Article ID: CJ-19-0511
Article ID: CJ-19-0511
Background: To clarify ventricular function in patients with asymptomatic coronary artery occlusion (ACAO) after Kawasaki disease (KD).
Methods and Results: We enrolled 65 patients with coronary artery lesions who had undergone cardiac magnetic resonance (CMR). Median age at CMR was 29 years. CMR was performed to evaluate only the transmural extent of late gadolinium enhancement (LGE) and ejection fraction (EF). Based on the depth of LGE, it was classified into 5 groups: 0% (G0), 1–25% (G1), 26–50% (G2), 51–75% (G3), and 76–100% (G4). We investigated the relationship of the degree of LGE and EF. Further, we also evaluated the EF among 3 groups [ACAO, myocardial infarction (MI), and noncoronary artery occlusion (Non-CO)]. The grade of LGE and the LVEF (mean±SD, %) were as follows: G0 (n=24, 52.6±4.8), G1 (n=13, 50.8±4.4), G2 (n=15, 49.1±5.6), G3 (n=9, 30.9±9.1), and G4 (n=9, 27.7±6.8). LVEF in patients with G3 and G4 was significantly low (P<0.05). LVEF (%) in patients with ACAO, MI, and Non-CO were 50.5±4.8 (n=38), 33.6±10.8 (n=17), and 53.0±5.7 (n=10), respectively. LVEF in the MI group was significantly low (P<0.0001).
Conclusions: LGE >50% can lead to LV dysfunction. The transmural extent of LGE in most of the study patients with ACAO was ≤50% and they had subendocardial infarction, with preserved LV function.
Kawasaki disease (KD) is an acute inflammatory syndrome of unknown origin in children that takes the form of systemic vasculitis. The acute inflammation and subsequent reparative process may lead to irreversible changes in the arterial structure.1 Although the administration of high-dose intravenous immunoglobulin during the acute phase of KD can reduce the occurrence of coronary artery lesions (CALs), destruction of arterial structures still continues, especially in nonresponders.2–5 Among the patients with CALs, some often have occlusions of the coronary arteries in the late period.6 The occurrence of acute myocardial infarction (AMI) can greatly affect the outcome of patients with CALs, because of left ventricular (LV) dysfunction and ventricular arrhythmias.7 In addition, as a characteristic of CALs caused by KD, either coronary artery occlusion that is unknown at the time of the complete occlusion or segmental stenosis (SS) suggestive of recanalization after a coronary artery occlusion exists.6–9 The cardiac function in patients with asymptomatic coronary artery occlusion (ACAO), including SS, have never been reported.
The value of cardiac magnetic resonance imaging (CMR) in the management of MI and heart failure is established for ventricular functional assessment and prognostication.10–13 Cardiac function is measured quantitatively by CMR. Inversion-recovery CMR after administration of an extracellular contrast agent allows direct visualization of the transmural extent of scars, because of the high spatial resolution and high contrast between infarcted and noninfarcted myocardium.14 Thus, the transmural extent of necrotic myocardium on late gadolinium enhancement (LGE) CMR can be assessed.15–19
We have found that approximately two-thirds of KD patients with coronary artery occlusions are asymptomatic.6 With the view of following these patients, it is important to clarify the difference in cardiac function between patients with ACAO and those with MI. We thought LGE might be useful in distinguishing LV dysfunction related to KD patients with CALs. Therefore, we investigated the morphologic changes in the myocardial wall and global ventricular function by CMR, using a LGE technique and quantification tool, in patients with CALs. Clarifying the cardiac condition of KD patients with ACAO will be useful for prognosis prediction and therapeutic intervention.
We enrolled consecutively 65 KD patients with CALs who had undergone CMR between April 2012 and June 2017. The retrospective study was approved by the Ethics Committee of the National Cerebral and Cardiovascular Center (M30-114) and performed in accordance with the Declaration of Helsinki. In this study, ACAO defined coronary artery occlusions or SS that were accidentally found on selective coronary angiography (CAG) or computed tomographic angiography (CTA) (Figure 1). All patients gave informed consent for CMR, CAG and CTA. MI was defined from the medical records as the occurrence of both symptoms during an attack and ECG changes after the attack. Patients’ information was also investigated from their records.

Coronary angiograms with in patients with asymptomatic coronary artery occlusion (ACAO). (Left) Right coronary artery (RCA) angiogram (Right) Left coronary angiogram. The 33-year-old female patient had acute Kawasaki disease at 9 months old. There is segmental stenosis of the RCA and asymptomatic occlusion of the left anterior descending artery (LAD). Collateral arteries from the RCA to the LAD are well developed. LVEDVI and LVEF were 87.1 mL/m2 and 53.5%, respectively. RVEDVI and RVEF were 76.3 mL/m2 and 49.9%, respectively. The transmural extent of LGE was <50% (Grade 2).
We evaluated only the transmural extent of LGE in the patients with CALs. Based on the depth of the LGE, it was classified into 5 grades: 0% (G0), 1–25% (G1), 26–50% (G2), 51–75% (G3), and 76–100% (G4) (Figure 2). We analyzed the relationship between the degree of LGE and ventricular function (end-diastolic volume index [EDVI], end-systolic volume index [ESVI], and ejection fraction [EF]) of the right and left ventricles (RV, LV). Further, the patients were classified into 3 groups based on native coronary artery occlusions on CAG or CTA that had been performed close to the time of the CMR examination. The 1st group had symptomatic MI, and the 2nd group ACAO, and the 3rd group were patients without complete coronary artery occlusion (Non-CO). In this study, SS that was accidentally found on coronary angiograms was also included in the ACAO group. We also investigated ventricular function among these 3 groups.

Transmural extent of late gadolinium enhancement (LGE) is shown by the yellow area. The grade of LGE is based on the transmural extent of hyperenhancement.
CMR was performed with a 1.5-Tesla unit (Sonata Magnetom; Siemens, Erlangen, Germany), and cardiac function was analyzed by an experienced observer who was blinded to patient information. Children younger than 10 years were given sodium thiopental sodium 3–5 mg/kg by intravenous infusion. Short-axis LGE images were obtained 10 min after the administration of 0.15 mmol/kg contrast agent with a segmented inversion-recovery steady-state free precession (SSFP) sequence. The range of short-axis images covered the entire length of both ventricles. All images were combined with ECG gating. In each short-axis cine image, the endocardial and epicardial borders of the ventricle were semi-automatically delineated at the end-diastolic volume and end-systolic phases to obtain the RV and LV function, with manual correction by the experienced radiologist. Parameters for LGE imaging were as follows: TR 3.5 ms, TE 1.7 ms, flip angle 60°, matrix 256×129, field of view 340 cm, section thickness 8 mm, gap between sections 2 mm. We obtained good images with the use of a single shot and increased time resolution in patients with a high heart rate.
We evaluated the transmural extent of LGE-CMR, and measured the EDVI (mL/m2), ESVI (mL/m2), and EF (%) in the RV and LV using a dedicated workstation (Ziostation2, Ziosoft Inc., Tokyo, Japan). The infarct mass was quantified using the Ziostation2 with a 6-SD threshold method on phase-sensitive inversion-recovery gradient echo imaging (Figure 2). The delayed enhancement area was overlaid in yellow for pixels with a signal intensity higher than the threshold value of 6-SD above the mean.12,20,21 Based on the overlaid pixels, the infarct area was automatically calculated. The regional transmural extent of the enhanced regions was calculated using the Ziostation2.
Statistical AnalysisMeasurements are presented as the mean±1SD. The statistical significance was determined by χ2-test, or one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons as a post-hoc analysis for the ANOVA. A P-value <0.05 was considered statistically significant. Graphs were drawn using GraphPad Prism version 6.04 software (GraphPad Software, Inc., La Jolla, CA, USA).
There were 49 male and 16 female patients (Table 1). Age at the time of CMR ranged from 5 to 47 years old, and the mean age was 27±10 years old. The interval from the onset of acute KD to the CMR examination ranged from 2 to 44 years, with a mean of 25±11 years. In the study group, 17 patients had had a MI and 5 of them had had a recurrent MI. The interval from the onset of acute KD to the time of the initial MI was 14 days to 9 years, with a median of 33±10 months. Creatine kinase concentration ranged from 1,895 to 8,415 U/L, with a median of 2,912 U/L. The interval from the initial MI to CMR was 7 months to 35 years, with a median of 6 years. A total of 32 patients (49%) had undergone coronary artery bypass grafting (CABG). The interval from the onset of acute KD to operation ranged from 51 days to 29 years, with a median of 15 years. The interval from the time of operation to CMR ranged from 7 months to 35 years, with a median of 15 years. The diagnosis of CALs in each patient was by selective CAG or CTA, and the median time between CMR and those examinations was 2 years. None of the patients had had cardiac events between CMR and the evaluation of CALs by CAG or CTA.
| KD patients with coronary artery lesions, n | 65 |
|---|---|
| Male sex, n (%) | 49 (75) |
| Previous myocardial infarction, n (%) | 17 (26) |
| Coronary artery bypass grafting, n (%) | 32 (49) |
| CMR examination | Median±SD (range) |
| Age at CMR (years) | 27±10 (5–47) |
| Age at the onset of KD (months) | 33±30 (2–120) |
| Interval from onset of KD to CMR (years) | 25±11 (2–44) |
| Height (cm) | 163±14 (112–180) |
| Weight (kg) | 58±17 (17–100) |
| Body surface area (mm2) | 1.6±0.3 (0.75–2.21) |
| Heart rate at CMR (beats/min) | 72±12 (47–95) |
CMR, cardiac magnetic resonance imaging; KD, Kawasaki disease.
The number of patients with ACAO was 38 (Table 2), of MI was 17 and of Non-CO patients was 10. The diagnosis of CALs in the Non-CO group and the number of native coronary artery occlusions are shown in Table 2. In the ACAO group, 17 patients had 1 vessel occlusion, 18 had 2 vessel occlusions, and 3 had 3 vessel occlusions. In the MI group, 7 patients had 1 vessel occlusion and 10 had 2 vessel occlusions. The number of patients after CABG in the ACAO group and MI group were 22 (58%) and 10 (59%), respectively.
| Non-CO group | ACAO group | MI group | |||
|---|---|---|---|---|---|
| No. of ACAO | No. of symptomatic CAO | ||||
| RCA LS | 3 (1) | RCA | 11 (4*) | RCA | 3 |
| LAD LS | 4 | LAD | 6 (2) | LAD | 3 |
| LCA AN | 1 | RCA LAD | 17 (14) | LCX | 1 |
| RCA AN LCA AN | 2 | RCA LCX | 1 (1) | LAD LCX | 2 (2) |
| RCA LAD LCX | 1 | RCA** LAD | 5 (5) | ||
| RCA LMT | 2 (2) | RCA LAD | 2 (2) | ||
| RCA LCX | 1 (1) | ||||
| Total | 10 | Total | 38 (22) | Total | 17 (10) |
No. of patients who had undergone CABG is shown in parentheses. *Four patients had CABG for LS of the LAD. **Five patients had ACAO of the RCA and symptomatic CAO of the LAD. ACAO, asymptomatic coronary artery occlusion; AN, aneurysm; LAD, left anterior descending [artery]; LCX, left circumflex [artery]; LMT, left main trunk; LS, localized stenosis; RCA, right coronary artery.
The LGE grades were as follows: G0 (n=24), G1 (n=13), G2 (n=15), G3 (n=9), and G4 (n=4). The grades in the ACAO group (n=38) were: G0 (n=14, 37.0%), G1 (n=12, 31.5%), G2 (n=11, 28.9%), and G3 (n=1, 2.6%); and in the MI group (n=17): G1 (n=1, 5.8%), G2 (n=4, 23.5%), G3 (n=8, 47.2%), and G4 (n=4, 23.5%). All patients in the Non-CO group had Grade 0. Regarding the grade of LGE, there was a significant difference between the ACAO and MI groups (P<0.0001) (Figure 3).

Grade of late gadolinium enhancement (LGE) in the ACAO and MI groups, showing a significant difference between groups. ACAO, asymptomatic coronary artery occlusion; MI, myocardial infarction. P<0.0001.
The measurements of RVEDVI (mL/m2), RVESVI (mL/m2), and RVEF (%) based on the LGE grade were as follows: RVEDVI, G0 86.8±21.4, G1 74.0±12.0, G2 85.4±16.9, G3 81.4±15.7, and G4 81.8±5.8; RVESVI, G0 46.7±10.9, G1 40.0±7.0, G2 45.1±10.4, G3 45.2±12.3, and G4 40.4±6.3; RVEF, G0 45.8±4.5, G1 45.8±4.6, G2 47.2±4.1, G3 44.9±6.9, and G4 50.7±4.4. There were no significant differences among the 5 groups regarding RV function (Table 3). In total, 4 patients had RVEF <40%, 3 had ACAO of the right coronary artery (RCA), and 1 had MI caused by occlusion of the RCA.
| LGE | P value | |||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| n | 24 | 13 | 15 | 9 | 4 | |
| Age (years) | 22.7±10.3 | 31.3±8.4 | 32.1±10.0 | 27.6±7.5 | 24.3±14.7 | NS |
| HR (beats/min) | 75±12 | 70±9 | 70±14 | 73±14 | 72±14 | NS |
| CI (L/min/m2) | 3.0±0.6 | 2.4±0.4 | 3.2±0.7 | 2.6±0.6 | 3.5±0.8 | NS |
| Right ventricle | ||||||
| EDVI (mL/m2) | 86.8±21.4 | 74.0±12.0 | 85.4±16.9 | 81.4±15.7 | 81.8±5.8 | NS |
| ESVI (mL/m2) | 46.7±10.9 | 40.0±7.0 | 45.1±10.4 | 45.2±12.3 | 40.4±6.3 | NS |
| EF (%) | 45.8±4.5 | 45.8±4.6 | 47.2±4.1 | 44.9±6.9 | 50.7±4.4 | NS |
| Left ventricle | ||||||
| EDVI (mL/m2) | 81.2±20.0 | 68.9±11.9 | 95.4±16.3 | 127.3±42.8 | 162.3±37.1 | <0.05 |
| ESVI (mL/m2) | 38.6±11.2 | 33.9±6.6 | 48.6±11.1 | 90.5±40.3 | 119.0±35.4 | <0.05 |
| EF (%) | 52.6±4.8 | 50.8±4.4 | 49.2±5.6 | 30.9±9.1 | 27.7±6.8 | <0.05 |
CI, cardiac index; EDVI, end-diastolic volume index; ESVI, end-systolic volume index; EF, ejection fraction; HR, heart rate; LGE, late gadolinium enhancement.
The measurements of LVEDVI (mL/m2), LVESVI (mL/m2), and LVEF (%) based on the LGE grade were as follows: LVEDVI, G0 81.2±20.0, G1 68.9±11.9, G2 95.4±16.3, G3 127.3±42.8, and G4 162.3±37.1; LVESVI, G0 38.6±11.2, G1 33.9±6.7, G2 48.6±11.1, G3 90.5±40.3, and G4 119.0±35.4; LVEF, G0 52.6±4.8, G1 50.8±4.4, G2 49.1±5.6, G3 30.9±9.1, and G4 27.7±6.8. The LVEDVI and LVESV in the G3 and G4 groups were significantly larger than in the other groups (P<0.05) (Figure 4, Table 3). The LVEDVI in the G3 group was significantly larger than that in the G2 group (P<0.05). LVEF in the G3 and G4 groups was significantly lower than in the other groups (P<0.05). In the patients with LGE >50% of the myocardial wall, LV function was significantly decreased.

Grade of late gadolinium enhancement (LGE) and left ventricular (LV) function. The median and standard error in each group are shown. (Left) Grade of LGE and the LVEDVI. The LVEDVI in the G3 and G4 groups was significantly larger than in the other groups (P<0.05). The LVEDVI in the G3 group was significantly larger than in the G2 group (P<0.05). (Right) Grade of LGE and the LVEF. The LVEF in the G3 and G4 groups was significantly lower than in the other groups (P<0.05).
The measurements of RVEDVI (mL/m2), RVESVI (mL/m2), and RVEF (%) based on CALs were as follows: RVEDVI, Non-CO 85.2±17.4, ACAO 81.0±19.6, and MI 85.7±13.0; RVESVI, Non-CO 45.4±10.0, ACAO 42.9±10.5, and MI 45.8±10.0; RVEF, Non-CO 46.6±4.8, ACAO 46.1±4.3, and MI 46.7±6.2. There were no significant differences among the 3 groups regarding RV function.
The measurements of LVEDVI (mL/m2), LVESVI (mL/m2), and LVEF (%) based on CALs were as follows: LVEDVI, Non-CO 81.0±22.3, ACAO 80.9±18.7, and MI 121.7±41.8; LVESVI, Non-CO 36.9±11.4, ACAO 40.3±11.5, and MI 87.4±41.2; LVEF, Non-CO 54.4±4.4, ACAO 50.4±5.1, and MI 34.7±11.6. The LVEDVI and LVESVI in the MI group were significantly larger than in the other groups (P<0.0001). The LVEF in the MI group was significantly lower than that in the other groups (P<0.0001). In the post-MI patients, LV function was significantly decreased. On the other hand, LV function in patients with ACAO was preserved (Figure 5).
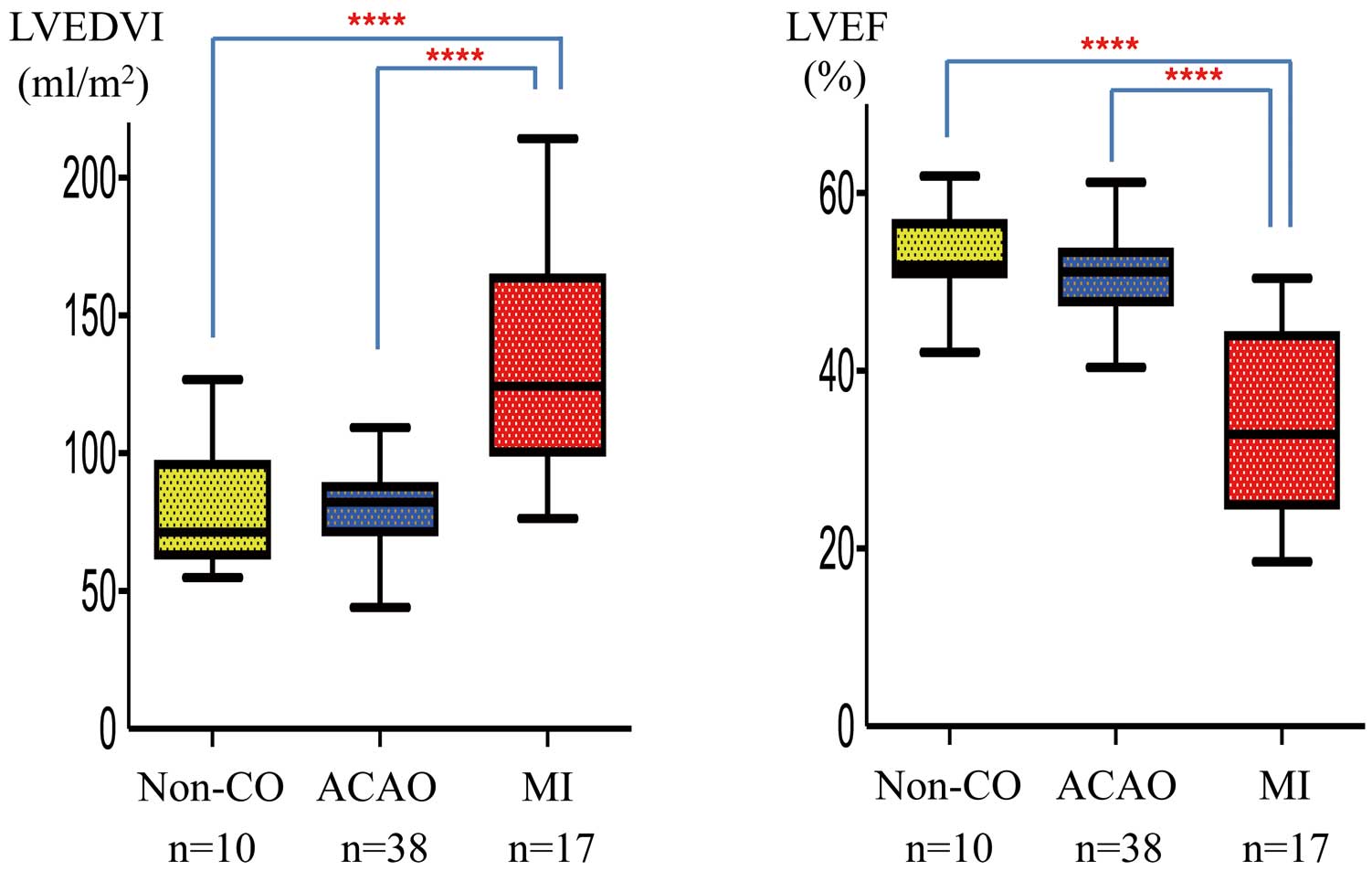
LVEDVI and LVEF in 3 groups These 3 groups based on native coronary artery occlusions found on CAG or CTA performed close to the time of CMR. The median and standard error in each group are shown. The LVEDVI in the MI group was significantly larger than in the other groups (P<0.0001). LVEF in the MI group was significantly lower than in the other groups (P<0.0001). ACAO, asymptomatic coronary artery occlusion; CAG, coronary angiography; CMR, cardiac magnetic resonance imaging; CTA, computed tomographic angiography; MI, myocardial infarction; Non-CO, noncoronary artery occlusion. ****P<0.0001.
Recently, LGE on CMR imaging has been used to distinguish between reversible and irreversible myocardial ischemic injury, and reversible myocardial dysfunction after coronary revascularization.22 The usefulness of LGE compared with other modalities is being able to evaluate the depth of scar in the wall of the LV. It is reported that infarcted segments with a transmural extent of LGE >75% show no significant change after revascularization. Thus, by assessing the transmural extent of necrotic myocardium by LGE, the recovery of dysfunctional segments after LV dysfunction can be predicted.23 The depth of LGE is related to the viability of the myocardial wall. Further, our study showed that the depth of LGE was related to global LV dysfunction.
Necrotic myocardium after AMI is replaced with fibrosis or scar tissue in the late period. The size of the infarct affects regional wall motion and global LV function. Regional fibrosis can be detected by CMR using LGE. A previous study described that infarct size as assessed with biomarkers after AMI with ST-elevation correlates strongly with LGE. Serum creatine kinase was elevated in the patients of the MI group, and those patients had a transmural extent of LGE >50%. On the other hand, most of the patients in the ACAO group had a transmural extent of LGE ≤50%. The difference between the MI and ACAO groups was the depth of the LGE. It is interesting that the appearance of symptoms was related to transmural impairment of the myocardial wall. Consequently, global LV function was preserved in most of the patients with ACAO, who had subendocardial infarction. It is considered that global LV function greatly depends on the outer wall rather than the inner wall.
LGE indicates non-viable, irreversibly damaged myocardium as a hyperenhanced or ‘bright’ area that closely correlates to the histopathologic findings.9 Therefore, it is suspected that the location, extent, and depth of LGE greatly relates to ventricular function. Recently, CMR has become the gold standard for quantification of biventricular global function. Our study showed the relation between the transmural grade of LGE and biventricular function. The existence of LGE >50% in the LV myocardial wall lead to an increased LVEDV and decreased LVEF. We considered that adverse remodeling of the LV after MI had occurred. We have reported that the survival rate after MI depended on the LVEF after acute MI.7 However, LGE ≤50% in the LV myocardial wall did not always impair global LV function, outcomes in patients with ACAO may be not as bad. On the other hand, the existence of LGE in the LV did not always affect RV function. Further, our data showed that global RV function in patients with CALs caused by KD was usually preserved. Although this finding was also interesting, the cause is unknown.
SS, which develops in new small vessels in large aneurysms, is a characteristic of CALs caused by KD.6 It is thought that recanalization occurs after a thrombotic occlusion of major coronary arteries. The cause of SS is suspected to be related to thrombosis and thrombolysis, and it often occurs within 2 years after the onset of KD. The prevalence of SS in the RCA is significantly higher than in the left coronary artery.9 Most patients with SS had no history of MI, and they were almost asymptomatic. It is possible that either mild symptoms or signs had not been always detected in the children. The degree of myocardial damage in them was unknown before. Our study showed that the extent of transmural damage was ≤50% in most of the patients with ACAO and that global LV function was preserved. Patients with ACAO have often developed collateral vessels from other coronary arteries. Further, ACAO including SS are likely to occur in the right rather than the left coronary artery.24 There was also a difference in the extent of the area that each coronary artery supplied, which might be one of the reasons why there was a decrease in LV impairment in most of the patients with ACAO.
Study LimitationsWe did not evaluate the CALs by CMR, because of the limited time for the CMR examination.25 It would be better to evaluate CALs and ventricular function by CMR at the same time. We did not analyze the location or extent of the area of LGE and those findings and regional wall motion, in addition to the results of this study, should be investigated to understand in detail the cause of LV dysfunction in this population.
The existence of LGE >50% in the myocardium suggests adverse remodeling of the LV. Although LGE was found in more than half of the study patients with ACAO, the transmural extent of LGE was ≤50% in most of them and their global LV function was preserved. Global RV function in the patients with CALs caused by KD was usually preserved.
None.
This retrospective study was approved by the Ethics Committee of the National Cerebral and Cardiovascular Center and performed in accordance with the Declaration of Helsinki.
H.N. collected the data and wrote the manuscript. E.T. had a role in the design of this retrospective study. Y.M. analyzed the CMR data. K.K. supervised this study.
The authors declare that they have no competing interests.