Article ID: CJ-19-0935
Article ID: CJ-19-0935
Intravascular optical coherence tomography (OCT) is a light-based imaging modality that enables detailed visualization of the intraluminal structures in coronary arteries. High-resolution (10–20 μm) cross-sectional images are generated by measuring the echo time delay and intensity of light that is reflected or backscattered from the internal structures in tissues.1,2
Article p ????
OCT is clinically useful during percutaneous coronary intervention (PCI) to assess lesion severity, optimize stent implantation, and characterize atherosclerotic plaques, including the identification of thin-cap fibroatheroma (TCFA) and lipid-rich plaques, which are reportedly responsible for periprocedural complications. OCT can also be used to assess the mechanism for stent thrombosis or restenosis.3,4
High-resolution imaging with OCT allows tissue characterization; however, penetration depth (the depth of a tissue at which OCT image data can be acquired) is considered a major limitation of OCT. As light passes through tissues, it is attenuated by scattering and/or absorption, which determines the penetration depth as well as the contrast and brightness of the OCT image.1,2 Penetration depth in OCT limits the ability to assess vessel size measured by the area of the external elastic lamina (EEL: the layer between the media and adventitia) and plaque burden. On the other hand, attenuation itself is one of the main factors in interpreting tissue characterization. Typically, fibrous tissue, calcium tissue, and white thrombus are characterized by low attenuation whereas lipidic tissue and red thrombus are identified by high-attenuation characteristics.
To overcome the invisibility of deeper tissue structures by attenuation of the OCT signal, attenuation-compensated OCT (AC-OCT) has been recently introduced.5 This technique corrects light attenuation and enhances the contrast and penetration depth of the conventional OCT (C-OCT) signal. Notably, this technique does not require additional data acquisition and can be retrospectively applied to existing C-OCT images. Teo et al reported a histological validation study of AC-OCT that showed improvement of the detection of plaque characteristics and the EEL contour compared with C-OCT.6
Further, in this issue of the Journal, Ramasamy et al7 demonstrate for the first time the efficacy and reproducibility of AC-OCT in a large set of images acquired in vivo. AC-OCT was superior to C-OCT for the detection of EEL and calcium in the native segment because it can improve penetration depth. Meanwhile, AC-OCT traded off tissue characterization, especially that of lipid plaque, and micro-characteristics such as the presence of macrophages and the thickness of the fibrous cap wherein signal attenuation itself plays an important role in detection. Moreover, AC-OCT was unsuitable for neointimal tissue characterization because of increased stent artifacts.
AC-OCT can be beneficial in the following aspect: better delineation of the EEL compared with C-OCT (Figure A) can help determine stent size and the necessity of post-dilatation for achieving optimal stent expansion.8,9 Enabling measurement of plaque burden allows us to observe plaque volume changes, and plaque progression or regression, which are almost impossible to be evaluated by C-OCT. Moreover, improved detection of calcium can be useful to identify calcified lesions that would benefit from plaque modification using atherectomy devices prior to stent expansion.10 Specifically, calcium thickness, one of the determinants of calcium fracture after balloon dilation and consequently better stent expansion,11 can be more visible with AC-OCT than with C-OCT. On C-OCT images, complete delineation of calcium is sometimes difficult, especially at the outer border, presumably because plaque content is not always homogeneous (Figure B), although calcium is usually described as a signal-poor or heterogeneous region with a sharply delineated border.1 An ex-vivo study showed that calcification with vague or invisible outer borders by OCT had lipid contents more frequently than those with a clear outer border.12 In return, assessment of lesion composition, especially of plaque vulnerability such as TCFA and macrophages, can be overlooked or misinterpreted by AC-OCT. TCFAs in culprit lesions have been reported as one of the independent predictors of periprocedural myocardial injury during PCI.13 In addition, the presence of lipid plaque in non-culprit lesions was reported to be associated with lesion progression14 or increased risk for future events.15
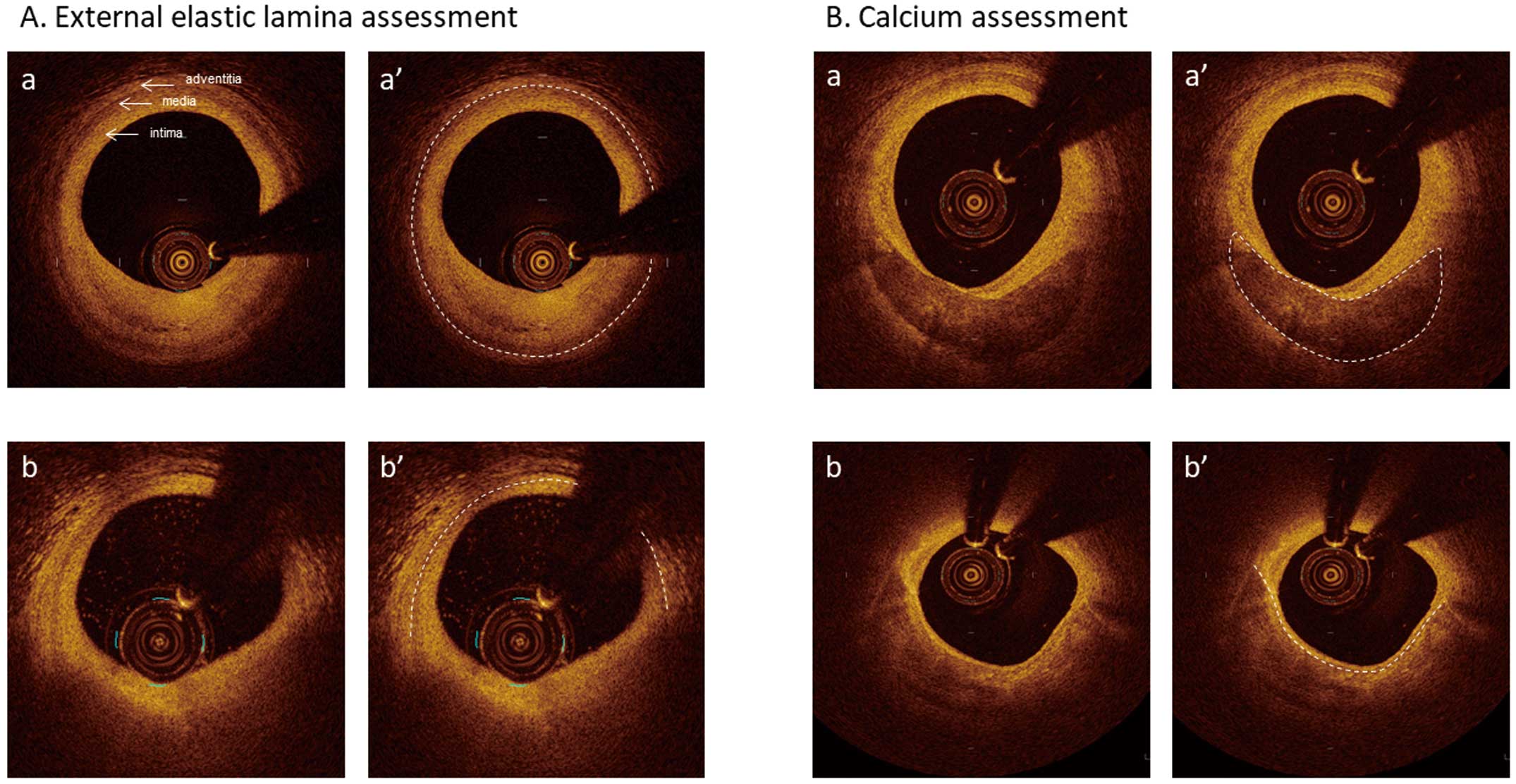
Conventional optical coherence tomography images. (A-a) 3-layer appearance. The external elastic lamina (EEL) border, the line between the media and adventitia, is clearly identifiable (dotted line in A-a’). (A-b) EEL border (dotted line in A-b’) is not identifiable at 1 o’clock position because of guide wire artifact and from 3 o’clock to 9 o’clock because of attenuation by inner lipidic tissue. (B-a) Calcium delineation, including the outer border, is clear (dotted line in B-a’). (B-b) Although the calcium inner border is visible (dotted line in B-b’), its outer border is vague.
Intravascular OCT imaging plays an important role in the research setting, as well as daily clinical practice. However, at the current stage of technological development in OCT, a trade-off between improving penetration depth and tissue characterization remains. Combining the advantages of C-OCT and AC-OCT (ideally, simultaneous visualization during PCI at the same console) might provide new insights of coronary plaque assessment. Yet to maximize the clinical utility of OCT, it is essential to clarify the purpose of OCT examination and identify the most relevant information that OCT can provide.
Dr. Shinke has served as a member of advisory boards of Abbott Vascular. There are no other potential conflicts to disclose that are associated with this manuscript.