Article ID: CJ-19-0593
Article ID: CJ-19-0593
Background: Ventricular septal defect (VSD) after myocardial infarction (MI) is a rare but fatal complication. We report patients’ characteristics and operative outcomes after surgical repair of post-MI VSD using a national database of Japan.
Methods and Results: This was a retrospective review of the Japan Adult Cardiovascular Surgery Database (JCVSD) to identify adults (age ≥18 years) who underwent surgical repair of post-MI VSD between 2008 and 2014. The primary outcome was operative death. We identified 1,397 patients (671 male [48%], 74.1±9.3 years old) undergoing surgical repair of post-MI VSD among 288,736 patients undergoing cardiac surgery enrolled in the JCVSD during the same period. Of these, 1,075 (77.0%) were supported preoperatively with an intra-aortic balloon pump. Surgical status was urgent in 391 (28.0%) and emergency/salvage in 731 (52.3%). Concomitant coronary artery bypass grafting was performed in 475 (34.0%). Overall 30-day and operative mortalities were 24.3% and 33.0%, respectively. Operative mortality varied according to surgical status: 15.6% in elective, 30.9% in urgent, and 40.6% in emergency/salvage cases. Multivariable analysis identified advanced age and emergency/salvage status as being strongly associated with increased odds of operative death.
Conclusions: Post-MI VSD remains a devastating complication in Japan as well as in the USA and Europe.
Ventricular septal defect (VSD) is a rare but lethal complication of acute myocardial infarction (MI).1 For patients treated medically, the mortality of post-MI VSD exceeds 90%.2 With the development of acute reperfusion strategies for MI, the incidence of post-MI VSD occurs in less than 1% of patients sustaining MI.1,2 Despite significant improvements over the past 2 decades in overall mortality for patients with acute MI, the outcome of patients who develop post-MI VSD remains poor. Even for patients who are treated surgically, mortality has been high, ranging between 19% and 60% in patients.1–7 However, most of these reports involved single-center experience with small sample sizes. To improve the outcomes, further study from a larger multicenter experience may be necessary.
Recently, several studies with large sample sizes from national databases have been reported from Europe and the USA. A single national registry report from Sweden reported 189 patients treated during a 7-year span.8 National registry data have been provided by the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) to examine risk factors for outcomes after surgical repair of post-MI VSD.9 In Japan, the Japan Adult Cardiovascular Surgery Database (JCVSD) was established in 2000.10 Thus, in the present study, we report a Japanese multicenter experience of surgical repair of post-MI VSD using the JCVSD.
The JCVSD includes 255 variables that are nearly identical to those in the database of the STS. Informed consent to register clinical data in the JCVSD was obtained from each patient. The JCVSD Organization Review Board approved the present study.
From the JCVSD, we identified adults (age ≥18 years) undergoing surgical repair of a post-MI VSD from January 2008 to December 2014. Eligible patients were identified by an affirmative response in the JCVSD data field for MI. Patients who underwent concomitant procedures such as coronary revascularization or valvular surgeries were included; however, patients with congenital heart disease were excluded.
An elective operation is one that is performed on a patient with cardiac function that has been stable in the days or weeks prior to operation. An urgent operation is one that is required within 24 h in order to minimize the chance of further clinical deterioration. Patients requiring emergency operations will have ongoing, refractory, unrelenting, cardiac compromise, with or without hemodynamic instability, and will not be responsive to any form of therapy except cardiac surgery. Salvage patients undergo CPR en route to the operating room or during induction of anesthesia.
OutcomesThe primary outcome was operative mortality, defined as death from any cause in-hospital or within 30 days of the index operation. Additional complications included length of ICU stay, reexploration for bleeding, and postoperative complications such as pneumonia.
Study DesignClinically relevant variables and previously reported predictors of operative death after post-MI VSD surgical repair according to the STS database were included as candidate variables in the full model of this analysis.9,11 Cardiogenic shock is defined as previously described.9 Anatomic site of VSD (anterior or posterior) was not contained in the JCVSD.
Statistical AnalysisSummary statistics for outcomes and baseline patient characteristics are presented as percentages for categorical variables and means with standard deviation for continuous variables. The Pearson χ2 test was used to compare categorical variables, and the Kruskal-Wallis test was used for continuous variables. Multivariable logistic regression modeling was used to estimate the risk of operative death as a function of patient baseline variables. The following variables were then included in the model predicting operative death after coronary artery bypass grafting (CABG), as detailed in an earlier STS report:9,11 age, sex, ejection fraction, body mass index, estimated glomerular filtration rate (eGFR), hemodialysis, diabetes mellitus, previous stroke, atrial fibrillation, NYHA class, immunosuppression therapy, previous cardiac surgery, previous percutaneous coronary intervention (PCI), left main disease, 3-vessel coronary artery disease (CAD), aortic stenosis, aortic regurgitation, mitral regurgitation, tricuspid regurgitation, chronic lung disease, peripheral artery disease, preoperative intra-aortic balloon pump (IABP), shock, surgical status (elective, urgent, emergency/salvage), and concomitant CABG. Additionally, to adjust time trends, the timing of the operation (categorized as every 2 or 3 years) was also included in the model. All statistical analyses were performed using SPSS, version 24.0 (SPSS, Chicago, IL, USA) and STATA, version 15 (STATA Corp., TX, USA).
We identified 1,397 patients (671 male [48%], 74.1±9.3 years old) undergoing surgical repair of post-MI VSD among 288,736 patients undergoing cardiac surgery enrolled in the JCVSD during the same period (0.48% of all cardiac surgeries).
Survivor vs. Non-SurvivorsThe 30-day and operative mortalities were 24.3% and 33.0%, respectively. A comparison of clinical characteristics between survivors and non-survivors (operative death) is presented in Table 1. Survivors tended to be younger and had higher eGFRs. Non-survivors had a higher prevalence of smoking history, hyperlipidemia, and chronic obstructive pulmonary disease.
| Total n=1,397 |
Survivors n=936 (67.0%) |
Non-survivors n=461 (33.0%) |
P value | |
|---|---|---|---|---|
| Age (years) | 74.1±9.3 | 72.9±9.5 | 76.7±8.4 | <0.01 |
| Male sex | 671 (48.0%) | 466 (49.8%) | 205 (44.5%) | 0.06 |
| Body mass index (kg/m2) | 22.8±3.7 | 22.8±3.6 | 23.0±4.1 | 0.36 |
| >30 | 52 (3.7%) | 31 (3.3%) | 21 (4.6%) | 0.25 |
| Current smoker | 304 (21.8%) | 217 (23.2%) | 87 (18.9%) | 0.07 |
| Diabetes mellitus | 481 (34.4%) | 316 (33.8%) | 165 (35.8%) | 0.45 |
| Undergoing therapy | 313 (22.4%) | 207 (22.1%) | 106 (23.0%) | 0.71 |
| eGFR (mL/min/1.73 m2) | 38.6±21.1 | 41.1±21.2 | 33.4±20.1 | <0.01 |
| <30 | 546 (39.1%) | 314 (33.5%) | 232 (50.3%) | <0.01 |
| Hemodialysis | 23 (1.6%) | 13 (1.4%) | 10 (2.2%) | 0.28 |
| Hyperlipidemia | 468 (33.5%) | 342 (36.5%) | 126 (27.3%) | <0.01 |
| Hypertension | 921 (65.9%) | 625 (66.8%) | 296 (64.2%) | 0.34 |
| Previous stroke | 136 (9.7%) | 89 (9.5%) | 47 (10.2%) | 0.68 |
| Chronic lung disease | 144 (10.3%) | 79 (8.4%) | 65 (14.1%) | <0.01 |
| Peripheral vascular disease | 54 (3.9%) | 34 (3.6%) | 20 (4.3%) | 0.52 |
| Neurological disorder | 129 (9.2%) | 52 (5.6%) | 77 (16.7%) | <0.01 |
| Previous cardiac surgery | 111 (7.9%) | 73 (7.8%) | 38 (8.2%) | 0.77 |
| CABG | 36 (2.6%) | 21 (2.2%) | 15 (3.3%) | 0.26 |
| Valve surgery | 12 (0.9%) | 4 (0.4%) | 8 (1.7%) | 0.01 |
| Thoracic aortic surgery | 61 (4.4%) | 36 (3.8%) | 25 (5.4%) | 0.18 |
| Previous PCI | 508 (36.4%) | 330 (35.3%) | 178 (38.6%) | 0.22 |
| MI <1 week | 646 (46.2%) | 399 (42.6%) | 247 (53.6%) | <0.01 |
| Shock | 859 (61.5%) | 495 (52.9%) | 364 (79.0%) | <0.01 |
| Cardiopulmonary resuscitation | 85 (6.1%) | 33 (3.5%) | 52 (11.3%) | <0.01 |
| Atrial fibrillation | 130 (9.3%) | 94 (10.0%) | 36 (7.8%) | 0.18 |
| Preoperative inotropic agents | 343 (24.6%) | 200 (21.4%) | 143 (31.0%) | <0.01 |
| Aortic stenosis | 30 (2.1%) | 22 (2.4%) | 8 (1.7%) | 0.46 |
| Surgical status | ||||
| Urgent | 391 (28.0%) | 270 (28.8%) | 121 (26.2%) | 0.31 |
| Emergency/salvage | 731 (52.3%) | 434 (46.4%) | 297 (64.4%) | <0.01 |
| NYHA class 4 | 987 (70.7%) | 619 (66.1%) | 368 (79.8%) | <0.01 |
| 3-vessel disease | 229 (16.4%) | 126 (13.5%) | 103 (22.3%) | <0.01 |
| Left main disease | 74 (5.3%) | 43 (4.6%) | 31 (6.7%) | 0.10 |
| Ejection fraction <3.0% | 248 (17.8%) | 128 (13.7%) | 120 (26.0%) | <0.01 |
| Aortic regurgitation >2 | 63 (4.5%) | 46 (4.9%) | 17 (3.7%) | 0.30 |
| Mitral regurgitation >2 | 190 (13.6%) | 123 (13.1%) | 67 (14.5%) | 0.48 |
| Tricuspid regurgitation >2 | 442 (31.6%) | 300 (32.1%) | 142 (30.8%) | 0.64 |
| Immunosuppression therapy | 41 (2.9%) | 26 (2.8%) | 15 (3.3%) | 0.62 |
| Preoperative IABP | 1,075 (77.0%) | 683 (73.0%) | 392 (85.0%) | <0.01 |
eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; MI, myocardial infarction; PCI, percutaneous coronary intervention; VSD, ventricular septal defect.
Operative characteristics and postoperative outcomes are listed in Table 2. Non-survivors were associated with longer cardiopulmonary bypass and aortic cross-clamp time. The rate of having concomitant CABG did not differ between groups. Non-survivors had a higher prevalence of additional treatment such as reexploration for bleeding and renal replacement therapy. Non-survivors were also associated with serious postoperative complications such as pneumonia, septicemia, heart block, and gastrointestinal complications.
| Total n=1,397 |
Survivors n=936 (67.0%) |
Non-survivors n=461 (33.0%) |
P value | |
|---|---|---|---|---|
| CPB time (min) | 198±81 | 183±65 | 228±98 | <0.01 |
| Aortic cross-clamp time (min) | 124±49 | 119±45 | 134±55 | <0.01 |
| Concomitant CABG | 475 (34.0%) | 317 (33.9%) | 158 (34.3%) | 0.88 |
| Concomitant mitral valve surgery | 49 (3.5%) | 20 (2.1%) | 29 (6.3%) | <0.01 |
| Pre- or intra-operative IABP | 1,200 (85.9%) | 769 (82.2%) | 431 (93.5%) | <0.01 |
| Pre- or intra-operative PCPS | 224 (16.0%) | 69 (7.4%) | 155 (33.6%) | <0.01 |
| ICU stay (days) | 15.2±17.4 | 18.0±21.5 | 13.8±14.9 | <0.01 |
| Reexploration for bleeding | 112 (8.0%) | 50 (5.3%) | 62 (13.4%) | <0.01 |
| Cerebrovascular accident | 64 (4.6%) | 37 (4.0%) | 27 (5.9%) | 0.11 |
| Renal replacement therapy | 295 (21.1%) | 91 (9.7%) | 204 (44.3%) | <0.01 |
| Heart block | 48 (3.4%) | 20 (2.1%) | 28 (6.1%) | <0.01 |
| Gastrointestinal | 105 (7.5%) | 49 (5.2%) | 56 (12.1%) | <0.01 |
| Atrial fibrillation | 334 (23.9%) | 208 (22.2%) | 126 (27.3%) | 0.04 |
| Septicemia | 104 (7.4%) | 34 (3.6%) | 70 (15.2%) | <0.01 |
| Pneumonia | 207 (14.8%) | 98 (10.5%) | 109 (23.6%) | <0.01 |
CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass time; IABP, intra-aortic balloon pump; ICU, intensive care unit; PCPS, percutaneous cardiopulmonary support.
Several clinical variables differed according to surgical status (Table 3). Those patients whose operations were considered to be emergency or salvage cases had worse renal function and were more likely to be male. Preoperative IABP was inserted in approximately 50% of elective patients and in more than 80% of urgent and emergency/salvage patients. Operative mortalities for elective, urgent, and emergent/salvage patients were 15.6%, 30.9%, and 40.6%, respectively (Table 4, P<0.01).
| Elective n=275 (19.7%) |
Urgent n=391 (28.0%) |
Emergency/salvage n=731 (52.3%) |
P value | |
|---|---|---|---|---|
| Age (years) | 73.3±8.7 | 74.3±9.8 | 74.4±9.3 | 0.22 |
| Male sex | 131 (47.6%) | 160 (40.9%) | 380 (52.0%) | <0.01 |
| Body mass index (kg/m2) | 22.7±3.6 | 23.1±3.8 | 22.8±3.8 | 0.24 |
| >30 | 9 (3.3%) | 20 (5.1%) | 23 (3.1%) | 0.23 |
| Current smoker | 54 (19.6%) | 80 (20.5%) | 170 (23.3%) | 0.35 |
| Diabetes mellitus | 104 (37.8%) | 131 (33.5%) | 246 (33.7%) | 0.42 |
| Undergoing therapy | 67 (24.4%) | 86 (22.0%) | 160 (21.9%) | 0.69 |
| eGFR (mL/min/1.73 m2) | 43.6±19.7 | 39.5±21.6 | 36.2±21 | <0.01 |
| <30 | 71 (25.8%) | 155 (39.6%) | 320 (43.8%) | <0.01 |
| Hemodialysis | 6 (2.2%) | 10 (2.6%) | 7 (1.0%) | 0.10 |
| Hyperlipidemia | 99 (36.0%) | 138 (35.3%) | 231 (31.6%) | 0.28 |
| Hypertension | 188 (68.4%) | 263 (67.3%) | 470 (64.3%) | 0.39 |
| Previous stroke | 25 (9.1%) | 36 (9.2%) | 75 (10.3%) | 0.79 |
| Chronic lung disease | 30 (10.9%) | 45 (11.5%) | 69 (9.4%) | 0.58 |
| Peripheral vascular disease | 11 (4.0%) | 12 (3.1%) | 31 (4.2%) | 0.69 |
| Neurological disorder | 10 (3.6%) | 20 (5.1%) | 99 (13.5%) | <0.01 |
| Previous cardiac surgery | 46 (16.7%) | 34 (8.7%) | 31 (4.2%) | <0.01 |
| CABG | 11 (4.0%) | 10 (2.6%) | 15 (2.1%) | 0.22 |
| Valve surgery | 4 (1.5%) | 5 (1.3%) | 3 (0.4%) | 0.16 |
| Thoracic aortic | 12 (4.4%) | 14 (3.6%) | 35 (4.8%) | 0.64 |
| Previous PCI | 99 (36.0%) | 150 (38.4%) | 259 (35.4%) | 0.62 |
| MI <1 week | 60 (21.8%) | 183 (46.8%) | 403 (55.1%) | <0.01 |
| Shock | 74 (26.9%) | 211 (54.0%) | 564 (77.2%) | <0.01 |
| Cardiopulmonary resuscitation | 5 (1.8%) | 11 (2.8%) | 69 (9.4%) | <0.01 |
| Atrial fibrillation | 36 (13.1%) | 34 (8.7%) | 60 (8.2%) | 0.53 |
| Preoperative inotropic agents | 45 (16.4%) | 116 (29.7%) | 182 (24.9%) | <0.01 |
| Aortic stenosis | 9 (3.3%) | 8 (2.0%) | 13 (1.8%) | 0.34 |
| Surgical status | ||||
| Urgent | 0 (0.0%) | 391 (100.0%) | 0 (0.0%) | <0.01 |
| Emergency | 0 (0.0%) | 0 (0.0%) | 731 (100.0%) | <0.01 |
| NYHA class 4 | 139 (50.5%) | 275 (70.3%) | 573 (78.4%) | <0.01 |
| 3-vessel disease | 50 (18.2%) | 69 (17.6%) | 110 (15.0%) | 0.36 |
| Left main disease | 12 (4.4%) | 20 (5.1%) | 42 (5.7%) | 0.67 |
| Ejection fraction <30% | 35 (12.7%) | 57 (14.6%) | 156 (21.3%) | <0.01 |
| Aortic regurgitation >2 | 16 (5.8%) | 20 (5.1%) | 27 (3.7%) | 0.28 |
| Mitral regurgitation >2 | 57 (20.7%) | 58 (14.8%) | 75 (10.3%) | <0.01 |
| Tricuspid regurgitation >2 | 103 (37.5%) | 137 (35.0%) | 202 (27.6%) | <0.01 |
| Immunosuppression therapy | 7 (2.5%) | 12 (3.1%) | 22 (3.0%) | 0.91 |
| Preoperative IABP | 140 (50.9%) | 324 (82.9%) | 611 (83.6%) | <0.01 |
Abbrvations as in Table 1.
| Elective n=275 (19.7%) |
Urgent n=391 (28.0%) |
Emergency/salvage n=731 (52.3%) |
P value | |
|---|---|---|---|---|
| CPB time (min) | 187±78 | 199±80 | 201±82 | 0.06 |
| Aortic cross-clamp time (min) | 123±51 | 123±46 | 125±50 | 0.74 |
| Concomitant CABG | 98 (35.6%) | 139 (35.5%) | 238 (32.6%) | 0.49 |
| Concomitant mitral valve surgery | 12 (4.4%) | 19 (4.9%) | 18 (2.5%) | 0.08 |
| Pre- or intra-operative IABP | 172 (62.5%) | 356 (91.0%) | 672 (91.9%) | <0.01 |
| Pre- or intra-operative PCPS | 13 (4.7%) | 47 (12.0%) | 164 (22.4%) | <0.01 |
| ICU stay (days) | 11.1±16.4 | 16.2±16.0 | 16.2±18.3 | <0.01 |
| 30-day death | 28 (10.2%) | 86 (22.0%) | 225 (30.8%) | <0.01 |
| Operative death | 43 (15.6%) | 121 (30.9%) | 297 (40.6%) | <0.01 |
| Reexploration for bleeding | 11 (4.0%) | 20 (5.1%) | 81 (11.1%) | <0.01 |
| Cerebrovascular accident | 11 (4.0%) | 16 (4.1%) | 37 (5.1%) | 0.67 |
| Renal replacement therapy | 21 (7.6%) | 93 (23.8%) | 181 (24.8%) | <0.01 |
| Heart block | 8 (2.9%) | 15 (3.8%) | 25 (3.4%) | 0.81 |
| Gastrointestinal | 14 (5.1%) | 41 (10.5%) | 50 (6.8%) | 0.02 |
| Atrial fibrillation | 50 (18.2%) | 98 (25.1%) | 186 (25.4%) | 0.05 |
| Septicemia | 9 (3.3%) | 36 (9.2%) | 59 (8.1%) | 0.01 |
| Pneumonia | 30 (10.9%) | 57 (14.6%) | 120 (16.4%) | 0.09 |
Abbrvations as in Table 2.
Advanced age was a strong predictor for operative death (Figure). Besides age, the factor with the highest magnitude of effect was emergency/salvage cases compared with elective ones. Ejection fraction ≤30%, renal failure, preoperative shock, and 3-vessel disease were also independently associated with greater odds of operative death. However, concomitant CABG procedure was not associated with operative death.
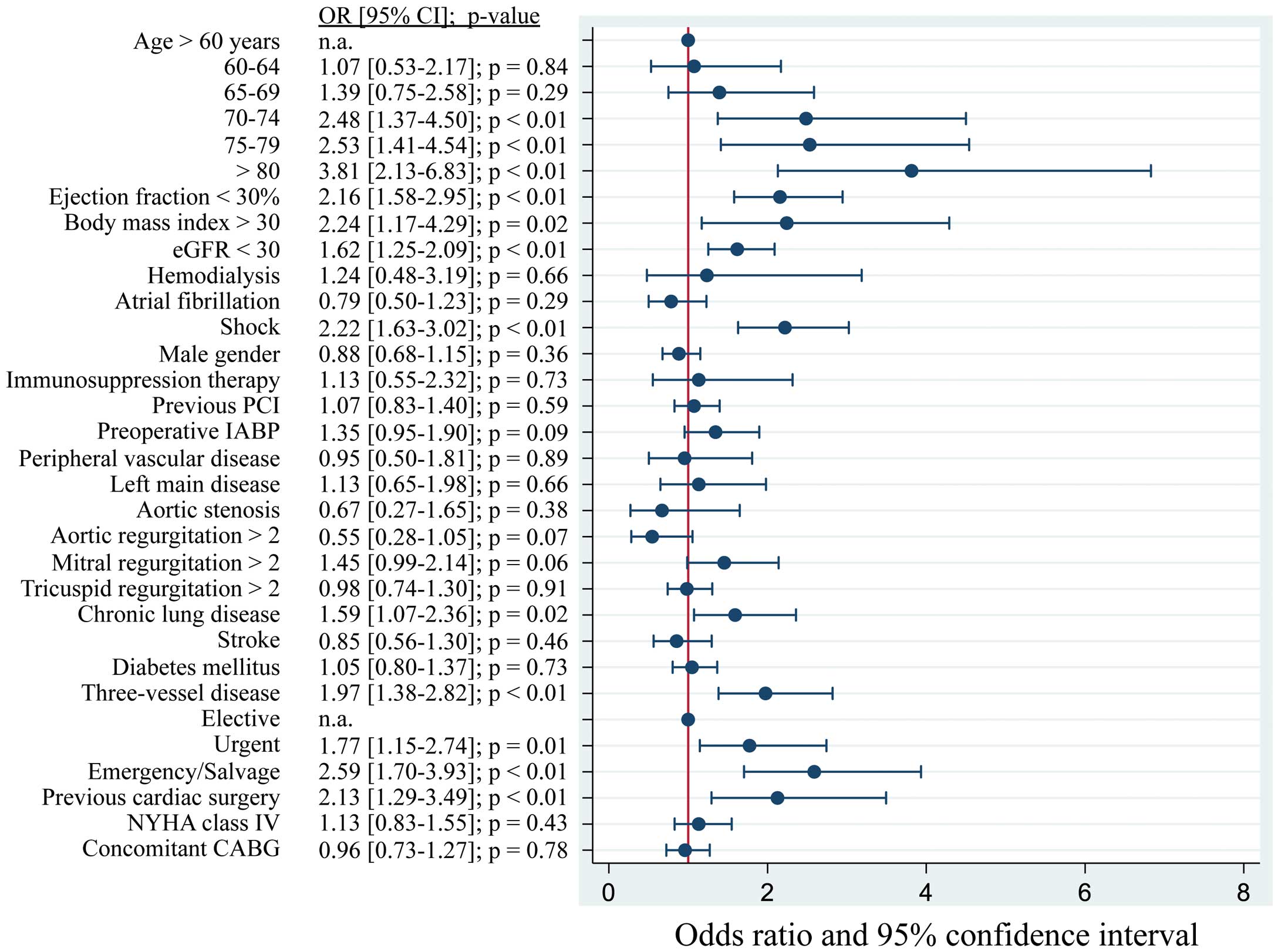
Forest plot of odds ratios for operative death after post-MI VSD repair. CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump; MI, myocardial infarction; PCI, percutaneous coronary intervention; VSD, ventricular septal defect.
The present study investigated in-hospital outcomes using data from the JCVSD in 1,397 patients undergoing surgical repair of post-MI VSD from 2008 to 2014. Most of the previous studies regarding outcome after surgical repair of post-MI VSD are single-center experiences with small numbers of patients collected over a long period of time. The advantages of the present study are that it is a multicenter experience that includes all patients operated upon in Japan during a relatively short period of time (7 years), and, to the best of our knowledge, the second largest study on in-hospital outcomes after surgical repair of post-MI VSD.
Surgical repair of post-MI VSD shows the highest mortality of all cardiac surgeries. The Global Utilization of Streptokinase and T-PA for Occluded Coronary Arteries (GUSTO) trial2 reported 84 patients who developed post-MI VSD out of 41,021 patients (0.2%) treated for MI; 34 patients with post-MI VSD selected for surgical repair had better outcomes than 35 patients treated medically (30-day mortality, 47% vs. 94%). In 2005, a national registry from Sweden reported short- and long-term outcomes in 189 patients undergoing surgical repair of post-MI VSD in 10 centers throughout Sweden during a 7-year period, with a 30-day mortality rate of 41%.8 The significance of that study is that it reported long-term survival following VSD repair. Total cumulative survival was 38% at 5 years. For patients who survived the first 30 days (n=112), the 5-year cumulative survival was 67%. In 2012, national registry data provided by the STS-ACSD examined risk factors for in-hospital outcomes after surgical repair of post-MI VSD in 2,876 patients from 1999 to 2010, with an operative mortality of 42.9% in the cohort overall, which represented the highest risk of all cardiac procedures recorded in the STS-ACSD.9 There was a nonlinear time trend with respect to operative mortality over the course of the study.
Operative mortality in the present study was 33.0%, which appears to be comparable to that in other recent reports on the subject (19–52%).1–9 Variations in patient selection, definitions of early mortality (30-day vs. hospital mortality), and whether or not patients operated upon long after diagnosis were excluded makes direct comparisons less meaningful. However, mortality in both ours and the other multicenter studies appears to be somewhat higher than that reported in single-center studies.8,9 One may suspect that centers reporting their own results have better than average outcomes because of superior patient selection and/or more refined surgical techniques.
We compared patients and operative characteristics between the STS data and that in the present study.9 Compared with the STS-ACSD, our registry included older patients (68±9 vs. 74±9 years old), fewer males (56.5% vs. 48.0%), and lower body mass index (27.8±5.3 vs. 22.8±3.7 kg/m2). Patients in the STS-ACSD had a higher incidence of previous cardiac surgery (12.2% vs. 7.9%) and a lower incidence of previous PCI (33.0% vs. 36.4%). Incidences of preoperative insertion of IABP and cardiogenic shock were lower in the STS-ACSD (65.0% vs. 77.0% and 51.7% vs. 61.5%, respectively). The STS-ACSD included many more patients with 3-vessel disease (33.6% vs. 16.4%). Concomitant CABG and mitral valve procedures were much more common in the STS-ACSD (63.9% vs. 34.0% and 7.3% vs. 3.5%, respectively). In the present study, a high operative mortality of 33.0% was also observed in the overall population. However, this mortality is lower than in the STS-ACSD (42.9%) and the Swedish registry (30-day mortality; 41%). The reason is unclear, but differences in patient selection, race, comorbidities, and ratio of inoperable patients might be associated with the different mortality rates.
Surgery early after diagnosis is a consistent risk factor for early mortality in the literature.7,12,13 In the present study, emergency/salvage status was the strong risk factor for operative death compared with elective status. Similarly, the mortality of patients in the STS database varied significantly depending on the timing of surgery.9 When examining operative mortality by surgical status, 13.2% patients undergoing elective operations died, compared with 56.0% of emergency and 80.5% of salvage patients. The better outcome with delayed surgery may be associated with healing and stabilization of the infarcted myocardium, which allows for a more effective repair. However, the fast deterioration of many patients with post-MI VSD often makes it impossible to delay surgery. Thus, the clinician must weigh the known risk of expedient surgery against the unknown risk of postponing surgery and developing further clinical deterioration. Based on these findings and current cardiology guidelines,14 surgical treatment should be initiated without delay after diagnosis, despite the high risk.
It is controversial whether concomitant CABG improves outcome after post-infarction-VSD repair. Most studies have shown no benefit of concomitant CABG on early-4–6,8,12,15–18 or long-term5,12,15–17 mortality, although CABG has been reported to reduce midterm survival in 30-day survivors.8 Other studies are associated with better early19 and later4,6,18,19 survival, which indicates that concomitant CABG can control the added risk of CAD. It can be argued that revascularization of transmurally infarcted myocardium is illogical because the culprit artery is often entrapped in the suture line of the ventriculotomy, which can make it impossible to bypass.3,19,20 Regarding operative outcomes, the STS-ACSD showed that concomitant CABG did not reduce the risk of operative death (odds ratio, 0.87; 95% confidence interval, 0.70–1.08; P=0.2).9 Similarly, in the present study, concomitant CABG was not associated with operative death. The Swedish national registry showed that concomitant CABG tended to result in lower early mortality (38 vs. 46%, P=0.29), while midterm survival (after exclusion of patients who died within the first 30 days) was significantly lower in patients undergoing CABG (P=0.03).8 However, when all postoperative deaths were included in the comparison, midterm mortality did not differ between patients undergoing concomitant CABG and those who did not (P=0.70). Furthermore, the number of anastomoses was an independent predictor of late mortality, with a risk ratio of 1.5 for each additional anastomosis. It is likely that the number of anastomoses reflects the extent of CAD at the time of repair.
Study LimitationsThere are several important limitations to this study. First, and most importantly, the observational study design precluded definitive conclusions regarding the timing of surgery and the significance of concomitant CABG after surgical repair of post-MI VSD because of selection bias and unmeasured potential confounders. Multivariate analysis may not adequately adjust for these biases. Second, the number of patients enrolled may still be considered small despite the data coming from a national large registry because preoperative conditions were quite different among the patients with post-MI VSD. Third, the national multicenter design necessitated a simplified data collection form with a limited number of variables to avoid missing data. Thus, the possibility that non-registered variables could have influenced the results of the multivariate analyses cannot be completely ruled out. For example, the database has no information on the interval between MI and VSD diagnosis, on the interval between VSD recognition and operative intervention, or on the location of VSD. Furthermore, large registry databases such as the JCVSD depend heavily on accurate coding. Fourth, lack of mortality data among patients who died while awaiting an operation is an added limitation. Finally, because this study is limited by the operative outcomes, it does not provide information on the durability of surgical repair of post-MI VSD.
In conclusion, this analysis of post-MI VSD repair using a Japanese national database showed that post-MI VSD remains a devastating complication. This study may be important because these results can serve as a benchmark for other therapeutic modalities.
The authors have no conflicts of interest to disclose.