deaths, heart failure readmissions or MR recurrences during follow-up (850±181 days). All patients remained in NYHA I or II.
Conclusions: Extended PML augmentation for ischemic MR showed excellent early results with deep leaflet coaptation through a “snuggling up” phenomenon, which would help prevent late MR recurrence.
Ischemic mitral regurgitation (MR) is a significant problem that influences the outcome of heart failure patients.1 Surgical management of ischemic MR for better outcomes remains controversial. Mitral valve replacement (MVR) in functional MR leaves much to be desired.2 Restrictive annuloplasty, proposed by Bolling et al,3 for non-ischemic functional MR in dilated cardiomyopathy was not shown to provide significantly different benefits from those of MVR in a recent randomized trial; however, importantly, the restrictive annuloplasty subgroup without recurrent MR showed better outcomes than the MVR group in that trial.4 Therefore, new adjunct and optimal surgical strategies to achieve better outcomes must provide mitral repair that prevents recurrent MR.
Several previous studies have elucidated the mechanism of functional MR.5,6 After infarction, localized or diffuse remodeling in left ventricular (LV) size and geometry (including papillary muscle displacement) increases the tethering forces, which results in incomplete mitral leaflet closure.5 It is known that an imbalance between the tethering force and the closing force causes ischemic MR, and the grade of ischemic MR fluctuates according to hemodynamic changes.5,6 He et al showed an inverse correlation between the severity of MR and the contact length of the anterior and posterior leaflets, the so-called “coaptation length” (CL), and the mobility of the posterior mitral leaflet (PML). A previous study showed that as the PML became less mobilized because of tethering and the CL became shorter, the MR increased.6 Furthermore, after restrictive annuloplasty, progression of PML tethering is known as a cause of late recurrence of functional MR.7 Therefore, the optimal surgical approach for ischemic MR needs to avoid progression of PML tethering and increase the CL to prevent recurrent MR.
In the past few years, we introduced an extended PML augmentation technique for heart failure patients with moderate or severe functional MR that detached the PML from the annulus almost completely and augmented the PML with a large 6×3-cm pericardial patch. This technique successfully decreased leaflet tethering and offered better leaflet coaptation, which reduced MR. The aim of this study was to clarify the echocardiographic features of leaflet configurations and the efficacy of our extended PML augmentation technique applied to ischemic MR.
This study was approved by the Institutional Review Board of Showa University Koto Toyosu Hospital. Data were collected from electronic medical records and scanned hospital records for use in this retrospective observational study. A total of 125 mitral valve repair surgeries were performed from March 2015 to July 2017. Among them, 17 heart failure patients underwent the extended PML augmentation technique to repair the mitral valve for moderate or severe ischemic MR. Although there were no 30-day mortalities, 2 patients died while in hospital because of low-output syndrome and sudden ventricular fibrillation. Because transthoracic echocardiography (TTE) data under stable hemodynamic conditions were not available after surgery, these 2 patients were excluded from the study. The other 15 patients who survived and were subsequently discharged from the hospital were included and evaluated. All patients had focal or global abnormal contraction of the LV and a previous history of percutaneous coronary intervention or ≥1 untreated significant coronary artery lesions. They were classified as New York Heart Association (NYHA) functional class ≥II and stage C or D according to the 2013 ACCF/AHA Guideline for the Management of Heart Failure.8 The patients had been receiving sufficient medical therapy before evaluation of the severity of MR. Patients with degenerative mitral valve disease and non-ischemic dilated cardiomyopathy were excluded.
The following preoperative variables were recorded: age, sex, body weight, height, body mass index, body surface area (BSA), NYHA class, presence or history of congestive heart failure, comorbidities (hypertension, dyslipidemia, diabetes mellitus, cerebral infarction and/or hemorrhage, coronary artery disease with or without previous intervention, peripheral artery disease, history of smoking, chronic obstructive pulmonary disease, previous sternotomy, liver cirrhosis, chronic renal failure with or without hemodialysis, endocarditis, and rheumatic disease), preoperative medications, and preoperative blood examination (including creatinine, estimated glomerular filtration ratio, serum albumin, cholinesterase, B-type natriuretic polypeptide (BNP), hemoglobin A1c). The EuroSCORE II for estimated surgical risk calculation was also acquired.9
TTE parameters within 40 days (24±8 days) before and after surgery were collected and evaluated. All patients were discharged and followed up annually. NYHA class and BNP were evaluated by attending physicians.
Inclusion CriteriaWe utilized patch augmentation for ischemic MR based on LV geometry and function. There were 3 typical types of ischemic MR that required patch augmentation. The first type was an enlarged LV with moderate to severely impaired LV function. The LV was significantly enlarged because of myocardial ischemia/infarction, and the LV function was severely impaired where typical leaflet tethering was observed. The second type was LV with a basal aneurysm. In general, patients had previous inferior (and/or posterior) myocardial infarction. In this second type, the LV was not always enlarged and sometimes it remained in the normal size range; however, typical leaflet tethering associated with the basal aneurysm was observed on echocardiography. The third type was large LV aneurysms from a previous anteroseptal myocardial infarction. LV geometry was characterized by dyskinetic apical aneurysms, which could displace the papillary muscle, leading to leaflet tethering. This type could be combined with the first type: enlarged LV with impaired LV function. However, the third type of ischemic MR had the most profound LV dysfunction.
Surgical TechniqueAll patients underwent mitral valve repair using the extended PML augmentation technique. After a full median sternotomy, a part of the pericardium was harvested and immersed in 0.625% glutaraldehyde for 10 min and then rinsed in normal saline solution 3 times. After cardioplegic cardiac arrest, a right-side left atriotomy was performed. The mitral valve was exposed, and leaflets were carefully observed and confirmed as having a typical ischemic configuration without degenerative changes (Figure 1A-a). The PML with a 2-mm margin was detached from the posterior annulus almost completely, leaving a small communication with the annulus around the commissure. The previously harvested glutaraldehyde-treated autologous pericardium was trimmed to a 3×6-cm oval shape. The pericardium was sutured to the detached clear zone of the PML with 5-0 polypropylene running sutures from the anterior commissure to the posterior commissure (Figure 1A-b). After the PML was augmented, the mitral annulus was stabilized with a semi-rigid complete ring (Carpentier-Edwards Physio Ring II; Edwards Lifescience, Irvine, CA, USA) (Figure 1A-c). Importantly, the ring size was selected according to the total surface area of the mitral valve in the water test and not adjusted for the anterior leaflet size or intertrigonal length, which led us to choose a ring more than 2 sizes larger than that in restrictive annuloplasty. All patients underwent concomitant tricuspid annuloplasty using an annuloplasty ring (MC3; Edwards Lifescience or Contour 3D, Medtronic, Minneapolis, MN, USA). At the surgeon’s discretion, some patients underwent coronary artery bypass grafting (CABG) or LV repair in the case of aneurysmal and scarring changes in the LV apex.
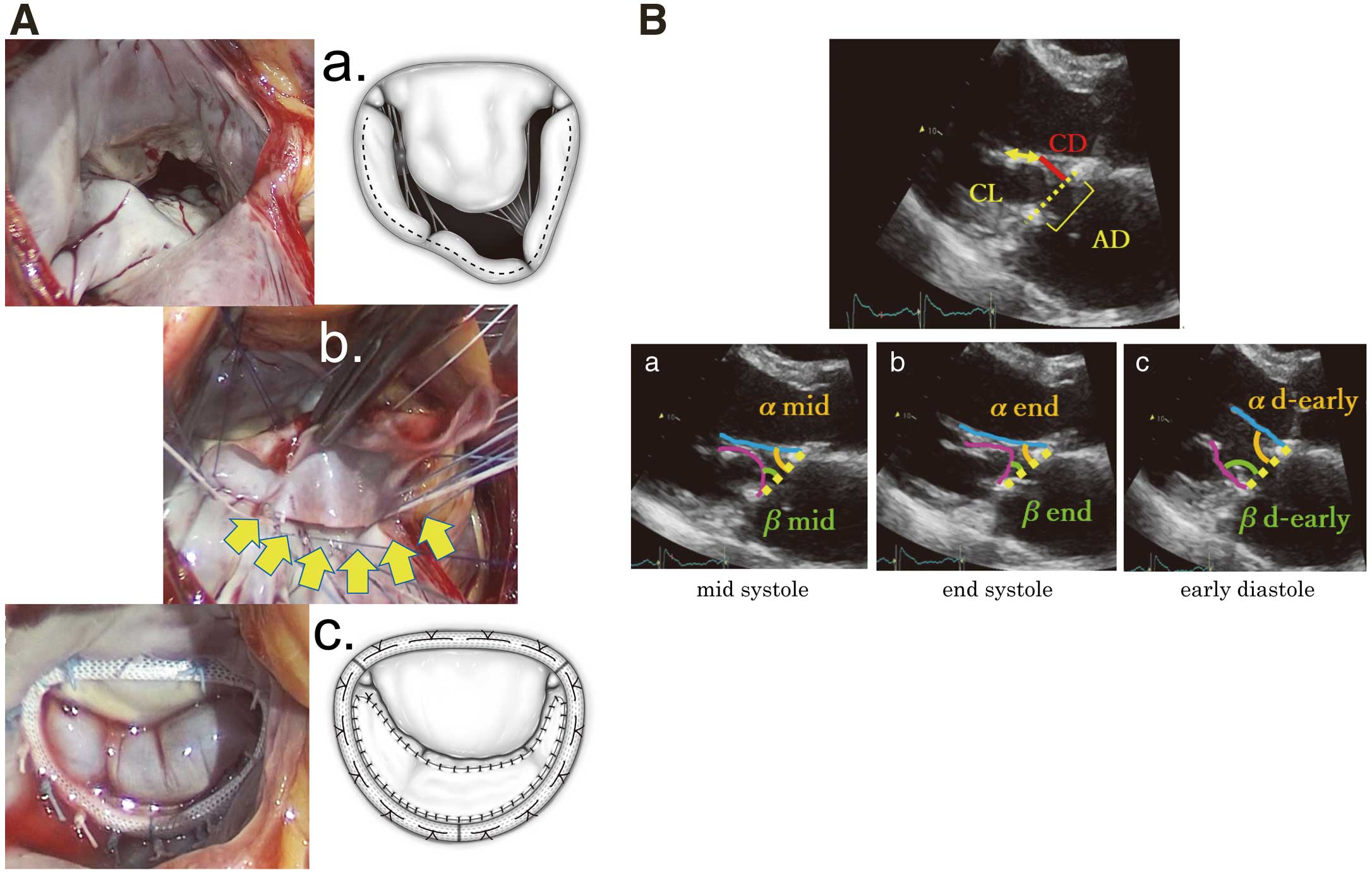
(A) Extended posterior mitral leaflet augmentation. (a) Surgeon’s view of the mitral valve showing typical ischemic mitral configuration with severe tethering at the posterolateral side of the posterior leaflet. Dotted line shows the incision line. (b) Intraprocedural view. The 3×6 cm glutaraldehyde-treated autologous pericardium (yellow arrows) is sutured to the clear zone of the whole posterior leaflet. (c) Postprocedural view at the water test. (B) Postoperative transthoracic echocardiographic measurements in the parasternal long-axis view. (a) The angles formed by the anterior and posterior leaflets and annulus line (yellow dotted line) in mid systole (αmid and βmid). (b) The angles in end systole (αend and βend). (c) The angles in the early diastolic phase (αd-early and βd-early). AD, annular distance; CL, coaptation length; CD, coaptation distance.
TTE was performed using the following ultrasonic inspection systems: Aplio 500 (Toshiba, Ohtawara, Japan) or Vivid E9 (General Electric Healthcare, Horten, Norway). Echocardiographic measurements were averaged over 2 cardiac cycles. Left atrial volume index (LAVI) was measured by the disk summation algorithm, and the right atrial (RA) area was traced in the apical 4-chamber view. Right ventricular systolic pressure (RVSP) was calculated by using the estimated RA pressure and the pressure gradient between the right ventricle (RV) and RA in a systolic phase. The pressure gradient between the RV and RA was obtained by an application of the modified Bernoulli equation to the peak velocity of the continuous-wave Doppler tricuspid regurgitation (TR) flow as follows: RVSP=estimated RA pressure+4×(TR peak velocity)2. The estimated RA pressure was obtained by inferior vena cava diameter and collapse.10 The LV end diastolic volume index (LVEDVI), LV end systolic volume index (LVESVI), and LV ejection fraction (LVEF) were obtained by the biplane modified Simpson’s method.11 The LV outflow tract (LVOT) velocity time integral was measured using a pulsed-wave Doppler method in the apical long-axis view. The LV sphericity index, defined as the LV short to long-axis dimension ratio, was measured in the end systolic apical 4-chamber view. The mitral leaflet configuration was evaluated in the parasternal long-axis view. MR severity was evaluated comprehensively according to the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease12 by quantifying the vena contracta width, effective regurgitant orifice area and regurgitant volume using color Doppler and the proximal isovelocity surface area method.13 The mean transmitral pressure gradient was calculated to evaluate the grade of functional mitral stenosis using integrated software and the trace of the Doppler diastolic mitral flow waveforms in the apical 4-chamber view.14 In the mid systolic parasternal long-axis view, the CL and coaptation depth (CD: the distance from the annulus line to the coaptation point) were measured, and the tenting area was calculated using the annular distance (AD) and CL (tenting area=AD×CD/2) (Figure 1B). The CL was indexed by BSA. We defined the angles formed by the annulus line and anterior mitral leaflet (AML) and PML as “α” and “β”, respectively. The α and β in the early systolic, mid systolic, end systolic and early diastolic phases were defined as αs-early and βs-early, αmid and βmid, αend and βend, and αd-early and βd-early, respectively (Figure 1B-a–c). Before and after surgery, the anterior leaflet length (AL) to the mitral valve contribution (annulus to coaptation), posterior leaflet length (PL) to the mitral valve contribution (annulus to coaptation), and distance from the septum to the mitral valve coaptation point (C-sept) were measured in the early systolic phase to evaluate the risk of systolic anterior motion (SAM) of the mitral leaflet (Figure 2).15,16
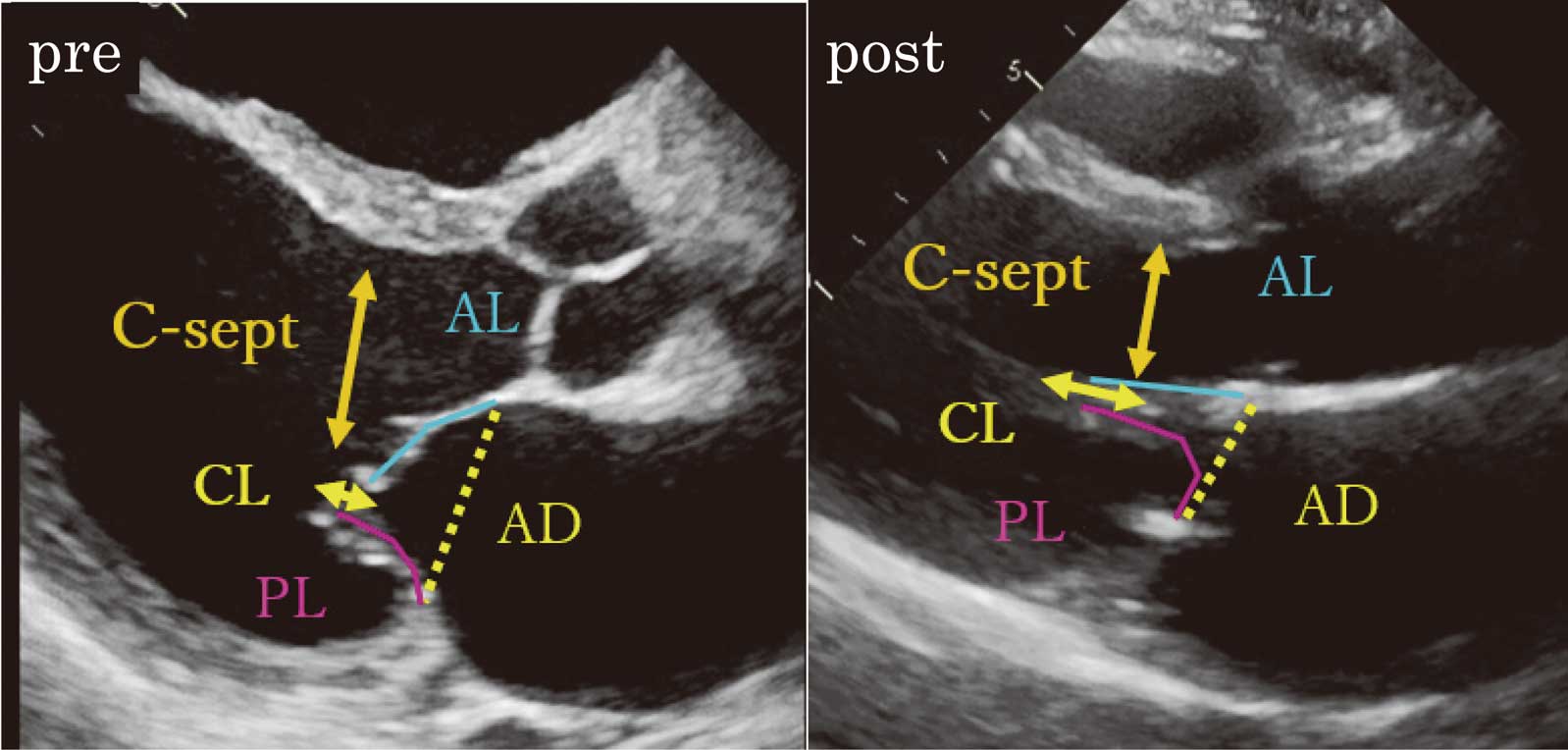
Pre- and postoperative transthoracic echocardiographic measurements related to systolic anterior movement. AL, anterior leaflet length to mitral valve contribution (annulus to coaptation), PL, posterior leaflet length to mitral valve contribution (annulus to coaptation), C-sept, distance from septum to mitral valve coaptation point in the early systolic phase.
Results are expressed as absolute values and percentages, continuous variables as the mean±standard deviation, and categorical variables as percentages. Continuous variables pre- and postoperatively were compared by paired t-test. Values of P<0.05 were considered as indicative of statistical significance.
The patients’ preoperative characteristics are shown in Table 1. The BNP level was 852±1,136 pg/mL, NYHA functional class II was 40%, class III was 47%, and class IV was 13%. Approximately 20% of the patients required hospitalization (≥2 times) because of heart failure within 1 year prior to surgery. The EuroSCORE II was high at 9.7±4.9, which was mainly related to renal impairment (40%), pulmonary hypertension judged by elevated estimated RVSP by echocardiography, and NYHA functional class. Although a β-blocker was administered to 67% of the patients, an angiotensin-converting enzyme inhibitor and angiotensin II receptor antagonists were administered in only 20% because of hypotension.
| Sex (male/female) | 12/3 |
| Age (years) | 66±10 |
| BSA (m2) | 1.62±0.17 |
| Heart rate on admission (beats/min) | 76±13 |
| Systolic BP on admission (mmHg) | 111±19 |
| Diastolic BP on admission (mmHg) | 68±12 |
| Prior hospitalization for HF (no. times) | 1.2±1.6 (0–6) |
| Comorbidities | |
| Dyslipidemia | 9 (60%) |
| Atrial fibrillation | 3 (20%) |
| Chronic renal failure (eGFR <40 mL/min/1.73 m2) | 6 (40%) |
| COPD | 1 (7%) |
| Diabetes mellitus | 5 (33%) |
| Past smoking | 14 (93%) |
| Euro score II | 9.7±4.9 |
| Medications | |
| Loop diuretics | 12 (80%) |
| Tolvaptan | 9 (60%) |
| β-blocker | 10 (67%) |
| ACEI or ARB | 3 (20%) |
| Anti-aldosterone | 9 (60%) |
| NYHA class | |
| II | 6 (40%) |
| III | 7 (47%) |
| IV | 2 (13%) |
| BNP level (pg/mL) | 852±1,136 |
| Cr (mg/dL) | 1.4±0.9 |
| Ischemic site | |
| Anterior | 6 (40%) |
| Inferior | 7 (47%) |
| Multiple | 2 (13%) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonists; BNP, B-type natriuretic peptide; BSA, body surface area; COPD, chronic obstructive pulmonary disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; HF, heart failure; NYHA, New York Heart Association; tolvaptan, selective oral vasopressin V2-receptor antagonist.
The patients’ operative data are shown in Table 2. All patients underwent mitral valve repair with techniques that included extended PML augmentation and mitral annuloplasty in addition to tricuspid annuloplasty. The mitral ring size was 32±1.4 mm. Concomitant procedures included CABG in 67% and LV repair in 27%.
| Posterior mitral leaflet augmentation | 15 (100%) |
| Mitral annuloplasty | 15 (100%) |
| Ring size (mm) | 32±1.4 |
| Concomitant procedure | |
| Coronary artery bypass grafting | 10 (67%) |
| No. of bypass grafts | 2.4±1.9 |
| Tricuspid annuloplasty | 15 (100%) |
| Left ventricular repair | 4 (27%) |
| Septal anterior ventricular exclusion procedure | 3 (20%) |
| Infarct exclusion procedure | 1 (7%) |
After discharge, all 15 patients were successfully followed up. The follow-up period was 850±181 days. None required readmission because of worsening heart failure. There were no recurrent cases of more than mild MR. All patients are now in NYHA functional class I or II (Figure 3). The serum BNP level was significantly decreased from 852±1,136 pg/mL to 218±165pg/mL (P<0.05).
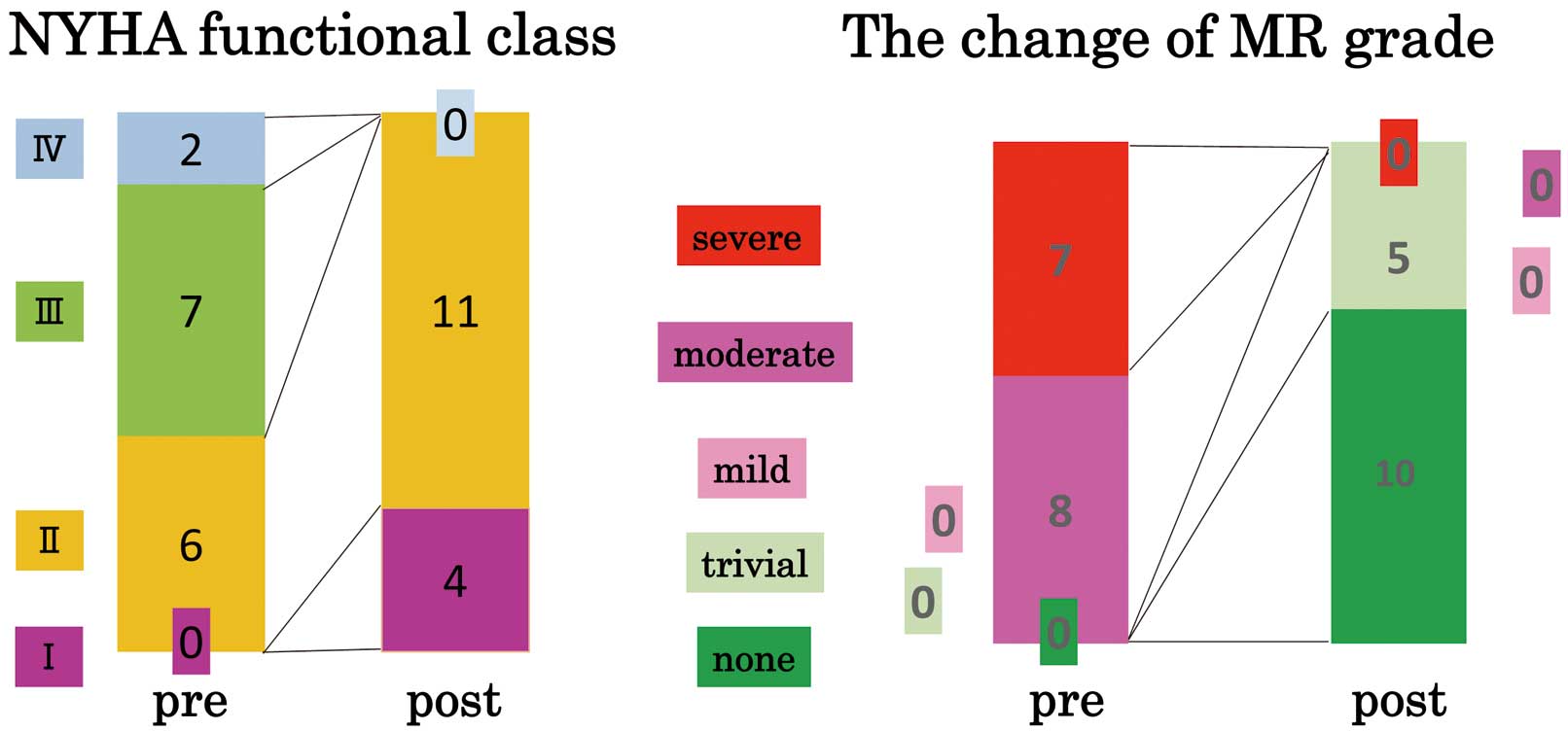
Grade of mitral regurgitation (MR) and patients’ symptoms after discharge from our clinic. All patients were in NYHA functional class I or II after surgery. The follow-up period was 850±181 days. MR grade improved to trivial or none in all cases. There were no cases of recurrent MR of more than mild degree during the follow-up period.
The TTE findings before and after surgery about patients without LV plasty (n=11) are shown in Table 3. We excluded patients with LV plasty because their LV geometric changes would have affected our results.
| Preoperative | Postoperative | P value | |
|---|---|---|---|
| LVDd (mm) | 62±8.3 | 56±6.6 | 0.006* |
| LVDs (mm) | 51±11 | 47±9.2 | 0.09 |
| LVEDVI (mL/m2) | 127±55 | 95±40 | 0.013* |
| LVESVI (mL/m2) | 79±47 | 61±38 | 0.051 |
| LVEF (%) | 39±13 | 39±14 | 1.0 |
| LVOT VTI (cm) | 15±4.4 | 16±3.7 | 0.71 |
| Sphericity index | 0.59±0.1 | 0.56±0.1 | 0.41 |
| LAVI (mL/m2) | 63±19 | 45±15 | 0.016* |
| RA area (cm2) | 19±4.2 | 15±5.7 | 0.018* |
| RVSP (mmHg) | 48±15 | 23±7.9 | 0.007* |
| MR VC (mm) | 6.5±1.4 | – | |
| MR EROA (cm2) | 0.37±0.2 | – | |
| MR grade | |||
| None | 0 | 6 | |
| Trivial | 0 | 5 | |
| Mild | 0 | 0 | |
| Moderate | 6 | 0 | |
| Severe | 5 | 0 | |
| CL (mm) | 4.6±0.8 | 9.8±2.5 | <0.001* |
| CL index (mm/m2) | 2.9±0.5 | 6.0±1.3 | <0.001* |
| CD (mm) | 9.5±1.8 | 5.9±1.4 | <0.001* |
| PSL AD (mm) | 32±5.0 | 19±3.7 | <0.001* |
| Tenting area (cm2) | 1.5±0.4 | 0.6±0.2 | <0.001* |
| MV mPG (mmHg) | – | 3.8±1.2, range: 2.1–5.8 | |
| α in early systole (degree) | 45±10.1 | 50±7.7 | 0.24 |
| α in mid systole (degree) | 40±7.9 | 46±5.2 | 0.09 |
| α in end systole (degree) | 41±8.8 | 42±5.7 | 0.74 |
| α in early diastole (degree) | 66±11 | 72±12 | 0.098 |
| β in early systole (degree) | 53±9.1 | 46±15 | 0.24 |
| β in mid systole (degree) | 45±18 | 27±6.7 | 0.015* |
| β in end systole (degree) | 41±21 | 14±3.7 | 0.002* |
| β in early diastole (degree) | 46±12 | 66±18 | 0.03* |
The tenting area was calculated from the equation: AD×CL/2 (cm2). We defined the angles formed by the annulus line and anterior and posterior mitral leaflets as “α” and “β”, respectively. The α and β in the early systolic, mid systolic, end systolic, and early diastolic phases were defined as αs-early and βs-early, αmid and βmid, αend and βend, and αd-early and βd-early, respectively. AD, annular distance; CD, coaptation depth; CL, coaptation length; EDVI, end diastolic volume index; ESVI, end systolic volume index; EROA, effective regurgitant orifice area; LAVI, left atrial volume index; LVDd, left ventricular diastolic dimension; LVDs, left ventricular systolic dimension; LVEF, left ventricular ejection fraction; LVVTI, left ventricular velocity time integral; MR, mitral regurgitation; MV mPG, mitral valve mean pressure gradient; PSL, parasternal long-axis view; RA, right atrium; RVSP, right ventricular systolic pressure; VC, vena contracta.
In all patients, MR grade improved to trivial or none (Figure 3). After surgery, the LVEDVI, LVESVI, LAVI, RA area, and RVSP were significantly decreased and LVEF was maintained. The CL in the parasternal long-axis view was significantly longer (Pre: 4.6±0.8 mm vs. Post: 9.8±2.5 mm; P<0.001), and the CD and tenting area were significantly decreased (Pre: 9.5±1.8 mm and 1.5±0.4 cm2 vs. Post: 5.9±1.4 mm and 0.6±0.2 cm2, respectively; P<0.001).
Features of Leaflet Configurations of Extended PML Augmentation for Ischemic MRBefore surgery, the AML and PML were tethered by displaced papillary muscles and could not gain sufficient coaptation in all systolic phases. Consequently, MR appeared and continued from the early systolic to end systolic phases (Figure 4A). After surgery, the augmented PML showed a unique movement within 1 cardiac cycle; it flexibly moved upward and contacted the AML in the systolic phase, especially in the mid to end systolic phases. The augmented PML moved towards and tightly contacted, as if “snuggling up” to the AML, which created a deep and tightly uniform coaptation (Figure 4B). The β in mid systole significantly decreased with a further significant decrease in the β in end systole (Table 3). In the mid and end systolic phases, as the angle β decreased, the CL increased (Figure 4C). Although trivial MR was observed in the early systolic phase, it gradually disappeared by the end systolic phase (Figure 4B).
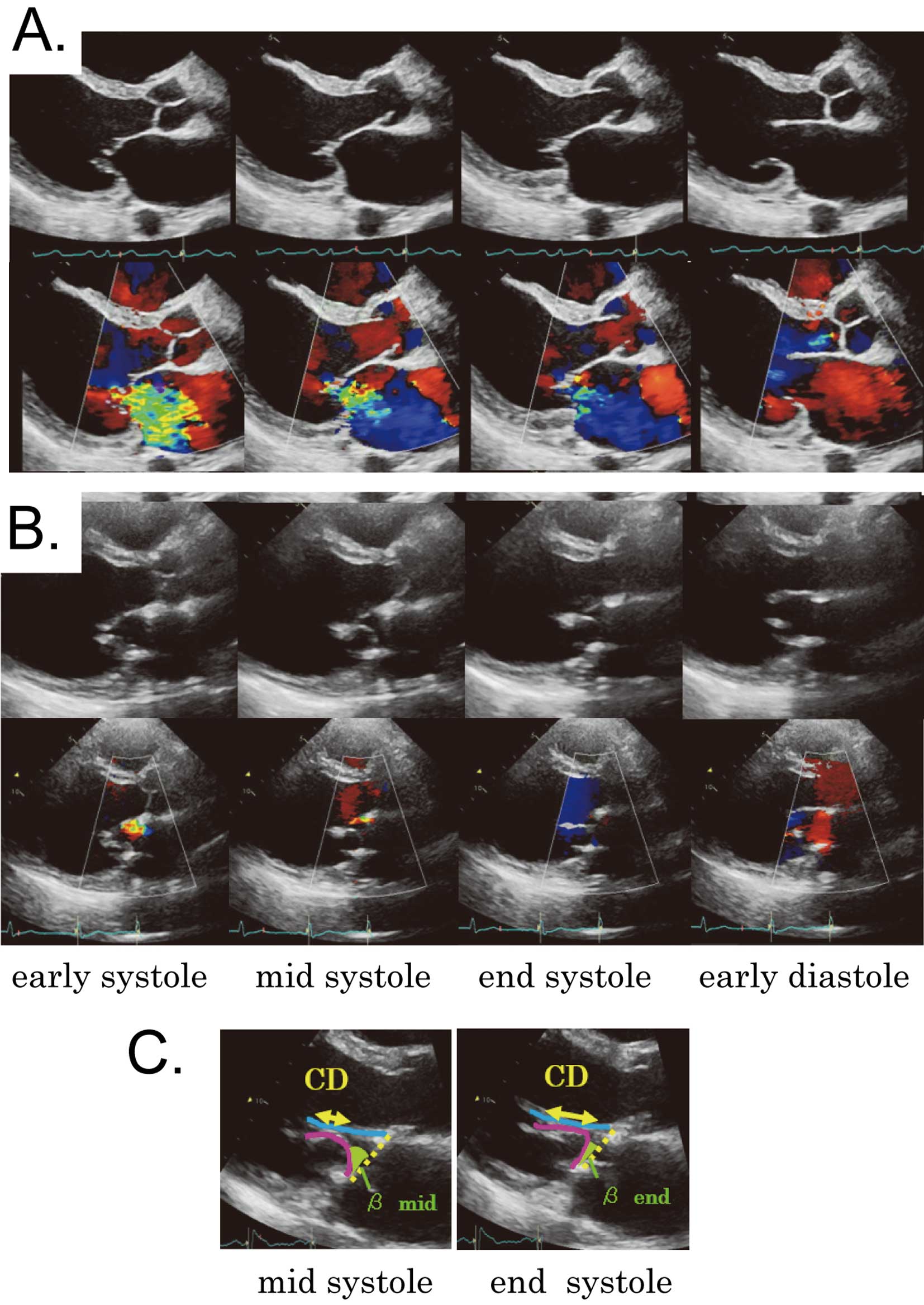
The “snuggling up” phenomenon of the posterior mitral leaflet after extended augmentation technique. The typical movement of the mitral valve and mitral regurgitation (MR) are shown in comparison with the movement of the preoperative configuration in each phase. (A) Before surgery, the anterior and posterior mitral leaflets were tethered by displaced papillary muscles and could not gain sufficient coaptation in all systolic phases. Consequently, MR occured and continued from the early systolic to the end systolic phases. (B) After surgery, the augmented posterior leaflet showed a unique movement within 1 cardiac cycle; it flexibly moved upward and contacted the anterior leaflet in the systolic phase, especially in the mid to end systolic phases. The movement of the augmented posterior leaflet created a deep and tightly uniform coaptation (i.e., “snuggling up”) with the anterior leaflet. Although trivial MR was observed in the early systolic phase, it gradually disappeared by the end systolic phase. (C) In the mid and end systolic phases, as the angle β decreased, the coaptation length increased.
The echocardiographic parameters used for evaluation of the risk of SAM are shown in Table 4. As described in Table 3, we excluded patients with LV plasty (n=11). Although AL, PL, and C-sept decreased in the early systolic phase after surgery, C-sept remained at >20 mm without changes in the AL/PL ratio, which indicated that the LVOT was wide open because of a typically large LV in the ischemic MR cases (Figure 2).
| Preoperative | Postoperative | P value | |
|---|---|---|---|
| AL (mm) | 23.6±4.3 | 16.6±3.2 | <0.001* |
| PL (mm) | 20. 3±4.4 | 15.4±3.1 | 0.0037* |
| AL/PL | 1.2±0.4 | 1.1±0.2 | 0.39 |
| C-sept (mm) | 29.5±5.8 | 25.1±5.2 | 0.0019* |
AL, anterior leaflet length to mitral valve contribution (annulus to coaptation); C-sept, distance from septum to mitral valve coaptation point; PL, posterior leaflet length to mitral valve contribution (annulus to coaptation).
Despite disappointing long-term results of restrictive annuloplasty in a recent randomized trial, the subgroup without recurrent MR showed significant LV reverse remodeling and better quality of life.4 Because we were not satisfied with our results for restrictive annuloplasty prior to this trial, we believed that there was a need for “mitral valve repair without late recurrence” to achieve better long-term survival. Worsening PML tethering and loss of sufficient CL were suggested as the main causes of recurrent MR.5,7 Furthermore, our unpublished data of restrictive annuloplasty patients showed that CL was a significant and independent risk factor of MR late recurrence by multivariate analysis. These data prompted us to introduce a leaflet augmentation technique for functional MR with severe leaflet tethering, instead of restrictive annuloplasty, as a procedure to gain sufficient coaptation. Anterior leaflet augmentation and limited PML augmentation performed much earlier had several acute or chronic MR recurrence, then we have developed an extended PML augmentation technique that detaches the PML from the posterior annulus almost completely (leaving a small communication with the annulus around the commissure), augments the PML with a large glutaraldehyde-treated autologous pericardial patch, and stabilizes the mitral annulus with a semi-rigid complete ring. This novel technique seemed to achieve a longer CL than with other techniques. We observed no 30-day mortality, which was obviously lower than the 5.3% mortality rate after restrictive annuloplasty reported by the Society of Thoracic Surgeons.17 Our mortality rate was even lower than the 1.6% of Goldstein et al,4 which reflected the expertise of experienced surgeons. Furthermore, no patients were converted to valve replacement. Although 2 patients died in hospital, no patients died or had recurrent moderate or severe MR during follow-up of 850±181 days (12% late mortality). Our rates of late death (0%) and minimal MR recurrence were much better than those of restrictive annuloplasty (58.8% and 19%, respectively, at 2 years).4 Furthermore, there were no heart failure readmissions during the follow-up period.
We used the same sized patch for all patients. The mean width of the adult mitral annulus is approximately 3 cm. If the mitral annulus is assumed to be a circle, the length of its circumference is approximately 9 cm. Because the PML occupies two-thirds of its circumference, the PML portion is approximately 6 cm. We detached the entire PML during our extended PML augmentation technique; therefore, we decided to use a patch with a width of 6 cm, which is usually sufficient when we suture the entire detached PML with an autologous patch. We initially used a 6×2-cm autologous patch. However, we experienced 1 case of MR recurrence after a few years in a patient who had severe MR with serious enlarged LV because of dilated cardiomyopathy. Since then, we have increased the height of the patch to 3 cm without any MR recurrence.
A previous study showed that functional MR dynamically changed within a cardiac cycle. Ischemic MR occurs because of an imbalance between the tethering force and the closing force,6 and the MR severity becomes minimal in mid-systole and maximal in early and late systole, reflecting a change in the closing force.5,18 The extended augmented PML technique resolved the imbalance with the substantial leaflet area. Although the chordae tendineae remained tethered without relocation of the papillary muscle, the augmented PML with its rich surface area seemed to obtain adequate closing force and was flexibly dragged upward and moved close to the AML in the mid to end systolic phase despite the increasing tethering force. Consequently, the PML and AML fitted tightly together to create a deep and uniform coaptation, which we described as “snuggling up”. Sufficient leaflet area enabled sufficient closing force to be obtained and improved the leaflet configuration in the systolic phase. After PML augmentation, the angle “β” formed by the annulus and PML, which represents the grade of PML tethering, became smaller and the CL became longer. The trivial MR observed in the early systolic phase disappeared by the end systolic phase. This is considered the most important and effective mechanism of the extended PML augmentation technique. We think that this mechanism, compared with that of restrictive annuloplasty in which PML tethering is known to be worsened, will help prevent residual and late recurrence of MR. In addition, it is also known that there are different tethering patterns according to the ischemic site: symmetric and asymmetric.19 Extended PML augmentation theoretically addresses any pattern of tethering with the enlarged posterior leaflet. We observed that this technique made the CL longer despite the residual tethering.
Another advantage of this technique is the low probability of SAM. We evaluated the risk of SAM in this study by C-sept. A large LV volume with insufficient LV contraction was observed in our typical ischemic MR patients preoperatively. Although a significant decrease in C-sept was observed after surgery, it was mainly caused by the reduction in the size of the LV and the upward change in the coaptation point caused by the “snuggling up” phenomenon. The angle “α”, formed by the annulus and AML, did not change in the systolic phase, and the AML did not move to the septum relatively. Consequently, the LVOT was kept wide open without increased LVOT flow velocities. The significant decrease in LV volume may have additional effects on the C-sept decrease. In any case, we did not observe MR because of SAM in our patients. We think that the risk of SAM in this technique was equivalent or even lower than that in other techniques of mitral valve repair.
Functional mitral stenosis has been another problem in restrictive annuloplasty.20,21 To assess this issue, we evaluated the mean transmitral pressure gradient. No patient showed a gradient >5.8 mmHg (3.8±1.2 mmHg, Table 3). Compared with restrictive annuloplasty, which may cause worsening PML tethering, especially in the enlarged LV,7 surgeons are able to choose larger size rings after extended PML augmentation. Augmented PML creates an adequate total surface area of the mitral leaflets and increases the zone that contributes to leaflet coaptation and the “snuggling up” phenomenon facilitated leaflet coaptation. Consequently, the augmented mitral valve did not require restriction by use of a small annular ring. In this study, we chose a larger ring (32±1.4 mm), and the mean transmitral pressure gradient between 2.1 and 5.8 mmHg was manageable by sufficient rate control. We think that choosing a larger size ring obviates the problem related to functional mitral stenosis.
Dal-Bianco et al showed that the ischemic milieu altered the mitral leaflet thicker histologically and the leaflet area enlarged to adapt to LV remodeling and tethering after myocardial infarction. However, if such changes were insufficient to prevent ischemic MR, considering the amount of LV remodeling, ischemic MR occured.22 Even from this perspective, we think that extended PML augmentation introduces a much larger PML area than the area in these self-adaptions and helps the mitral leaflet react against tethering and LV remodeling in the long-term, which we believe makes sense from the viewpoint of biological compensation.
Study LimitationsFirst, this was a single-center retrospective study, and the number of patients was small. It is obvious that we need to accumulate more patients. However, the echocardiographic changes after the described procedure were quite uniform. The angles to evaluate these uniform echocardiographic features reached statistical significance even with the small number of cases. Second, we performed concomitant procedures: CABG and LV repair. The effects of these procedures might improve mitral configuration. Third, we did not evaluate the preoperative viability of the myocardium. A procedure to assess the ischemic state might improve LV systolic function and lead to LV reverse remodeling by improving ischemia. We need to investigate the effects of residual ischemia in our future study. Finally, long-term follow-up is needed to determine whether this procedure leads to improved prognosis.
Extended PML augmentation for ischemic MR showed excellent early results with deep coaptation through a “snuggling up” phenomenon, which would help prevent long-term MR recurrence.
The authors report no relationships that could be construed as a conflict of interest.