Abbreviations
| 2D |
two-dimensional |
| 3D |
three-dimensional |
| ACC |
American College of Cardiology |
| AF |
atrial fibrillation |
| AHA |
American Heart Association |
| AR |
aortic regurgitation |
| AS |
aortic stenosis |
| AVA |
aortic valve area |
| BMI |
body mass index |
| BNP |
brain natriuretic peptide |
| BSA |
body surface area |
| CABG |
coronary artery bypass grafting |
| CO |
cardiac output (mL/min) |
| CQ |
clinical question |
| CT |
computed tomography |
| DOAC |
direct oral anticoagulant |
| EACTS |
European Association of Cardiology and Thoracic Surgery |
| EOA |
effective [valve] orifice area |
| EROA |
effective regurgitant orifice area |
| ESC |
European Society of Cardiology |
| FAC |
fractional area change |
| FED |
fibroelastic deficiency |
| HF |
heart failure |
| HR |
heart rate (beats/min) |
| JCS |
Japanese Circulation Society |
| LA |
left atrium/left atrial |
| LV |
left ventricle/left ventricular |
| LVEDD |
LV end-diastolic diameter |
| LVEF |
left ventricular ejection fraction |
| LVESD |
LV endsystolic dimension |
| mPG |
mean pressure gradient |
| MELD |
Model for End-stage Liver Disease |
| MR |
mitral regurgitation |
| MRI |
magnetic resonance imaging |
| MS |
mitral stenosis |
| MVA |
mitral valve area |
| NYHA |
New York Heart Association |
| OMC |
open mitral commissurotomy |
| PASP |
pulmonary artery systolic pressure |
| PCI |
percutaneous coronary intervention |
| PISA |
proximal isovelocity surface area |
| PPM |
patient–prosthesis mismatch |
| PR |
pulmonary regurgitation |
| PS |
pulmonary stenosis |
| PT-INR |
international normalized ratio of prothrombin time |
| PTMC |
percutaneous transseptal mitral commissurotomy |
| RA |
right atrium/right atrial |
| RCT |
randomized controlled trial |
| RV |
right ventricle/right ventricular |
| SAM |
systolic anterior motion of the mitral leaflet |
| SAVR |
surgical aortic valve replacement |
| SVD |
structural valve deterioration |
| TAPSE |
tricuspid annular plane systolic excursion |
| TAVI |
transcatheter aortic valve implantation |
| TEE |
transesophageal echocardiography |
| TR |
tricuspid regurgitation |
| TS |
tricuspid stenosis |
| TTE |
transthoracic echocardiography |
| VHD |
valvular heart disease |
I. Introduction
1. Preface to the Revision
The “Guidelines on the Management of Valvular Heart Disease” were initially published as the “Guidelines on Non-pharmacological Treatment of Valvular Heart Disease” in 2002, revised in 2007, and again partially revised in 2012. However, advancements in the diagnosis and treatment of VHD have been rapid. The ACC/AHA guidelines were revised in 2014, with some updates in 2017, and the ESC/EACTS guidelines were revised in 2012 and again in 2017.
TAVI was introduced in October 2013, and in April 2018 transcatheter treatment was introduced for MR. Evidence from these new transcatheter treatments has accumulated. In July 2018, valve-in-valve implantation became covered by insurance for valve dysfunction after bioprosthetic valve replacement. Regarding surgical treatment, progress has been remarkable over the past few years, including the spread of minimally invasive surgery, valve repair, or valve-sparing surgery for patients with AR, as have changes in the selection of prosthetic valves owing to improvements in prosthetic valve technology. In addition, new insights have been gained into the pathophysiology and prognosis of VHD, including low-flow/low-gradient severe AS, MR associated with AF, and isolated functional TR. Treatment for HF is essential for VHD with LV dysfunction, and many advances have also been made in the treatment of HF.
Thus, the increased range of treatments for VHD makes it necessary to determine the indications and optimal timing of interventions for each patient. At present, we are in the era of team-based medicine, and the importance of the “Heart Valve Team” is emphasized.
There are very few randomized trials on the treatment of VHD, and there is a lack of high-quality evidence regarding the surgical indications and timing. The accumulation of evidence from Japan is limited, but based on the insights and clinical experience gained from previous observational studies, the present guidelines have been developed to reflect actual, current clinical practices.
New treatment techniques for VHD are expected to be introduced in the future, and a large amount of evidence is expected to emerge. The present guidelines should be understood to include the most recent data, as well as conventional evidence, to continue to develop the practice guidelines currently available.
This English version is a translated, abbreviated form of the Japanese version; the sections were selected based on their importance, with some parts omitted from the English version.
2. About the Recommendations
The Medical Information Network Distribution Service (Minds), which is operated by the Japan Council for Quality Health Care, has provided different levels of recommendation and evidence. However, we have applied the classes of recommendation and levels of evidence followed in conventional guidelines and are similar to those used in the present ACC/AHA and ESC guidelines (Tables 1,2), because emphasis is placed on widespread use of conventional recommendations and evidence levels within the field of cardiovascular disease, and these easily align with international guidelines.
Table 1.
Class of Recommendation
| Class I |
There is evidence and/or general agreement that a given procedure or treatment is effective and/or useful |
| Class II |
There is no consistent evidence and/or general agreement that a given procedure or treatment is
effective and/or useful |
| Class IIa |
Weight of evidence and opinion is in favor of effectiveness and/or usefulness |
| Class IIb |
Effectiveness or usefulness is not fully established by evidence or opinion |
| Class III |
There is evidence and/or general agreement that a given procedure or treatment is not effective and/or
useful or may be harmful |
Table 2.
Level of Evidence
| Level A |
Demonstrated with multiple randomized controlled studies or meta-analyses |
| Level B |
Demonstrated with a single randomized intervention clinical study or non-randomized, non-intervention
study |
| Level C |
Only consensus opinion of experts and/or small-scale clinical studies (including retrospective studies and
registration) |
Some have recommended the use of more scientific methods, such as systematic review, to determine recommendation guidelines.1
The present guidelines cover 5 CQs and comprise a systematic review. For each CQ systematic review, all evidence was assessed to form recommendations in accordance with the methods outlined in Minds 2014.1
■ Strength of Recommendation
1: Strongly recommended
2: Weakly recommended (proposed)
■ Strength of Overall Evidence
A (strong): Strong confidence in the estimated effect
B (moderate): Moderate confidence in the estimated effect
C (weak): Limited confidence in the estimated effect
D (very weak): Very little confidence in the estimated effect
Table 3
lists the 5 CQs and recommendations with the systematic review.
Table 3.
Clinical Questions Covered in the Present Guidelines
| |
Evaluation by systematic review |
Strength of
recommendation |
Strength of
overall evidence |
| CQ 1 |
Should early surgery be recommended for asymptomatic patients with severe primary MR with an LVEF >60% and an LVESD
<40 mm who do not have new onset AF or pulmonary hypertension? |
Early surgery is reasonable when a successful and durable repair surgery can be safely
performed. |
2 |
C |
| CQ 2 |
Should early surgery be conducted for asymptomatic patients with severe AR with an LVEF ≥50% and an LVESD index >25 mm/m2? |
Early surgery may be considered for asymptomatic patients with severe AR with an LVEF
≥50% and an LVESD index >25 mm/m2. |
2 |
C |
| CQ 3 |
Should early operation be conducted for asymptomatic patients with very severe AS and preserved LVEF? |
If the transvalvular peak velocity is ≥5.0 m/s, the mean transvalvular pressure gradient is ≥
60 mmHg, or the AVA is ≤0.6 cm2, operation is recommended for asymptomatic patients
at low surgical risk. |
2 |
B |
| CQ 4 |
Should concomitant tricuspid valve repair be conducted for patients with mild TR and tricuspid annular dilation (>40 mm or 21 mm/m2
in diameter)? |
Concomitant tricuspid valve repair may be considered for patients with mild TR and tricuspid
annular dilation (>40 mm or 21 mm/m2 in diameter). |
2 |
C |
| CQ 5 |
Can direct oral anticoaglants (DOACs) be used in patients with AF after bioprosthetic valve replacement? |
DOACs can be used in patients with AF and bioprosthesis after the 3rd postoperative
month. |
2 |
C |
AF, atrial fibrillation; AS, aortic stenosis; AVA, aortic valve area; LVEF, left ventricular ejection fraction; LVESD, LV end-systolic dimension; MR, mitral regurgitation; TR, tricuspid regurgitation.
II. General Comments
1. Symptoms, Physical Findings, and Blood Tests
1.1 Medical History
The therapeutic strategies for VHD are determined by combining clinical evaluation and quantitative assessment. It is essential to take a detailed medical history, because the presence or absence of symptoms greatly affects the therapeutic strategy. In general, the clinical course of chronic VHD is gradual, so patients may not be aware of symptoms because they subconsciously avoid activities that cause the symptoms. Such a trend is seen most commonly in the elderly, and thus, needs to be paid careful attention. Therefore, rather than simply assessing a patient’s subjective symptoms, questioning regarding signs similar or related to those being reported by the patient may help detect the slow progression of symptoms in daily life. If a patient has a history of HF and is now asymptomatic with medication, the patient should be considered as symptomatic. Documenting the history of complications and treatment is also crucial in differentiating symptoms and determining treatment strategies.
1.2 Physical Findings
The physical findings need to be detailed in order to diagnose VHD and assess its severity.2
When a heart murmur is heard, the phase, strongest point, strength, pitch and other characteristics, and the effects of respiration should be assessed. Although each VHD has its characteristic auscultatory findings, the phase of the murmur should be examined most precisely. In a patient with HF, the intensity of a heart murmur may be low, even in severe VHD. In addition, inspection and palpation are useful for evaluating VHD. Inspection and analysis of the jugular vein, which are useful in the evaluation of HF, are important. Evaluation of the location and characteristics of the apex beat and the characteristics of the carotid pulse by palpation are useful for the diagnosis of VHD.
1.3 Biomarkers
It has been reported that serum levels of BNP are useful for both assessing the severity of VHD and predicting the prognosis.3,4
The level may also be useful in determining therapeutic interventions, especially for patients with asymptomatic VHD.5–8
However, the optimal cutoff value for determining the therapeutic strategy in each VHD has not been clarified.8
2. Echocardiography (Tables 4,5)
Table 4.
Recommendations of Echocardiographic Evaluations
| Recommendations |
COR |
LOE |
| TTE is indicated in the initial evaluation of patients with known or suspected VHD |
I |
B |
TTE is indicated in patients with known VHD with any change in symptoms or physical
examination findings |
I |
C |
| Periodic monitoring with TTE is indicated in asymptomatic patients with known VHD |
I |
C |
| TEE is recommended in suboptimal evaluation with TTE or if further examinations are required |
I |
B |
COR, Class of Recommendation; LOE, Level of Evidence; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VHD, valvular heart disease.
Table 5.
Frequency of Echocardiographic Examinations in Asymptomatic Patients With Valvular Heart Disease
| Stage |
Valve lesion |
| Aortic stenosis |
Aortic regurgitation |
Mitral stenosis |
Mitral regurgitation |
| Mild |
Every 3–5 years |
Every 3–5 years |
Every 3–5 years |
Every 3–5 years |
| Moderate |
Every 1–2 years |
Every 1–2 years |
Every 1–2 years |
Every 1–2 years |
| Severe |
Every 6–12 months |
Every 6–12 months
Dilating LV: more frequently |
Every 1 year |
Every 6–12 months
Dilating LV: more frequently |
LV, left ventricle; MVA, mitral valve area; Vmax, maximum velocity.
TTE is an essential imaging modality for the definitive diagnosis of VHD, evaluation of hemodynamics, and determination of therapeutic strategies. Therefore, TTE should be performed in all patients with known or suspected VHD (Class I).9–28
A comprehensive examination is recommended, including assessment of the mechanism of VHD, qualitative and quantitative evaluation of regurgitation and stenosis, and evaluation of chamber size, cardiac function, and hemodynamics. For patients with asymptomatic VHD, regular echocardiographic follow-up is recommended (Class I).
Table 5
shows the approximate follow-up period according to severity.29
If the TTE examination provides a suboptimal evaluation or further examinations are required, TEE is recommended (Class I).8,29–31
TEE is particularly useful for observing the mitral valve and LA. Recommendations for each VHD are described in the following sections.
2.1 Evaluation of Valvular Regurgitation
Valvular regurgitation is classified into 2 types: organic (primary regurgitation) accompanied by structural changes such as leaflet perforation and prolapse, and functional (secondary regurgitation) caused by ventricular and atrial remodeling despite no organic changes in the valve structure itself. Echocardiography is the first choice for assessing the mechanism and severity of regurgitation. When interpreting the mechanism and severity of regurgitation, a single index may not be sufficient to make an accurate diagnosis. Therefore, evaluation in combination with multiple findings or indices is needed.8,29–31
Color Doppler is the primary tool for evaluation and is commonly used in the clinical setting to assess the site, direction, and severity of the regurgitant flow. However, there are issues with this evaluation method, based on the regurgitant area. Therefore, a semiquantitative evaluation method measuring the width of the vena contracta of the regurgitant flow and quantification by the EROA, regurgitant volume, and regurgitant fraction are recommended.30,31
These quantitative indices are measured using the PISA method (Figure 1) and the volumetric method based on Doppler imaging (Figure 2). However, each method has multiple assumptions and sources of error that necessitate careful interpretation of the measurements.30,31

LV size and LVEF are important in determining the therapeutic strategy in regurgitant valvular diseases.8,29
It is recommended that the LV diameter is measured at the top of the mitral leaflet.32
The LVEF should be measured using the modified Simpson’s method.
2.2 Evaluation of Valvular Stenosis
With the increase in the number of patients with AS, due to the aging population, and the rapid spread of TAVI use, echocardiography is required to make a more accurate diagnosis of AS. Estimation of the AVA is essential in the diagnosis of AS and there are 2 methods of measurement: (1) trace the 2D image using the planimetry method or (2) use the continuity equation using the Doppler method and 2D images.33,34
The continuity equation estimates the area of the functional valve orifice where the blood flow passes through the aortic valve at its most contractile point, whereas the planimetry method measures the anatomical valve orifice area. The functional valve orifice is located on the aortic side relative to the location of the anatomical valve orifice, and the AVA measured by the continuity equation is smaller than that with the planimetric method. Because it is often difficult to draw accurate cross-sections if there is advanced calcification, the use of the planimetric method is not particularly recommended for TTE.33,34
Conversely, it is necessary to keep in mind that errors may occur due to several factors in the continuity equation. For this reason, when there is a discrepancy between the estimated AVA and the clinical findings, echocardiographic remeasurement and AVA measurement by CT should be considered. In addition, these indices are affected by stress-related parameters, such as aortic pressure and CO, so careful interpretation of the measurements is necessary, considering the diversity of each pathophysiology.33
For MS, evaluations using the mPG at the mitral valve and the pressure half-time method using the continuous-wave Doppler method are recommended.33
As with evaluation of the aortic valve, the planimetry method may cause errors in estimation of the MVA.
In the evaluation of regurgitation and stenosis, we need to understand the pitfalls of each measurement method. Each evaluation should be performed using multiple indices. If there are discrepancies among the results, the cause should be investigated, and evaluation using other modalities should be considered depending on the situation.
2.3 Evaluation of Pulmonary Hypertension and RV Function
Left-sided VHD may be accompanied by secondary pulmonary hypertension due to increased LA pressure. Pulmonary hypertension at rest and during exercise has been known to be associated with symptoms and prognosis, especially in AS and MR.35–41
Therefore, the PASP should be estimated for VHD. The estimated PASP can be obtained by calculating the RV systolic pressure using the simplified Bernoulli equation, which is based on the maximum velocity of the TR, the RA pressure estimated using the inferior vena cava maximum diameter, and the respiratory variability.10,42
An estimated PASP >35 mmHg is suggestive of pulmonary hypertension. However, in cases of an unclear TR waveform, PASP may be over- or underestimated. It should also be noted that in severe TR, the laminar regurgitant flow often results in an inaccurate estimation of the RV systolic pressure using the simplified Bernoulli equation. In patients without TR or with an unclear TR waveform, the mean pulmonary artery pressure can be estimated from the acceleration time of the pulmonary artery blood flow and the maximum velocity of the pulmonary regurgitant velocity waveform.42
Pulmonary hypertension and tricuspid or pulmonary valve disease can cause RV dysfunction. Thus, RV function should be assessed in combination with the TAPSE, tissue Doppler-derived tricuspid annular systolic velocity, and FAC (Figure 3).42
However, in cases of significant TR, these indices may overestimate RV systolic function, so it may be challenging to evaluate RV systolic function correctly.
3. Other Noninvasive Investigations
3.1 Stress Testing
In patients with VHD, the severity of the condition may not match the clinical symptoms or findings. The purpose of stress testing is to clarify the need for further intervention in such cases. There are 2 types of stress tests: exercise or pharmacological. Exercise stress tests that can assess symptoms and hemodynamic changes associated with exertion are preferred.43–45
Evidence has accumulated regarding the usefulness of exercise stress echocardiography, which can evaluate cardiac function and hemodynamics during exercise stress testing.46–53
A method that uses a supine ergometer and can allow for recording of echocardiograms during exercise is desirable. Pharmacological stress echocardiography is used mainly for low-flow/low-gradient AS with LV systolic dysfunction, as described below.54–57
Stress echocardiography allows for the assessment of changes in various parameters such as ventricular volume, ventricular function, the pressure gradient in stenotic valves, regurgitant severity, and hemodynamics such as PASP. However, it is difficult to evaluate all parameters during stress testing. Therefore, the recording of indices necessary for the diagnosis in each individual case should be given priority.43–45
Table 6
shows the recommended level of stress testing that is indicated for each type of VHD.
Table 6.
Recommendations of Valvular Stress Echocardiography
| Valve disease |
Type |
Indications |
COR |
LOE |
Mitral
regurgitation |
Chronic primary MR |
In asymptomatic patients with severe MR or symptomatic patients with
moderate MR, exercise testing is reasonable to confirm the absence/presence
of symptoms, to assess changes of PASP and LV function with exercise |
IIa |
C |
| Secondary MR |
In asymptomatic patients with severe MR or symptomatic patients with
moderate MR, exercise testing is reasonable to confirm the absence/presence
of symptoms, to evaluate the changes in MR severity, and systolic pulmonary
artery pressure during exercise, and to determine the indications for mitral
valve surgery |
IIa |
C |
Mitral
stenosis |
|
In patients with discordance between symptoms and stenosis severity, exercise
testing is indicated to reveal symptoms and assess haemodynamic consequences
of MS based on the increase in gradient and PASP during stress |
IIa |
C |
Aortic
stenosis |
Asymptomatic severe
AS |
Exercise testing is reasonable to confirm the absence of symptoms, to assess
hemodynamic changes with exercise, and to stratisfy the risk of cardiovascular
events |
IIa |
B |
Symptomatic low-flow/
low-gradient severe AS
with LVEF <50% |
Low-dose dobutamine stress echocardiography is reasonable to differentiate
true- from pseudo-severe AS and assess LV contractile reserve |
IIa |
B |
Aortic
regurgitation |
|
In patients with discordance between symptoms and AR severity, exercise
testing is used to assess symptoms, exercise tolerance, and LV response to
stress |
IIb |
C |
AS, aortic stenosis; AR, aortic regurgitation; CABG, coronary artery bypass grafting; COR, Class of Recommendation; LOE, Level of Evidence; LV, left ventricular; LVEF, LV ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; PASP, pulmonary artery systolic pressure.
In AS, stress echocardiography is recommended for patients with asymptomatic severe AS to confirm asymptomatic status, assess the hemodynamic response during exercise, or for risk stratification for cardiac events (Class IIa).8,29,46–49
Further, in patients with low-flow/low-gradient AS and LV dysfunction, low-dose dobutamine stress echocardiography is recommended to distinguish true-severe AS from pseudo-severe AS and for confirming the LV contractile reserve.54–57
In MR, stress testing is recommended for asymptomatic patients with severe primary chronic MR and for symptomatic patients with non-severe MR during resting echocardiographic examination (Class IIa).50,58,59
Because secondary MR changes dynamically, exercise stress echocardiography is useful for understanding the pathophysiology and predicting the prognosis.60
However, there is still insufficient evidence for determining the therapeutic strategies for such cases, and the only related prospective study to date has been the TIME trial.61
Based on the results of that study, exercise stress testing is recommended to determine a possible surgical intervention for moderate MR in patients referred for CABG based on the development of symptoms and worsened pulmonary hypertension during exercise stress testing. Exercise echocardiography data suggests that pulmonary hypertension (≥60 mmHg) during stress is associated with the prognosis of MR, but there is insufficient evidence to determine the surgical indications.8,50
In MS, exercise stress echocardiography is recommended for patients with a discrepancy between the Doppler echocardiographic findings at rest and the clinical symptoms, for evaluation of the mPG at the mitral valve and the pulmonary artery pressure response during exercise (Class IIa).51
In AR, because the stress-induced increase in heart rate shortens diastole, AR severity may decrease during stress testing. Therefore, stress testing is not recommended for evaluating AR severity.52
However, in patients with a discrepancy between the severity of their condition and their symptoms, a stress test might be used to evaluate the hemodynamic response and LV contractile reserve during exercise (Class IIb).
3.2 CT and MRI
Although the morphological and functional evaluation of VHD is usually conducted by TTE or TEE, in recent years CT and MRI have also been used for a more accurate evaluation.
In addition to evaluating valvular anatomy, CT can effectively evaluate calcification.62–65
Preoperative screening and procedure planning with CT is important, especially for TAVI. CT is also useful for ruling out coronary artery disease prior to surgery, because of its high negative predictive value in coronary stenosis.66
CT is also useful for assessing the bicuspid aortic valve and the ascending aortic diameter.67
In recent years, CT has been able to more accurately assess valve leaflet mobility during the cardiac cycle and is also useful for the diagnosis of valve thrombosis following SAVR and TAVI.68–70
MRI can be used for the morphological evaluation of the entire heart, including the function of the valves, RV,71,72
and LV, as well as for quantitative evaluation of ventricular volume, regurgitation volume, and fibrosis of the myocardium. MRI is useful for determining the etiology of functional MR and for evaluating RV contraction and dilatation in TR.73
4. Invasive Investigations (Table 7)
Table 7.
Recommendations of Invasive Examination for Valvular Heart Disease
| Recommendations |
COR |
LOE |
Preoperative coronary angiography is recommended for patients with severe valvular
disease who have any of the following:
• History of coronary artery disease
• Findings that suggest myocardial ischemia
• Deterioration of left ventricular function
• Males over 40 years old, postmenopausal females
• One or more cardiovascular risk factors |
I |
C |
Assessment of severity and hemodynamics by cardiac catheterization is reasonable for
patients who are difficult to evaluate by echocardiography or for patients showing
discrepancy between clinical symptoms and echocardiographic findings |
IIa |
C |
Coronary angiography is reasonable for patients with moderate to severe secondary mitral
regurgitation and suspected involvement of myocardial ischemia |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
Because the diagnosis and severity of VHD can usually be assessed with TTE and TEE, invasive cardiac catheterization is not always necessary. However, assessment of severity and hemodynamics using cardiac catheterization is reasonable for patients who are difficult to evaluate by echocardiography or for patients showing a discrepancy between their clinical symptoms and the echocardiographic findings (Class IIa).
In addition to coronary angiography, left ventriculography is used to evaluate LV size as well as LV contractility, wall motion abnormalities, and MR. This method also evaluates the cardiac load by measuring LV end-diastolic pressure and the severity of AS by measuring the pressure gradient between the aorta and the LV. Aortography is used in the evaluation of AR and the ascending aortic diameter. The severity of regurgitation is graded on a 4-level scale. It is important to place the pigtail catheter in the appropriate position and acquire images with the correct amount of contrast agent to be able to precisely examine the degree of regurgitation. In VHD, right cardiac catheterization is important for evaluating the pulmonary artery pressure and the pulmonary capillary wedge pressure. In MS, the MVA is calculated by measuring the pressure gradient between the LV and the pulmonary capillary wedge pressure. Severe MR may be characterized by an increased V wave of the pulmonary capillary wedge pressure.
Preoperative coronary angiography is recommended for patients with severe VHD with a history of coronary artery disease, suspected myocardial ischemia, or impaired LV function; males or post-menopausal females over 40 years of age; and patients with at least 1 cardiovascular risk factor (Class I). This procedure is also recommended for patients with suspected ischemia in moderate-to-severe functional MR (Class IIa).8
5. Risk Assessment
5.1 JapanSCORE and Comprehensive Risk Assessment
Risk assessment prior to heart valve surgery is important to ensure “quality control in surgery” (improvement of the quality of cardiac surgery and medical safety for patients).74
Predicted operative mortality can be calculated using existing risk calculators such as the JapanSCORE,75
EuroSCORE II,76
and STS score.77
Risk assessment before transcatheter treatment for VHD is traditionally conducted with the foreign risk calculators, but should also be performed with the JapanSCORE, a similar assessment based on Japanese clinical data.74,78–80
In addition to the 30-day operative mortality, the JapanSCORE 2 can predict the incidence of 7 major complications, namely, stroke, reoperation for bleeding, new requirement of dialysis, deep sternum infection, prolonged ventilation >24 h, gastrointestinal complication, and intensive care unit stay >7 days. These predictive data are useful in perioperative management. The JapanSCORE2 is now available via a mobile software application and can be easily accessed on a smartphone. Although the input data used to calculate the score can vary, frailty (see
Section I.6: Special Considerations for Elderly Patients), procedure-specific factors, and alternative approaches, such as right minithoracotomy, are not included in this scoring system. In this regard, the 2014 AHA/ACC “Guideline for the Management of Patients With Valvular Heart Disease” proposes a “comprehensive risk assessment”, including evaluation of frailty, major organ damage, and procedure-specific factors in addition to predicted operative mortality.29
Figure 4
shows the comprehensive risk assessment according to the present guideline. In the clinical setting, the surgical risk assessment should be based on multidisciplinary and comprehensive discussions among the Heart Valve Team. It is important to note that there is no absolute way to evaluate frailty or cognitive function.

Figure 4.
Comprehensive risk assessment.
· Surgical risk should be evaluated by the Heart Valve Team.
· Representative assessment tools are shown for both Frailty and Cognitive function.
*1Patient data include age, body surface area, sex, body mass index, current smoker status, history of diabetes mellitus and its treatment, chronic kidney disease (CKD) and hemodialysis, hypertension, infective endocarditis, chronic lung diseases, carotid artery disease, extracardiac vascular disease, history of cerebrovascular accident, consciousness disturbance within 24 h, history of heart valve surgery, previous coronary intervention, myocardial infarction, congestive heart failure New York Heart Association (class 0–II, III, IV), angina pectoris, cardiogenic shock, history of arrhythmia, use of inotropic agents, mitral valve stenosis, aortic valve stenosis, number of diseased vessels in coronary artery , left ventricular function, valve regurgitation sites and the degree or regurgitation, emergency status, concomitant coronary artery bypass grafting, unpredicted coronary artery surgery, surgical methods (e.g., replacement, repair, aortic root replacement), and double or triple valve surgery.
*2GOLD=Global Initiative for Chronic Obstructive Lung Disease, Stage I=mild (%FEV1 ≥80%), Stage II=moderate (50%≤%FEV1<80%), Stage III=severe (30%≤%FEV1<50%), Stage IV=very severe (%FEV1 <30%).
*3CKD=chronic kidney disease
eGFR categories (mL/min/1.73 m2) description and range: G1=normal or high (≥90), G2=mildly decreased (60–89), G3a=mildly to moderately decreased (45–59), G3b=moderately to severely decreased (30–44), G4=severely decreased (15–29), G5=kidney failure (<15), 5D=dialysis.
*4Frailty: 1=very fit, 2=well, 3=managing well, 4=vulnerable, 5=mildly frail, 6=moderately frail, 7=severely frail, 8=very severely frail, 9=terminally ill.
An excerpt from Geriatric Medicine Research.81
©2009. Version 1.2_EN. All rights reserved. Geriatric Medicine Research, Dalhousie University, Halifax, Canada. Permission granted to copy for research and educational purposes only.
Please refer to Clinical Frality Scale:CFS Version 1.2_EN (https://www.dal.ca/sites/gmr/our-tools/clinical-frailty-scale.html).
*5HDS-R=revised Hasegawa dementia scale, MMSE=Mini-Mental State Examination, MoCA=Montreal Cognitive Assessment.
5.2 Cautions Regarding Comorbidities, Steroid Users, and Anticoagulation Drugs
5.2.1 Cognitive Impairment
With the emergence of a “super-aged” society in Japan, an increasing number of patients with cognitive impairment are expected to undergo cardiac surgeries. Preoperative cognitive impairment indicates the risk of delirium after surgery,82–84
with a frequency of onset exceeding 10–20%.85–87
Delirium is reported to increase operative mortality,87,88
prolong the duration of hospital stay,82
and increase the risk of postoperative cardiac events.85
Therefore, the preoperative assessment of cognitive function and postoperative delirium care are crucial to improve outcomes in elderly patients. Shortened operation time, early rehabilitation, perioperative pain management, and high-quality sleep are important in preventing postoperative delirium.85,89
5.2.2 Diabetes Mellitus
Patients with diabetes mellitus are at high risk of surgical site infections such as mediastinitis. The perioperative blood glucose level should be maintained between 140 and 180 mg/dL to prevent both surgical site infections and hypoglycemia.90
The preoperative HbA1c level is a useful risk assessment marker to predict surgical site infection: ≥7.0% has been reported to increase the risk of mediastinitis.91
5.2.3 Liver Cirrhosis
The surgical indications for patients with cirrhosis should be carefully considered because surgical intervention may lead to fatal liver dysfunction. An indocyanine green retention rate at 15 min of over 25%, Child–Pugh classification of B or C,92–94
MELD (Model for End-stage Liver Disease) score of over 13,94–96
and a low cholinesterase level97
are reported as markers of poor prognosis after surgery.
5.2.4 Blood Diseases
Blood diseases are reported to be related to perioperative infections and bleeding. To prevent perioperative bleeding, patients with idiopathic thrombocytopenic purpura can be treated with γ-globulin before surgery,98
and those with hemophilia B can be treated with factor IX before and after surgery.99
The risk of side effects, such as thromboembolism and acute renal failure, should be considered when administering γ-globulin.
5.2.5 Malignancies
Cardiopulmonary bypass in patients with malignancies is suspected to alter the immune response, tumor progression, and cancer cell metastasis;100–103
however, there is no evidence supporting these concerns.104–106
Heart valve surgery is therefore not contraindicated in patients with malignancies and should be considered comprehensively based on each patient’s life expectancy and general condition. In cases of hematological malignancy, the rates of postoperative bleeding and infection are as high as 23–57%.104,107–110
5.2.6 Steroid Users
Patients with a long-term history of steroid use have a greater risk of compromised immunity, delayed wound healing, gastrointestinal ulcers, hyperglycemia, and acute adrenal insufficiency after surgery.111
In order to prevent these complications, decreasing the oral steroid dosage may be considered only if the underlying diseases can be properly managed. Perioperative steroid coverage is recommended for patients unable to restart oral steroids soon after surgery.
5.2.7 Oral Anticoagulation Drugs
Preoperative discontinuation of anticoagulation drugs is recommended.112–114
Warfarin should be discontinued 3–5 days before surgery and may be replaced with heparin if necessary. Heparin administration is recommended in patients with a high risk of thromboembolism, such as those with mechanical valve implantation, as well as those with a history of cerebral infarction or a CHA2DS2-VASc score ≥2.29,115,116
The heparin dosage should be adjusted to increase the activated partial thromboplastin time to 1.5–2.5-fold the normal control value, but the risk of bleeding complications should also be carefully considered. Unfractionated heparin should be discontinued 4–6 h before surgery. If prompt correction of the PT-INR is required in emergency cases, administration of vitamin K, factor IX complex, and fresh frozen plasma is recommended. Dabigatran should be discontinued 1–2 days before surgery if the creatinine clearance rate is >50 mL/min or 2–4 days before surgery if it is between 30 and 49 mL/min. If necessary, heparin replacement should be administered 12 h after the termination of dabigatran. Apixaban and edoxaban should be discontinued 48 h and 24 h before surgery, respectively, and replaced with heparin if necessary. Rivaroxaban should be discontinued at least 24 h before surgery.
5.2.8 Dialysis
The 3 major causes of death after surgery in patients with dialysis are infection, low cardiac output syndrome, and respiratory complications.117
To improve outcomes, infection control, slow dehydration during dialysis and intensive respiratory management including rehabilitation are imperative. Nonobstructive intestinal ischemia is one of the critical complications after surgery,118–120
and its association with norepinephrine administration has been reported.119,120
6. Special Considerations for Elderly Patients
The Japanese average life expectancy has been increasing with recent improvements in health awareness, lifestyle, and medical standards. According to statistics from the Ministry of Health, Labor, and Welfare in 2017, the life expectancy was 87.26 years for females and 81.09 years for males,121
both being among the highest in the world with respect to longevity. According to a 2016 report, the healthy lifespan is 74.79 years for females and 72.14 years for males. Treatment options for the elderly are changing as the healthy lifespan increases, and it is necessary to actively evaluate frailty and cognitive function rather than simply classifying patients as elderly.
6.1 Assessing Frailty
This section is omitted from the English version.
6.2 Preoperative Frailty and Outcomes in Cardiac Surgery
This section is omitted from the English version.
6.3 Special Treatment Strategies for the Elderly
For elderly patients, not simply age and comorbidity, but also frailty (Figure 4) should be evaluated when predicting treatment outcomes. New, minimally invasive treatments with undetermined long-term but favorable short-term results are often beneficial for the elderly, given their relatively shorter remaining life expectancy compared with younger people. However, such treatment is generally expensive. Cost-effective treatment is an important issue for many elderly patients with a relatively short life expectancy. In elderly patients, VHD is often associated with other diseases. In such cases, it is necessary to consider which diseases define the prognosis in order to determine the order of priority to treat. Physicians should not hesitate to provide invasive treatment based on advanced age alone, but should also consider palliative alternatives. A treatment strategy for elderly patients should be fully discussed among the Heart Valve Team after a detailed evaluation of frailty, cognitive function, comorbidity, life expectancy determinants, and the patient’s own desires.
7. Importance of the Heart Valve Team (Heart Team)
Even experienced cardiologists sometimes hesitate when making decisions about treatment for VHD. In particular, for patients with asymptomatic, severe VHD or high surgical risk, difficult decision-making may be required for selecting treatment options, which include transcatheter therapy. There is also a risk of biased decisions, with physicians favoring their own specialty.
Decision-making in treating such complex and severe VHD cases must be done with the cooperation of cardiologists and cardiac surgeons. For some diseases, the extended Heart Valve Team may be required, including cardiologists, interventional specialists, cardiac imaging specialists, cardiac surgeons, anesthesiologists, radiologists, sonographers, and other healthcare professionals. The role of the Heart Valve Team is to consider the risks and benefits of different treatment options and to determine the best method without bias towards their own expertise. For example, determining the surgical indications for elderly patients with AS or choosing between TAVI and SAVR requires the Heart Valve Team to thoroughly discuss the possible surgical risks and treatment effects, as well as the patient’s age, severity of condition, frailty, cognitive function, and comorbidities. The Heart Valve Team must also fully explain the results of their discussion to the patients and families.
A well-balanced Heart Valve Team should be composed of experienced specialists from multiple fields and must be able to determine treatment strategies based on the guidelines after thorough evaluations and consultations. The Heart Valve Team is also required to provide highly specialized medical, transcatheter, or surgical treatment in a timely manner. Furthermore, the accumulation of case-related data and the creation of a national database will enable statistical evaluation of the effects and associated risks of the treatments. It is also important to register the medical results in the Japan Cardiovascular Surgery Database, as the information will help determine future treatment policies and lead to better medical care. In addition, it is desirable that the Heart Valve Team provide a systematic training program for cardiac surgeons, interventional specialists, and imaging specialists.
Although the term “Heart Team” is commonly used, it derives from discussions by cardiac surgeons and cardiologists on whether to choose CABG or catheter interventions for coronary artery diseases. With regard to valvular diseases, it is more important to discuss the severity of the disease, the features of valvular lesions, indications for intervention, and choice of surgical procedures. Therefore, it is essential to discuss these issues within an experienced and well-balanced team with diverse specialties as described above. The Heart Team for VHD described in the European and American guidelines is herein referred to as the “Heart Valve Team”, which means a group of experts who possess knowledge and experience related to VHD.
III. Mitral Regurgitation (MR)
1. Classification and Etiology
1.1 Primary (Organic) and Secondary (Functional) MR
MR is one of the most common VHDs in developed countries.122,123
In the USA, 9.3% of the population aged over 75 years is reported to have moderate or severe MR.123
Mitral valve function is determined by the LV, papillary muscle, chordae tendineae (tendinous cords), valve leaflet, annulus, and LA, collectively known as the “mitral valve complex”. An abnormality at any of these sites may result in MR (Table 8). MR arising from an abnormality in the valve leaflets, chordae tendineae, and papillary muscles is referred to as primary (organic MR), including degenerative and rheumatic MR. MR associated with dilatation or dysfunction of the LV or left atrium is called secondary (functional MR). The underlying causes of MR are listed in
Table 9.
Table 8.
Etiologies of Mitral Regurgitation
| Lesion |
Etiology |
Mitral leaflet |
Carpentier type |
| LV |
Tethering due to LV dilatation and/or systolic dysfunction* |
Reduced mobility |
IIIb |
| Papillary muscle |
Rupture due to myocardial infarction |
Prolapse |
II |
| Cordae tendineae |
Elongation or rupture due to degeneration (FED) |
Prolapse |
II |
| Rupture due to infective endocarditis |
Prolapse |
II |
| Mitral leaflet |
Myxomatous change due to Barlow’s disease |
Prolapse |
II |
| Infective endocarditis |
Perforation |
I |
Mitral leaflet and/or
cordae tendineae |
Senile or rheumatic sclerosis and/or calcification |
Reduced mobility |
IIIa |
| LA and mitral annulus |
LA and mitral annular dilatation mainly due to atrial fibrillation† |
Reduced coaptation
(+reduced mobility‡) |
I (+IIIb‡) |
*,†Etiologies of secondary (functional) MR. The others are etiologies of primary (degenerative) MR. ‡Atriogenic tethering of posterior mitral leaflet is also suggested as an etiology of functional MR occurring due to LA and mitral annular dilatation.124–126 FED, fibroelastic deficiency; LA, left atrial or left atrium; LV, left ventricular or left ventricle; MR, mitral regurgitation.
Table 9.
Classification and Causes of Mitral Regurgitation
| Primary (degenerative) |
| • Prolapse |
| Idiopathic, Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, trauma |
| • Rheumatic disease |
| • Sclerosis or calcification of mitral leaflets and/or annulus |
| • Infective endocarditis |
| • Papillary muscle rupture due to myocardial infarction |
| • Congenital lesion |
| Mitral cleft, parachute anomaly |
| • Autoimmune disease |
| Systemic lupus erythematosus, antiphospholipid syndrome (Libman-Sacks endocarditis) |
| • Drugs |
| Secondary (functional) |
| • Coronary artery disease, dilated cardiomyopathy |
| • Atrial fibrillation |
The incidence of rheumatic MR has declined in developed countries because of the decreased occurrence of rheumatic fever, although the incidence of mitral prolapse and valve leaflet/annular sclerosis or calcification in the elderly is increasing.123
Mitral valve prolapse involves myxomatous degeneration through accumulation of mucopolysaccharides in the leaflets and chordae tendineae. It may be associated with abnormal connective tissue diseases, such as Marfan syndrome, but is often idiopathic. When rupture or elongation of the chordae tendineae is the primary cause, and degeneration of the leaflet itself is minimal, the prolapse and MR are often localized. These abnormalities are pathologically defined as FED (Figure 5). Conversely, when myxomatous degeneration of a valve leaflet is the primary cause, the leaflet thickens and expands, and billowing occurs (with the leaflet belly inflating toward the LA during systole). Multiple leaflet prolapse sites and multiple MR jets are seen in severe cases. These patients are diagnosed with Barlow’s disease, which is distinct from FED (Figure 5). The intermediate state between FED and Barlow’s disease is called “
forme fruste
” (French for “incomplete form”).127
Note that prolapse is defined as the tip of the leaflet exceeding the mitral valve annular plane during systole. By contrast, billowing is a term for the mitral valve morphology described above, irrespective of the presence or absence of prolapse.

Mitral valve leaflet tethering due to LV dilatation and systolic dysfunction is the most well-known cause of secondary MR.128–132
Secondary MR associated with acute or prior myocardial infarction may be referred to as “ischemic MR”. However, that term may include primary MR due to papillary muscle rupture associated with acute myocardial infarction. Cases of LA dilatation due to AF indicate that secondary MR can also occur without LV systolic dysfunction, referred to as “atrial functional MR” and another type of secondary MR.124–126,133–135
Although there are multiple reports of the mechanism underlying this type of MR, a consensus remains to be established. However, “atrial functional MR” is commonly found with both LA and mitral annular dilatation.
The Carpentier classification considers MR origin based on leaflet motion.136
Normal leaflet motion, such as that seen with leaflet perforation due to infective endocarditis, congenital cleft, and/or AF-associated annular dilatation, is involved in type I. MR resulting from excessive leaflet motion (i.e., prolapse) is considered as type II. MR resulting from restricted leaflet motion and incomplete valve closure is considered as type III, which includes age-related or rheumatic lesion leading to restricted motion during systole and diastole (type IIIa), and valve tethering causing restricted motion during only systole (type IIIb) (Table 8).
In long-term chronic cases and in the elderly, several factors may occur simultaneously, such as changes in the leaflet, enlargement of the annulus, and LV dysfunction.
1.2 Acute and Chronic MR
The etiology of acute MR includes chordae tendineae rupture caused by idiopathic or infective endocarditis, trauma, or papillary muscle rupture associated with acute myocardial infarction. Acute severe MR often causes low CO and pulmonary congestion, and in severe cases, shock.
Conversely, chronic MR involves LV dilatation leading to a compensatory increase in the LV total stroke volume, which avoids low CO and/or pulmonary congestion. LV afterload in patients with significant MR is low because a proportion of the LV ejection flows into the LA, a low-pressure chamber. Therefore, LVEF is maintained at a higher than normal level. As such, even LVEF less than the lower limit of normal can be considered to indicate the occurrence of myocardial damage. Over several years, a noncompensatory period occurs during which the LVEF falls, reducing forward stroke volume and leading to chronic heart failure (HF). Moreover, a chronic rise in LA pressure can cause LA enlargement and AF.
2. Assessment of Severity
MR is classified as mild, moderate, or severe, which is evaluated mainly by TTE (Table 10).30
Various qualitative and quantitative evaluation methods have been proposed for assessing the severity of MR, each with certain advantages and disadvantages. Appropriate methods may vary by case. Therefore, the appropriate method for each case should be applied after understanding its strengths and weaknesses, and severity should be assessed comprehensively.
Table 10.
Grading the Severity of Mitral Regurgitation (MR) by Echocardiogaphy
| |
MR severity |
Pitfalls |
| Mild |
Moderate |
Severe |
| Structural |
| LV and LA zize |
Normal |
– |
Dilated |
LV and LA size can be within the
normal range for patients with
acute severe MR. The grading of
secondary MR cannot be
estimated from LV and/or LA size |
| Qualitative Doppler |
| Color flow jet area |
Small, central, narrow,
often brief |
– |
Large central jet (>50% of LA) |
Regurgitant grading tends to be
underestimated from eccentric
wall-impinging jet |
Proximal flow
convergence |
Not visible, transient or
small |
– |
Large throughout systole |
|
Continuous wave
Doppler |
Faint or partial |
– |
Holosystolic and dense |
|
| Semiquantitative |
Vena contracta
width (cm) |
<0.3 |
0.3–0.69 |
≥0.7 |
Vena contracta width measured
from a single plane is not suitable
for the grading of secondary MR |
| Pulmonary vein flow |
– |
– |
Minimal to no systolic flow or
systolic flow reversal |
|
| Transmitral flow |
– |
– |
E-wave elevation (>1.2 m/s) |
|
| Quantitative |
EROA derived from
PISA method (cm2) |
<0.20 |
0.20–0.39 |
≥0.40 |
PISA method is not suitable for
the grading of secondary MR |
Regurgitant volume
(mL) |
<30 |
30–59 |
≥60 |
Regurgitant volume may be lower
in patients with low-flow conditions
in secondary MR due to LV
dysfunction |
Regurgitant fraction
(%) |
<30 |
30–49 |
≥50 |
Doppler-derived volumetric
method comparing LV inflow and
outflow is not suitable for MR
grading in patients with cocomitant
significant aortic regurgitation |
EROA, effective regurgitant orifice area; LA, left atrial; LV left ventricular; MR, mitral regurgitation; PISA, proximal isovelocity surface area. Produced by reference to Zoghbi et al. J Am Soc Echocardiogr 2017; 30: 303–371.30
Qualitative evaluation is often used to assess the size of the MR jet using the color Doppler method. Color Doppler is easy to use, although it depends on the cross-sectional settings and the etiology and direction of the jet.137
Moreover, eccentric jets along the LA wall appear smaller than true regurgitations on color Doppler (due to the Coandă effect), and thus, MR will be underestimated.138
Other disadvantages of the color Doppler method include significant effects of the machine settings, such as color gain and pulse repetition frequency (velocity range). Quantitative evaluation methods include the PISA method and the Doppler-derived volumetric method. The PISA method is a quantitative method to calculate the EROA and the regurgitant volume, assuming that the proximal flow convergence is hemispherical. The Doppler-derived volumetric method is a quantitative method to determine the LV diastolic filling and systolic ejection volumes. Regurgitant volume is calculated as the difference between these volumes, whereas the regurgitant fraction is calculated using the LV diastolic filling volume. The PISA method should be used for the assessment of primary MR when the regurgitant orifice is localized. However, in the case of multiple MR jets, the PISA method is not suitable because calculating the EROA using only one of the proximal flow convergence points would underestimate the true EROA. The PISA calculations include measuring the MR velocity, and thus, it is not suitable when the MR jet velocity cannot be properly measured from a given angle due to eccentricity. Conversely, with secondary MR, a transverse gap along the coaptation of the anterior and posterior leaflets leads to a regurgitant orifice.139
Therefore, determination of the EROA, which is calculated assuming that the proximal flow convergence is hemispherical, by the PISA method is often inaccurate. Furthermore, the EROA in secondary MR shows dynamic changes during systole.140
This is also one of the causes of incorrect quantitative evaluation of secondary MR by the PISA method. Therefore, it is better to use the Doppler-derived volumetric method to quantify regurgitation in secondary MR. The disadvantage of the Doppler-derived volumetric method is that calculating MR concomitant with AR often results in inaccuracies.
3. Primary (Organic) MR
3.1 Pathophysiology and Natural History
This section is omitted from the English version.
3.2 Diagnostics
3.2.1 Symptoms and Physical Findings
This section is omitted from the English version.
3.2.2 Echocardiography
If MR is suspected from the physical findings, the next required examination is TTE (Class I). TTE is used to diagnose the etiology and severity of MR, and to evaluate cardiac function and hemodynamics.
The Carpentier classification is used for MR mechanism evaluation (see section III. 2). Primary MR corresponds to types I, II, and IIIa.136
Classification of the MR mechanism is strongly linked to the choice of surgical treatment. In particular, it is important to identify the location of the prolapse or ruptured tendinous cord with type II.
Evaluation of the location and extent of the prolapse can be performed using multiple cross-sectional images. The regurgitation signal direction obtained by color Doppler is also essential in diagnosing the site of prolapse. In principle, the regurgitation jet flows opposite to the side of the prolapse. It is essential to select a cross-section that shows both the accelerated flow and the regurgitation jet in the same plane.30,141
The grading of severity is shown in
Table 10. The EROA (cm2), regurgitant volume (mL/beat), and regurgitation fraction (%) are calculated by the Doppler-derived volumetric method and the PISA method. The quantitative evaluation should then be compared to the semiquantitative findings, with the vena contracta width of the regurgitation jet and regurgitation jet area being compared with the LA area.
TEE is recommended as an alternative to TTE for patients with moderate or severe MR in whom conventional TTE is technically not feasible for assessing the severity and mechanism of the MR (Class I). TEE can provide higher image quality and is useful in cases wherein images cannot be obtained by TTE because of acoustic shadow. Detailed and systematically recorded images provide essential information for evaluation of the mechanism of regurgitation, as well as for estimating reparability. In particular, 3D TEE can provide more information in the preoperative evaluation of mitral valve repair (Class IIa),31,142,143
allowing for the surgeon’s view (or
en face
view) of the mitral valve.
However, it should be noted that due to sedatives administered changes in the hemodynamic status during the TEE tend to result in an underestimation of the degree of regurgitation. For example, with intraoperative echocardiography, the effects of general anesthesia and the decrease in blood flow because of extracorporeal cardiopulmonary bypass reduce the amount of regurgitation.144
Exercise stress echocardiography is reasonable for patients with asymptomatic severe MR or symptomatic moderate MR to evaluate the hemodynamic physiology (Class IIa). It is also useful for evaluating hemodynamic changes and predicting the prognosis.50,53,145,146
3.2.3 Other Diagnostic Tests
In recent years, cardiac MRI has been used to assess the size and contraction of the LV and RV as well as the severity of MR (Class IIb). This method is useful when TTE records are insufficient. However, there are limitations to the evaluation of the regurgitation mechanism.147–149
Cardiac catheterization is often performed to diagnose the presence of coronary artery disease, and sometimes a pressure study is performed to estimate hemodynamic severity. Increased pulmonary capillary wedge pressure, notably an increased V wave, is closely related to shortness of breath symptoms. Left ventriculography may not accurately reflect the severity of regurgitation because it is sometimes affected by the size of the LA. Cardiac catheterization is reasonable if there is a discrepancy between the noninvasive tests and clinical findings or if evaluation with noninvasive tests is not technically feasible (Class IIa).
Cardiac CT may also help to diagnose mitral valve calcification, but currently, there are no clear data on its efficacy or accuracy.
Table 11
lists the recommendation for diagnostic testing for primary MR.
Table 11.
Recommendations for Diagnostic Testing for Primary Mitral Regurgitation (MR)
| Recommendations |
COR |
LOE |
TTE is indicated for patient with primary MR to assess severity and mechanism of the
regurgitation, evaluate LV size, LV function, LA size, and pulmonary hypertension |
I |
B |
TEE is recommended as an alternative to TTE for patients with moderate or severe MR in whom
conventional TTE is technically not feasible to assess the severity and mechanism of MR |
I |
B |
Periodical TTE is indicated for asymptomatic severe MR to determine the timing of surgical
intervention |
I |
B |
Exercise stress test is reasonable for patients with asymptomatic severe MR to clarify the
symptoms |
IIa |
C |
Exercise stress echocardiography is reasonable for patients with asymptomatic severe MR or
symptomatic moderate MR to evaluate hemodynamic physiology |
IIa |
B |
Cardiac catheterization is reasonable if there is a discrepancy between the noninvasive tests
and clinical findings or if evaluation with noninvasive tests is not technically feasible |
IIa |
C |
3D-TEE is reasonable for patients with mitral valve prolapse to evaluate the mechanism and
location of the prolapse. |
IIa |
C |
Cardiac MRI may be an alternative to TTE in patients with MR in whom TTE evaluation is
technically not feasible |
IIb |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
3.3 Surgical Treatment
3.3.1 Surgical Procedures and Results
This section is omitted from the English version.
3.3.2 Indications and Timing of Surgery (Figure 6, Table 12)

Table 12.
Indications of Surgery for Severe Chronic Primary Mitral Regurgitation (MR)
| Recommendations |
COR |
LOE |
MV repair is recommended in preference to MV replacement, when a durable repair can be
safely accomplished |
I |
B |
Concomitant MV repair or replacement is recommended for patients undergoing cardiac
surgery for other indications |
I |
C |
| MV surgery is recommended for symptomatic patients with LVEF >30% |
I |
B |
MV surgery is reasonable for symptomatic patients with LVEF ≤30%, when the Heart
Valve Team judges that surgery is beneficial |
IIa |
C |
MV surgery is recommended for asymptomatic patients with LVEF ≤60% or LVESD ≥40 mm
(LVESDI ≥24 mm/m2) |
I |
B |
MV surgery is reasonable for asymptomatic patients with LVEF >60% and LVESD <40 mm
(LVESDI <24 mm/m2) who have new onset of AF or resting PASP >50 mmHg |
IIa |
B |
MV repair is reasonable for asymptomatic patients with LVEF >60% and LVESD <40 mm
(LVESDI <24 mm/m2) who have neither new onset of AF nor resting PASP >50 mmHg, when
durable repair can be safely accomplished |
IIa |
C |
MV repair may be considered for asymptomatic patients with LVEF >60% and LVESD <40 mm
(LVESDI <24 mm/m2) who have neither new onset of AF nor resting PASP >50 mmHg, in
the following cases:
*progressive LV dysfunction, or
*PASP >60 mmHg during excercise, or
*left atrial dilatation (left atrial volume index ≥60 mL/m2) |
IIb |
C |
AF, atrial fibrillation; COR, Class of Recommendation; LOE, Level of Evidence; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic dimension; LVESDI, LVESD index (=LVESD/body surface area); MV, mitral valve; PASP, pulmonary artery systolic pressure.
a. Chronic Primary MR
Careful evaluation of the symptoms is necessary because the indications for surgical intervention are mainly determined by the presence of symptoms. Exercise testing can be helpful when the presence of symptoms is unclear, or the symptoms are atypical. Exercise stress echocardiography is recommended to evaluate the symptoms and severity of pulmonary hypertension during exercise.
i) Surgical Indications for Symptomatic MR
Symptomatic severe MR with LVEF >30% is indicated for mitral valve repair or replacement (Class I).150,151
In cases of severe LV dysfunction with LVEF ≤30%, mitral valve surgery is reasonable when adequate medical treatment is not effective and mitral valve surgery is expected to improve symptoms (Class IIa). In cases of high surgical risk, transcatheter mitral valve edge-to-edge repair surgery can be considered as an alternative interventional strategy. Surgical intervention for severe LV dysfunction requires particularly careful discussion by the Heart Valve Team.
ii) Surgical Indications for Asymptomatic MR
For asymptomatic severe MR resulting from mitral valve prolapse, prophylactic intervention has been recommended based on recent technical developments and favorable results of mitral valve repair surgery for prolapsed mitral valve. Careful assessment of some important triggers that affect the postoperative prognosis is required during discussion by the Heart Valve Team.
1) LVEF and LVESD
Poor postoperative LV function and prognosis has been demonstrated with preoperative LVEF ≤60% and/or LVESD ≥40 mm, and surgical intervention is indicated by the presence of these triggers (Class I).18,152,153
Because the cutoff value of the LV diameter is selected according to the European and American literature, it is reasonable for patients with a small body size to refer to the adjusted values. In the present guideline, an LVESD index ≥24 mm/m2
has been offered as a BSA indexed value for the LVESD ≥40 mm in patients with a BSA of <1.7 m2.
Although the evidence is not definitive, it has been reported that an LV end-systolic volume of 40 mL/m2
measured by 3D echocardiography reflects the postoperative prognosis better than conventional 2D measurements.154
In addition, surgery can be considered when progressive reduction of LVEF or enlargement of LVESD is noted on serial echocardiographic examinations even if the LVEF 60% or the LVESD ≤40 mm criterion is not met (Class IIb).
2) Pulmonary Hypertension and AF
In cases of preserved LV function, new-onset AF or pulmonary hypertension (PASP >50 mmHg) at rest are indications for mitral valve surgery (Class IIa).40,155,156
The actual onset of AF is often unclear in chronic severe MR. Surgical intervention should be considered when main etiology of MR can be definitely determined as organic lesion such as flail leaflet, and improvement of prognosis is highly expected by intervention.
3) Early Surgery for Asymptomatic MR Without Triggers
It is still controversial whether early surgery is indicated for asymptomatic chronic severe MR caused by mitral valve prolapse without any triggers for surgical intervention. Early surgical intervention is recommended on the basis of an adverse long-term prognosis in patients with severe MR, even though cardiac function is maintained.17
Some clinical studies have suggested a “watchful waiting” strategy with regular follow-up for asymptomatic severe MR when sinus rhythm can be maintained with LVEF >60% or LVESD <40 mm in the absence of pulmonary hypertension.157
In recent cohort studies, however, surgery following the onset of symptoms was associated with a worse prognosis than that of early asymptomatic surgery. At this time, early surgery is preferred for asymptomatic severe MR if successful mitral valve repair with long-term durability can be expected.152,158–160
Early surgery for asymptomatic severe MR cases with complex lesions, such as with Barlow’s disease and bileaflet prolapse, may not be recommended, and indications for early surgery should be carefully considered.
Accordingly, mitral valve repair is reasonable for asymptomatic patients with LVEF >60% and LVESD <40 mm (LVESD index <24 mm/m2) without new-onset AF or a resting PASP >50 mmHg when durable repair can be safely accomplished (Class IIa). Repairability, durability, and surgical risk in each individual case should be carefully discussed by an experienced Heart Valve Team. When a “watchful waiting” strategy is applied for primary severe MR, semi-annual or annual evaluation of symptoms and echocardiographic findings are recommended to judge the proper timing of surgery.157,161,162
CQ1 should also be referenced regarding early surgery in asymptomatic severe MR.
4) Other Parameters
For asymptomatic severe MR, exercise stress echocardiography is useful for hemodynamic assessment in cases without resting pulmonary hypertension. An estimated PASP >60 mmHg during exercise stress is a benchmark for considering early surgery50
(Class IIb). However, PASP during exercise should be interpreted with consideration of the effects of other factors, such as age and LV diastolic function. The BNP level may be helpful if the presence of symptoms associated with severe MR is uncertain or if early surgery is borderline suitable.5
LA enlargement (≥60 mL/m2) has been reported to predict a poor prognosis in patients even if they are asymptomatic and may be an indication of surgery (Class IIb).163
When open-heart surgery is planned for treatment of other cardiac diseases, mitral valve surgery for severe MR should be performed at the same time (Class I). For moderate MR, concurrent surgery with surgery for other cardiac diseases is reasonable when the valve lesion is diagnosed as being repairable (Class IIa). If the valve lesion is not suitable for repair, indications for concurrent surgery should be carefully discussed by an experienced Heart Valve Team.
b. Acute MR
In acute MR, unlike in chronic MR, there is a sudden volume overload on the small, less compliant LA, possibly resulting in a rapid increase in LA pressure followed by acute HF. Sudden-onset HF due to acute severe MR is more difficult to control than HF caused by chronic severe MR and generally requires surgical intervention.
CQ1
Should early surgery be recommended for asymptomatic patients with severe primary MR with LVEF >60% and LVESD <40 mm who do not have new onset AF or pulmonary hypertension?
[Conclusion]
Early surgery is reasonable when a successful and durable repair surgery can be safely performed (2C).
[Strength of Recommendation]
2: Weakly recommended (proposed)
[Strength of Evidence]
C (weak): Limited confidence in the estimated effect
[Comment]
Patients with chronic severe primary MR, even without symptoms and LV dysfunction, have a high likelihood of developing “triggers” such as symptoms, LV dysfunction, AF, or pulmonary hypertension over the course of 6–10 years and require mitral valve surgery.17,157,162,164,165
Previous studies have reported that the overall survival of patients with the “watchful waiting” strategy (follow-up monitoring until the aforementioned triggers are reached and thereafter referred for surgery) was not statistically different from the expected survival.157,162
Conversely, several studies have reported that early surgery prior to the appearance of the triggers improves the prognosis.153,159,160
The advantages of mitral valve repair over prosthetic valve replacement166–169
are widely recognized and therefore repair surgery should be indicated for early surgery, which should be successful and durable and performed safely. The repair feasibility depends on the anatomy of the lesions. For early surgery, therefore, complex lesions that have a high likelihood of requiring mitral valve replacement should be avoided. Lesions limited to one scallop of the posterior leaflet may be a good candidate for early surgery. The ACC/AHA guidelines indicate that mitral repair is reasonable in patients without any triggers in whom the likelihood of a successful and durable repair is >95% with a mortality rate of <1% when performed at highly experienced facilities.29
It is important to recognize that the results of mitral valve repair depend on the experience of the Heart Valve Team, especially the surgeon’s skill and experience.170,171
[Evidence]
There are several reports supporting early surgery before the triggers are reached.
① Kang et al160
divided patients with asymptomatic severe MR into 2 groups: an early surgery group with no triggers (LVESD <40 mm, LVEF >60%, absence of AF, and absence of pulmonary hypertension) and a group of patients who were referred for surgery when the triggers were reached. For the 207 propensity-matched pairs, the early surgery group had a significantly lower cardiac mortality rate (1% vs. 6% at 12 years; P=0.010) and cardiac event rate (4% vs. 19% at 12 years; P=0.001). The authors also reported in an age-based subanalysis that early surgery was particularly beneficial for patients aged ≥50 years.
② In research from the Mitral Regurgitation International Database registry from 6 centers in 4 countries, a subanalysis (790 patients in total) compared patients with early surgery with no triggers and patients who underwent medical treatment until the onset of triggers.159
At 10 years after the diagnosis, the survival rate was 87% and 82% (P=0.04) for the early surgery and triggered groups, respectively, and the heart failure incidence was 5% and 20% (P<0.001), respectively. This study suggested early surgery is associated with a better prognosis compared with initial medical management.
③ Enriquez-Sarano et al153
defined the triggers as class I (symptoms, LVESD ≥40 mm, and LVEF <60%) or class II (AF and pulmonary hypertension) and stratified 1,512 primary MR patients into 3 groups: patients who underwent mitral valve surgery with a class I trigger; mitral valve surgery with a class II trigger; and early surgery with no trigger but with a high probability of valve repair. The postoperative hospital stay was the shortest for patients with early surgery. Survival rates at 15 years were 42%, 53%, and 70%, respectively, and the incidence of HF for each group was 35%, 27%, and 15%, respectively.153
[Note]
As mentioned, early surgery can be indicated for patients with severe MR with no triggers on the premise that a successful and durable repair is safely feasible. It has been reported that a recurrence of moderate or more than moderate MR after mitral valve repair is associated with adverse LV remodeling and an increased likelihood of death.172
Therefore, careful assessment is mandatory for determining the indications for early surgery. In addition, early surgery is indicated for patients with severe MR on the premise that the MR severity is adequately evaluated. When the severity of MR cannot be confidently diagnosed, or there are difficulties in making a clear decision about early surgery, other triggers such as LA size,163,173,174
changes in pulmonary hypertension on exercise echocardiography,175
exercise capacity by cardiopulmonary exercise testing,59,176
myocardial strain,177,178
BNP,5
and change in LV function over time can be considered.
As the cutoff value for LV diameter, one of the triggers that is an indication for surgery, is selected based on European and American guidelines, it is reasonable for patients with a small body size to refer to the appropriate adjustment values. In the present guideline, an LVESD index ≥24 mm/m2
for patients with BSA ≤1.7 m2
is offered as a BSA-indexed value for an LVESD ≥40 mm, which is one of the triggers that indicates LV remodeling.
3.4 Medical Therapy and Follow-up
This section is omitted from the English version.
4. Secondary (Functional) MR Associated With LV Dysfunction
4.1 Pathophysiology and Natural History (Figures 7,8)
This section is omitted from the English version.
4.2 Diagnostics
4.2.1 Symptoms and Physical Findings
This section is omitted from the English version.
4.2.2 Echocardiography
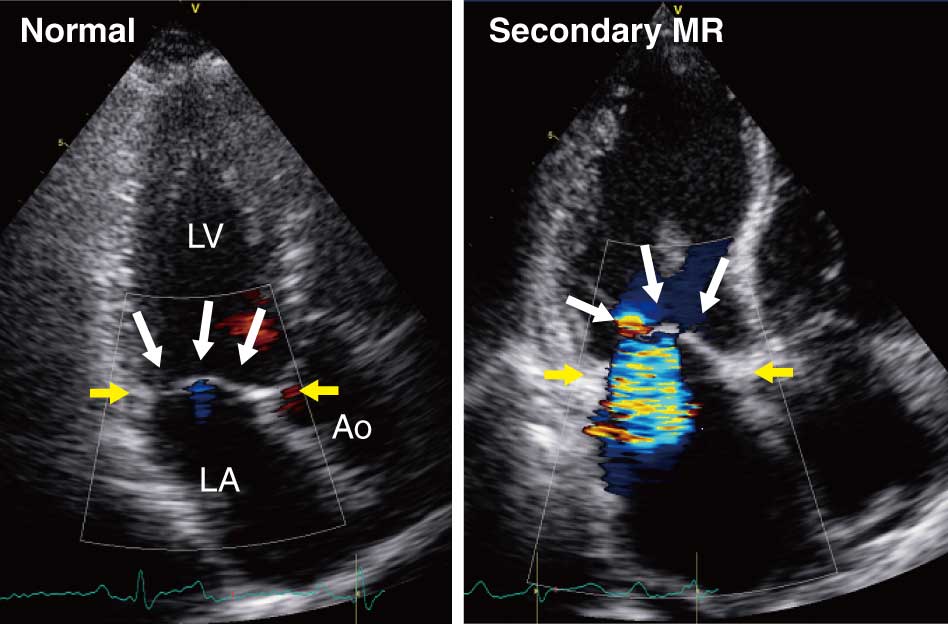
In the management of HF, it is important to assess the possible etiology and hemodynamic status using the medical history, physical findings, ECG, or chest X-ray, and TTE. TTE plays a crucial role in the diagnosis of baseline heart disease and hemodynamic conditions in patients with HF. TTE can evaluate regional or diffuse LV wall motion abnormality, the degree of LV dysfunction with LV dilatation, and characteristic leaflet tenting/tethering without structural abnormality (Class I). The degree of tethering is generally evaluated by measuring the tenting area and height (or length) using 2D echocardiography (Figure 9). Although there is not a definitive recommendation regarding the measurement site, the tenting area and tenting height are commonly measured in the parasternal long-axis view at the center of the annulus in mid-systole.179
The greater the tethering degree, the greater the amount of MR.129
Severe tethering is associated with recurrence after mitral annuloplasty. Because of the multiple factors that can affect the postoperative result after mitral valve repair surgery, it is difficult to set a threshold of tenting area or length that accurately predicts postoperative recurrence.180–182

Commonly in secondary MR, color Doppler echocardiography shows a regurgitant jet toward the center of the LA, in a slightly posterior direction. The regurgitant jet generally has a wide regurgitant orifice together with a coaptation line; it is well-observed in the bicommissural view (along the commissure–commissure plane) from the apical approach.
Multiplane TEE is useful for detailed observation of the valve’s structure, regurgitation sites, and regurgitant severity. In addition, 3D observation is helpful in the assessment of valve morphology and regurgitation, and 3D TEE is mandatory for perioperative assessment of transcatheter mitral valve edge-to-edge repair surgery (Class I).
In the assessment of secondary MR, stress echocardiography is useful for evaluating the severity of the MR and the RV pressure during exercise (Class IIa).60,183
According to some reports, pharmacological stress echocardiography may be useful when reperfusion therapy is being considered for ischemic MR.
4.2.3 Other Diagnostic Tests
Cardiac MRI may be useful when the assessment of ventricular size and function by echocardiography is not conclusive (Class IIb). In functional MR, delayed enhancement may contribute to the assessment of myocardial fibrosis. Myocardial scintigraphy would also be useful to evaluate myocardial viability in patients with secondary MR due to LV remodeling.
Coronary angiography is required for the diagnosis of coronary artery disease, but it is not necessary if the number of coronary risk factors is low and the coronary CT scans are negative for coronary lesions. Because the degree of regurgitation and hemodynamics can be diagnosed by Doppler echocardiography, left ventriculography and right cardiac catheterization are not necessarily performed. These invasive tests (Class IIa) are recommended when noninvasive tests are not conclusive in the evaluation of MR severity or hemodynamic assessment.
Table 13
lists the tests recommended for secondary MR.
Table 13.
Recommendations for Diagnostic Testing for Secondary Mitral Regurgitation (MR) Caused by LV Systolic Dysfunction
| Recommendations |
COR |
LOE |
TTE is indicated to evaluate the etiology and severity of regurgitation, LV size and function,
chamber sizes and pulmonary hypertension, when secondary MR is clinically suspected |
I |
C |
| Intraoperative TEE is indicated during transcatheter mitral valve surgery |
I |
C |
Assessment of coronary artery disease by invasive and/or noninvasive examinations
(scintigraphy, stress echo, coronary CT and coronary angiography) is reasonable when
moderate or severe secondary MR is suspected |
IIa |
C |
Exercise stress echocardiography is reasonable to determine hemodynamic significance of
functional MR in symptomatic but moderate MR |
IIa |
C |
Cardiac catheterization is reasonable for determination of severity of MR and
hemodynamic evaluation when noninvasive data are nondiagnostic or if there is a
descrepancy between clinical and echocardiographic evaluations |
IIa |
C |
Cardiac MRI may be considered when TTE is not conclusive in the evaluation of LV size and
function and myocardial damage |
IIb |
C |
COR, Class of Recommendation; LOE, Level of Evidence; MRI, magnetic resonance imaging; TEE, trasesophageal echocardiography; TTE, transthoracic echocardiography.
4.3 Surgical Treatment
4.3.1 Surgical Procedures and Results
This section is omitted from the English version.
4.3.2 Indications and Timing of Surgery (Figure 10, Table 14)

Table 14.
Recommendations for Treatment for Secondary Mitral Regurgitation (MR) With LV Systolic Dysfunction
| Recommendations |
COR |
LOE |
Optimal medical therapy (ACEIs, ARBs, β-blockers, mineralcorticoid receptor antagonists)
of heart failure should be administered |
I |
A |
Cardiac resynchronization therapy should be performed for heart failure, if indicated (refer to
Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure (JCS 2017/
JHFS 2017)) |
I |
A |
Mitral valve surgery is recommended for patients with severe MR and LVEF >30% who
have indication for CABG |
I |
C |
Mitral valve surgery may be considered for patients with severe MR and LVEF ≤30% who
have indication for CABG |
IIb |
C |
Mitral valve surgery may be considered for patients with moderate MR who have indication
for CABG and no viability in the posteroinferior wall |
IIb |
C |
CABG without mitral valve surgery may be considered for patients with moderate MR who
have indication for CABG and have viability in the postero-inferior wall |
IIb |
B |
Surgical or catheter intervention may be considered for patients with LVEF >30% and
severe MR who have no indication for coronary revascularization |
IIb |
B |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CABG, coronary artery bypass grafting; COR, Class of Recommendation; LOE, Level of Evidence; LVEF, left ventricular ejection fraction.
Eliminating MR may not be a perfect solution for secondary MR because it is a ventricular disease, not a valvular disease. Therefore, adequate medical treatment for HF is mandatory (Class I). In terms of surgical treatment, ischemic cardiomyopathy, in which recovery of LV function by simultaneous revascularization can be expected, and non-ischemic cardiomyopathy, in which surgery helps manage the MR only, should be considered separately. For ischemic MR without indications for revascularization, surgical indications should be considered using the same strategy as for non-ischemic cardiomyopathy.
PCI and CABG are revascularization methods for the treatment of ischemia. The difference between them is that CABG allows a concomitant valvular procedure and other surgical procedures whereas PCI can only improve the ischemia. The choice between CABG and PCI is based on whether it is necessary to perform a simultaneous operation on the mitral valve and whether CABG is safe for high-risk patients even if no mitral valve surgery is indicated. If CABG is not suitable because of patient risk, PCI should be selected to treat myocardial ischemia. Transcatheter mitral edge-to-edge repair should also be considered if indicated. Each case should be discussed individually in this regard.
a. Severe MR
i) When Revascularization by CABG Is Indicated
Isolated CABG may improve LV function and reduce MR if the posteroinferior region is viable; however, in cases of severe MR, isolated CABG is usually not sufficient to eliminate MR. Secondary MR has been reported to worsen the prognosis after myocardial infarction, even in cases with mild or moderate regurgitation.184
Therefore, if the risk of using extracorporeal circulation is not high, mitral valve surgery is recommended for patients with LVEF >30% and severe MR with a high likelihood of persistence after CABG (Class I). If the LVEF is <30%, mitral valve intervention may be indicated according to LV myocardial viability, the risk of using extracorporeal circulation, and the extent to which the MR is involved in HF (Class IIb).
Regarding mitral valve surgery, valve replacement or ring annuloplasty with subvalvular procedures can be performed in patients at high risk of MR recurrence (Table 15)185–191
and isolated ring annuloplasty can be performed in cases of low risk of MR recurrence.
Table 15.
Echocardiographic Parameters for Prediction of Recurrence After Valve Repair for Secondary Mitral Regurgitation (MR)
| |
Parameter |
View |
Phase |
Cutoff
values |
Sensitivity
(%) |
Specificity
(%) |
Reference |
Transthoracic
echocardiography |
Tenting area |
Apical 4-chamber view |
Mid-systole |
≥2.5 cm2 |
64 |
95 |
185 |
| Tenting height |
Apical 4-chamber view |
Mid-systole |
≥1 cm |
64 |
90 |
185 |
Posterior tethering
angle |
Apical 4-chamber view |
Mid-systole |
≥45° |
100 |
95 |
185 |
| Anterior tethering angle |
Parasternal long-axis
view |
Mid-systole |
≥39.5° |
98 |
97 |
186 |
Distal anterior leaflet
angle |
Parasternal long-axis
view |
Mid-systole |
>25° |
88 |
94 |
187 |
Interpapillary muscle
distance |
Parasternal short-axis
view |
End-systole |
>20 mm |
96 |
97 |
188 |
| MR grade |
|
|
Severe |
|
|
189 |
Systolic sphericity
index |
Apical view |
End-systole |
≥0.7 |
100 |
100 |
190 |
Transesophageal
echocardiography |
Mitral annular diameter |
4-chamber view |
Systole |
≥37 mm |
84 |
76 |
191 |
| Tenting area |
Long-axis view |
Systole |
≥1.6 cm2 |
80 |
54 |
191 |
| MR grade |
|
Systole |
≥3.5 |
42 |
81 |
191 |
ii) When Revascularization by CABG Is Not Indicated
If the patient is asymptomatic, careful periodic observation is recommended. If HF symptoms are present, careful and comprehensive medical treatment, including PCI, and cardiac resynchronization therapy, if indicated, should be considered (Class I). If controlling HF symptoms is difficult even after comprehensive medical treatments, MR intervention may be considered if the LVEF is >30% (Class IIb).192
The patient’s general status and LV function193
should be considered by the Heart Valve Team when discussing the indications for transcatheter or surgical interventions. If the LVEF is ≤30%, some reports indicate that prognosis is not improved by invasive treatments compared with medical intervention.194
The Heart Valve Team should consider the patient’s age and general condition to determine whether to perform LV assist device treatment, heart transplantation, mitral valve intervention (transcatheter or surgical), or rigorous medical treatment.
b. Moderate MR
i) When Revascularization by CABG Is Indicated
For moderate MR, it is highly likely that an isolated CABG will improve MR for patients in whom the posteroinferior region is viable or dyssynchrony is not observed.195,196
Other considerations include the severity of the LV dysfunction and the probability of MR improvement by revascularization alone. CABG without mitral valve surgery may be considered for patients who have a viable posteroinferior wall (Class IIb).195,196
Conversely, mitral valve surgery may be considered for patients without a viable posteroinferior wall or those with a viable posteroinferior wall but without an adequate right or left circumflex coronary artery for bypass,196,197
if there is no increased risk for extracorporeal circulation (Class IIb).195,196,198
If mitral valve surgery is indicated, ring annuloplasty is the first choice.
ii) When Revascularization by CABG Is Not Indicated
If CABG is not indicated for revascularization, careful periodic observation and medical treatment are the first choice if there are no signs of HF. In cases of medical treatment not controlling the HF, especially when pulmonary hypertension or MR worsens by exercise stress echocardiography, the treatment policy should be determined by discussion in the Heart Valve Team.
4.4 Medical Therapy and Follow-up (Table 14)
This section is omitted from the English version.
5. Secondary (Functional) MR Associated With LA Dilatation: Atrial Functional MR
5.1 Pathophysiology, Natural History, and Mechanism
Significant functional MR and functional TR without ventricular dysfunction can occur in patients with atrial dilatation and mitral/tricuspid annular dilatation accompanied by persistent AF. There is little systematic research on this type of TR and MR. However, in light of recent studies on MR due to atrial dilatation in persistent AF cases, this type of MR has garnered more attention.124–126,133–135,199–207
Many Japanese reports on this condition have contributed greatly to formulating the concept of this disease.124–126,134,135,206,207
The condition is often referred to as “atrial functional MR” or simply “atrial MR”. To distinguish this disease, the conventional functional MR associated with LV dysfunction can be referred to as “ventricular functional MR”. It is rare, but atrial dilatation due to LV diastolic dysfunction can also cause atrial functional MR in a sinus rhythm.
The frequency of moderate or severe atrial functional MR in patients with AF varies by population, but is generally low (2.8–15%).125,133–135,205
However, a higher proportion (28%) of moderate or severe atrial functional MR was observed in cases of longstanding, persistent AF over 10 years than in those with a duration of <10 years.135
In that report, the severity of both atrial functional MR and TR was an independent predictor of cardiac events. Moreover, the event-free rate was low, especially when moderate or severe MR and TR coexisted. Some other studies have reported that patients with AF who were hospitalized due to HF frequently showed significant atrial functional MR (37–44%), even at discharge after medical treatment, and that atrial functional MR was associated with readmission for HF during the post-discharge follow-up.206,207
There are a number of studies on the mechanism of atrial functional MR125,126,133,134,199,202–205
Failed coaptation of the anterior and posterior leaflets with LA dilatation and mitral annular dilatation is common across all studies. Disruption of the mitral annular saddle shape,125,204,205
reduction in mitral annular contractility,125,203
inadequate compensation for mitral annular dilatation resulting from the lack of leaflet remodeling,134,204,205
and atriogenic tethering of the posterior mitral leaflet124–126
have been proposed, together with other etiologies. Atriogenic tethering of the posterior mitral leaflet has been traditionally referred to as hamstringing of the posterior mitral leaflet observed in the giant LA (Figure 11). The main determinant and the relationship among the various etiologies still need to be established.
5.2 Diagnosis
MR can be diagnosed as “atrial functional MR” when associated with LA dilatation without global/regional LV systolic dysfunction nor apparent structural abnormalities in the mitral leaflets and chordae tendineae. Cases of LV dilatation or dysfunction (LVEF <50%) may be excluded when atrial functional MR is strictly defined.124,125,134,199,202–204
However, even with atrial functional MR, mild LV dilatation and reduced LVEF may occur in longstanding chronic or advanced cases. Therefore, if the main mechanism is atrial dilatation, the MR can be referred to as atrial functional MR. As mentioned above, most cases of atrial functional MR occur in the context of AF, especially longstanding persistent AF.135
If posterior leaflet tethering or hamstringing is present, poor coaptation between the anterior and posterior leaflets may arise. The tip of the anterior leaflet will then deviate to the atrial side, overriding the posterior leaflet.125,126
This phenomenon can be referred to as anterior leaflet pseudoprolapse or overriding, which has been originally reported as a characteristic of ventricular functional MR (Figure 11).208–210
5.3 Medical Therapy (Table 16)
Table 16.
Recommendations for Treatment of Atrial Functional Mitral Regurgitation (MR)
| Recommendations |
COR |
LOE |
Standard medical therapy for heart failure, including diuretics, should be performed for
symptomatic patients with atrial functional MR |
I |
C |
AF catheter ablation is reasonable for symptomatic patients with persistent AF and severe
atrial functional MR if successful ablation and maintenance of sinus rhythm can be expected
from the duration of AF and the left atrial size |
IIa |
C |
Mitral valve surgery is reasonable for patients with severe atrial functional MR who are
consistently symptomatic despite standard medical therapy for heart failure*,† |
IIa |
C |
*This recommendation can also be applied to patients with chronic moderate atrial functional MR if the MR is severe at the worsening of heart failure or on exercise stress tests. †Atrial functional MR is likely to accompany secondary atrial functional TR, and concomitant tricuspid surgery should be performed in patients with both atrial functional MR and TR. AF, atrial fibrillation; COR, Class of Recommendation; LOE, Level of Evidence; TR, tricuspid regurgitation.
Symptomatic patients with atrial functional MR should first receive standard medical therapy for HF, including diuretics (Class I). Invasive treatments should also be considered if patients with severe atrial functional MR are consistently symptomatic despite standard medical therapy for HF. According to a previous study, successful maintenance of sinus rhythm after catheter ablation resulted in reduction in the size of both the LA and mitral annulus, which in turn led to significant improvement in atrial functional MR.133
Therefore, the maintenance of sinus rhythm by catheter ablation may be an effective treatment for atrial functional MR (Class IIa). However, there are many cases in which LA dilatation is prominent and the probability of maintaining sinus rhythm by catheter ablation is low. In such cases, surgical treatment for MR is reasonable (Class IIa). However, the appropriate cutoff value of LA size for selecting catheter ablation or surgery remains undetermined.
5.4 Surgical Treatment
5.4.1 Surgical Procedures and Results
This section is omitted from the English version.
5.4.2 Indications and Timing of Surgery (Table 16)
Conventional treatment guidelines for functional MR with LV dysfunction cannot be applied to atrial functional MR because it is a completely different disease entity. Further, the data supporting treatment selection are still lacking. Consequently, consideration of surgery should be limited to patients with HF symptoms. Mitral valve surgery is reasonable if rhythm control therapy is not indicated and severe MR with HF symptoms persists despite medical therapies in patients with persistent AF (Class IIa). This recommendation can also be applied to patients with chronic moderate atrial functional MR if the MR becomes severe with worsening HF or during stress tests.
Atrial functional MR is likely to accompany secondary atrial functional TR. Patients with both atrial functional MR and atrial functional TR therefore require concomitant tricuspid surgery when they undergo mitral surgery. Mitral leaflet augmentation to increase the coaptation, the Cox maze procedure, or LA plication can be considered as a concomitant procedure based on a case-by-case evaluation. However, more evidence is needed to determine whether conservative or surgical interventions are superior for avoiding cardiac events. Future clinical research should involve discussion of surgical indications, procedures, and optimal timing for this condition.
6. Transcatheter Treatment
6.1 Current Status of Transcatheter Treatment
This section is omitted from the English version.
6.2 Indications for Transcatheter Treatment
6.2.1 Primary MR
Transcatheter mitral valve edge-to-edge repair and conventional surgery were compared in the multicenter randomized EVEREST II trial.211
Participants suffered from primary symptomatic severe MR. Mitral valve surgery within 1 year because of recurrent or residual MR occurred significantly more often after transcatheter treatment at 20% compared with after conventional surgery at 2%. In patients with low perioperative risk, surgery is the first choice and transcatheter treatment cannot replace surgery, although transcatheter treatment can be used in high-risk patients. The indications should be determined after discussion in the Heart Valve Team, considering the surgical risk and the presence of any concomitant conditions such as TR.
6.2.2 Secondary MR
There is high expectation of transcatheter mitral valve edge-to-edge repair for secondary MR. In fact, about 70% of the patients undergoing transcatheter treatment are those with secondary MR.212
In the EVEREST II high-risk cohort study, the survival rate was favorable and the recurrence rate of HF was lower in the transcatheter treatment group than in the medical treatment group.213
Two prospective multicenter RCTs, the COAPT study214
and the MITRA-FR study,215
were conducted to compare conventional medical therapy alone and in combination with the transcatheter mitral valve edge-to-edge repair for secondary MR with LV dysfunction. In the COAPT study, the transcatheter treatment group showed not only a reduction in the HF rate, but also a reduction in mortality. However, the MITRA-FR study showed no improvement in either the HF rate or the prognosis. The conflicting results of these studies are believed to be due to differences in MR severity and LV function, together with insufficient medical therapy for HF in the MITRA-FR study. At present, transcatheter mitral valve edge-to-edge repair should be considered for symptomatic patients with severe secondary MR despite optimal medical therapy and cardiac resynchronization therapy, if indicated. Severity of secondary MR changes depending on the load conditions, and thus, evaluation with stress echocardiography is useful. The Heart Valve Team should thoroughly review and discuss this issue to determine the proper indications.
6.2.3 Current Indications in Japan
A prospective multicenter study216
was conducted in Japan to confirm the safety and efficacy of transcatheter mitral valve edge-to-edge repair in patients with symptomatic severe MR. The participants included those with primary and secondary MR with LVEF >30% and who were considered as inoperable by the Heart Valve Team. The average participant age was 80 years, and approximately half of the participants had secondary MR. After treatment with transcatheter mitral valve edge-to-edge repair, 87% of the participants had MR ≤2+. There were no post procedure major adverse events or device complications at 30 days. Based on the results of this study, the indications for this treatment were determined in Japan (Table 17).217
The difference in indications between Japan and Europe/USA is that the LVEF lower limit is 15% in Europe and 20% in North America, whereas it is 30% in Japan. In response to the results of the COAPT study, the LVEF lower limit may be reduced from 30% in Japan, but currently remains unchanged.
Table 17.
Indication of MitraClip
®
Suitable for MitraClip®
therapy |
• Symptomatic chronic moderate-to-severe (3+) or severe (4+) primary or secondary MR, LVEF ≥30% and
deemed difficult for mitral valve surgery by the local site heart team (MR severity can be assessed at rest or at
stress state).
• Reduction of MR would lead to symptom relief.
• Anatomically suitable for MitraClip® therapy. |
| Excluding condition |
• Secondary MR patient who have not undergone JCS guideline oriented medical therapy.
• Acute decompensated heart failure.
• Inotrope dependent patient.
• Patient with mechanical support device. |
| Unsuitable for MitraClip® therapy |
• Unable to insert sheath through femoral vein, or deep venous thrombus at the device insertion site.
• Leaflet anatomy which may preclude MitraClip® implantation, proper positioning on the leaflets or sufficient
reduction in MR by the MitraClip® (listed below).
- Calcification of leaflet where MitraClip is planned to grasp.
- Wide indentation where MitraClip® is planned to grasp.
- Multiple origin of MR jet which may be difficult to reduce MR by MitraClip® therapy.
- Rheumatic mitral valve or mitral valve with restricted motion which may create iatrogenic mitral stenosis by
MitralClip® therapy.
- Posterior leaflet length <7 mm or its morbidity highly restricted.
- Mitral valve orifice area <3.0 cm2.
• Active endocarditis or active rheumatic heart disease or leaflets degenerated from rheumatic heart disease.
• Prior mitral valve leaflet surgery or any currently implanted prosthetic mitral valve.
• Echocardiographic evidence of intracardiac mass, thrombus or vegetation.
• Unable to perform transesophageal echocardiography.
• Life expectancy <1 year due to associated non-cardiac comorbid conditions.
• Contraindication for antiplatelet or anticoagulation therapy. |
Produced by reference to the guidance on transcatheter edge-to-edge repair (http://www.j-circ.or.jp/MitraClip/tekisei_shishin.pdf).
IV. Mitral Stenosis (MS)
1. Etiology
The incidence of rheumatic MS has decreased significantly in developed countries;218
however, MS due to annular calcification is increasing, especially in the elderly,219
though the condition rarely becomes severe.220
In some rare cases, MS is associated with congenital mitral valve abnormalities, such as undifferentiated papillary muscle, parachute mitral valves, mitral arcade, or double-orifice mitral valve, which are usually diagnosed during infancy or childhood.
2. Pathophysiology and Natural History
This section is omitted from the English version.
3. Assessment of Severity
In the 2014 AHA/ACC guidelines,221
severe VHD is defined as “the severity at which symptoms may appear and improve with intervention”; therefore, an MVA of 1.0–1.5 cm2
is defined as severe MS, and MVA <1.0 cm2
has been newly defined as very severe MS. In contrast, the conventional classification (moderate MVA 1.0–1.5 cm2; severe MVA <1.0 cm2) continues to be used in the 2017 ESC guidelines,8
and MS with an MVA <1.5 cm2
is considered “clinically significant”.
Accordingly, this guideline defines an MVA <1.0 cm2
as severe MS and an MVA 1.0–1.5 cm2
as moderate MS (Table 18).
Table 18.
Grading the Severity of Mitral Stenosis (MS)
| |
Mild |
Moderate |
Severe |
| MVA |
1.5–2.0 cm2 |
1.0–1.5 cm2 |
<1.0 cm2 |
| Mean transmitral PG |
<5 mmHg |
5–10 mmHg |
>10 mmHg |
| PHT |
<150 ms |
150–220 ms |
>220 ms |
*Mitral valve PG and PHT should be used as a reference because of the influence of hemodynamics. MVA, mitral valve area; PG, pressure gradient; PHT, pressure half time.
Given that the mean transmitral pressure gradient and diastolic pressure half-time are highly affected by valvular flow, LA/LV compliance, and heart rate, these parameters should be used as reference.
4. Diagnosis and Follow-up
4.1 Symptoms and Physical Findings
This section is omitted from the English version.
4.2 Echocardiography
Echocardiography is useful in diagnosing MS and assessing the severity (Table 18) and hemodynamics; thus, TTE is indicated when the symptoms or physical findings change (Class I). It is recommended that TTE should be performed every 3–5 years for mild MS, every 1–2 years for moderate MS, and annually for severe MS.221
The reduction in the mitral valve cusp opening and thickening of the leaflet are considered signs of MS. In particular, characteristic doming of the anterior leaflet, commissural fusion, and subvalvular tissue changes such as chordal shortening/fusion, can be observed in rheumatic MS.222
The MVA can be measured by tracing the valve orifice with a short-axis view of the mitral valve level (using the planimetry method). The mean transmitral pressure gradient and pressure half-time are measured by the continuous-wave Doppler method. The MVA can be calculated from the pressure half-time, but it is noted that this measurement can be used in rheumatic MS and that the pressure half-time is affected by conditions other than MS that modify the LV or LA pressure. In addition, it cannot be used for evaluation immediately after PTMC. The planimetry method is the standard for MVA measurement, but it sometimes overestimates according to the measurement views used. Therefore, it is also helpful to use the continuity equation and/or the PISA method when there is a significant discrepancy between the measurement in planimetry and the pressure half-time methods.
TTE is useful in determining whether the mitral valve morphology is suitable for PTMC. Because it is a procedure that cleaves the commissural fusion, PTMC is indicated for rheumatic MS. Commissurotomy is difficult if calcification is present, and if the valve leaflet or subvalvular lesion is advanced, it is unlikely that a sufficient increase in the MVA can be obtained. When significant MR is present, PTMC should be avoided because it may exacerbate MR. The Wilkins score (Table 19)223
is known to be an objective method of assessing whether the mitral valve morphology is suitable for a PTMC. If the score is less than 8 points, it is appropriate for PTMC. However, because the Wilkins score has the disadvantage of a lack of information on the commissure fusion, a score >8 does not necessarily indicate that PTMC cannot be performed.
Table 19.
Wilkins Score
| Thickening |
Calcification |
Subvalvular thickening |
| Leaflet near normal |
Only a single area |
Minimal thickening just below the mitral leaflets |
Mid leaflets normal, considerable
thickening of margins |
Scattered areas of leaflet
margins |
Thickening of chordal structures extending to one-
third of the chordal length |
Thickening extending through
the entire leaflets |
Extending into the mid-
portions of the leaflets |
Thickening of chordal structures extending to two-
thirds of the chordal length |
Considerable thickening of all
leaflet tissure |
Extensive throughout much
of the leaflet tissues |
Extensive thickening and shortening of all chordal
structures extending down to the papillary muscles |
Modified from Wilkins GT, et al. 1988223 ©1988 BMJ Publishing Group Ltd. & British Cardiovascular Society.
TTE is usually sufficient for evaluation of valve morphology and severity, and TEE does not need to be performed routinely. However, TEE is recommended for the evaluation of LA thrombus in cases of AF or when considering PTMC (Class I).
Exercise stress echocardiography is reasonable for evaluating the change in hemodynamics during exercise if there is a discrepancy between clinical symptoms and the severity of MS by echocardiography at rest (Class IIa).
4.3 Cardiac Catheterization
In recent years, the significance of cardiac catheterization for MS has decreased because the transvalvular pressure gradient, MVA, hemodynamics, and LV function can be evaluated by TTE. However, cardiac catheterization is reasonable for evaluating the severity and hemodynamics of MS when echocardiography is nondiagnostic or if there is a discrepancy between the clinical and echocardiographic evaluations (Class IIa). Ideally, the transvalvular pressure gradient is evaluated by simultaneously measuring the pulmonary capillary wedge pressure and the LV pressure. The MVA is calculated using the Gorlin formula after measuring CO by the thermodilution method or the Fick method (in the case of severe TR or intracardiac shunt).
MVA (cm2) = CO / (DFP * HR) / 37.7 
where DFP is the diastolic flow period (s/beat) and MVG is the mean ventricular gradient (mmHg).
The transvalvular pressure gradient is the result of the total mitral valve blood flow, so it is necessary to note that the valve area is underestimated in patients with significant MR. In addition, hemodynamic measurement by catheter before and after exercise loading may be useful when there is a discrepancy between the symptoms and pressure data at rest.
Table 20
lists the recommendations for diagnostic testing for MS.
Table 20.
Recommendations for Diagnostic Testing for Mitral Stenosis (MS)
| Recommendations |
COR |
LOE |
TTE is indicated for patients with MS to assess the severity of stenosis, mitral valve
morphology, the size and function of the LV and LA, degree of pulmonary hypertension,
and other valvular diseases |
I |
B |
TEE is recommended for patients with MS to evaluate LA thrombus in cases for which
PTMC is being considered |
I |
B |
Exercise stress echocardiography is reasonable for patients with MS to evaluate the change of
hemodynamics during excersise if there is a discrepancy between clinical symptoms and
severity of MS by echocardiography at rest |
IIa |
B |
Cardiac catheterization is reasonable to evaluate severity and hemodynamics of MS
when echocardiography is nondiagnostic or if there is a descrepancy between clinical and
echocardiographic evaluations |
IIa |
C |
COR, Class of Recommendation; LA, left atrium; LOE, Level of Evidence; LV, left ventricle; PTMC, percutaneous tansseptal mitral commissurotomy; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
5.1 Surgical Procedures and Results
This section is omitted from the English version.
5.2 Results of PTMC
This section is omitted from the English version.
5.3 Indications, Timing, and Selection of Intervention
5.3.1 Indications and Timing of Intervention (Figure 12, Table 21)

Table 21.
Indications of PTMC/Mitral Valve Surgery for Mitral Stenosis (MS)
| Recommendations |
COR |
LOE |
PTMC is recommended in symptomatic patients with moderate or severe MS and without
unfavorable valve morphology for PTMC |
I |
B |
Surgery is recommended in symptomatic patients with moderate or severe MS who have
unfavorable valve morphology for PTMC and without a contraindication or high risk for surgery |
I |
B |
Concomitant mitral valve surgery is recommended for patients with severe MS
undergoing other cardiac surgery |
I |
C |
PTMC or surgery is reasonable for asymptomatic patients with moderate or severe
MS who show symptoms or mPG >15 mmHg or pulmonary hypertension (PASP >60 mmHg)
during exercise |
IIa |
B |
Concomitant mitral valve surgery is reasonable for patients with moderate MS undergoing
other cardiac surgery |
IIa |
C |
PTMC or surgery may be considered for asymptomatic patients with moderate or severe
MS and new onset AF or history of embolism due to LA thrombus |
IIb |
C |
PTMC or surgery may be considered for symptomatic patients with mild MS who show
mPG >15 mmHg or PASP >60 mmHg during exercise |
IIb |
C |
Concomitant mitral valve surgery may be considered for patients with mild MS undergoing
other cardiac surgery |
IIb |
C |
AF, atrial fibrillation; COR, Class of Recommendation; LA, left atrium; LOE, Level of Evidence; mPG, mean pressure gradient; PASP, pulmonary artery systolic pressure; PTMC, percutaneous tansseptal mitral commissurotomy.
The indications for intervention for MS are determined by symptoms and the MVA. Intervention is recommended for moderate and severe MS (MVA ≤1.5 cm2) with symptoms such as dyspnea on effort despite medical therapy (Class I).224–226
Intervention may be considered for mild MS (MVA >1.5 cm2) with symptoms and when meeting certain criteria (mean pressure gradient >15 mmHg or PASP >60 mmHg) during exercise stress testing (Class IIb).227
Intervention is reasonable for asymptomatic patients with moderate or severe MS who develop new-onset AF or with recurrent embolization despite appropriate anticoagulant therapy (Class IIb). The progression of MS is very slow; thus, patients with MS limit their activity unconsciously and are often asymptomatic. Therefore, exercise testing with echocardiography is useful in determining the appropriate intervention. Intervention is reasonable for asymptomatic patients with moderate or severe MS when they become symptomatic or the transvalvular pressure gradient exceeds 15 mmHg or the PASP exceeds 60 mmHg during exercise stress228
(Class IIa). PTMC may be considered, irrespective of anatomical suitability, for critical symptomatic patients who are at high risk and contraindicated for surgery.229
Surgical intervention is recommended during open-heart surgery for other indications (severe MS: Class I, moderate MS: Class IIa, Mild MS: Class IIb).
The etiology of MS is predominantly rheumatic; however, nonrheumatic MS due to age-related calcification in the leaflets or annulus is seen in the elderly and hemodialysis patients.220
In such cases, PTMC is often contraindicated because it causes MS without commissural fusion. It remains controversial whether nonrheumatic MS progresses more slowly than rheumatic MS.220
5.3.2 Selection Criteria for PTMC and Surgery
PTMC is relatively less invasive and several randomized comparative studies have shown results comparable to those of OMC. Conversely, cases suitable for PTMC are also well suited to OMC. If the MR is more than mild, OMC may be chosen, though it depends on the experience of the surgeon. Patients for whom PTMC or OMC are deemed inappropriate are often indicated for valve replacement. The choice of PTMC versus surgical treatment is determined by a complex weighing of the anatomical suitability and the surgical risk (Table 22).
Table 22.
Choice of PTMC or Mitral Valve Surgery
| |
PTMC favorable |
PTMC possible |
PTMC inappropriate |
| Low surgical risk |
PTMC |
Surgery or PTMC |
Surgery |
Intermediate surgical
risk |
PTMC |
PTMC or Surgery |
Surgery |
High surgical risk or
inoperable |
PTMC |
PTMC |
Heart Team discussion (surgery
or PTMC or medical Tx) |
*Suitability for PTMC is determined by multiple factors such as Wilkins score, Iung score, existence of LA thrombus, and degree of MR. *Surgery: MVR or OMC is determined according to surgeons’ experience and patients’ clinical background. PTMC, percutaneous tansseptal mitral commissurotomy; MVR, mitral valve repair/replacement; OMC, open mitral commissurotomy.
The most important factor affecting the success or failure of PTMC is the valve morphology, and it is necessary to evaluate this in detail by TTE or other modalities. Evaluation of the valve morphology using the Wilkins score223
is well established, but physicians should also consider whether PTMC is appropriate by referring to other scoring systems such as the Iung score230
(Table 23). Although the mechanism of balloon dilatation of valve stenosis is considered to involve the splitting of the rheumatic, fused commissures and stretching of the entire valve orifice, the procedure may not have a proper effect and may lead to unfavorable splitting when the commissural fusion is very severe. In cases of restricted leaflet motion because of rheumatic changes or in cases of severe subvalvular lesion, the effect of valvotomy is limited. The pathophysiologies for which PTMC is inappropriate include (1) atrial thrombus, (2) MR >grade III, and (3) severe or bilateral commissural calcification. In cases of LA thrombus, dislodgement of the thrombus during the intervention is possible, and thus, these cases are strictly contraindicated for PTMC. PTMC should be performed following confirmation that the thrombus has been dissolved by anticoagulation therapy for 1–3 months. Associated MR >grade III may be exacerbated by PTMC and should undergo surgical treatment.
Table 23.
Scoring Mitral Valve Morphology for Suitability of PTMC
| Wilkins score |
Lung and Cormier score |
| Leaflet mobility (1–4)* |
Group 1: Pliable noncalcified anterior mitral leaflet and mild subvalvular
disease (thin chordae ≥10 mm long) |
| Leaflet thickneing (1–4)* |
Group 2: Pliable noncalcified anterior mitral leaflet and severe subvalvular
disease (thickened chordae <10 mm long) |
| Subvalvular ThickEning (1–4)* |
Group 3: Calcification of mitral valve of any extent, as assessed by
fluoroscopy, whatever the state of the subvalvular apparatus |
| Leaflet calcification (1–4)* |
|
<8 points: PTMC appropriate
>12 points: PTMC inappropriate |
Group 1: PTMC appropriate, Group 3: PTMC inappropriate |
*See Table 19. PTMC, percutaneous transseptal mitral commissurotomy. Produced by reference to Wilkins GT, et al. 1988,223 Bouleti C, et al. 2012.230
Although the majority of aforementioned nonrheumatic MS conditions are not suitable for PTMC based on anatomical requirements, some cases may involve a combination of rheumatic and nonrheumatic etiologies. Treatment strategies for such cases should be determined after considering the surgical risks.229
5.3.3 Treatment Selection for Restenosis After PTMC
MS recurrence because of commissural fusion after PTMC or surgical commissurotomy should also be considered for PTMC if the above anatomical conditions are met.229
Typically, commissural calcification is more pronounced in such cases, and the procedural risk is higher than in the previous PTMC. In such cases, valve replacement is usually selected. However, patients may be elderly or have multiple coexisting diseases, thus treatment suitability should be determined by taking a comprehensive approach with consideration of the surgical risk and anatomical requirements.
6. Medical Therapy and Follow-up
This section is omitted from the English version.
V. Aortic Regurgitation (AR)
1. Classification and Etiology
1.1 Valvular AR and AR Due to Aortic Root Dilatation
AR is caused by insufficient coaptation of the 3 cusps because of congenital or acquired abnormalities of the aortic valve cusps or the aortic root. The mechanism is structural change of the valve cusp itself such as rheumatic or age-related degenerative changes, or aortic root dilatation with normal cusps (Table 24). AR associated with a ventricular septal defect is caused by valve prolapse due to right coronary cusp herniation in patients with outlet type (supracristal type) despite no structural changes of the aortic valve itself. Outlet type (supracristal type) is commonly found in Asian populations and should be recognized as a congenital disease and a potential cause of AR. Uncommonly, AR can also be seen in patients with perimembranous ventricular septal defect. Valve prolapse can be seen in patients with not only congenital bicuspid valves, but also acquired abnormalities such as myxomatous change of the aortic valve cusp, rupture of a fenestration that is located in a marginal region of the aortic valve cusps, rupture of fibrous strands, and localized aortic dissection.231
Table 24.
Etiology and Mechanism of Aortic Regurgitation
| Mechanism |
Etiology |
| Congenital abnormalities |
Bicuspid, unicuspid or quadricuspid aortic valve |
| Acquired abnormalities |
Senile degeneration |
| Infective endocarditis |
| Rheumatic disease |
| Leaflet prolapse |
| Leaflet herniation associated with ventricular septal defect |
| Radiation-induced valvulopathy |
| Drug-induced valvulopathy |
| Carcinoid |
| Trauma |
Congenital/genetic aortic root
abnormalities |
Annuloaortic ectasia |
Connective tissue disease: Loeys Deitz syndrome, Ehlers-Danlos syndrome,
Marfan syndrome, osteogenesis imperfecta |
Acquired aortic root
abnormalities |
Idiopathic aortic root dilatation |
| Systemic hypertension |
Autoimmune disease: systemic lupus erythematosis, ankylosing spondylitis,
Reiter’s syndrome |
| Aortitis: syphilitid, Takayasu’s arteritis |
| Aortic dissection |
| Trauma |
Produced by reference to Zoghbi WA, et al. 2017.30
A functional classification of AR mechanisms has been proposed, and AR is classified as types I–III based on the cusp motion232,233
(Figure 13). In patients with type I, the cusp motion is normal, and AR is caused by aortic root dilatation or cusp perforation. This type can be subdivided: type Ia: dilatation from the sinotubular junction to the ascending aorta; type Ib: dilatation of the sinuses of Valsalva and the sinotubular junction; type Ic: dilatation of the ventriculoaortic junction; and type Id: cusp perforation. Type Ia is often caused by arteriosclerosis, type Ib is often caused by Marfan syndrome, type Ic is caused by arteriosclerosis or annuloaortic ectasia, and type Id is caused by infective endocarditis or trauma. Type II is due to leaflet prolapse, and type III is due to leaflet restriction, which may be found in bicuspid, degenerative, or rheumatic valvular disease as a result of calcification, thickening, and fibrosis of the aortic valves. These classifications are considered by surgeons for surgical procedures.

1.2 Acute and Chronic AR
Acute AR is caused by a sudden onset of insufficient valve closing resulting from infective endocarditis, aortic dissection, trauma, or post-catheter intervention. Acute AR is often severe, requiring an urgent diagnosis and intervention because the rapid increase in LV end-diastolic pressure causes pulmonary congestion and low CO. Conversely, in patients with chronic AR, the disease course is slow, with gradually increasing LV volume and pressure overload with LV adaptation via chamber dilatation and hypertrophy. Unlike in patients with acute AR, the onset of symptoms with chronic AR is late; thus, physicians should take care not to miss the appropriate timing for surgery.
2. Pathophysiology and Natural History
This section is omitted from the English version.
3. Assessment of Severity
AR is classified as mild, moderate, or severe and is evaluated primarily by TTE. Qualitative, semiquantitative, and quantitative assessments are available using TTE, and severity is finally determined in combination with each result. However, if the results differ among those indices and TTE alone is insufficient to determine AR severity, results from other modalities such as TEE, cardiac MRI, and cardiac catheterization should be combined to definitively determine the severity (Table 25).
Table 25.
Grading the Severity of Aortic Regurgitation (AR)
| |
AR severity |
| Mild |
Moderate |
Severe |
Transthorathic
echocardiography |
Structual
parameters |
Aortic leaflets |
Normal or mildly
abnormal |
Normal or mildly
abnormal |
Abnormal, flail, or
wide coaptation |
| LV size |
Normal |
Normal or
enlarged |
Enlarged (except
acute AR) |
Qualitative
parameters |
AR jet width, (color flow) |
Small in central
jets |
Intermediate |
Large in central
jets: variable in
eccentric jets |
| AR flow convergence, (color flow) |
None or very
small |
Intermediate |
Large |
| Jet density (color wave Doppler) |
Incomplete or
faint |
Dense |
Jet deceleration rate (color wave
Doppler), PHT, ms |
>500 |
500–200 |
<200 |
Diastolic flow reversal (descending
aorta) (pulse wave Doppler) |
Brief, early
diastolic reversal |
Intermediate |
Prominent holodiastolic
reversal |
Semiquantitative
parameters |
Vena contracta width (cm) |
<0.3 |
0.3–0.6 |
>0.6 |
Jet width/LVOT width (%) (central
jet only) |
<25 |
25–64 |
≥65 |
Jet area/LVOT area (short axis)
(%) (central jet only) |
<5 |
5–59 |
≥60 |
Quantitive
parameters |
Rvol (mL/beat): volumetric or
PISA |
<30 |
30–59 |
≥60 |
| RF (%): volmetric |
<30 |
30–49 |
≥50 |
| EROA (cm2): PISA |
<0.10 |
0.10–0.29 |
≥0.30 |
Transesopharyngeal
echocardiography |
Semiquantitative
parameters |
3D vena contracta width (cm) |
<0.3 |
0.3–0.6 |
>0.6 |
| Cardiac MRI |
|
RF (%): phase contrast |
<30 |
30–49 |
≥50 |
Cardiac
catheterization |
Aortography |
Sellers criteria |
I |
II |
III–IV |
EROA, effective regurgitant orifice area; LVOT, left ventricular outflow tract; PHT, pressure half time; PISA, proximal isovelocity surface area.
With TTE, the most frequent index for evaluating AR severity is the size of the AR jet (jet length and width) using the color Doppler method. This method is simple and easy to use, but tends towards underestimation in the case of an eccentric AR jet. Furthermore, AR severity evaluated by jet length is affected by diastolic blood pressure. The vena contracta method, which measures the width of the narrowest portion of the AR jet, is useful as a semiquantitative evaluation. In addition, the ratio of the width of the AR jet to the LV outflow tract and the ratio of area of the AR jet to the LV are also parameters of AR severity, but they are difficult to evaluate in cases of an eccentric jet or multiple jets. Quantitative evaluation includes the PISA and volumetric methods. The PISA method in AR is often difficult to measure in cases of severe calcification of the aortic valve or the presence of multiple jets. However, because the volumetric method is also affected by concomitant MR or an incorrect measurement of the annulus diameter, it is not optimal for determining AR severity using a single parameter.
It is also important to determine AR severity through combining the results of other modalities when the TTE results are inconclusive. TEE is generally better for evaluating aortic valve morphology than for AR severity, but it is possible to measure the vena contracta by 3D imaging for evaluation of AR severity. In addition, the phase-contrast method with cardiac MRI can be used to calculate regurgitant volume and fraction. The Sellers classification with aortography in cardiac catheterization is also an important method for determining the severity.
4. Diagnosis
4.1 Symptoms and Physical Findings
This section is omitted from the English version.
4.2 Echocardiography
TTE is an indispensable imaging test for assessing the severity and etiology of AR, evaluating LV size and function, and determining the timing of valve intervention (Class I).
4.2.1 Acute AR
Acute AR requires immediate identification of the severity and etiology, whereas severe AR is often difficult to diagnose because of the lack of LV enlargement and the attenuated color Doppler signal because of the rapid increase in LV end-diastolic pressure. Findings such as a shorter pressure half-time of the continuous-wave Doppler AR signal (<300 ms), shorter deceleration time of the mitral inflow E wave (<150 ms), and early closure of the mitral valve with subsequent diastolic MR indicate a marked rise in LV end-diastolic pressure.234
The degree of pulmonary hypertension can be estimated by the continuous-wave Doppler signal. Possible underlying causes of acute AR, such as infective endocarditis or aortic dissection, should be investigated, and TEE should be performed if diagnosis with TTE alone is difficult.
4.2.2 Chronic AR
The aortic valve/root morphology, etiology/severity of AR, size of the LV, and cardiac function should all be assessed.
Table 25
lists the key TTE indices. If TTE provides enough information, no other imaging tests are required for follow-up evaluation. Exercise stress echocardiography is useful for evaluating subclinical cardiac dysfunction or symptoms in asymptomatic patients with severe AR.28,45,235,236
Exercise stress echocardiography allows for evaluation of the LV contractile reserve, and lack of contractile reserve has been found to predict LV systolic dysfunction development postoperatively or at follow-up.237
In addition, exercise stress echocardiography can reveal another cause for symptoms, such as ischemic heart disease, functional MR, LV diastolic dysfunction, or pulmonary hypertension.45
TTE is a central diagnostic modality, and TEE is used for detailed evaluation of the leaflet, regurgitation, and aortic root morphology (Class I).238
In particular, in cases for which aortic valve repair or valve-sparing root replacement can be indicated, the cause of regurgitation should be clarified and detailed measurements are required to determine the suitability for repair surgery and the surgical design, including the selection of the annuloplasty ring or vessel graft type and size. The most important preoperative measurement is the leaflet length (geometric height). Insufficient geometric height is not suitable for aortic valve repair.239
Effective height is the vertical distance from the annular plane to the leaflet tip at leaflet closure and is an index of leaflet prolapse and tethering240
(Figure 14). The diameter of the annulus, sinus of Valsalva, and sinotubular junction are also measured. The diameter of the annulus is calculated by measuring the annular perimeter or area, because the annulus is not circular. Evaluation of the geometric height and effective height and root measurements may not be possible by the 2D approach, and the optimal cross-section should be extracted and measured using a 3D approach. Cardiac CT is also useful, with image resolution higher than that obtained with 3D TEE (Class IIa).241

4.3 Cardiac Catheterization
When the echocardiographic evaluation is suboptimal or when there is a discrepancy between the clinical assessment and the echocardiographic findings, cardiac catheterization is useful for evaluating the severity of both AR and hemodynamics (Class IIa).
The hemodynamic characteristics include wide pulse pressure. In severe AR, the LV diastolic pressure rises rapidly, and the LV pressure and aortic pressure are almost equal at end-diastole. AR severity is evaluated by the Sellers classification, which is based on the amount of contrast that appears in the LV after aortography. Left ventriculography allows for measurement of LVEF and LV size and is useful when other imaging modalities do not provide adequate information. Coronary angiography is used to assess coronary lesions, but it is not mandatory if the risk of coronary artery disease is low and coronary CT is negative for coronary lesions.
4.4 CT/MRI
Although it is possible to evaluate the aortic root and the ascending aorta by plain CT, detailed morphological evaluation of the leaflets and aortic root requires the use of contrast agents. Multidetector CT offers significantly improved spatial and temporal resolution and allows for evaluation of the coronary arteries and cardiac function in addition to evaluation of the aortic valve complex. Cine MRI allows imaging with a high contrast between the blood and the myocardium without the use of contrast agents,242
enabling accurate measurement of the size of the LV and LVEF.243
In addition, a phase-contrast method can be used to calculate the blood flow in the aortic root to determine the regurgitant volume and the regurgitant fraction. Thus, MRI is a useful modality in cases of suboptimal echocardiographic images for assessment of the severity of AR, LV function, and LV size (Class IIa). Cardiac MRI with late gadolinium enhancement has been established as a method of detecting myocardial fibrosis. It has been reported that a greater amount of myocardial fibrosis quantified using delayed-contrast MRI was associated with a worse prognosis after aortic valve replacement.244
However, there is limited evidence to support this finding.
Table 26
lists the recommendations for diagnostic testing for AR.
Table 26.
Recommendations for Diagnostic Testing for Aortic Regurgitation (AR)
| Recommendations |
COR |
LOE |
TTE is indicated for patients with signs or symptoms of AR to evaluate the etiology and
severity of regurgitation, LV size and systolic function, and to determine the timing of
intervention |
I |
B |
TTE is indicated for patients with dilated aortic sinuses or ascending aorta or with a bicuspid
aortic valve to evaluate the presence and severity of AR |
I |
B |
TEE is recommended for patients with moderate or severe AR and suboptimal TTE
images for the assessment of etiology or severity of AR |
I |
B |
TTE is indicated for patients with a bicuspid aortic valve to evaluate valve morphology, the
severity of AS and AR, and to assess the shape and diameter of the aortic sinuses and ascending
aorta for prediction of clinical outcome and to determine the timing of intervention |
I |
B |
Aortic magnetic resonance angiography or CT angiography is indicated for patients with a
bicuspid aortic valve when morphology of the aortic sinuses, sinotubular junction, or
ascending aorta cannot be assessed accurately or fully by TTE |
I |
C |
Cardiac MRI is reasonable for patients with moderate or severe AR and suboptimal TTE
images for the assessment of etiology or severity of regurgitation |
IIa |
B |
Cardiac catheterization is reasonable for patients with AR to assess hemodynamics and
severity of regurgitation if TTE images are suboptimal for the assessment of AR and/or
there is a discrepancy between clinical symptoms and echocardiographic findings |
IIa |
C |
Cardiac CT is reasonable for patients undergoing aortic valve repair to evaluate
leaflets and aortic root |
IIa |
C |
AS, aortic stenosis; COR, Class of Recommendation; CT, computed tomography; LOE, Level of Evidence; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
5.1 Surgical Procedures and Results
This section is omitted from the English version.
5.2 Indications and Timing of Surgery (Figure 15, Table 27)

Table 27.
Indications of Surgery for Chronic Aortic Regurgitation (AR)
| Recommendations |
COR |
LOE |
| Surgery is recommended for symptomatic patients with severe AR |
I |
B |
| Surgery is recommended for asymptomatic patients with severe AR with LVEF <50% |
I |
B |
Surgery is recommended for patients with severe AR undergoing CABG or surgery of the
ascending aorta or of other heart valves |
I |
C |
Surgery is reasonable for asymptomatic patients with severe AR with preserved LVEF
(≥50%) but with LVESD >45 mm |
IIa |
B |
Surgery is reasonable for patients with moderate AR undergoing CABG or surgery of
the ascending aorta or of other heart valves |
IIa |
C |
Surgery may be considered for asymptomatic patients with severe AR with preserved LVEF
(≥50%) but with LVEDD >60 mm |
IIb |
C |
Surgery may be considered after careful follow-up of asymptomatic patients with severe AR
with LVEF ≥50% but with LVESDI >25 mm/m2
|
IIb |
C |
CABG, coronary artery bypass grafting; COR, Class of Recommendation; LOE, Level of Evidence; LVEDD, LV end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESDI, LV endsystolic dimension index.
5.2.1 Acute AR
Considering the causes (e.g., infective endocarditis, aortic dissection, or traumatic valve destruction), acute AR is often difficult to treat medically, thus physicians should consider performing surgery as soon as possible.
5.2.2 Chronic AR
The surgical indications for chronic AR are determined by the presence of symptoms, LVEF reduction, LV dilatation, and performing other open-heart surgery (Figure 15).
a. Symptoms
When symptoms due to severe AR appear, aortic valve surgery is indicated regardless of LV function and size (Class I), except in cases of high surgical risk and contraindications for the procedure.27,245
Even in patients with preoperative severe LV dysfunction, surgery is recommended with maximal medical therapy before the operation.246–249
b. LV Dysfunction: LVEF <50%
Early aortic valve surgery is indicated in asymptomatic patients with severe AR and LV dysfunction (LVEF <50%) if other causes of LV dysfunction such as coronary artery disease can be ruled out (Class I). In patients with LV dysfunction due to AR, surgery improves the postoperative prognosis compared with waiting for the appearance of symptoms or progression of LV dilatation.246,247,250–252
Conversely, as mentioned above, physicians should consider whether the cause of LV dysfunction is AR, and it is important when using this criterion of LVEF to exclude the other causes of LV dysfunction. In patients with severe LV dysfunction (LVEF <30%), surgical indications should be determined for each case based on surgical risk, degree of myocardial damage, and the clinical course of the patient.
c. LV Dilatation: LVESD >45 mm, LVEDD >65 mm
LVESD is a more useful index than LVEDD for surgical indication in patients with asymptomatic severe AR and preserved LV function (LVEF ≥50%). This is because an increase in afterload as well as in preload is the main factor in the LV dysfunction and dilatation in patients with chronic severe AR. In Europe and the USA, it has been often reported that LVESD >50 mm is a reasonable cutoff value when considering the postoperative prognosis.253–255
However, some studies in Japan have reported that LVESD <50 mm is a reasonable cutoff value for Japanese.250,256
Therefore, considering body size, it is appropriate to use a cutoff value for LVESD of 45 mm as a surgical indication (Class IIa) in this guideline.
Conversely, regarding LVESD with a BSA correction, the LVESD index of 25 mm/m2
is used in the ESC and ACC/AHA guidelines, which is different from the LVESD index of 28 mm/m2
calculated from the LVESD cutoff value of 45 mm in the present guideline and the average BSA of 1.6 m2
for Japanese.250,256
In a Japanese study, an LVESD index of 26.7 mm/m2
was used as the cutoff value for the occurrence of postoperative LV dysfunction.256
However, as discussed in CQ2, considering several reports on the LVESD index of 25 mm/m2, an LVESD index of 25 mm/m2
is a warning criterion for detailed follow-up,27,257–259
and the surgical indication related to the LVESD index value is not Class IIa, but Class IIb.
Regarding LVEDD, there is little evidence for surgical indications, and it has been reported in a Japanese study that the LVEDD was not associated with postoperative prognosis.250
In the present guideline, an LVEDD >65 mm is used as a criterion for surgery (Class IIb), considering the difference in average body size between Western and Japanese populations.250,255,260
d. Indications for Other Open-Heart Surgeries Such as Ascending Aortic Replacement, Coronary Artery Bypass, and Mitral Valve Surgery
When other open-heart procedures such as an ascending aortic replacement, coronary artery bypass, or mitral valve surgery are performed, aortic valve surgery is indicated in severe AR (Class I) and should be considered in moderate AR (Class IIa) to prevent perioperative hemodynamic failure due to AR and avoid reoperation for AR in the near future. In particular, with regard to ascending aortic dilatation, physicians need to follow up with a focus on the increase of the ascending aortic diameter as well as the surgical indications for AR itself in patients with a bicuspid valve or Marfan syndrome. The surgical indications depend mainly on the ascending aorta and aortic root dilatation and are described in the following sections.
CQ2
Should early surgery be conducted for asymptomatic patients with severe AR, LVEF ≥50%, and LVESD index >25 mm/m2?
[Conclusion]
Early surgery may be considered for asymptomatic patients with severe AR, an LVEF ≥50%, and an LVESD index >25 mm/m2 (2C).
[Strength of Recommendation]
2: Weakly recommended (proposed)
[Strength of Evidence]
C (weak): Limited confidence in the estimated effect
For asymptomatic severe AR, LVEF <50% is a Class I indication and LVEF >50% with obvious LV enlargement is a Class IIa indication for surgical intervention.29,261
Studies have shown that a BSA-corrected LVESD (LVESD index) is more important for prognostic prediction than the single value of LVESD.27,257
Dujardin et al27
reported a worse prognosis with LVEF <55% (5.8% risk/year) or an LVESD index >25 mm/m2
(7.8% risk/year) in asymptomatic patients. Sambola et al257
reported that the significant predictive power of BSA-corrected LV size was observed only in those with a BSA <1.68 m2. Only 23% of the patients in the study by Sambola et al were asymptomatic, versus 50% of the patients in the study by Dujardin et al.
The ACC/AHA 2014 guidelines29
state that LVESD >50 mm is a Class IIa indication for SAVR, and LVEDD >65 mm is a Class IIb indication. However, those guidelines also suggest that it is appropriate to consider BSA-corrected values in females or smaller patients. The 2017 ESC/EACTS guidelines261
state that Class IIa is warranted for LVEDD >70 mm or LVESD >50 mm. For smaller patients with BSA <1.68 m2, a LVESD index >25 mm/m2
is also indicated as Class IIa.
Mentias et al258
investigated patients with severe AR and normal EF, using the data of 933 patients from the Cleveland Clinic who underwent SAVR and 484 conservatively treated patients. Among the total cases, 87% were asymptomatic. For patients with a LVESD index of 20–25 mm/m2, which is below the conventional threshold, long-term survival was better in the surgery group than in the non-surgery group. Among the study population, 75% were males in their late 50 s or older with an average BSA of ≈2.0 m2. It should be noted that the study population had a relatively large body size, but this is the first study showing that surgery may be preferable even for patients with a LVESD index <25 mm/m2. In addition, Yang et al259
studied patients with severe AR and compared 361 patients who underwent SAVR and 387 conservatively treated patients. Among the total cases, 58% were asymptomatic. Multivariate analysis revealed a significant correlation between prognosis and a LVESD index >25 mm/m2
as well as conventional Class I indications. Maeda et al262
analyzed 268 patients with severe AR (162 asymptomatic patients). Patients with a LVESD index <25 mm/m2
and LVEDD <65 mm (61 cases) demonstrated superior prognoses after 10 years as compared with patients with a LVESD index >25 mm/m2
or LVEDD >65 mm (101 cases). This was a Japanese observational study with the average age being over 55 years, and males constituting a 70–80% proportion with a mean BSA of 1.63 m2.
There are no RCTs comparing early surgical intervention and conservative treatment for patients with asymptomatic severe AR with normal LVEF and LV enlargement. The number of observational studies is also limited. There is no study regarding the optimal cutoff value of the LVESD index. All of the cited studies were retrospective, and therefore the evidence class was considered to be 2C.
6. Indications and Timing of Surgery for Aortic Dilatation and Specific Diseases (e.g., Marfan Syndrome, Bicuspid Valve) (Table 28)
Table 28.
Indications of Surgery for Aortic Root Disease
| Recommendations |
COR |
LOE |
| A. Tricuspid valve |
A-1. When surgey is not
indicated for AR itself: |
Replacement of the aortic root or tubular ascending aorta is
reasonable in the presence of a maximal ascending aortic
diameter ≥55 mm |
IIa |
B |
Replacement of the aortic root or tubular ascending aorta is
reasonable in the presence of a maximal ascending aortic
diameter <55 mm and aortic size increase >5 mm per year |
IIa |
C |
A-2. When surgery is indicated
for severe AR: |
Replacement of the aortic root or tubular ascending aorta
concommitant with aortic valve surgery is reasonable in the
presence of a maximal ascending aortic diameter ≥50 mm |
IIa |
C |
| B. Bicuspid valve |
B-1. When surgey is not
indicated for AR itself: |
Replacement of the aortic root or tubular ascending aorta is
reasonable in the presence of a maximal ascending aortic
diameter ≥50 mm with additional risk factors* or coarctation or
in younger patients |
IIa |
C |
Replacement of the aortic root or tubular ascending aorta is
reasonable in the presence of a maximal ascending aortic
diameter ≥55 mm without additional risk factors* or coarctation |
IIa |
C |
B-2. When surgery is indicated
for severe AR: |
Replacement of the aortic root or tubular ascending aorta
concommitant aortic valve surgery is reasonable in the
presence of a maximal ascending aortic diameter ≥45 mm |
IIa |
C |
| C. Marfan syndrome |
C-1. When surgey is not
indicated for AR itself: |
Replacement of the aortic root or tubular ascending aorta is
recommended in the presence of a maximal ascending aortic
diameter ≥50 mm |
I |
C |
Replacement of the aortic root or tubular ascending aorta is
reasonable in the presence of a maximal ascending aortic
diamater ≥45 mm with additional risk factors* or a TGFBR1 or
TGFBR2 mutation (including Loeys-Dietz syndrome) |
IIa |
C |
C-2. When surgery is indicated
for severe AR: |
Replacement of the aortic root or tubular ascending aorta
concommitant with aortic valve is recommended in the presence
of a maximal ascending aortic diameter ≥45 mm |
I |
C |
*Additional risk factors: family history of aortic dissection or spontaneous vascular dissection, severe AR or MR, desire for pregnancy, systemic hypertension and/or aortic size increase >3 mm per year. AR, aortic regurgitation; COR, Class of Recommendation; LOE, Level of Evidence; MR, mitral regurgitation.
6.1 Ascending Aortic Dilatation With a Tricuspid Aortic Valve
6.1.1 When Surgery Is Not Indicated for AR
Regardless of AR severity or aortic valve morphology, a maximum ascending aortic diameter ≥55 mm suggests that a preventative ascending aortic replacement should be considered263,264
(Class IIa). Preventative ascending aortic replacement should be considered in patients with >5 mm/year increase of maximum ascending aortic diameter even if the maximum ascending aortic diameter is <55 mm (Class IIa). Moreover, concomitant aortic valve surgery should be considered if the AR severity is more than moderate (Class IIa).
6.1.2 When Surgery Is Indicated for Severe AR
If surgery is indicated for severe AR, simultaneous ascending aortic surgery should be considered in patients with a maximum ascending aortic diameter ≥50 mm (Class IIa). However, if the patient is elderly or the overall invasiveness and risk of surgery with an additional procedure is high, the indications for an ascending aortic replacement should be discussed by the Heart Valve Team.
6.2 Ascending Aortic Enlargement With a Bicuspid Aortic Valve
6.2.1 When Surgery Is Not Indicated for AR
In the presence of a bicuspid valve with additional risk factors or coarctation, an ascending aortic replacement should be considered in patients with an ascending aortic diameter ≥50 mm (Class IIa).265
Additional risk factors include a family history of aortic dissection, personal history of spontaneous vascular dissection, severe AR or MR, desire for pregnancy, systemic hypertension, and/or an aortic size increase >3 mm/year. Moreover, concomitant aortic valve surgery should be considered if the AR severity is more than moderate (Class IIa).
6.2.2 When Surgery Is Indicated for Severe AR
If surgery is indicated for severe AR, simultaneous ascending aortic surgery should be considered in patients with a maximum ascending aortic diameter ≥45 mm (Class IIa).
6.3 Ascending Aortic Enlargement in Marfan Syndrome
6.3.1 When Surgery Is Not Indicated for AR
In the presence of Marfan syndrome, a preventative ascending aortic replacement is indicated regardless of background diseases in patients with an ascending aortic diameter ≥50 mm (Class I).266
In the presence of additional risk factors or genetic mutations, including Loeys–Dietz syndrome, an ascending aortic replacement should be considered for an ascending aortic diameter ≥45 mm (Class IIa).267
Additional risk factors include a family history of aortic dissection, personal history of spontaneous vascular dissection, severe AR or MR, desire for pregnancy, systemic hypertension, and/or an aortic size increase >3 mm/year. Moreover, among females with a small BSA or with the presence of a
TGFBR2
mutation (related to familial thoracic aortic aneurysm or dissection) or severe extra-aortic features, an ascending aortic replacement may be considered in those with a maximum ascending aortic diameter ≥40 mm (Class IIb).267,268
Concomitant aortic valve surgery should be considered if the AR severity is more than moderate (Class IIa).
6.3.2 When Surgery Is Indicated for Severe AR
If surgery is indicated for severe AR, simultaneous ascending aortic surgery is indicated in patients with a maximum ascending aortic diameter ≥45 mm (Class I). Moreover, among females with a small BSA or with the presence of a
TGFBR2
mutation (related to familial thoracic aortic aneurysm or dissection) or severe extra-aortic features, an ascending aortic replacement may be considered in those with a maximum ascending aortic diameter of ≥40 mm (Class IIb).267,268
6.4 Consideration of Body Size
In patients with a low BSA, slightly earlier timing for the surgery than that with the above criteria should be considered. In addition, serial dilatation of the aortic diameter should be taken into consideration. However, there is no evidence for criteria regarding smaller diameter or lower progression rate, and thus this is not defined in this guideline.
7. Medical Therapy and Follow-up
This section is omitted from the English version.
VI. Aortic Stenosis (AS)
1. Etiology
The primary etiology of AS varies greatly depending on the region and age. In developed countries such as Japan, where the average life expectancy is longer, age-related degeneration of the aortic valve accounts for more than 80% of severe AS requiring surgery.122
Rheumatic AS, which was once frequently seen, hardly occurs nowadays because adequate treatment for rheumatic fever is provided in childhood. Because age-related degeneration of the aortic valve is the primary etiology in developed countries, the frequency of AS increases with age. Although the incidence of severe AS in patients aged <70 years is <1%, it is approximately 7% in patients aged ≥80 years.123,269,270
Causes other than age-related and rheumatic AS include congenital factors such as unicuspid, bicuspid, and quadricuspid valves. Among these, bicuspid valves occur most frequently, with a prevalence of 0.5–2% and a sex ratio of 3:1, weighted towards males.271
Previous studies have reported that there are more patients with bicuspid aortic valves272
than with tricuspid valves among patients aged <70 years who receive aortic valve replacement. The prevalence of unicuspid valves is approximately 1/10 to 1/30 of that of bicuspid valves,272,273
and quadricuspid valves are very rare.274
2. Pathophysiology and Natural History (Table 29)25,275–278
Table 29.
Previous Reports on Prognosis of Asymptomatic Patients With Severe Aortic Stenosis (AS)
Reference
number
(published
year) |
Definition of
severe AS |
No.
subjects |
Male
(%) |
Mean
age
(years) |
Outcome |
Survival rate without events (%) |
| 1 year |
2 years |
3 years |
4 years |
5 years |
6 years |
7 years |
8 years |
275
(2000) |
Vmax ≥4 m/s |
128 |
53.9 |
60±18 |
All-cause
death or
AVR |
67±5 |
56±5 |
|
33±5 |
|
|
|
|
25
(2005) |
Vmax ≥4 m/s |
622 |
61.7 |
72±11 |
Cardiac
death or
AVR |
80 |
63 |
|
|
25 |
|
|
|
276
(2010) |
Vmax ≥5 m/s |
116 |
50.9 |
67±15 |
Cardiac
death or
AVR |
64±4 |
36±5 |
25±4 |
12±4 |
|
3±10 |
|
|
278
(2010) |
AVA ≤0.75 cm2 and
Vmax ≥4.5 m/s or m
PG ≥50 mmHg |
95 |
46.3 |
63±12 |
Cardiac
death or
AVR |
|
71±5 |
|
47±5 |
|
28±6 |
|
|
277
(2018) |
AVA <1.0 cm2 |
861 |
57.7 |
72±12 |
All-cause
death or
AVR |
|
54±2 |
|
32±3 |
|
|
|
12±3 |
279
(2015) |
AVA <1.0 cm2 or
Vmax >4.0 m/s or
mPG >40 mmHg |
1,517 |
39.8 |
77.8±9.4 |
All-cause
death |
87.7 |
|
72.4 |
|
58.3 |
|
|
|
AVA, aortic valve area; AVR, aortic valve replacement; mPG, mean pressure gradient; Vmax, maximum velocity.
This section is omitted from the English version.
3. Assessment of Severity (Table 30)
Table 30.
Grading the Severity of Aortic Stenosis (AS)
| |
AS |
Mild AS |
Moderate AS |
Severe AS |
Very severe AS |
| Vmax (m/s) |
≤2.5 |
2.6–2.9 |
3.0–3.9 |
≥4.0 |
≥5.0 |
| Mean PG (mmHg) |
– |
<20 |
20–39 |
≥40 |
≥60 |
| AVA (cm2) |
– |
>1.5 |
1.0–1.5 |
<1.0 |
<0.6 |
| Indexed AVA (cm2/m2) |
– |
>0.85 |
0.60–0.85 |
<0.6 |
– |
| Velocity ratio |
– |
>0.50 |
0.25–0.50 |
<0.25 |
– |
AVA, aortic valve area; PG, pressure gradient; Vmax, maximum velocity; Velocity ratio, ratio of velocity at LV outflow tract to maximum velocity.
When a limitation to the opening of the aortic valve is confirmed, an AVA ≥1.0 cm2
corresponds to mild or moderate AS. Severe AS is defined by an AVA of <1.0 cm2
or an AVA indexed to BSA of <0.6 cm2/m2.
Severe AS can be roughly divided into a high-pressure gradient type, with a peak transvalvular velocity ≥4.0 m/s and a mean pressure gradient ≥40 mmHg, and a low-pressure gradient type with a peak velocity <4.0 m/s and a mean pressure gradient <40 mmHg. Low-flow/low-gradient severe AS is further divided into 2 types: low stroke volume with reduced LVEF or preserved LVEF and small LV cavity. Low-flow/low-gradient severe AS should be differentiated from pseudo-severe AS, which is discussed later (see section
VI.4.4).
In addition to the objective data, patients with severe AS are classified as symptomatic or asymptomatic according to whether they have symptoms due to AS such as HF symptoms, chest pain, and syncope. Symptomatic patients have worse prognoses than asymptomatic cases and should be considered more carefully.
4. Diagnosis and Follow-up
4.1 Symptoms and Physical Findings
This section is omitted from the English version.
4.2 Echocardiography
The diagnostic and severity assessments are primarily performed by echocardiography. Valve morphology (number of valve leaflets, presence of cusp fusion), the presence, distribution, and degree of calcification, and the valve opening are evaluated by TTE. When the findings indicate AS, the Doppler method should be used to assess severity (Class I). AVA is calculated using the continuity equation, for which measurements of peak transvalvular velocity, LV outflow tract flow velocity, and diameter are needed. The transvalvular velocity and pressure gradient are simple indices but may change due to hemodynamics; therefore, the AVA is also required for assessing severity. The AVA is also measured using the planimetry method, but it is often difficult to trace accurately if there is calcification. The Doppler beam must be parallel to the direction of the blood flow for accurate velocity measurements. In addition to the apical approach, multiple windows such as the right parasternal, the upper intercostal, and the suprasternal or other approaches should be considered to obtain the highest velocity. In particular, if the angle between the ascending aorta and the LV axis is relatively sharp (≤115°), as is the case with the sigmoid septum, a right parasternal approach is often useful for recording the peak velocity.280
LV function, wall thickening, dilatation of the proximal portion of the ascending aorta, other valve diseases, and the presence of pulmonary hypertension should also be evaluated. Because the bicuspid aortic valve may involve aortic lesions (dilatation, aneurysm, dissection), the aorta must be observed within the visible range. Grading for severity is shown in
Table 30.34
Other parameters, such as valvuloarterial impedance and aortic valve resistance, are also important, but they are not routinely measured.34
The valve area measured by the continuity equation tends to be smaller than the valve area obtained by cardiac catheterization. One reason for this is the pressure recovery phenomenon, whereby the hydrostatic pressure rises at the distal site of stenosis, and is theoretically observed when the valve opening is not orifice-like or when the ascending aorta is small (<30 mm in diameter), resulting in no significant distal turbulence. However, in calcific AS, which accounts for the majority of cases, the valve opening is usually considered orifice-like and turbulent flow is prominent; therefore, the valve area is less affected by the pressure recovery phenomenon. A formula for correcting for the pressure recovery phenomenon has also been proposed,34
and should be considered in the case of congenital AS, a small ascending aorta, or when there is a discrepancy between the valve area measured by the continuity equation and the clinical findings. TEE is recommended when the image quality of the TTE is poor and the TTE assessment is inconclusive (Class IIa). TEE is useful for evaluating the number of valve leaflets, valve morphology, calcification distribution, and dilatation of the ascending aorta.
Stress echocardiography should be performed when the findings of TTE at rest are inconclusive for an assessment of severity (Class IIa). Exercise stress echocardiography is reasonable for asymptomatic or equivocally symptomatic patients with severe AS to evaluate symptoms. In addition, subsequent cardiovascular events have been reported when the mean transvalvular pressure gradient exceeds 20 mmHg or the PASP exceeds 60 mmHg during exercise.281
In low-flow/low-gradient severe AS, dobutamine stress echocardiography is recommended for distinguishing between true-severe AS and pseudo-severe AS (Class IIa). Further details are provided in section
V.4.4.
4.3 Cardiac Catheterization, CT, and MRI
Cardiac catheterization is important for a preoperative diagnosis of AS. Coronary artery disease concomitant with AS should be closely assessed when determining the treatment strategy, and coronary angiography is the standard diagnostic method to achieve this.282
It is important to evaluate the presence of myocardial ischemia in patients with AS with coronary angiography and recognize the stenosis, though any interpretation should be made with caution in the presence of LV hypertrophy.283
A fractional flow reserve measurement may be difficult to perform in patients with AS because of concerns about blood pressure drop induced by coronary vasodilators. However, a nonpharmacological method has been recently reported to be useful.284
In addition, when the patient is elderly, or the risk of coronary angiography is high, coronary CT can be performed more safely with a high negative predictive value.66
Coronary angiography does not necessarily need to be performed before surgery if the patient does not have chest pain and the coronary CT shows no significant stenosis.
The concept of low-flow/low-gradient severe AS has been advanced in recent years, and an accurate severity assessment is important in determining the AS treatment. If severity is difficult to determine by echocardiography, hemodynamic assessment with a right cardiac catheter and simultaneous aortic and LV pressure measurements may be useful (Class IIa). Calcification scores based on cardiac CT are also useful in determining the severity.62,285
Cardiac CT with ECG synchronization is useful for accurate valve morphology evaluation (especially bicuspid valves) (Class IIa).67
Evaluation of the aortic valve and aortic root are also important in the evaluation of the procedural risk of TAVI, as well as determining the type and size of valve and the approach (Class IIa).63–65
Cardiac MRI may be useful in the detection and quantification of fibrosis in the myocardium.244
CT is useful for detecting valve thrombosis and vascular complications after SAVR and TAVI.68–70,286
Table 31
lists the recommendations for diagnostic testing for AS.
Table 31.
Recommendations for Diagnostic Testing for Aortic Stenosis (AS)
| Recommendations |
COR |
LOE |
TTE is indicated for patients with known or suspected AS to evaluate severity and ethiology
of AS, LV size and LV function |
I |
B |
Periodic TTE is indicated for patients with moderate AS to evaluate severity of stenosis
and for asymptomatic patients with severe AS to decide the timing of intervention |
I |
B |
Cardiac CT is indicated for patients who are candidates for TAVI to evaluate anatomy of
aortic valve and aortic root |
I |
C |
Low-dose dobutamin stress echocardiography is reasonable to diagnose severity of stenosis
and to evaluate contractile reserve for patients with low-flow/low-gradient AS and reduced LVEF |
IIa |
B |
Exercise stress test is reasonable for asymptomatic patients with severe AS to
assess symptoms |
IIa |
C |
Exercise stress echocardiography is reasonable for asymptomatic patients with severe AS or
symptomatic patients with moderate AS to evaluate symptoms and hemodynamics during
exercise |
IIa |
C |
Cardiac catheterization is reasonable for patients with AS to evaluate severity of stenosis
and hemodynamics if TTE assessment is inconclusive or there is a discrepancy between
TTE and clinical findings |
IIa |
C |
TEE is reasonable to evaluate aortic valve and aortic root if TTE assessment is inconclusive
due to technical difficulty |
IIa |
C |
Cardiac CT is reasonable for patients with low-flow/low-gradient AS with preserved EF to
evaluate calcification of aortic valve |
IIa |
C |
COR, Class of Recommendation; CT, computed tomography; EF, ejection fraction; LOE, Level of Evidence; LV, left ventricle; TAVI, transcatheter aortic valve implantation; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
4.4 Low-Flow/Low-Gradient AS
The diagnosis of severe AS is generally based on the AVA (<1.0 cm2), the mean transvalvular pressure gradient (≥40 mmHg), and peak transvalvular velocity (≥4.0 m/s).
If the measurements of valve area and pressure gradient/velocity are concordant, the assessment of severity is easy. However, these measurements are often discordant.287
In addition to possible measurement error, the velocity/pressure gradient may change depending on hemodynamics. Therefore, the pressure gradient does not increase if the stroke volume is low in severe AS, which is known as low-flow/low-gradient severe AS. Low-flow conditions include afterload mismatch, myocardial damage, and concomitant cardiac disease where the LVEF is reduced to <50%. Another condition is characterized by LVEF >50% but with a hypertrophied small LV leading to reduced stroke volume (paradoxical low-flow severe AS).
4.4.1 Low-Flow/Low-Gradient Severe AS With Reduced LVEF (<50%)
In patients with low stroke volume due to LV systolic dysfunction and an AVA <1.0 cm2, dobutamine stress echocardiography can help distinguish between true-severe AS and pseudo-severe AS.288
When dobutamine infusion (maximum dose: 20 μg/kg/min) increases stroke volume by >20%, true-severe AS is diagnosed if the AVA is <1.0 cm2
and the peak velocity is >4.0 m/s or the mean pressure gradient is >30–40 mmHg. A diagnosis of pseudo-severe AS is made if the AVA is >1.0 cm2. If the stroke volume does not increase by >20% after the administration of dobutamine, it indicates the absence of contractile reserve. In this condition, it is difficult to distinguish between true-severe AS and pseudo-severe AS. Although the calcification score obtained by CT is a guide to such differentiation,289
the prognosis in this group has been shown to be poor even when SAVR is performed. However, recent reports indicate that the prognosis following TAVI is not influenced by the presence or absence of contractile reserve.290
Further consideration should be given to surgical indications in patients without contractile reserve.
4.4.2 Low-flow/Low-Gradient Severe AS With Preserved LVEF (≥50%) (Paradoxical Low-Flow Severe AS)
Paradoxical severe AS is defined as an AVA <1.0 cm2, peak velocity <4.0 m/s, and a mean pressure gradient <40 mmHg despite preserved LVEF. A hypertrophied and small LV is characteristic of this group, resulting in low stroke volume (stroke volume index <35 mL/m2). The prognosis for this disease varies in published reports and is controversial.291–294
In cases of low-gradient severe AS with preserved LVEF, the prognosis has been reported to vary greatly depending on whether the stroke volume index is maintained or decreased.295
Therefore, in order to diagnose this group of patients, it is important to ensure that there are no errors in the measurement of stroke volume index or AVA. Many cases are accompanied by a history of hypertension. We should comprehensively determine whether or not true-severe AS using the calcification score,62
the degree of LV hypertrophy,296
relatively high mean pressure gradient (30–40 mmHg), and small valve area (AVA <0.8 cm2).
Figure 16
presents a flowchart for AS severity assessment.
5. Surgical and Transcatheter Treatment
5.1 Surgical Procedures and Results
This section is omitted from the English version.
5.2 Current Status of Transcatheter Treatment
This section is omitted from the English version.
5.3 Indications and Timing of Intervention (Figure 17, Table 32)

Table 32.
Indications of Intervention for Aortic Stenosis (AS)
| Recommendations |
COR |
LOE |
| Intervention (SAVR or TAVI) is recommended for symptomatic patients with severe AS |
I |
B |
Intervention (SAVR or TAVI) is recommended for asymptomatic patients with severe AS and
LV systolic dysfunction (LVEF <50%) |
I |
C |
SAVR is recommended for asymptomatic patients with severe AS undergoing other
cardiac surgery |
I |
C |
Intervention (SAVR or TAVI) is recommended for asymptomatic patients with severe AS and
symptoms on exercise test |
I |
C |
Intervention (SAVR or TAVI) is reasonable in asymptomatic patients with severe AS and a
decrease in blood pressure on exercise test |
IIa |
C |
Intervention (SAVR or TAVI) is reasonable for asymptomatic patients with very severe AS
(Vmax ≥5 m/s, mPG ≥60 mmHg, or AVA <0.6 cm2) at low surgical risk* |
IIa |
B |
Intervention (SAVR or TAVI) is reasonable for asymptomatic patients with severe AS and
severe pulmonary hypertension (PASP at rest ≥60 mmHg) due to AS at low surgical risk* |
IIa |
C |
Intervention may be considered for asymptomatic patients with severe AS and a
rapid progression (Vmax increase ≥0.3 m/s per year) at low surgical risk* |
IIb |
C |
SAVR is reasonable for asymptomatic patients with moderate AS undergoing other cardiac
surgery** |
IIa |
C |
*Risk of procedure (SAVR or TAVI) is considered to be low based on patient’s characteristics and anatomy. **Should be decided after discussion with Heart Valve Team, taking into account age and surgical risk. AVA, aortic valve area; COR, Class of Recommendation; LOE, Level of Evidence; LVEF, left ventricular ejection fraction; mPG, mean pressure gradient; PASP, pulmonary artery systolic pressure; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; Vmax, peak velocity.
5.3.1 Indications for Intervention for Symptomatic Severe AS
Intervention is recommended for patients diagnosed with symptomatic severe AS unless they have contraindications to the intervention or predicted survival <1 year (Class I). Cases of AS with angina, dyspnea, or syncope have a poor survival prognosis,297
and surgical treatment is expected to improve symptoms, survival, and exercise tolerance.297–300
Symptomatic AS also carries a risk of sudden death, thus surgical intervention is required when symptoms occur.
SAVR for severe AS has been the gold standard treatment and the first choice for younger patients with lower surgical risk, conferring a positive long-term prognosis.299,300
However, with the clinical application of TAVI since 2002, it has become necessary to decide whether SAVR or TAVI should be selected. Although TAVI was initially limited to high-risk cases among the elderly,8,297,301
the adoption of TAVI has increased as the technology has advanced and the procedure has been further improved. In 2017, 2 RCTs comparing TAVI and SAVR were conducted with intermediate-risk patients and demonstrated the clinical outcomes and survival with TAVI as superior to those of SAVR.302–304
In 2019, 2 more RCTs on TAVI and SAVR with low-risk patients were published that demonstrated the superior or noninferiority of TAVI to SAVR with regard to composite endpoints such as death, cerebral infarction, and heart failure-associated hospital readmission.305,306
The 30-day mortality rate of isolated SAVR in Japan was 1.9% and that of re-do surgery for AVR was 5.0%.307
The 30-day mortality rate of TAVI in Japan was ≤2% despite the fact that TAVI is indicated for higher-risk groups such as the elderly and patients with a history of previous open-heart surgery.308,309
On the other hand, the current challenge for TAVI is the lack of long-term bioprosthesis durability data over a period >10 years. Accordingly, the ESC/EACTS guidelines set the age cutoff at 75 years, and the average age of the patients in the 2 low-risk RCTs was 73–74 years. In Japan, the average life expectancy is known to be 3–5 years longer than that in Europe and the USA, and life expectancy of the elderly in Japan aged ≥75 years is 1–2 years longer than that in the USA and Europe.
The selection of SAVR or TAVI should be done after considering the patient’s age, the durability data available for each surgical valve or TAVI valve, surgical risk (STS score, EuroSCORE, and JapanSCORE, etc.), TAVI procedure risks, anatomical characteristics, comorbidities, and frailty,297,299,303
as well as the level of expertise of the physician in the required techniques (Table 33). Informed consent should be obtained after supplying patients with all the latest information on both the SAVR and TAVI treatments. Finally, the Heart Valve Team should be consulted prior to any decision being made. Given the various factors to consider and the lack of definitive evidence, the present guidelines do not establish a clear cutoff value of age for TAVI or SAVR. Rather, the present guidelines offer an index of prioritization that suggests using TAVI in patients aged ≥80 years and SAVR in those aged <75 years. Because progress is being made every year in this field of clinical practice, a focused update should be implemented in accordance with these guidelines.
Table 33.
Factors to Be Considered by the Heart Valve Team When Deciding Between SAVR and TAVI for Patients With Aortic Stenosis
| |
SAVR should be considered |
TAVI should be considered |
Clinical patients’
factors |
• Younger patients
• Suspicion of endocarditis
• Cardiac conditions in addition to aortic stenosis that
reguire consideration for concomitant surgical intervention
Severe coronary artery disease requiring
revascularization by CABG
Severe mitral valve disease that could be treated
surgically
Severe tricuspid regurgitation
Aneurysm of the ascending aorta requiring surgical
intervention
Septal hypertrophy requiring myectomy, etc. |
• Older patients
• Frailty
• Presence of severe comorbidity
• Unfavorable conditions (except cardiac disease) for open
heart surgery
Liver cirrhosis
Respiratory disease
Chronic obstructive pulmonary disease (FEV1.0 <1L)
Suspicion of worsening interstitial pneumonia
Bleeding tendency |
Anatomical and
technical factors
related to SAVR
and TAVI |
• Unfavorable access for TAVI
Severe calcification, tortuosity, stenosis, obstruction of
vessel
• High risk for coronary artery obstruction during TAVI
Short distance between coronary ostia and annulus,
relatively long cusp, small Valsalva, etc
• High risk for annulus rupture during TAVI
Severe calcification in left ventricular outflow tract etc
• Unfavorable valve morphology and size (annulus, aortic
root, ST junction) for TAVI
• Presence of thorombi in left ventricle |
• Favorable access for trans-femoral TAVI
• Unfavorable surgical access for SAVR
Sequelae of chest radiation (severe adhesion in
mediastinum)
Previous cardiac surgery
Presence of intact coronary bypass grafts at risk when
sternotomy is performed
Severe chest deformation or scoliosis
• Unsafe aortic cross-clamping (porcelain aorta)
• Small annulus highly expected patient-prosthesis mismatch |
*Patient’s preference should be fully considered when making the decision between SAVR and TAVI. CABG, coronary artery bypass grafting; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Both SAVR and TAVI are becoming increasingly safer every year, but with minimal improvements in the prognosis or activities of daily life, especially in the elderly. Invasive procedures for bedridden patients or those with dementia also result in a poor prognosis.310,311
Intervention in such cases should be strictly avoided. Therefore, based on an ethical and medical economic perspective, the use of such procedures should be determined after careful discussion with the Heart Valve Team.
When intervention is indicated for severe AS, simultaneous surgery of the ascending aorta is reasonable if the ascending aortic diameter is ≥50 mm in the tricuspid aortic valve and ≥45 mm in the bicuspid aortic valve. More details are provided in the AR section (V.6).
5.3.2 Indications for Intervention for Asymptomatic Severe AS
Whether early surgical intervention should be provided for asymptomatic severe AS has been controversial for decades, but no clear conclusion has yet been reached. In order to recommend early surgery for asymptomatic patients with severe AS, the benefits of early surgery, such as avoidance of sudden death or irreversible LV myocardial damage, must outweigh the risks of early surgery, such as operative mortality and postoperative complications associated with prosthetic valves. Previous observational studies have reported poor prognoses for asymptomatic patients with severe AS who received conservative treatment,25,312
and have supported early surgery.278,279,313,314
RCTs such as the AVATAR study315
and the RECOVERY study, which compare “watchful waiting” and early AVR, are ongoing. RCTs comparing “watchful waiting” and early TAVI for asymptomatic severe AS are also in progress (the EARLY TAVR study) or planned (the EVE-TAVI study).316
Until the results of these studies are available, the Heart Valve Team is advised to discuss and decide the optimal therapeutic strategy based on each individual patient.
However, early surgery is recommended for some groups of asymptomatic patients with severe AS. Patients with reduced LVEF (<50%)312–314
and patients with symptoms on exercise testing46,47,317
have poor prognoses compared with those without these findings if managed conservatively; therefore, early surgery is recommended for these groups (Class I). LVEF <50% is known to be associated with poor prognosis, but recent studies have reported that LVEF <60% is also associated with a poor prognosis.277,318–321
Early surgery is reasonable in patients with a decrease in blood pressure on exercise testing46,47
(Class IIa). Among asymptomatic patients with severe AS, patients whose peak transvalvular velocity is highly increased (very severe AS) have a poor prognosis if managed conservatively.276,278,322
Conservative management of patients with a peak transvalvular velocity ≥5.0 m/s results in poorer prognoses than for those with a peak transvalvular velocity <5.0 m/s; therefore, early surgery is reasonable for patients with a peak transvalvular velocity ≥5.0 m/s278,322
(Class IIa) (see
CQ3). A rapid increase in the peak transvalvular velocity is also considered a prognostic factor.21,275,323
Early surgery may be considered if rapid progression of ≥0.3 m/s per year is observed (Class IIb). Regarding parameters other than peak aortic jet velocity, early surgery is reasonable in patients with a mean pressure gradient ≥60 mmHg324
or with an AVA ≤0.6 cm312,325
because the prognoses of such patients are poor if managed conservatively (Class IIa). Early surgery is also reasonable if there is marked pulmonary hypertension (systolic pulmonary artery pressure ≥60 mmHg) caused by AS35,326
(Class IIa).
The biomarker BNP can predict symptoms and cardiovascular events in asymptomatic severe AS.7,327–329
BNP is a simple, inexpensive, and repeatable test in outpatients, making it suitable for follow-up. The evaluation of the chronological change in the BNP level has been reported to be useful for prognosis prediction.330
Therefore, early surgery may be considered in patients with BNP level elevation. However, it is difficult to identify specific BNP levels that indicate surgical intervention because the cutoff values vary across published reports and no consensus has been reached.
In addition to AS severity, it is important to assess surgical risk when determining the surgical indications. Especially when the recommendation is Class II, surgery may be considered in patients with low surgical risk (as determined by the STS score, EuroSCORE, JapanSCORE, or catheter treatment procedure risk), whereas continued follow-up should be considered as an option in patients with high surgical risk.
The choice of either SAVR or TAVI for patients requiring intervention should be determined according to the criteria described in the previous section on symptomatic severe AS. According to the guidelines of the ACC/AHA and ESC, TAVI is indicated only for symptomatic AS. However, the definitions of symptoms are often vague, and it is difficult to determine whether AS is symptomatic or asymptomatic, especially in elderly patients. Given the current status of TAVI treatment in Japan, the Heart Valve Team is also required to determine the type of intervention when determining the indications for intervention in asymptomatic severe AS.
Concomitant SAVR is recommended in asymptomatic patients with severe AS undergoing coronary artery bypass surgery, ascending aortic surgery, or other valve surgery (Class I). In addition, concomitant SAVR is reasonable in patients with moderate AS if they are aged <70 years or if they are aged ≥70 years with a low risk of concomitant surgery (Class IIa).331,332
6. Medical Therapy and Follow-up
This section is omitted from the English version.
CQ3
Should early operation be conducted for asymptomatic patients with very severe AS and preserved LVEF?
[Conclusion]
If the transvalvular peak velocity is ≥5.0 m/s, the mean transvalvular pressure gradient is ≥60 mmHg or the AVA is ≤0.6 cm2, operation is recommended for asymptomatic patients at low surgical risk (2B).
[Strength of Recommendation]
2: Weakly recommended (proposed)
[Strength of Evidence]
B (medium): Moderate confidence in the estimated effect
According to current guidelines in Europe and the USA, severe AS defined as an AVA <1.0 cm2, transvalvular peak velocity (Vmax) ≥4.0 m/s, and a mean pressure gradient (mPG) ≥40 mmHg warrants consideration of surgical intervention when symptoms are present or when the LVEF is <50%. In other words, an asymptomatic patient with severe AS and preserved LVEF does not necessarily warrant surgery. This is because the sudden death rate is ≈1% per year, even with severe AS, and if each patient is monitored carefully, survival during the asymptomatic period is similar to that of healthy people.333
What then should we do for patients with more severe AS (very severe AS) that occurs in the absence of symptoms? Rosenhek et al divided 116 patients with asymptomatic AS and a Vmax
≥5.0 m/s into 2 groups according to Vmax
≥5.5 m/s or <5.5 m/s and found significantly poorer prognoses for patients with a Vmax
≥5.5 m/s. During an additional 6 months of follow-up, 6 patients suffered cardiac-related death (sudden death, n=1; heart failure, n=4; myocardial infarction, n=1).276
This indicates that even asymptomatic AS can result in cardiac-related death if the patient has very severe AS. Kang et al studied 197 cases of asymptomatic very severe AS (AVA ≤0.75 cm2, Vmax
≥4.5 m/s, and mPG ≥50 mmHg) divided into early surgery and conventional treatment groups. They found that the early surgery group had a superior prognosis and that the independent predictor associated with cardiac-related death in the conventional treatment group was Vmax
≥5.0 m/s.278
In a study that classified patients by mPG, 559 AS cases with preserved LVEF and no or minimal symptoms revealed a significantly lower 4-year survival rate when the mPG was >60 mmHg.324
Regarding AVA, the smaller the AVA, the lower the 4-year cardiac events-free rate (34% for patients with AVA between 0.8 and 1.0 cm2, 26% between 0.6 and 0.8 cm2, and 11% at ≤0.6 cm2), even in asymptomatic cases. Mortality was significantly higher when the AVA was <0.6 cm2, as opposed to >0.6 cm2.325
There are also a number of similar reports from Japan. In a single-institute retrospective study, the prognoses were compared between 166 cases of severe AS (Vmax
≥4.0 m/s, mPG ≥40 mmHg, AVA <1.0 cm2) and 58 cases of very severe AS (Vmax
≥5.0 m/s, mPG ≥50 mmHg, AVA <0.6 cm2). Very severe AS with symptoms had the worst prognosis, being similar to those with asymptomatic very severe AS and symptomatic severe AS, and a comparatively favorable prognosis was shown for asymptomatic severe AS.322
These results suggest that in Japan, early surgery should be considered for very severe asymptomatic AS. In addition, Japan’s multicenter CURRENT AS Registry shows that among 1,808 patients with asymptomatic severe AS, 291 who underwent early surgery experienced better prognoses compared with 1,517 patients who underwent a conservative course of observation. These results may be partly explained by the fact that they included 114 early surgery cases of very severe AS (Vmax
≥5.0 m/s).279
Indeed, some studies have reported that early surgery yields better prognoses in cases of very severe AS when the LVEF is preserved without symptoms.334,335
Considering these data, the ACC/AHA guidelines recommend surgery for patients with low surgical risk, Vmax
≥5.0 m/s, and mPG ≥60 mmHg as Class IIa.335
The ESC/EACTS guidelines recommend surgery for patients with low surgical risk that present with Vmax
≥5.5 m/s, prominent valve calcification, annual Vmax
progression ≥0.3 m/s, marked increase in BNP (although the cutoff value has not been established), and pulmonary hypertension with a PASP >60 mmHg as Class IIa.221
The ACC/AHA guidelines note that an annual Vmax
progression rate ≥0.3 m/s is classified as Class IIb, and no recommendations are given for pulmonary hypertension with a PASP ≥60 mmHg. In addition, the standards for very severe AS differ across the guidelines from Europe and the USA. The ACC/AHA guidelines indicate a Vmax
≥5.0 m/s, while the ESC/EACTS guidelines indicate a Vmax
≥5.5 m/s.8
Bohbot et al studied 558 patients with no or minimal symptoms, Vmax
>4.0 m/s, and LVEF >50% and found a significant difference in survival between those with a Vmax
<5.0 m/s and those with a Vmax
≥5.0 m/s. They did not find any difference between the Vmax
5.0–5.49 m/s group and the Vmax
≥5.5 m/s group.336
Accordingly, the present guidelines refer to cases with a Vmax
≥5.0 m/s, mPG ≥60 mmHg, and an AVA ≤0.6 cm2
as very severe AS. If any of these parameters are met and the risk of surgery is low, surgery should be considered even if the patient is asymptomatic.
VII. Tricuspid Regurgitation (TR)
It is reported that TR is a prognostic factor for various heart diseases,337–339
and the importance of evaluating TR is emphasized.
1. Classification and Etiology
TR is classified as primary (organic) or secondary (functional), with secondary TR accounting for 70–90% of all cases.340–344
1.1 Primary TR (Organic TR)
TR due to changes in the tricuspid leaflet and subvalvular tissue itself is referred to as primary TR; causes include infective endocarditis, trauma, iatrogenic causes (e.g., after pacemaker/defibrillator implantation, after RV biopsy), rheumatic, prolapse due to myxomatous change, congenital tricuspid valve lesions such as an Ebstein anomaly, carcinoids, and radiation therapy.
1.2 Secondary TR (Functional TR)
TR due to RV dilatation/dysfunction or RA dilatation without tricuspid leaflet abnormality is referred to as secondary TR. RV dilatation due to pressure and/or volume overload, dilatation of the RA due to AF, or disorders of the RV myocardium can result in tricuspid annular dilatation or tethering of the tricuspid valve, thereby worsening coaptation.
The most common condition that causes pressure overload to the RV is post-capillary pulmonary hypertension associated with left-sided heart disease, but it may also occur with pulmonary arterial hypertension or cor pulmonale (precapillary pulmonary hypertension). Atrial septal defect is a representative disease that causes volume overload to the RV. As the prevalence of AF increases with aging, AF-induced annular dilatation is a major cause of secondary TR.340,345–347
Secondary TR may often appear and progress late after left-sided valve surgery (mainly mitral valve surgery), without left-sided valve dysfunction. AF and preoperative hemodynamics are considered to be related to this process.348–351
As a mechanism for TR, tethering is more pronounced in TR with pulmonary hypertension, whereas annular dilatation is the main mechanism in TR without pulmonary hypertension such as TR due to AF.342,343
However, tethering may occur after a long period of time as RV dysfunction develops. Valve morphology and RV function352,353
are important factors in determining the surgical indications, procedure type, and prognosis.
2. Pathophysiology and Natural History
This section is omitted from the English version.
3. Assessment of Severity
TR severity is primarily determined by echocardiography. However, there are several problems with TR severity assessment,30,31,354
as follows. Although the severity of TR varies according to the pressure and/or volume loading conditions, such as in the treatment of HF, it is uncertain at what time the surgical indications should be determined. Many cases show multiple regurgitation jet signals, and the EROA is not circular. Quantitative evaluation of TR is difficult because of the high prevalence of AF and high respiratory variability. Quantitative evaluation of RV volume is also difficult with 2D echocardiography because the RV has complex geometry.
More accurate evaluation of RV volume can be achieved with cardiac MRI.
Grading the severity of TR is shown in
Table 34.30,31
The severity of TR should be evaluated and determined using multiple parameters.
Table 34.
Grading the Severity of Tricuspid Regurgitation (TR)
| |
Mild TR |
Moderate TR |
Severe TR |
| Qualitative |
| Color flow regurgitant jet |
1+ |
2+ |
3+ |
| CW signal of regurgitant jet |
|
|
Triangular with early peaking |
| Semiquantitative |
| Regurgitaion jet area (cm2) |
< 5 |
5–10 |
>10 |
| Jet area/RA area (%) |
|
|
≥50 |
| Vena contracta width (cm) |
<0.3 |
0.3–0.69 |
≥0.7 |
| PISA radius (cm) |
<0.6 |
0.6–0.9 |
>0.9 |
| Hepatic venous flow |
|
|
Systolic reverse flow |
| Quantitative |
| EROA (cm2) (PISA method) |
<0.2 |
0.2–0.39 |
≥0.4 |
| Regurgitant volume (mL) |
<30 |
30–44 |
≥45 |
CW, continuous wave; EROA, effective regurgitant orifice area; PISA, proximal isovelocity surface area; RA, right atrium.
In addition to confirming the symptoms and physical findings, imaging is important for diagnosis and the therapeutic strategy in TR (Table 35). Although TTE is the main imaging modality to evaluate TR, TEE, exercise testing, and cardiac MRI may also be used if necessary.355
Table 35.
Recommendations for Diagnostic Testing for Tricuspid Regurgitation (TR)
| Recommendations |
COR |
LOE |
TTE is indicated to evaluate the severity of TR, determine etiology, assess RV and RA,
estimate PASP, and assess concomitant left-sided heart disease |
I |
C |
Cardiac catheterization is reasonable for patients with TR to assess pulmonary artery
pressures, pulmonary vascular resistance and RA pressure if there is a discrepancy between
clinical findings and noninvasive tests |
IIa |
C |
| TEE may be considered when TTE images are inadequate |
IIb |
C |
CMR or real-time 3D echocardiography may be considered to assess RV volume and
systolic function |
IIb |
C |
Exercise echocardiography or CPX may be considered for patients with severe TR with no
or minimal symptoms to assess exercise capacity |
IIb |
C |
COR, Class of Recommendation; CMR, cardiac magnetic resonance imaging; CPX, cardiopulmonary exercise test; LOE, Level of Evidence; PASP, pulmonary artery systolic pressure; RA, right atrium; RV, right ventricle; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
4.1 Symptoms and Physical Findings
This section is omitted from the English version.
4.2 Echocardiography
Echocardiography is the primary imaging technique for evaluating the severity and etiology of TR (Class I). In most cases, TTE alone is enough to assess TR, but TEE may be used as needed (Class IIb).
4.2.1 TTE
The main focus of TTE evaluation in patients with TR is to assess the etiology and severity of the TR, RV size, and RV function, and to estimate the pulmonary artery and RA pressures. In secondary TR, the cause of regurgitation, such as left-sided heart disease or pulmonary hypertension, should also be assessed. These evaluations of TR follow the American Society of Echocardiography guidelines for the evaluation of native valvular regurgitation,30
cardiac chamber quantification,32
and the assessment of the right heart42
by echocardiography.
The first step is to determine whether the TR is primary or secondary, especially noting the presence of any structural abnormalities of the tricuspid valve. The presence of structural valvular abnormalities, annular dilatation, or valvular tethering should be carefully assessed in the multiple windows.
The regurgitant jet area measured by the color Doppler method is widely used for assessing the severity of TR. However, measurement of the regurgitant jet area is often limited by multiple jets or a complex regurgitant orifice of the TR. In addition, in patients with elevated RA pressure from severe TR, the regurgitant jet area may decrease as the RV–RA pressure gradient decreases, resulting in an underestimation of TR severity. For this reason, the width of the TR jet vena contracta is often used as a semiquantitative parameter for TR grading. Continuous-wave Doppler and quantitative PISA are also used to assess TR severity. However, because each parameter has its intrinsic limitations (Table 36),30
an integrative assessment of TR severity using multiple parameters is recommended, especially in cases where moderate or severe TR is suspected (Table 34).30
Table 36.
Echocardiography in Evaluating Severity of TR: Advantages and Pitfalls
| Parameter |
Advantages |
Pitfalls |
| Color Doppler |
Proximal flow
convergence |
• Rapid qualitative assessment |
• Multiple jets
• Nonhemispheric shape |
Vena contracta
width |
• Surrogate for regurgitant orifice size
• Independent of flow rate and driving pressure for a
fixed orifice
• Less dependent on technical factors
• Good at identifying severe TR |
• Problematic in the presence of multiple jets
• In order to measure it, convergence zone needs to be
clearly visualized |
| Jet area |
• Qualitative |
• Dependent on the driving pressure and jet direction
• Direction and shape of jet may cause overestimation or
underestimation of jet area |
| Pulsed wave Doppler |
Hepatic vein flow
reversal |
• Simple supportive sign of severe TR
• Can be obtained with both TTE and TEE |
• Depends on compliance of the RA
• May not be reliable in patients with AF, paced rhythm
with retrograde atrial conduction |
| Continuous wave Doppler |
Density of
regurgitant jet |
• Simple
• Density is proportional to the number of red blood cells
reflecting the signal
• Faint or incomplete jet is compatible with mild TR |
• Qualitative
• Perfectly central jets may appear denser than eccentric
jets of higher severity
• Overlap between moderate and severe TR |
| Jet contour |
• Simple
• Specific sign of pressure equalization in low velocity,
early-peaking dense TR jet |
• Qualitative
• Affected by changes in RV and RA pressures |
| Quantitative Doppler |
| PISA |
• Quantitative assessment of EROA and regurgitant
volume |
• Not valid for multiple jets, less accurate in eccentric jets
• Limited evidence
• Typically lower RV pressures lead to greater contour flattening
and underestimation of PISA |
AF, atrial fibrillation; EROA, effective regurgitant orifice area; LV, left venticle; PISA, proximal isovelocity surface area; RA, right atrium; RV, right ventricle; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography. Produced by reference to Zoghbi et al.30
Evaluation of RV enlargement and function is essential in determining the optimal TR treatment strategy. The main reason for the difficulty in assessing RV function and volume by 2D echocardiography is the complex geometry of the RV. The echocardiographic parameters commonly used for evaluating RV systolic function are RV FAC, TAPSE, and the systolic wave velocity of the tricuspid annulus (s′) by tissue Doppler imaging in the RV free wall.42
TAPSE and tissue Doppler-derived s′ values356
are parameters of RV longitudinal systolic function and correlate well with CO in the RV. However, in patients with moderate or severe TR, the tricuspid annulus shows exaggerated longitudinal motion, which can result in overestimation of RV systolic function.357
Therefore, the FAC is commonly used to assess RV function in patients with significant TR. However, it should be recognized that the FAC may also overestimate RV systolic function in patients with significant TR because of regurgitant blood flow to the RA low-pressure chamber during systole, as seen in LVEF in patients with significant MR. For assessing RV dilatation, RV diameter and area are measured in the apical 4-chamber view,32
while the presence of a D-shaped LV cavity due to RV volume and/or pressure overload should be confirmed in the short-axis view. Furthermore, 3D echocardiography is useful for accurately measuring the RV volume and the ejection fraction;32,358
the complexity of estimating RV volume and function with 2D echocardiography has been well documented. Currently, cardiac MRI is the standard method for RV volume evaluation, but 3D echocardiography may also be considered (Class IIb). Strain derived from 2D speckle-tracking echocardiography also provides excellent parameters of RV systolic function compared with conventional parameters. RV free wall longitudinal strain derived from 2D speckle-tracking imaging is calculated as the percentage of systolic shortening of the RV free wall from the base to the apex and is commonly used for assessing RV systolic function.42
In particular, measurement of the tricuspid annular diameter is important in determining the indications for intervention in secondary TR. According to the ACC/AHA144
and ESC8
guidelines, the indications for surgical intervention are based on tricuspid annular dilatation (diameter >40 mm or >21 mm/m2
indexed to BSA) assessed by echocardiography in the apical 4-chamber view. However, it has not been elucidated whether this value is appropriate for the Japanese population, which has a smaller body size than that of Western populations.
In secondary TR, tethering of the tricuspid valve should be also evaluated during mid-systole at the time of maximal valve closure in the apical 4-chamber view. In this view, the anterior leaflet (posterior leaflet in 20% of cases) is visualized at the side of the RV free wall, and the septal leaflet is visualized at the septal side. If the leaflets of the tricuspid valve are retracted in the direction of the apex by RV dilatation and valve leaflet coaptation is reduced, the valve is considered to have tethering. Preoperative tricuspid valve tethering height >8 mm was reported as an important determinant of recurrence after tricuspid valve repair.359
4.2.2 TEE
For evaluation of the tricuspid valve, TEE has limitations such as limited echocardiographic windows and a larger distance from the probe to the tricuspid valve, compared with TTE. Therefore, TEE is not always required to evaluate TR.30
However, TEE is effective in cases where optimal images cannot be obtained with TTE (Class IIb) and 3D TEE allows for simultaneous visualization of the 3 valve leaflets to evaluate valve coaptation and TR, as well as accurately measure the 3D tricuspid annular diameter and perimeter.142
Therefore, the indications for TEE should be considered as necessary.
4.2.3 Exercise Stress Echocardiography
In asymptomatic or equivocally symptomatic patients, despite severe primary TR, exercise stress echocardiography may be effective in determining the surgical indications (Class IIb). Exercise stress echocardiography allows us to evaluate the relationship between TR severity and stress-induced symptoms or exercise tolerance. Studies on the Ebstein anomaly have shown that severe TR reduces CO from the RV during exercise stress, which can be improved by surgery.360
4.3 Cardiac Catheterization
There are several limitations to the accuracy of echocardiographic estimations of pulmonary artery pressure, pulmonary vascular resistance, and RA pressure. Therefore, invasive right heart catheterization is useful and can be beneficial for assessing these hemodynamic parameters to determine the surgical indications for TR (Class IIa). Although right ventriculography can be used for evaluating TR severity and RV volume, these assessments are adequately made using echocardiography or cardiac MRI in most cases. Therefore, routine right ventriculography is not recommended for evaluating TR. However, right ventriculography might be useful in patients for whom echocardiography or cardiac MRI does not adequately determine the severity of the TR. In patients with severe TR, CO obtained based on thermodilution is inaccurate and the Fick method should be used instead.
4.4 Other Diagnostic Tests
Cardiac MRI permits accurate evaluation of RV volume and systolic function,358
and is the current gold standard. Cardiac MRI has also been reported as useful for assessment of hemodynamic parameters such as quantitative TR severity, tricuspid valve tethering, and pulmonary vascular resistance.355
Although it is not always necessary to perform cardiac MRI for the assessment of TR severity, it may be performed as needed if it is available (Class IIb). Evaluation of systemic venous congestion is also important for assessing the clinical severity of TR. Liver dysfunction due to congestion is a risk for perioperative mortality, and irreversible liver cirrhosis is a contraindication for surgery.361
Moreover, pancytopenia resulting from hypersplenism is associated with a risk of infection and bleeding and should be assessed in the consideration of the indications for surgical intervention. Some patients may not recognize their symptoms and in such patients, cardiopulmonary exercise testing is useful for the assessment of symptoms and exercise tolerance360
(Class IIb).
Table 35
lists the recommended TR tests.
5. Surgical Treatment
5.1 Surgical Procedures and Results
This section is omitted from the English version.
5.2 Indications and Timing for Surgery (Figures 18–20, Tables 37–39)


Table 37.
Indications of Surgery for Primary Tricuspid Regurgitation (TR)
| Recommendations |
COR |
LOE |
Tricuspid valve repair (or replacement) is recommended for patients with severe TR
undergoing left-sided heart surgery |
I |
B |
Tricuspid valve repair (or replacement) is recommended for patients who suffer from
recurrent right heart failure due to severe TR despite medical therapy (or a lack of response
to medical therapy is highly suspected)* |
I |
C |
Tricuspid valve repair (or replacement) is reasonable for patients with severe TR and
progressive RV dilation and/or systolic dysfunction regardless of the presence of
symptoms |
IIa |
C |
Tricuspid valve repair is reasonable for patients with moderate TR undergoing left-sided valve
surgery |
IIa |
C |
*Except patients with irreversible severe RV dysfunction. COR, Class of Recommendation; LOE, Level of Evidence; RV, right ventricle.
Table 38.
Indications of Surgery for Secondary Tricuspid Regurgitation (TR) at Time of Left-Sided Valve Surgery
| Recommendations |
COR |
LOE |
Concomitant tricuspid valve repair (or replacement) is recommended for patients with
severe TR |
I |
C |
| Concomitant tricuspid valve repair is reasonable for patients with moderate TR |
IIa |
C |
Concomitant tricuspid valve repair may be considered for patients with mild TR and
tricuspid annular dilation (>40 mm or 21 mm/m2) |
IIb |
C |
Concomitant tricuspid valve repair may be considered for patients with mild TR and
persistent AF |
IIb |
C |
AF, atrial fibrillation; COR, Class of Recommendation; LOE, Level of Evidence.
Table 39.
Indications of Surgery for Isolated Secondary Tricuspid Regurgitation (TR)
| Recommendations |
COR |
LOE |
| Isolated secondary TR after previous cardiac surgery |
Tricuspid valve repair (or replacement) is reasonable for patients with severe TR who
suffer from recurrent heart failure despite medical therapy and without either of severe
RV dysfunction, irreversible pulmonary hypertension, or irreversible liver dysfunction |
IIa |
C |
| Isolated secondary TR due to AF |
Tricuspid valve repair (or replacement) is reasonable for patients with serere TR who
suffer from recurrent heart failure despite medical therapy |
IIa |
C |
Tricuspid valve repair may be considered for patients with severe TR and progressive RV
dysfunction and/or dilation despite medical therapy regardless of the presence of
symptoms |
IIb |
C |
AF, atrial fibrillation; COR, Class of Recommendation; LOE, Level of Evidence; RV, right ventricle.
With a growing number of patients with TR, the number of patients who require surgical treatment has also been increasing.344
However, the indications for surgical intervention for TR and its optimal timing remain controversial and there are still many issues on which broad consensus has not been reached. One of the main reasons for this is that the etiology of TR is usually multifactorial and various clinical factors are associated with the pathophysiology and prognosis of TR. Another reason is that the clinical evidence that supports the indications for surgery is not sufficient, even though surgical treatment is considered to be effective for TR.362
Delayed surgical intervention for severe TR results in irreversible RV dysfunction,363
and worsening of the patient’s physical condition, such as hepatic dysfunction, renal dysfunction, and cachexia due to right heart failure.361
This ultimately results in a poor prognosis.364
A recent retrospective observational study365
suggested that surgical treatment for isolated TR did not improve the prognosis in comparison with medical treatment; however, the study included multifactorial etiologies and, in addition, the symptoms and RV function/size at the timing of the surgery were not provided, so it is unclear whether the optimal timing for surgery was selected for all of the study patients. Thus, the study could not conclude on denying the surgical benefits for TR. This section describes the indications for surgical intervention for TR and its optimal timing, with reference to opinions from experts, ACC/AHA144
and ESC guidelines.8
5.2.1 Primary TR (Figure 18, Table 37)
This section describes the surgical indications for primary TR induced by structural abnormalities of the leaflets, including tricuspid valve prolapse, infective endocarditis, Ebstein anomaly, and blunt chest wall trauma. The indications for surgical intervention for functional TR associated with AF are described separately in another section.
TR may progress over time, even late after left-sided valve surgery, including mitral valve surgery, which is known to be one of the poor prognostic factors.351,366–368
In addition, reoperation for isolated TR after left-sided valve surgery is associated with a high perioperative mortality rate.369
Therefore, tricuspid valve repair (or replacement) is recommended for patients with severe primary TR undergoing left-sided valve surgery regardless of symptoms (Class I). Several studies report that tricuspid valve repair provides a better postoperative prognosis than replacement;370–372
thus tricuspid valve repair is preferable to replacement if feasible. Because moderate TR may have a risk of progression over time, tricuspid valve repair, if feasible, can be beneficial for patients with moderate TR at the time of left-sided valve surgery (Class IIa).
Meanwhile, right heart failure due to TR is known to respond well to medical therapy. Therefore, medical therapy is recommended in patients with severe primary TR in whom left-sided valve surgery is not being planned or considered. Tricuspid valve repair (or replacement) is recommended in cases with recurrent or persistent right heart failure despite optimal medical therapy or in cases where a lack of response to medical therapy for right HF is highly suspected (Class I). However, patients who have already developed severe irreversible RV dysfunction may not benefit from tricuspid valve surgery because of high perioperative risk, and the surgical indications should be carefully assessed in such patients. Similarly, tricuspid valve repair (or replacement) should be considered in cases of progressive RV dysfunction or dilatation regardless of the presence of symptoms (Class IIa), because the postoperative prognoses are poor for patients with advanced RV dysfunction before surgery.337,363,373,374
However, the specific cutoff values for RV dilatation or dysfunction have not yet been clarified.
5.2.2 Secondary TR at Time of Left-Sided Valve Surgery (Figure 19, Table 38)
The severity of secondary TR associated with left HF often changes, depending on the hemodynamics, and may be reduced together with improvement in left HF. However, many clinical studies have shown that TR often progresses even after left-sided valve surgery, particularly late after mitral valve surgery, and is associated with a poor prognosis.351,367,368,375–378
Conversely, it has been shown that concomitant tricuspid valve repair at the time of left-sided valve surgery does not increase the surgical risk.379
Therefore, the indications for concomitant surgical intervention for secondary TR should be considered in patients undergoing left-sided valve surgery.
Because severe TR may not improve after treatment of left-sided valve disease, aggressive surgical intervention, either involving valve repair or replacement, is recommended for severe secondary TR at the time of left-sided valve surgery (Class I). For moderate secondary TR, concomitant tricuspid valve repair should be considered even without tricuspid annular dilatation or right HF symptoms (Class IIa). This is because, in addition to the fact that concomitant tricuspid valve surgery does not increase the perioperative risk,379,380
it has been shown that moderate secondary TR may worsen after left-sided valve surgery even in patients without preoperative tricuspid annular dilatation.376–378
According to the ACC/AHA144
and ESC8
guidelines, tricuspid annular dilatation is an important determinant for surgical intervention381,382
with a cutoff value of 40 mm (in diameter) or 21 mm/m2
(indexed to BSA) as measured by echocardiography in the apical 4-chamber view. However, to date, only one RCT has validated this value,383
and a cutoff value of the tricuspid annular diameter was not adopted for moderate secondary TR in this guideline. Refer to CQ4 for further information.
The evidence supporting surgical intervention for mild secondary TR is even more limited. However, because mild TR may progress after left-sided valve surgery in patients with tricuspid annular dilatation,382
tricuspid valve repair may be considered for patients with mild secondary TR and tricuspid annular dilatation at the time of left-sided valve surgery (Class IIb). Because the criteria for tricuspid annulus dilatation are not supported by definitive evidence as discussed in CQ4, this guideline adopts the cutoff value of 40 mm (in diameter) or 21 mm/m2
(indexed to BSA), following the ACC/AHA and ESC guidelines.8,144
Recently, it was reported that persistent AF is associated with TR progression late after left-sided valve surgery.349,351,375
Thus, patients with persistent AF may benefit from concomitant tricuspid valve repair for mild TR (Class IIb). The benefits of tricuspid valve replacement for mild or moderate secondary TR have not been proven and valve replacement is not recommended in this guideline.
5.2.3 Isolated Secondary TR (Figure 20, Table 39)
Secondary TR is often ameliorated by medical therapy, so it should be tried first in patients with symptomatic secondary TR. However, secondary TR is also characterized by frequent worsening and recurrent right HF even when HF was previously improved by medical therapy. In such cases of secondary TR, venous congestion may cause liver and renal dysfunction,361
resulting in increased perioperative risk. Moreover, tricuspid valve tethering due to progressive RV dilatation may present difficulties in tricuspid valve repair.359
Therefore, for secondary TR, it is important to consider the surgical indications at the optimal timing while monitoring the response to medical therapy, as reported by Latib et al.384
However, it should be recognized that optimal medical therapy and timing of surgical intervention have not been established and accumulation of more clinical evidence is needed.
The current major challenge regarding isolated secondary TR in clinical practice involves 2 presentations: progressive TR after left-sided valve surgery such as mitral valve surgery, and TR in elderly patients with AF. The present guideline provides recommendations for the surgical indications in these cases of isolated secondary TR. As for secondary TR with pulmonary hypertension, pulmonary hypertension itself is a major prognostic factor rather than the TR, and treatment should focus on the pulmonary hypertension.
As described above, the severity of secondary TR may vary depending on the hemodynamics. Here, we describe the indications for surgical intervention for secondary TR based on TR severity at the time when right HF worsens.
a. Isolated TR After Previous Cardiac Surgery
Although many studies have demonstrated the risk of progression of secondary TR after left-sided valve surgery,351,367,368,375–378
surgical intervention for secondary TR after left-sided valve surgery was thought to be associated with high perioperative mortality. However, in recent years, the high perioperative mortality rate has been attributed to a deterioration in systemic conditions and RV dysfunction because of delayed surgical treatment.373,374
Therefore, surgical intervention at optimal timing is required to improve the prognoses of patients who developed significant TR after left-sided valve surgery. On the other hand, because severe secondary TR following heart surgery often responds to medical therapy, medical therapy should be attempted first. Surgical indications may be considered in patients who experience recurrent right HF despite medical therapy. Tricuspid valve repair (or replacement) is reasonable for patients with severe TR who suffer from recurrent HF despite medical therapy without severe RV dysfunction, irreversible pulmonary hypertension, or irreversible liver dysfunction (Class IIa). In patients with severely impaired RV function, the postoperative outcomes are poor.373,374,385
In cases of irreversible advanced pulmonary hypertension or liver dysfunction or in those where improvement in RV function after surgery cannot be expected, the balance between surgical risks and benefits should be comprehensively evaluated. Patients in whom no benefit of surgery is expected should be treated conservatively. The indocyanine green clearance test, Child-Pugh classification, and the MELD score are commonly used to evaluate liver function.361
However, the optimal test of liver function for predicting perioperative risk in TR surgery has not been established so far.
b. Isolated TR Associated With AF
AF causes tricuspid annular dilatation, often resulting in the development of significant TR.343
In severe TR associated with AF, medical therapy should be first attempted. Surgical intervention can be beneficial for patients who suffer from recurrent right HF despite medical therapy (Class IIa). Severe RV dysfunction and dilatation are associated with poor postoperative outcome.373,374
Thus, tricuspid valve repair may be considered before the development of right HF in patients with progressive RV dysfunction or dilatation despite medical therapy (Class IIb). In patients who have already developed pulmonary hypertension or liver dysfunction, the surgical indications should be carefully assessed based on the balance between the risks and benefits of surgery.
c. Future Directions in TR
Currently, open-heart surgery is the only available treatment option for TR in Japan. In recent years, TR treatment based on minimally invasive transcatheter techniques using several tools has been reported,384,386
and the indications for invasive treatment for TR may be more proactively assessed when these techniques are introduced in Japan.
CQ4
Should concomitant tricuspid valve repair be conducted for patients with mild TR and tricuspid annular dilatation (>40 mm or 21 mm/m2 in diameter)?
[Conclusion]
Concomitant tricuspid valve repair may be considered for patients with mild TR and significant tricuspid annular dilatation (>40 mm or 21 mm/m2
in diameter) (2C).
[Strength of Recommendation]
2: Weakly recommended (proposed)
[Strength of Evidence]
C (weak): Limited confidence in the estimated effect
[Comment]
Many observational studies have shown that even mild TR left uncorrected at the time of left-sided valve surgery may progress over time, resulting in a poor prognosis.337,349,351,367,368,375,387–390
On the other hand, if left HF improves after left-sided valve surgery, TR often improves.391
Therefore, it is necessary to identify patients at risk of postoperative TR progression among those with mild TR undergoing left-sided valve surgery and to perform prophylactic concomitant tricuspid valve repair. To date, tricuspid annular dilatation, decreased RV function, concomitant AF, and LA enlargement have been reported as risk factors for progression of TR following left-sided valve surgery.349,351,367,368,375,387–390
According to the current ACC/AHA144
and ESC8
guidelines for valvular disease, concomitant tricuspid valve repair is recommended as a Class IIa indication in patients with mild TR at the time of left-sided valve surgery and with tricuspid annular dilatation of >40 mm or >21 mm/m2
in diameter. The tricuspid annular diameter is assessed by echocardiography in the apical 4-chamber view, which is well correlated with the actual diameter measured intraoperatively.392
This cutoff value for tricuspid annular dilatation was first described in the 2007 ESC guideline for valvular disease393
and was based on 2 previous studies. Colombo et al381
reported 50 cases with a tricuspid annular diameter of ≥21 mm/m2
who underwent tricuspid annuloplasty with the De Vega method regardless of the TR severity at the time of mitral valve surgery. In that series of patients, none developed severe TR during the follow-up period and their clinical outcomes were favorable. However, the study lacked control cases and has limited significance as clinical evidence. In another study, Dreyfus et al382
retrospectively examined the association between tricuspid annular diameter by direct intraoperative measurements and progression of TR in patients who underwent mitral valve surgery. They concluded that a tricuspid annuloplasty should be performed at the time of mitral valve surgery to prevent progression of TR if the tricuspid annular diameter was greater than twice the normal size (>70 mm) regardless of the grade of TR. Their study did not examine the measurements of tricuspid annular diameter by echocardiography.
On the other hand, only a few RCTs have examined the validity of the 40 mm or the 21 mm/m2
tricuspid annular diameter cutoff values. Benedetto et al383
conducted a prospective study enrolling 44 patients with less-than-moderate TR (≤+2) and a tricuspid annular diameter ≥40 mm who underwent mitral valve surgery, randomizing them to receive or not receive tricuspid annuloplasty. At the 12-month follow-up, TR was absent in 71% versus 19% of patients in the tricuspid annuloplasty and control groups, respectively (P=0.001). Moderate to severe TR was present in 0% versus 28% of patients in the tricuspid annuloplasty and control groups, respectively (P=0.02). Although there was no significant difference in the improvement in NYHA functional class, the RV reverse remodeling was marked and the improvement in the 6-minute walk test was greater in the tricuspid annuloplasty group. Based on that study, if concomitant tricuspid valve repair is performed at the time of left-sided valve surgery in accordance with ESC or ACC/AHA guidelines, we may expect an improvement of symptoms or prognosis with improvement in RV function during the long-term follow-up, even if prognostic or symptomatic improvement over the short-term period of 12 months is not observed. In a report by Shi et al,394
167 patients with moderate or less than moderate TR undergoing mitral valve surgery were randomized to 70 patients who received tricuspid annuloplasty and 97 patients who did not. During the long-term follow-up, the severity of TR was similar between the groups for patients with an annular diameter ≤21 mm/m2, but the severity of TR was significantly less in the annuloplasty group in patients with a diameter >21 mm/m2. However, that study included not only patients with mild TR but also those with moderate TR, and selection bias might have affected the results because the randomization was uneven and there were differences in the operating periods between groups. Therefore, we conclude that the evidence from this report has limited reliability with regard to the scope of this CQ.
Several observational studies of the cutoff values of the tricuspid annular diameter have been published.366,387,395–397
For example, Van De Veire et al found that concomitant tricuspid annuloplasty at the time of mitral valve surgery significantly prevented long-term TR progression and right ventricular remodeling in patients with mild TR and with an annular diameter >40 mm.396
On the other hand, David et al found that a tricuspid annular diameter of 40 mm was not a predictive factor for the development of moderate or severe TR in the long-term among 312 patients who underwent mitral valve repair without tricuspid annuloplasty.397
However, all of these studies were retrospective and included various potential biases, such as differences in operative techniques. In addition, there are studies that have reported contradicting results. It was suggested that mild TR (≤1+) may progress to moderate or severe if concomitant tricuspid annuloplasty is not performed, even in patients with an tricuspid annular diameter <40 mm.387
Another study showed no significant difference in the preoperative tricuspid annular diameter between patients who showed and did not show significant TR during long-term follow-up.375
It was also reported that tricuspid annular dilatation was a modest contributing factor for TR progression.367
Therefore, there are significant limitations in determining the indications for concomitant tricuspid valve repair based solely on the tricuspid annular diameter.
Additionally, many previous investigations have suggested that mitral valve replacement has a higher risk of worsening postoperative TR than does mitral valve repair, implying that a variety of left-sided valve surgeries may not have the same effect on postoperative TR. For example, in patients after mitral valve replacement, it was reported that tricuspid annular dilatation was a high risk factor for worsening of postoperative TR,388
or that the mitral valve replacement itself was a contributing factor for the progression of postoperative TR.378
As for mitral valve replacement, it was shown in a propensity matched study that concomitant tricuspid valve repair for mild TR, regardless of the tricuspid annular diameter, might prevent progression of TR during long-term follow-up without increasing perioperative risk.379
As described above, there is enough data showing that mild TR left uncorrected at the time of left-sided valve surgery may progress to moderate or severe TR during long-term follow-up, resulting in a poor prognosis, and that tricuspid annular dilatation may be associated with postoperative progression of TR. However, the evidence is considered insufficient to strongly recommend the indication of tricuspid valve repair for mild TR based only on the annulus diameter, and we concluded that this indication is “weakly recommended”.
6. Medical Therapy and Follow-up
This section is omitted from the English version.
VIII. Tricuspid Stenosis (TS)
1. Diagnosis
The most common etiology of TS is rheumatic disease. Isolated TS is rare, and TS is often found with other valvular diseases, particularly MS. Other etiologies include congenital disease, carcinoid syndrome, and drug-induced valvular disease. TS is usually accompanied by TR of varying degrees of severity. In addition, similarly to TR, the pathophysiology of TS includes decreased cardiac output due to impaired RV filling and systemic congestion due to increased venous pressure.
The most common diagnostic technique for TS is echocardiography (Class I). Echocardiographic findings in TS include sclerotic change, restricted motion, and doming of the tricuspid valve. However, TS may be overlooked if the tricuspid valve is not evaluated carefully due to the rarity of the disease. Although a mean tricuspid pressure gradient >5 mmHg is generally considered to indicate significant TS,398
there is little evidence demonstrating an association between the severity of isolated TS and prognosis, and the classification of TS severity has not been well validated.
Table 40
shows the echocardiographic parameters indicating severe TS.398
Table 40.
Findings of Severe Tricuspid Stenosis (TS)
| Valve anatomy |
Doppler parameters by
echocardiography |
Other |
• Thickened, sclerotic, calcified
leaflets. Restricted motion of
leaflets. |
• PHT ≥190 ms
• Valve area ≤1.0 cm2
• Tricuspid mean pressure gradient
≥5 mmHg
• Tricuspid TVI >60 cm |
• Dilation of right atrium and inferior
vena cava |
Valve area can be measured by the continuity equation or planimetry method by 3D echocardiography. PHT, pressure half time; TVI, time-velocity integral.
The tricuspid valve area can be calculated using the continuity equation or 3D echocardiography. In the continuity equation method, the valve area is calculated as stroke volume divided by the time–velocity integral of the transtricuspid flow using continuous-wave Doppler. Alternatively, this can be measured using the planimetry method using 3D echocardiography. Because of limited echocardiographic windows, the tricuspid valve area is rarely measured by 2D echocardiography. Routine cardiac catheterization only for the purposes of assessing TS severity is not recommended. Right heart catheterization may be considered for assessing the pressure gradient between the RV and RA when there is a discrepancy between the TS severity as determined by echocardiography and the clinical findings (Class IIb).
2. Surgical Treatment
Very few cases require surgical treatment for severe TS, and few reports have been published on the topic. Surgical treatment is recommended for symptomatic severe TS. In many cases, tricuspid valvuloplasty is difficult because the valve is highly impaired and tricuspid valve replacement is required. As mentioned in the TR surgical treatment section (VII.5), there are no clear criteria for selecting the artificial valve replacement (bioprosthetic or mechanical valve). Rather, physicians must make their determination based on individual cases.
3. Medical Therapy
This section is omitted from the English version.
IX. Pulmonary Regurgitation (PR)
1. Diagnosis
1.1 Etiology
Mild PR is frequently seen with normal pulmonary valves, but severe PR is a rare VHD.399,400
The structural abnormalities of the pulmonary valves that cause severe PR are usually congenital, such as in tetracuspid valves, bicuspid valves, and hypoplasia. However, significant PR is frequently observed after pulmonary valvuloplasty in cases of tetralogy of Fallot. In addition, infective endocarditis, carcinoid syndrome, rheumatic heart disease, and use of the dopamine D1
and D2
agonist pergolide (often used in Parkinson’s disease) can cause PR. Rarely, blunt thoracic trauma may cause pulmonary valve prolapse or crushing, leading to PR. Pulmonary hypertension can be a cause of secondary PR involving no apparent abnormality of the pulmonary valve.
1.2 Assessment of Severity by Echocardiography
There is little evidence for assessing the severity of PR by echocardiography, and its usefulness is limited.30,31
RV enlargement reflects PR severity, but pulmonary hypertension causes RV enlargement and dysfunction depending on the pressure overload. Therefore, RV enlargement is unlikely to reflect the severity of PR in patients with pulmonary hypertension. In contrast, acute PR may not cause RV enlargement. Although there is a limitation in the evaluation of PR severity based on RV enlargement, a basal RV diameter ≥42 mm and a middle diameter ≥35 mm in the apical 4-chamber view are proposed as criteria for RV enlargement in tetralogy of Fallot.401
The usefulness of RV function assessment using TAPSE or tissue Doppler in PR has not been recognized.30,31,401
The measurement of RV volume and RV ejection fraction by 3D echocardiography may be useful, but further accumulation of evidence is required. Color Doppler, pulsed Doppler, and continuous-wave Doppler at the RV outflow tract are commonly used to evaluate PR severity. The most reliable method of PR evaluation is to evaluate the ratio between the width of the regurgitation jet and the diameter of the pulmonary valve annulus. A ratio ≥0.7 indicates severe PR (Figure 21).402
The continuous-wave Doppler waveform of severe PR has a high signal intensity and shows signs such as rapid deceleration and early termination (Figure 21). As a result, the pressure half-time of the PR waveform is shortened, suggesting severe PR in less than 100 ms of pressure half-time. However, because pressure half-time depends on the pressure gradient between the pulmonary artery and the RV, the specificity of the shortened pressure half-time decreases when RV diastolic pressure increases.403
There is also the PR index, which is the ratio of PR duration to diastolic time, and a PR index <0.77 indicates more than moderate PR (Table 41).30,31
The usefulness of TEE is limited in assessing PR.

Table 41.
Parameters Used in Assessing Severity of Pulmanary Regurgitation (PR)
| Parameter |
Mild |
Moderate |
Severe |
| RV size |
Normal |
Normal or dilated |
Dilated
RV basal end-diastolic dimension ≥42 mm
RV mid end-diastolic dimension ≥35 mm |
| Ratio of PR jet width to PV annulus diameter |
|
|
>0.7 |
| Density and contour of PR continuous Doppler flow |
Faint or imcomplete |
Dense |
Densel, early termination of diastolic flow |
| Pressure half time of PR Doppler flow |
|
|
<100 ms |
| PR index |
|
<0.77 |
<0.77 |
Pulsed wave Doppler flow reversal in the main or
branch PA |
|
|
Prominent |
Pulmonic systolic flow VTI compared with systemic
flow (LVOT) VTI |
Slightly increased |
Intermediate |
Greatly increased |
| Regurgitant fraction by CMR |
<20% |
20–40% |
>40% |
CMR, cardiovascular magnetic resonace; LVOT, left ventricular outflow tract; PA, pulmonary artery; PV, pulmonary valve; RV, right ventricular; VTI, velocity-time integral. Produced by reference to Zoghbi WA, et al. 2017.30
1.3 Assessing PR by Cardiac MRI
Cardiac MRI is the most reliable quantitative evaluation method for PR. If PR evaluation is difficult by echocardiography, PR evaluation by MRI can be useful. MRI enables the measurement of stroke volume and regurgitant volume at the pulmonary valve using the phase-contrast method, and the regurgitant fraction can be obtained. A regurgitant fraction determined by MRI to be ≥40% is classified as severe, 20–40% is moderate, and <20% is mild (Table 41).404
In addition, the RV ejection fraction and RV volume measured by MRI are used as reference values to determine the indications for pulmonary valve reoperation in tetralogy of Fallot.
1.4 Comprehensive Evaluation of PR Severity
Because there is no established method for the quantification of PR by echocardiography, a comprehensive assessment should be performed using multiple indices (Table 41). It is reasonable to diagnose severe PR if all criteria for severe are met.30,31
However, if echocardiography cannot evaluate the severity or if the evaluation is inconclusive, especially in the late stages after pulmonary valve repair for tetralogy of Fallot, the severity of PR and RV function should be evaluated by MRI.30,31,401,404–407
2. Surgical Treatment (Table 42)
Table 42.
Indications of Intervention for Pulmonary Regurgitation (PR)
| Recommendations |
COR |
LOE |
Pulmonary valve replacement is recommended for symptomatic patients with moderate or
severe PR |
I |
C |
Pulmonary valve replacement is reasonable for asymptomatic patients with moderate or severe
PR with RV dilation and/or RV dysfunction |
IIa |
C |
Pulmonary valve replacement may be considered for asymptomatic patients with
moderate or severe PR with ventricular tachycardiac arrhythmia |
IIb |
C |
COR, Class of Recommendation; LOE, Level of Evidence; RV, right ventricle.
Surgical treatment is indicated for patients with symptomatic PR. Pulmonary valve replacement is recommended for patients with symptomatic moderate or severe PR after transcatheter or surgical intervention for pulmonary stenosis (PS) (Class I).408
Surgical treatment is reasonable for asymptomatic patients with moderate or severe PR with RV dilatation and/or RV dysfunction (Class IIa). Surgical treatment may be reasonable in asymptomatic patients with moderate or severe PR with a ventricular tachycardia arrhythmia (Class IIb), in which case, concomitant surgical ablation should also be considered. Surgical treatment may also be considered in patients showing moderate or severe TR due to RV dilatation. According to some reports, pulmonary valve replacement before exceeding an RV end-diastolic volume of 170 mL/m2
and an RV end-systolic volume of 80 mL/m2
is recommended;409
however, the cutoff value for RV size that has not been established to date.
The advantage of using a homograft for pulmonary valve replacement has been shown,410
though it is rarely used in Japan. Because the durability of current bioprosthetic valves has improved and anticoagulant therapy is not required, some reports have revealed that the long-term results are better than those for mechanical valves.411,412
Other reports have shown that the reoperation rate is lower in patients with mechanical valves than in those with bioprosthetic valves, if adequate anticoagulation treatment is given.413
In Japan, bioprosthetic valves are more frequently used.414
Because pulmonary valve replacement is expected to improve symptoms, decrease RV end-diastolic and end-systolic volumes, improve exercise tolerance, and decrease the incidence of ventricular tachycardia, it is important to perform pulmonary valve replacement at the appropriate timing.415,416
3. Medical Therapy
This section is omitted from the English version.
X. Pulmonary Stenosis (PS)
1. Diagnosis
RV outflow tract obstruction is classified as valvular, subvalvular, and supravalvular PS based on the stenotic location, and is most commonly caused by a congenital abnormality, accounting for 8% of all congenital heart disease cases.417
Valvular PS accounts for 80 to 90% of all RV outflow tract obstruction cases.418
Valvular PS is most commonly found as isolated valvular PS, but is also often associated with other congenital heart disease such as atrial septal defect, ventricular septal defect, or patent ductus arteriosus. In addition, multiple stenotic lesions are often present, such as valvular and subvalvular PS seen in tetralogy of Fallot.
The age of onset and symptoms depend on the degree of stenosis, unless there is other congenital heart disease. Patients with critical PS require emergency treatment during the neonatal period, whereas patients with mild PS remain asymptomatic throughout their lives. Most patients with mild or moderate PS are usually diagnosed as an incidental finding on auscultation.
Valvular PS is diagnosed based on thickened leaflets showing systolic doming on 2D echocardiography, and an accelerated jet across the stenotic valve on Doppler echocardiography. However, it is often difficult to distinguish valvular PS from subvalvular or supravalvular PS in adult patients. Careful observation of the location of a mosaic pattern of blood flow using color Doppler echocardiography and assessment of the location of the accelerated blood flow using pulsed Doppler echocardiography are useful for identifying an accurate stenotic lesion. The subxiphoid approach is useful in distinguishing valvular PS from subvalvular PS, but in adult patients there is often poor visualization in the subxiphoid view. Echocardiography cannot always accurately diagnose the stenotic lesion; cardiac MRI, cardiac CT, or cardiac catheterization is required in such patients. The severity of PS is graded by the peak transvalvular pressure gradient using Doppler echocardiography. A peak transvalvular pressure gradient <36 mmHg (peak jet velocity <3.0 m/s), 36–64 mmHg (3.0–4.0 m/s), or ≥64 mmHg (≥4.0 m/s) is graded as mild, moderate, or severe, respectively (Table 43).408,418
In addition, it is necessary to estimate the RV systolic pressure using the TR peak jet velocity. Whether or not there is a large discrepancy between the RV systolic pressure and the pressure gradient measured at the stenotic lesion should be confirmed. Especially when there are multiple stenotic lesions, the estimation of the pressure gradient at the stenotic lesions may be inaccurate; therefore, the severity of PS should be assessed by RV systolic pressure calculated from the TR peak jet velocity.33
Table 43.
Grading the Severity of Pulmonary Stenosis
| |
Mild |
Moderate |
Severe |
| Doppler peak gradient (mmHg) |
<36 |
36–64 |
>64 |
| Doppler peak velocity (m/s) |
<3 |
3–4 |
>4 |
Table 44.
Indications of intervention for Pulmonary Stenosis (PS)
| Recommendations |
COR |
LOE |
Balloon valvuloplasty is recommended for symptomatic patients with severe PS (heart
failure, cyanosis from right-to left shunt, and/or exercise intolerance), surgical repair is
recommended for patients who are ineligible for or who failed balloon valvuloplasty |
I |
C |
Balloon valvuloplasty or surgical repair is reasonable for symptomatic patients with
moderate PS (heart failure, cyanosis from right-to left shunt, and/or exercise intolerance) |
IIa |
C |
Balloon valvuloplasty or surgical repair is reasonable for asymptomatic patients with
severe PS |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
It is difficult to standardize the indications for surgical intervention for patients with PS because there is insufficient evidence. Although each guideline has slightly different recommendations, intervention is recommended for patients with severe PS (symptomatic Class I, asymptomatic Class IIa) or symptomatic moderate PS (Class IIa). The indications for intervention in patients with PS are shown in
Figure 22
and
Table 44.
Since the first closed pulmonary commissurotomy in 1948, surgical intervention has been the only option for the treatment of patients with PS. However, since the first transcatheter intervention for PS in 1982, this intervention has rapidly become popular than surgical treatment. Transcatheter intervention is available for most patients with PS, except those with annular hypoplasia, cusp dysplasia, or severe PR. Surgical intervention is only indicated in cases where the catheter intervention is difficult or unsuccessful, or for those who need surgical repair for concomitant congenital heart disease.
The safety of the transcatheter intervention has been established, and both the mortality and major complication rates are lower than 1%.419,420
In terms of efficacy, the transcatheter intervention for adults as well as pediatric patients has been reported to be effective.421–423
Care should be taken for complications such as PR after transcatheter intervention. In cases of concomitant infundibular stenosis (subvalvular stenosis), a sudden reduction in RV afterload may cause a transient deterioration in the infundibular stenosis. However, the stenosis usually improves after several months in many such cases.421,423
When surgical intervention is indicated, open pulmonary commissurotomy or pulmonary valve replacement is performed. Pulmonary valve replacement is often necessary in adults with calcification or structural deterioration of the valves. In terms of the type of prosthetic valve used, mechanical valves, which are generally considered durable, provide favorable results if sufficient anticoagulant therapy is provided.413
Conversely, some reports have revealed that bioprosthetic valves are effective without anticoagulation treatment because of low right heart pressure,412
and others have reported that the type of prosthetic valve used has no relationship to the long-term outcome.424
The freedom from reintervention rates 20 years after surgical intervention and catheter intervention are favorable at 88% and 84%, respectively.425
3. Medical Therapy
This section is omitted from the English version.
XI. Combined or Multiple Valvular Disease
1. Problems in Combined or Multiple Valvular Disease
According to the 2016 Annual Report of the Japanese Society of Thoracic Surgery, 28.2% of valvular surgery was performed for combined valvular disease.307
The most common combination was mitral and tricuspid valves (55.8%), followed by mitral and aortic valves in 20.6%, and the aortic, mitral, and tricuspid valves in 15.9%.307
Thus, the combination of tricuspid valve and left-sided valvular diseases is common, accounting for 79.4% of surgeries for combined valvular disease.
Though combined valvular diseases are common, there is a lack of evidence for their diagnosis and treatment because of the various combinations. Especially in the combined valvular diseases of the aortic and mitral valves, the hemodynamic interactions between both valvular diseases may promote or conversely suppress the appearance of clinical findings in each lesion. Therefore, the physical findings, imaging, and catheterization data that have been validated for a single-valve disease may not provide accurate information in combined valvular diseases. It is necessary to be aware of the pitfalls for each combination (Table 45).426,427
Table 45.
Echocardiographic Caveats in the Diagnosis of Multivalvular Disease
Combination of
valve lesions |
AS |
AR |
MR |
MS |
| AS |
– |
• PHT method is unreliable |
• High MR volume can occur
• Mitral effective regurgitant
orifice is less affected than
MR volume |
• PHT method is unreliable
• Low-flow/low-gradient
mitral stenosis can occur |
| AR |
• Simplified Bernoulli formula
might not be applicable if
LVOT velocity is elevated
• Gorlin formula using
thermodilution is invalid
• Continuity equation is
applicable
• Peak aortic jet velocity
reflects severity of both AS
and AR |
– |
• Doppler volumetric method
is invalid |
• AR jet can be mistaken for
MS jet
• Continuity equation is
unreliable |
| MR |
• Low-flow/low-gradient AS
is common
• MR jet should not be
mistaken for the AS jet |
• Doppler volumetric method
is inapplicable
• PHT method can be
unreliable |
– |
• Continuity equation is
unreliable
• PHT method is unreliable |
| MS |
• Low-flow/low-gradient AS
is common |
• MS can blunt the increase
in pulse pressure
associated with AR |
• Mitral valvular area might
be underestimated due to
valvular calcification |
– |
| TR |
• Gorlin formula using
thermodilution is invalid |
• Not affected |
• Not affected |
• Gorlin formula using
thermodilution is invalid |
This table presents a given valvular lesion (horizontal columns) in the presence of a concomitant valvular lesion (vertical rows). AR, aortic regurgitation; AS, aortic stenosis; LVOT, left ventricular outflow tract; MR, mitral regurgitation; MS, mitral stenosis; PHT, pressure half time; TR, tricuspid regurgitation. Modified from Unger P, et al. 2011,426 2016.427 ©2011 BMJ Publishing Group Ltd. & British Cardiovascular Society ©2016 Springer Nature. https://www.nature.com/nrcardio/
In addition to the combinations of severe and moderate valvular diseases, combined valvular diseases with multiple moderate valvular diseases also may cause reduced exercise tolerance, HF symptoms, LV dilatation and dysfunction, and pulmonary hypertension. Because each valvular disease is moderate, there is no evidence for the therapeutic strategies. However, the indications for surgery should be determined by comprehensively judging the effects of all lesions. Therefore, exercise tolerance, maximum oxygen uptake, evaluations of ventricular function and pulmonary hypertension at rest and during exercise, and serum BNP levels may be useful in determining therapeutic strategies.428,429
2. Disease-Specific Considerations
2.1 Mixed AS and AR (ASR)
Studies on ASR are few and therapeutic guidelines have not been proposed. Therefore, it is assumed that the treatment strategy for ASR will be based on treatment guidelines for the predominant disease. However, ASR may have a different clinical course from that of a case of only AS or only AR. Previous studies have reported that the prognosis of ASR with moderate AS and moderate AR is the same as for severe AS.430–432
Myocardial mass in ASR increases compared with AS only and AR only, because AS causes concentric hypertrophy by pressure overload, and AR causes eccentric hypertrophy by volume overload.430–432
The relationship between increased myocardial mass and poor prognosis of heart diseases is known.433,434
LV diastolic dysfunction accompanied by advanced LV hypertrophy is considered to be the primary cause of symptoms in ASR. Namely, a rapid increase in LV diastolic pressure due to both LV diastolic dysfunction and volume load by AR may manifest as HF symptoms.435
Thus, conventional AS or AR guidelines are not sufficient for managing patients with ASR. Patients with ASR with moderate AS and moderate AR must receive careful and detailed follow-up as a severe aortic valvular disease, with evaluations of the myocardial mass and LV diastolic function in addition to the evaluation of valvular disease severity.
2.2 MS and MR (MSR)
The mechanism of concomitant MS and MR (MSR) is known to be rheumatic changes or mitral annular calcification due to aging. The mechanism of degenerative change due to aging is mitral annular calcification, in which fusion of the commissures rarely occurs. However, the differential diagnosis between degenerative change and rheumatic change is sometimes tricky among elderly patients. In order to detect the distribution of the calcification clearly, CT scans have been used as well as echocardiography.
MSR affects quantitative evaluation of MR using the Doppler method. MR increases the transmitral flow during diastole, resulting in an overestimation of MS severity. A comprehensive evaluation, including symptoms, rather than relying on a single Doppler index, is needed. Invasive pressure measurements by cardiac catheterization, not just evaluation by echocardiography, may help in assessing the severity. Evidence as to the optimal timing for surgery is limited. A comprehensive diagnosis from multiple viewpoints by the Heart Valve Team is required to determine the optimal timing of the operation.
Careful consideration should also be given to the choice of the surgical method. If rheumatic MS is the primary condition and less than moderate MR is present, the patient can be treated with PTMC. However, severe MR is a contraindication for PTMC. Furthermore, advanced techniques are indeed necessary in valvuloplasty for rheumatic mitral lesions. In addition to the preoperative evaluation, it is also necessary to include evaluation using intraoperative TEE.
2.3 MR and AS
2.3.1 Pathophysiology
In recent years, in addition to SAVR, TAVI has been available for patients with severe AS. However, the presence of more than moderate MR affects the selection of SAVR or TAVI.436
It is important to evaluate this combined valvular disease, considering the tendency to “underestimate AS” and “overestimate MR”. MR is exacerbated and overestimated due to AS because of increasing the LV systolic pressure. Conversely, AS is underestimated because of decreasing aortic valve forward flow due to MR.437
2.3.2 Diagnosis and Surgical Indications
Taking the pathophysiology into consideration, the severity of AS should also be assessed by the degree of aortic valve calcification evaluated by CT. However, although AS does not improve with intervention for the mitral valve, MR may improve with intervention for the aortic valve; therefore, in this combined valvular disease, evaluating the severity and mechanism of MR is of particular importance. In other words, when surgery is indicated for severe MR, it is recommended to perform a dual valve replacement if the severity of AS is moderate or worse. However, in patients with severe AS, the indications for surgical intervention for MR (especially in the moderate stage) are determined by predicting the degree of improvement in MR with intervention for AS alone.436,438,439
The cause of MR is not only secondarily associated with LV dysfunction due to AS, but also primarily associated with valve prolapse, rheumatic change, and annular calcification. In the presence of decreased LV function due to severe AS, MR may improve after intervention for AS associated with improvement of LV function. It is important to carefully evaluate the preoperative mitral valve morphology on echocardiography because MR may not improve postoperatively in patients with significant degenerative changes of the mitral valve or mitral annular dilatation with AF.440,441
2.4 MR and AR
2.4.1 Pathophysiology
In this combined valvular disease, both AR and MR cause a volume overload to the LV resulting in LV dilatation. The degree of pressure overload depends on the predominant valvular disease, MR or AR.
2.4.2 Diagnosis and Surgical Indications
Regarding evaluation of the regurgitant severity, the volumetric method on echocardiography cannot be used for this combined valve disease, though the PISA method is appropriate. The phase-contrast method with cardiac MRI can be used to obtain more accurate data about regurgitant volume and fraction.
Because the surgical criteria for LV size and LVEF are different between asymptomatic AR and MR, the surgical indications should be determined by the predominant valvular disease. A dual valve surgery is recommended for more than moderate AR if surgical intervention is indicated for severe MR. Conversely, if surgical intervention is indicated for severe AR in patients with moderate MR, it is important to diagnose the cause of the MR, primary or secondary, as in the case of AS and MR. For secondary MR caused by LV dilatation due to severe AR, the MR may improve after surgery for the AR alone. The possibility of mitral valve repair should be determined by TEE.
2.5 AS and MS
2.5.1 Pathophysiology
Combined AS and MS is mostly caused by rheumatic disease, but the number of patients with degenerative AS and MS has been increasing in the aging population. In this combined valvular disease, AS tends to be “underestimated” because the aortic valve forward flow reduces, caused by reduced LV filling due to the MS. In addition, the MS tends to be “underestimated” because the transmitral pressure gradient is underestimated by the increased LV end-diastolic pressure due to the severe AS.
2.5.2 Diagnosis and Surgical Indications
Regarding the evaluation of MS severity, it is important to measure the mitral valve area by the continuity equation and the planimetry method using 2D/3D cardiac echocardiography in addition to measuring the pressure gradient. The degree of aortic valve calcification evaluated by CT should also be considered. The extent of LV hypertrophy also helps assess the severity of the AS.
As for treatment options, PTMC should be considered first if the AS is mild and the morphology of the mitral valve is appropriate for PTMC in patients with rheumatic MS. Re-evaluation of AS severity should be done after performing PTMC.
With degenerative MS, the mitral annulus is severely calcified, not the valve leaflet. In patients with severe mitral annular calcification, it is difficult to suture a prosthetic valve to the mitral annulus, thus the surgical risk is high. The MS severity should be carefully assessed, and intervention for only the AS should also be considered if the severity of MS is mild or moderate in elderly patients. In this case, performing TAVI alone is also a treatment option. However, in patients with severe MS, intervention for AS alone leads to a poor prognosis.442
XII. Prosthetic Valve Replacement
1. Types and Selection of Prosthetic Valves
Prosthetic valves can be mechanical or biological. Mechanical valves are more durable, but anticoagulation is required for life. In contrast, anticoagulants are not required for bioprostheses, but there is an issue with durability. Durability depends on the patient’s age at the time of surgery. However, progress in the production of prosthetic valves has been made, and other factors such as the emergence and popularization of transcatheter interventions for valvular disease, and the aging and longevity of the Japanese population may affect valve selection, which must be considered in the light of a variety of factors, and the final decision should be made by the patient with fully informed consent.8,443
1.1 Types and Progress of Mechanical Valves
Historically, ball valves and tilting disc valves have been used, but all current mechanical valves are bileaflet. In terms of durability, mechanical valves are significantly superior to a bioprosthesis. However, the biggest disadvantage of mechanical valves is the requirement for continuous anticoagulant therapy for life. Therefore, there is a higher risk of bleeding and thromboembolic events than with a bioprosthesis.
Recent advances in mechanical valves include the low-thrombotic On-X aortic valves. An RCT was performed comparing low-dose (PT-INR: 1.5–2.0) and standard-dose (PT-INR: 2.0–3.0) warfarin groups of high thromboembolism risk patients, showing that bleeding complications were significantly reduced in the low-dose group, but cerebral infarction and mortality rates were comparable in both groups.444
In patients with a low risk of thromboembolism, an RCT comparing dual antiplatelet therapy and standard warfarin therapy was discontinued because of the high incidence of thromboembolism and valve thrombosis in the dual antiplatelet group.445
1.2 Types and Progress of Bioprostheses
Bioprostheses are made of either bovine pericardial leaflets or porcine leaflets. A 20-year durability of bioprostheses was reported in patients ≥60 years of age.299
Many studies have compared the hemodynamics of bovine pericardial valves and porcine valves, showing that the pressure gradient across the valve was lower and the EOA was larger in bovine pericardial valves compared with porcine valves.446
However, few studies showed a difference in LV mass regression, and the 10-year survival was comparable.447
There are 2 types of bovine pericardial valve: (1) ordinary internally mounted and externally mounted valves; (2) internally mounted valve with pericardium on the inside of the stent post and an externally mounted valve with pericardium on the outside. Although the externally mounted valves have an advantage in hemodynamics,448
there are few reports on the long-term results beyond 10 years and reports of longer-term studies are pending. In addition, when performing the valve-in-valve procedure with TAVI for externally mounted valves, the risk of coronary obstruction is considered to be higher than with internally mounted valves, and it is important to consider various factors, such as the distance between the annulus and the coronary artery orifice and the size of the Valsalval sinus, for the valve-in-valve procedure.449
The leaflet of the bioprosthesis is treated with various anticalcification media to prevent SVD. Recently, a bioprosthesis using specially treated bovine pericardium has become available. The pericardium is usually exposed to unstable aldehydes during fixation and storage, which binds calcium in the body. With this new bioprosthesis, a new method of preparation permanently blocks unstable aldehydes with capping, and excellent anticalcification effects have been found in animal experiments.450
Although clinical results are still scant,451
this valve is expected to be effective in avoiding SVD, especially in young patients.
1.3 Valve Selection (Table 46)
Table 46.
Recommendations for Prosthetic Valve Selection
| Recommendations |
COR |
LOE |
| Valve choice should be finally determined by patients with fully informed consent |
I |
C |
Bioprosthesis is recommended when anticoaglant therapy is contraindicated or
cannot be fully controlled (poor compliance or remote area) |
I |
C |
| Bioprosthesis is reasonable for females who desire childbirth |
IIa |
C |
Mechanical valve is reasonable for patients <60 years in the aortic position and <65 years
in the mitral position |
IIa |
C |
Bioprosthesis is reasonable for patients ≥65 years in the aortic position and ≥70 years in
the mitral position |
IIa |
C |
Bioprosthesis is reasonable in reoperation for mechanical valve thrombosis despite good
anticoaglation control |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
There are several studies comparing the long-term results between mechanical valves and bioprostheses in SAVR. In younger patients, mechanical valves are reported to have a survival benefit over bioprosthesis,452–454
although some studies showed no difference in survival between mechanical valves and bioprostheses by age group.455,456
Reoperation was performed more frequently after bioprosthesis implantation, but the incidence of bleeding and embolic events was reported to be higher with mechanical valves.
In terms of actual usage, the proportion of bioprosthesis use increases as age increases, and the proportion of bioprosthesis use overall has increased in recent years, according to a report from the UK.457
In Japan, 2013–14 data from the Japan Cardiovascular Surgery Database also showed that the ratio of bioprosthesis use was 20% among patients in their 50 s, 60% in the 60 s, and >90% among those aged ≥70 years.458
The ACC/AHA guidelines updated in 2017443
recommend mechanical valves for those under 50 years of age and bioprostheses for those over 70 years of age. Valve selection should be individualized for patients between these ages. The 2017 ESC/EACTS guidelines recommend mechanical valves in the aortic position for patients aged ≤60 years, and in the mitral position for those aged ≤65 years of age, and they recommended bioprostheses in the aortic position for patients aged ≥65 years, and in the mitral position for those aged ≥70 years. As in the 2017 ESC/EACTS guidelines, the present guidelines recommend a bioprosthesis in the aortic position for patients aged ≥65 years, and in the mitral position for those aged ≥70 years; a mechanical valve in the aortic position in those aged ≤60 years, and in the mitral position for those aged ≤65 years (Class IIa). Physicians should be familiar with the advantages and disadvantages of each prosthetic valve type and consider not only age but also patient factors (e.g., lifestyle such as athletic preferences, hope of childbirth, etc.), comorbidities (e.g., bleeding disorders, thromboembolic disease), hospital access, and drug compliance. Patients who do not have adequate warfarin control (medication compliance, remote location) (Class I) or females who desire childbirth (Class IIa) are recommended for bioprostheses, but if a mechanical valve has already been implanted in the other position, a mechanical valve is often selected. It is also recommended that a bioprosthesis be used in patients who require re-do surgery for a thrombosed mechanical valve despite good anticoagulant control (Class IIa). In all cases, sufficient information should be provided to the patient and discussed among the patient, family members, and physicians before the final decision is made by the patient (Class I).443
As data on the long-term durability of TAVI valves, the durability of bioprostheses in young people, and long-term antithrombotic use for mechanical valves are revealed, the age cutoff values for artificial valves may change. It is also important to inform patients of the need for secondary and tertiary interventions when selecting bioprostheses for young patients.
The choice of artificial valves for patients on dialysis is controversial. According to the 2006 ACC/AHA guidelines,459
bioprostheses for patients with renal dysfunction, especially for patients on hemodialysis, are known to have durability issues. Although SVD by calcification is known to occur in bioprostheses and bleeding complications are common in mechanical valves, recent studies reported no difference in the surgical results between bioprostheses and mechanical valves and that it is not necessary to avoid bioprostheses for patients on hemodialysis.460,461
However, no conclusions have been reached on this topic. Physicians can only discuss the issues with patients on a case-by-case basis, and the present guidelines do not recommend either valve.
Transcatheter intervention for SVD after SAVR (valve-in-valve) is now common, and the age cutoff for bioprostheses may become lower in the future. In addition, the selection of valve (size and type) at first SAVR is important, given the potential for future valve-in-valve procedures.
2. Diagnosis, Treatment, and Follow-up After Prosthetic Valve Replacement
2.1 Prosthetic Valve Assessment and Follow-up
2.1.1 Basic Assessment of Follow-up
After prosthetic valve replacement, all patients must be followed for life for the early detection of prosthetic valve dysfunction, impaired cardiac function, or progressive disease of other heart valves. Therefore, regular follow-up TTE is necessary even in the absence of symptoms. Postoperative follow-up TTE is conducted within approximately 30 days postoperatively and 1 year postoperatively, then annually thereafter.462
In particular, follow-up on valve deterioration by echocardiography is important in cases where more than 10 years have passed since the surgery. If a new symptom presents, TEE should be used immediately to determine the cause.
TEE should be used in cases of suspected artificial valve malfunction or infective endocarditis.463,464
Both cinefluoroscopy of the mechanical valve and cardiac CT are useful in the event of suspected stenosis caused by prosthetic valve thrombus or pannus formation.464
2.1.2 When Prosthetic Valve Thrombus Is Suspected
If prosthetic valve thrombosis is suspected in patients with new-onset dyspnea, then TTE, TEE, cinefluoroscopy, and cardiac CT may be used to quickly confirm the diagnosis. Prosthetic valve thrombosis is a problem often associated with mechanical valves, though they are also reported to occur with bioprosthetic valves.465,466
Bioprosthetic valve thrombosis with no clinical symptoms is often observed on CT,68
and in recent years has been reported after TAVI. Valve thrombus increases the transvalvular pressure gradient.467
2.1.3 Hemolysis and Paravalvular Regurgitation
TTE should be used in cases of suspected hemolysis after prosthetic valve replacement, even if valve regurgitation is not observed. Re-do surgery should be considered if paravalvular regurgitation is caused by infective endocarditis or if the hemolysis causes refractory anemia or HF.
2.2 Antithrombotic Therapy
2.2.1 Selection and Control of Antithrombotic Drugs in Mechanical Valve Patients (Table 47)
Table 47.
Antithrombotic Therapy for Prosthetic Valve Patients
| Recommendations |
COR |
LOE |
| Mechanical valve |
Oral anticoagulat therapy with warfarin is recommended lifelong for all patients
Target INR of warfarin control
• Aortic position: INR 2.0–2.5
• Aortic position and thrombotic risks: INR 2.0–3.0
• Mitral position: INR 2.0–3.0 |
I |
B |
Warfarin control of INR 2.5–3.5 is reasonable for patients with thrombotic event despite
adequate anticoagulation therapy |
IIa |
C |
Aspirin combination therapy may be considered for patients with thrombotic event
despite adequate anticoagulation therapy |
IIb |
C |
| Single aspirin therapy is contraindicated |
III |
B |
| DOAC usage is contraindicated |
III |
B |
| Bioprosthetic valve |
Anticoagulation therapy with warfarin control of INR 2.0–2.5 is reasonable for the first 3
months after surgery |
IIa |
B |
DAPT (aspirin 75–100 mg+clopidogrel 75 mg) is reasonable for the first 6 months after
TAVI, followed by lifelong single antiplatelet therapy (aspirin or clopidogrel) |
IIa |
C |
COR, Class of Recommendation; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant; INR, international normalized ratio; LOE, Level of Evidence; TAVI, transcatheter aortic valve implantation.
Anticoagulant therapy with warfarin is required in all patients after mechanical valve replacement (Class I). However, thromboembolic events may appear at a rate of ≈1–3% per year despite using warfarin.468
In the ACC/AHA261
and ESC/EACTS301
guidelines for 2nd-generation mechanical bileaflet valves (e.g., Carbomedics valves, ATS valves, and St. Jude Medical valves), a target PT-INR of 2.5 (2.0–3.0) in the aortic valve position and a target PT-INR 3.0 (2.5–3.5) in the mitral valve position is recommended. A history of embolism, presence of AF, MS, or an LVEF <35% are considered risk factors for embolism and require a target PT-INR 0.5 higher than the above ranges. For patients with a ball valve, a target PT-INR of 3.0 (2.5–3.5) in the USA and 3.5 (3.0–4.0) in Europe are recommended. In addition to warfarin, these guidelines also recommend the use of aspirin (75–100 mg). However, Japanese are known to have a higher risk of bleeding and a lower risk of embolism compared with Western populations. The optimal therapeutic range of warfarin or combinations of antiplatelet drugs should be modified from the US and European guidelines and should be considered for each individual case rather than for all cases in Japanese patients. In the present guideline, a PT-INR of 2.0–2.5 is recommended for the aortic valve position, and a PT-INR of 2.0–3.0 is recommended for the mitral valve position or the aortic valve position with a risk of embolism. However, the use of concomitant antiplatelet drugs is not recommended. For patients with apparent thromboembolic events, even during appropriate anticoagulant therapy, a PT-INR of 2.5–3.5 may be used (Class IIa) or a combination with aspirin may be considered (Class IIb).
The use of DOACs is not recommended for mechanical valve patients because in an RCT using warfarin and DOACs for mechanical valve patients,469
both embolism and bleeding events were higher in the DOAC group (Class III).
2.2.2 Indications and Selection of Antithrombotic Therapy in Bioprosthetic Valve Patients (Table 47)
The risk of thromboembolism is high within 3–6 months after bioprosthetic valve implantation.470–472
The incidence of cerebral infarction and death is significantly reduced with anticoagulant therapy for 6 months compared with 3 months.470
Anticoagulant therapy with warfarin (PT-INR: 2.0–2.5) is recommended for the first 3 months (or 6 months if the patient has no risk of bleeding) after bioprosthetic valve implantation (Class IIa). Continuous anticoagulant therapy (PT-INR: 2.0–2.5) after 6 months is not recommended but may be considered in patients with thrombotic risk factors. After TAVI, dual antiplatelet drugs should be administered for 6 months followed by single-antiplatelet therapy for life (Class IIa). Recently, post-TAVI prosthetic valve thrombosis and restricted valve opening were reported at 7–40%467,473,474
and 18%, respectively.474
The 2017 AHA/ACC guidelines recommended anticoagulant therapy for 3 months after TAVI in patients with a low risk of bleeding.475
However, a study in Japan also reported that although 9.3% of TAVI patients experienced valve thrombosis within 30 days’ post-TAVI, the long-term prognosis did not differ from a group without valve thrombosis.70
The use of additional anticoagulants should be done with caution, because the risk of falling is higher among the elderly and the incidence of bleeding events is higher in the Japanese population than in Western populations. As with previous guideline, dual antiplatelet therapy for 6 months (class IIa) followed with single antiplatelet therapy for rest of their life is recommended in this guideline. This recommendation may change as with future coming evidence.
2.2.3 Excessive Dosage of Anticoagulant Therapy and Bleeding Complications (Table 48)
Table 48.
Treatment for Overdose of Anticoagulant Therapy or Bleeding Complication in Patients With a Prosthetic Valve
| Recommendations |
COR |
LOE |
| General emergency management is indicated for bleeding complication |
I |
C |
Dose reduction or discontinuance of warfarin is indicated depending on bleeding severity and
Vitamin K administration as required |
I |
C |
Dose reduction or discontinuance of heparin or reversal with protamine is indicated depending
on bleeding severity |
I |
C |
Administration of fresh frozen plasma is reasonable when reversal of anticoagulation is
urgent |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
If the PT-INR exceeds 4.5 during warfarin administration, the risk of bleeding increases. If the PT-INR exceeds 6.0, the risk increases dramatically. In such cases, consider rapid reversal. If the PT-INR is 4.5–6 without active bleeding, stop warfarin or administer vitamin K depending on the risk of bleeding. If serious bleeding is observed, consider the administration of vitamin K and prothrombin complex concentrate.476
The recommendation of the present guideline follows the 2009 JCS guidelines for the management of anticoagulant and antiplatelet therapy in cardiovascular disease. Restarting anticoagulant therapy should be determined by the location of the bleeding, the type of intervention that has been done to stop the bleeding, and by establishing the underlying cause of the bleeding.477
2.2.4 Interruption of Anticoagulant Therapy During Noninvasive Treatment or Noncardiac Surgery
Interruption of warfarin may increase the risk of developing a thromboembolism during dental treatments such as a tooth extraction.478
Prospective studies have shown that dental extraction can be done without serious bleeding under warfarin if the PT-INR is between 2.0 and 4.0,479,480
although some reports recommend dental extraction at an PT-INR <2.5.481
As per the 2009 JCS guidelines for the management of anticoagulant and antiplatelet therapy in cardiovascular disease, we recommended that dental extraction should be done under continued use of warfarin controlling the PT-INR within the optimal therapeutic range.
Antiplatelet drugs for patients undergoing noncardiac surgery should be discontinued 1 week prior to the procedure. If major surgery is performed, warfarin should be discontinued 72 h prior to the procedure and the PT-INR should be controlled to <1.5. Continuous heparin administration is recommended during the perioperative period, while the PT-INR is <2.0. The heparin dose should be adjusted to maintain the activated partial thromboplastin time (aPTT) at 55–70 s and should be discontinued 4–6 h before surgery. After confirming there is no active bleeding after surgery, heparin should be restarted as soon as possible and then changed to warfarin. Anticoagulant and antiplatelet therapies should be continued during gastrointestinal endoscopy if performed only for observation. For endoscopic low-risk procedures such as biopsy and high-risk procedures such as polypectomy, warfarin should be discontinued for 3–4 days and adjust the dose to achieve a PT-INR of ≤1.5. Consider using heparin in cases of a high risk of thrombosis or embolism.
2.3 Diagnosis and Treatment of Thrombosis
Thrombosis in mechanical valves can cause fatal conditions such as HF or cardiogenic shock due to valve stenosis/obstruction. Patients may be asymptomatic but may show dull systolic or mechanical valve closure sounds on auscultation, and valve opening restriction or abnormal Doppler findings on TTE. Diagnosis should be made comprehensively, detected by increased pressure gradient and mobile tumor-like images on TTE, and a reduction in leaflet movement on fluoroscopy or cardiac CT. Pannus is another condition involving restricted valve closure similar to valve thrombosis. Valve thrombosis may be suspected when the control of anticoagulant therapy is poor, symptoms appears early after valve replacement, or the onset is relatively rapid. It may, however, be difficult to differentiate between thrombosis and pannus and they may be concomitant.
Because the prognosis for valve thrombosis is poor for left-heart mechanical valves, symptoms suspected to be from valve thrombosis require an emergency initial action including low-dose fibrinolytic treatment or emergency surgery (Class I:
Table 49). The 30-day mortality rate of emergency surgery was found to be high at 10–15% overall, though it was favorable for <5% of patients in the New York Heart Association (NYHA) I/II category.482–486
Until 2012, the 30-day mortality rate of obstructive left-heart mechanical valve thrombolytic therapy was 8%, with a hemodynamic success rate of 70%. However, the rate of concurrent thromboembolism and bleeding complications was high at 14%.485
In recent years, studies of low-dose fibrinolysis using the slow infusion protocol under echocardiographic guidance have been published, with mortality rates of <2% and success rates > 90%. Moreover, both thromboembolism and bleeding complications were reported to be <2%.487,488
Using that protocol, the study also showed favorable results even in cases of severe symptoms and with a large thrombus. However, whether to perform surgery or select fibrinolysis therapy should be determined on a case-by-case basis, considering the capability of each facility. Surgery is recommended in patients with low surgical risk, contraindication to thrombolysis, large thrombus (e.g., >0.8 cm2
or >10 mm), high level of severity (hemodynamic instability, NYHA IV), and other concomitant VHD requiring cardiac surgery. Fibrinolytic therapy should be considered when skilled surgeons are unable or unavailable to perform immediate surgery, in cases of small thrombus or in hemodynamically stable patients.
Table 49.
Treatment for Prosthetic Valve Dysfunction
| Recommendations |
COR |
LOE |
| Valve thrombosis |
Low dose thrombolytic therapy or urgent/emergency valve replacement is
recommended for symptomatic patients due to obstructive thrombosis of left-sided
mechanical prosthesis |
I |
C |
| Valve stenosis/regurgitation |
Surgical replacement is recommended for severe prosthetic valve stenosis with heart
failure symptoms |
I |
C |
Surgical replacement is recommended for severe prosthetic valve regurgitation with
heart failure symptoms or hemolysis |
I |
C |
Transcatheter valve-in-vave implantation is reasonable for high-risk symptomatic
patients with severe bioprosthetic valve stenosis or transvalvular regurgitation in the
aortic position |
IIa |
C |
COR, Class of Recommendation; LOE, Level of Evidence.
2.4 Nonthrombotic Prosthetic Valve Dysfunction (Stenosis/Regurgitation)
2.4.1 Definition and Pathophysiology of Prosthetic Valve Dysfunction
The definition of prosthetic valve dysfunction differs across reports, as does the frequency of dysfunction. As a result, the need to unify the definition of prosthetic valve dysfunction or failure has increased and several statements on this matter have been released from European and American academic societies.462,464,489
In both statements, SVD was clearly differentiated from infective endocarditis, paravalvular regurgitation, and patient–prosthesis mismatch (PPM), and SVD was described as a structural abnormality of the prosthetic leaflet.
With the new definition, it is important to correctly evaluate the severity of the stenosis and regurgitation, mainly based on echocardiographic assessment and the presence of symptoms.
A mechanical prosthetic valve is highly durable, but occasionally formation of a thrombus or pannus can cause leaflet malfunction. Formation of pannus is caused by various inflammatory responses to the materials of the prosthetic valve and thrombus can form on the surface of pannus. For bioprosthetic valves, SVD usually begins 5–7 years after implantation, mainly through degeneration and calcification, and no useful medical therapy has yet been established to prevent SVD. A bioprosthetic valve at the mitral position tends to be more prone to valve degeneration.
Porcine aortic valve leaflets and bovine heart pericardium are the main materials used for bioprosthetic valves. Externally-mounted leaflet valves such as the Trifecta, Mitroflow, and stentless Solo Smart, and sutureless valves such as the Perceval and Intuity valves are relatively new, and it remains unclear whether they can produce results comparable to those of other surgical bioprosthetic valves. Attention should be paid to the development of leaflet lacerations, particularly in the externally-mounted leaflet valves.490
Current transcatheter bioprosthetic valves are limited to aortic valve placement in Japan, and there are 2 types: balloon-expandable and self-expandable valves. Both valves can be affected by prosthetic valve failure from damage associated with crimping, asymmetric expansion, and valve-frame interaction.491
Paravalvular regurgitation is a complication that can be observed after valve replacement with either a mechanical or bioprosthetic valve. It can be caused by infective endocarditis, suture failure, mechanical stress, etc. Paravalvular regurgitation is a complication that occurs at a rate of 5–10% after aortic valve replacement and 10–20% after mitral valve replacement. The majority of cases are asymptomatic, and ≈10% of patients with paravalvular regurgitation have symptoms of HF or hemolytic anemia.492
2.4.2 Diagnosis of Prosthetic Valve Dysfunction
Diagnosis of prosthetic valve dysfunction is mainly made by echocardiography, but CT and MRI sometimes play a significant role. CT can help distinguish between a thrombus and pannus and could help in recognizing paravalvular regurgitation.493
The quantification of prosthetic valve regurgitation using MRI is particularly important for prosthetic valves at the pulmonic valve position.494
Fluoroscopy is useful when evaluating the movement of the mechanical valve leaflets, the stent strut form, and calcification of a bioprosthetic valve.495
Each mechanical valve has a predefined threshold for the opening and closing angles. Excessive valve ring movement (>15°) is also recognized as dehiscence and usually accompanies a high degree of paravalvular regurgitation. Gallium scintigraphy or positron emission tomography CT can be useful for assessing the presence of infection.496
a. Diagnosis of Prosthetic Valve Stenosis
The opening of the prosthetic valve is mainly assessed by echocardiography to determine the EOA. However, it is difficult to accurately measure the diameter of the LV outflow in some situations and the degree of stenosis can be alternatively assessed using the Doppler velocity index or other methods. There is a predetermined normal EOA reference value for each valve type and size, with moderate prosthetic valve stenosis defined as <−1 standard deviation (SD) from the reference value and severe prosthetic valve stenosis as <−2 SD from the reference value. It is recommended to diagnose severe prosthetic valve stenosis when there is a peak transvalvular velocity ≥4 m/s and an EOA ≤0.8 cm2
for the aortic position, and a peak transvalvular velocity ≥2.5 m/s and an EOA ≤1.0 cm2
for the mitral position (Table 50).
Table 50.
Grading the Severity of Prosthetic Valve Stenosis
| |
Mild |
Moderate |
Severe |
| Aortic valve prosthesis |
| Acceleration time (ms) |
<80 |
80–99 |
≥100 |
| Peak velocity (m/s) |
<3.0 |
3.0–3.9 |
≥4.0 |
| Mean PG (mmHg) |
<20 |
20–34 |
≥35 |
| Increase in mean PG during follow-up (mmHg) |
<10 |
10–19 |
≥20 |
| EOA (cm2) |
>1.1 |
0.8–1.1 |
<0.8 |
| Measured EOA vs normal reference value (cm2) |
Reference±1SD |
<Reference−1SD |
<Reference−2SD |
| DVI (VTILVOT/VTIAV) |
≥0.30 |
0.25–0.30 |
<0.25 |
| Mitral valve prosthesis |
| Pressure half time (s) |
<130 |
130–200 |
>200 |
| Peak velocity (m/s) |
<1.9 |
1.9–2.5 |
>2.5 |
| Mean gradient (mmHg) |
≤5 |
6–10 |
>10 |
| Increase in mean PG during follow-up (mmHg) |
<5 |
5–12 |
>12 |
| EOA (cm2) |
>2.0 |
1.0–2.0 |
<1.0 |
| Measured EOA vs normal reference value (cm2) |
Reference±1SD |
<Reference−1SD |
<Reference−2SD |
| DVI (VTIMV/VTILVOT) |
<2.2 |
2.2–2.5 |
>2.5 |
AV, aortic valve; DVI, Doppler velocity index; EOA, effective [valve] orifice area; LVOT, left ventricular outflow tract; PG, pressure gradient; VTI, velocity time integral.
PPM is not a valve failure itself, but may occur when an undersized valve compared to the patient’s body size has been implanted. Therefore, if a relatively high-pressure gradient is recorded early after surgery, physicians should suspect PPM. Moderate PPM is relatively common (20–70%), but severe PPM is rare (2–10%). Severe PPM is defined as an EOA index <0.65 cm2/m2
(for BMI <30 kg/m2) or <0.55 cm2
(BMI >30 kg/m2) at the aortic position, or an EOA index <0.90 cm2/m2
(BMI <30 kg/m2) or <0.75 cm2/m2
(BMI >30 kg/m2) at the mitral position.
b. Diagnosis of Prosthetic Valve Regurgitation
With mechanical valves, although small amounts of physiological regurgitation jet can usually be seen at the rotating axis (pivot) and inside the sewing cuff, pathological transvalvular regurgitation may be observed due to the formation of pannus, a thrombus, or vegetation. Although small amounts of physiological regurgitation may also be seen at the center of the bioprosthetic valve, advanced leaflet degeneration can lead to pathological regurgitation.
The severity of regurgitation is determined by the criteria shown in
Table 51
for both mechanical and bioprosthetic valves.463,464
In addition, because of the increase in the number of TAVI cases in recent years, independent diagnostic criteria have been proposed for paravalvular regurgitation following TAVI.497
The localization of paravalvular regurgitation is numbered in a clockwise manner from the LA side with “12 o’clock” at the center of the aortomitral continuity. The severity of regurgitation is mainly determined by the echocardiographic findings, but the acoustic shadow caused by the metal structure of the prosthetic valve often complicates the evaluation and TEE can be useful in such cases. In particular, a diagnosis of mitral valve paravalvular regurgitation can be exclusively achieved by TEE alone in the majority of cases. Conversely, aortic valve regurgitation can be more accurately detected by TTE, depending on the location. Quantification of artificial valve regurgitation is often difficult with TTE or TEE, and blood flow quantification using MRI often helps to identify the severity.
Table 51.
Grading the Severity of Prosthetic Valve Regurgitation
| |
Mild |
Moderate |
Severe |
| Aortic valve prosthesis |
| AR jet width |
Small |
Intermediate |
Large (>65% of LVOT) |
| Pressure half time of AR jet (ms) |
>500 |
200–500 |
<200 |
| Circumferential extent of PVL (%) |
<10 |
10–29 |
≥30 |
| Vena contracta width (cm) |
<0.3 |
0.3–0.6 |
>0.6 |
| EROA (cm2) (PISA method) |
<0.10 |
0.1–0.29 |
≥0.3 |
| RV vol (mL) |
<30 |
30–59 |
≥60 |
| Mitral valve prosthesis |
| Color flow MR jet |
Small |
Intermediate |
Large |
| Pulmonary vein flow |
Systolic dominance |
Systolic blunting |
Systolic flow reversal |
| Mitral inflow (m/s) |
– |
– |
≥1.9 |
| DVI (VTIPrMV/VTILVOT) |
<2.2 |
2.2–2.5 |
>2.5 |
| VC width (cm) |
<0.30 |
0.30–0.59 |
≥0.6 |
| Circumferential extent of PVL (%) |
<10 |
10–29 |
≥30 |
| EROA (cm2) |
<0.20 |
0.20–0.39 |
≥0.40 |
| RV vol (mL) (volumetric or PISA method) |
<30 |
30–59 |
≥60 |
AR, aortic regurgitation; DVI, Doppler velocity index; EROA, effective regurgitant orifice area; LVOT, left ventricular outflow tract; PVL, paravalvular leakage; RV, right ventricle; VTI, velocity-time integral.
2.4.3 Indications for Surgery and Transcatheter Intervention for Prosthetic Valve Dysfunction
Severe prosthetic valve stenosis/regurgitation causes HF and hemolysis. If these symptoms are present, surgery or transcatheter treatment should be considered.
a. Surgical and Transcatheter Interventions
Surgery was originally the only treatment option available for severe prosthetic valve dysfunction, including paravalvular regurgitation. In recent years, however, the option of transcatheter treatment has emerged, including valve-in-valve treatment for bioprosthetic valve stenosis or transvalvular regurgitation, and transcatheter closure for paravalvular regurgitation.
Favorable results have been shown for re-do SAVR,498,499
but the operative mortality was reported to be higher than for the initial SAVR.500
The Valve-in-Valve International Data Registry report indicates that the surgical mortality rate among 459 patients who underwent the valve-in-valve procedure with a high surgical risk was lower than the expected mortality rate predicted by the STS score.501
Conversely, the outcomes of re-do SAVR were reported to be comparable to those for valve-in-valve treatment.502,503
There are also reports of poor prognoses when valve-in-valve is performed for small-sized bioprosthetic valve cases of PPM just after initial SAVR.501,504
Re-do SAVR or valve-in-valve options should be determined on a case-by-case basis by the Heart Valve Team with consideration of the size and type of bioprosthesis, anatomical compatibility of the size of the Valsalval sinus, surgical risk, etc.
Valve-in-valve for mitral bioprostheses has not been approved in Japan as of March 2020. However, the 1-year survival rate of this treatment was comparable to that for re-do valve replacement, despite involving higher risk patients compared with re-do valve replacement according to overseas retrospective observational studies.505
Surgical procedures for paravalvular regurgitation include re-do mitral valve replacement and partial repair using a patch. These approaches may be selected based on the risk of surgery and the extent of the leak. In a long-term follow-up study involving 190 patients who underwent surgical procedures for paravalvular regurgitation, the operative mortality was 7%, 10-year survival was 56%, and the recurrence rate at 10 years was 32%.506
Among 122 long-term follow-up cases, the 30-day mortality was 10.7% and 12-year survival was 39%.507
Meanwhile, the long-term outcomes of transcatheter closure have not yet been shown, although short-term results have been reported as favorable. Among 308 cases, 91% had success in placing closure devices and the mortality rate was 3.9%. The median follow-up period was 110 days, with 25.3% of patients showing moderate or greater paravalvular regurgitation.508
Transcatheter closure of paravalvular regurgitation has not been approved in Japan as of March 2020.
b. Indications for Intervention for Prosthetic Valve Dysfunction (Table 49)
Re-do valve replacement is performed for severe prosthetic valve stenosis and/or regurgitation with HF symptoms (Class I). Re-do valve replacement should be performed for prosthetic valve regurgitation (often paravalvular) that causes severe hemolysis (Class I). In addition, transcatheter valve-in-valve treatment is performed for symptomatic severe aortic bioprosthetic valve stenosis and/or regurgitation with a high surgical risk (Class IIa).
For asymptomatic severe prosthetic valve stenosis/regurgitation, the Heart Valve Team should carefully determine the indications based on guidelines for native valve diseases, considering the risks with re-do surgery.
2.4.4 Medical Therapy
A moderate degree of stenosis due to PPM or SVD or a moderate degree of paravalvular or transvalvular regurgitation can be managed with medical therapy, including common HF drug therapy, especially diuretics and antihypertensive agents.
Conversely, mechanical hemolysis may present with severe hemolytic anemia in cases of paravalvular regurgitation even at less than a severe degree.492
Severe hemolytic anemia may require surgical treatment, but is often challenging because of re-do surgery and coexistent comorbidities. In such cases, transfusion is the only therapeutic option, but frequent transfusions pose a risk of developing hemochromatosis and irregular antibodies. Hematopoiesis is usually amplified in cases of hemolytic anemia, but erythropoietin and iron administration are often effective. Because hemolysis occurs when blood passes through a small tunnel between the prosthetic valve and the tissue, beta-blockers may be effective for preventing progression of anemia by reducing stress on blood cells.509
If bradycardia is caused by beta-blockers, permanent pacemaker implantation may be required.
XIII. Management After Valve Repair
1. Mitral Valve Repair
1.1 Postoperative Assessment and Follow-up
The evaluation of mitral valve repair is performed by echocardiography and should be done with prior information of preoperative cardiac function, anatomical lesion, etiology of MR, selected surgical procedures and when the surgery was performed.
1.1.1 Intraoperative TEE After Mitral Valve Repair
Assessment should be performed with the patient in a hemodynamically stable state after removal of the aortic cross-clamp. Even in cases of mild residual regurgitation, the reoperation rate is higher than without residual regurgitation, which suggests the MR should be completely controlled.166
A maximum residual regurgitant jet area of ≥2 cm2
by color Doppler TEE indicates an unsuccessful repair and requires revision of the repair.510
However, the jet area needs to be carefully evaluated, because it is influenced by blood pressure and the settings of the echocardiographic system. A vena contracta width ≥3 mm indicates moderate or more than moderate MR,30
and requires correction of the residual MR.511
In cases of more than mild regurgitation, less than mild but with a rapid regurgitation jet striking the annuloplasty ring, which is a risk of hemolysis, and MS with a transmitral mean pressure gradient ≥7 mmHg or maximum pressure gradient ≥17 mmHg,512
also require a revision of repair. SAM causes LV outflow tract obstruction and MR, and if the SAM is not resolved by LV volume expansion, discontinuation of inotropic drugs or administration of beta-blockers, a revision of repair should be considered.513
1.1.2 Postoperative Follow-up
Periodic monitoring is done by TTE, focusing on the changes in cardiac function and recurrence of regurgitation or appearance of stenosis. Recurrent mitral valve dysfunction after mitral valve repair can be classified as either procedure-related or valve-related.514,515
Procedure-related failure includes suture dehiscence, problems with artificial chords, SAM, and incomplete repair. Valve-related failure includes progressive valve degeneration, infective endocarditis, and leaflet retraction. There is a tendency for procedure-related repair failure and post-repair hemolysis to typically occur within 1–2 postoperative years and rapidly diminish, but the occurrence of valve-related repair failure is relatively constant after the operation.172,514–517
Therefore, TTE should be performed before hospital discharge, at 1–3 months after discharge, and thereafter at 6-month intervals for 2 years, and then once every 2–3 years if there are no symptoms and the TTE findings remain unchanged. If there is residual regurgitation or leaflet restriction, annual TTE observation should be continued, together with observation for symptoms and auscultation for heart murmur. There are no clear criteria for the interval of follow-up and so it should be adjusted according to the clinical status of the patient.
1.2 Indications for Reoperation
Reoperation is indicated for patients with severe recurrent/residual MR, progressive anemia caused by repair-related hemolysis, or severe MS. Patients with less than severe but moderate or more than moderate MR, who show symptoms such as exertional dyspnea, progressive enlargement of the LV and/or LA, progressive pulmonary hypertension, or new-onset of AF should be carefully evaluated, taking reoperation into consideration, because the severity of MR was possibly underestimated. TEE is indicated for patients with suboptimal TTE images for detailed assessment of the etiology of the regurgitation or stenosis. When the decision is difficult to make regarding reoperation due to a discrepancy between the clinical assessment and the echocardiographic findings, exercise stress echocardiography is useful for revealing symptoms and changes in pulmonary artery pressure. To determine the indications for reoperation in cases of severe LV dysfunction, discussion by the Heart Valve Team is needed to choose the best individualized approach.
2. Aortic Valve Repair and Valve-Sparing Root Replacement
2.1 Postoperative Assessment and Follow-up
The postoperative evaluation of aortic valve repair is performed mainly by echocardiography, which should be done with the prior information of preoperative cardiac function, anatomical lesion, etiology of AR, and selected surgical procedures.
2.1.1 Intraoperative TEE After Aortic Valve Repair
Assessment needs to be performed with the patient in a hemodynamically stabilized state after removal of the aortic cross-clamp. Coaptation length ≥4 mm518
and effective height ≥9 mm519
have been reported as indicators for preventing recurrent AR late after aortic valve repair. Because the effective height correlates with BSA,520
in patients with small body size appropriate adjustment may be needed when these indices are used. Other predictors of recurrent AR include a coaptation occurring below the level of the aortic annulus and residual AR.518
Previous studies have identified that even a mild residual regurgitation is a risk factor for recurrent AR.518
However, if the residual AR appears centrally with sufficient coaptation, mild AR (vena contracta <3 mm) can be acceptable. More than moderate regurgitation, or mild regurgitation but appearing eccentrically along the leaflet surface, indicates an incomplete repair such as residual leaflet prolapse, which requires revision of the repair or valve replacement. In cases of annuloplasty, the annular diameter needs to be measured by TEE to determine whether plication of the annulus has been adequately performed. Moderate or more than moderate AS (AVA <1.5 cm²), which may occasionally result from excessive plication of the leaflet or the aortic root, requires consideration of re-repair or valve replacement. In cases of coronary artery reconstruction, the patency of the coronary arteries should be checked by TEE with visualization of the coronary blood flow and the wall motion of the LV. In cases of deep root dissection, the presence of an interchamber fistula should be ruled out.
2.1.2 Postoperative Follow-up
After repair surgery, a change in the pulse pressure and a diastolic murmur on auscultation should be carefully evaluated. LV function, residual regurgitation, and postoperative stenosis should be checked by TTE, usually 1 week postoperatively or before discharge. When the TTE image is suboptimal for visualizing postoperative hematoma around the aortic root, evaluation by CT before discharge is recommended. In patients who underwent coronary artery reconstruction, cardiac CT is appropriate to rule out anastomotic failure. Leaflets and the aortic root can be visualized and evaluated by cardiac CT. Unlike the occurrence of recurrent MR after mitral valve repair, which plateaus 1–2 years postoperatively, that of recurrent AR tends to be consistent after surgery. Recurrent AR due to suture dehiscence or perforation at the repair site can lead to a rapid change in the severity of regurgitation. There are no clear criteria for follow-up intervals. It may be adequate to follow-up with TTE at discharge, 1–3 months after discharge, then every 6 months thereafter. If the TTE findings remain unchanged, annual follow-up may be reasonable.
2.2 Indications for Reoperation
The cause of recurrent AR depends on the repair procedure, and its incidence varies according to the institution. Residual leaflet prolapse and its progression, suture dehiscence, perforation at the repair site, scarring or calcification of the leaflet, annular redilatation, infective endocarditis, and aortic dissection are reported as causes of recurrent AR.518,521–523
Re-repair is rarely feasible and AVR is selected for reoperation in most cases. The decision for reoperation can be made in reference to the initial AR surgery guideline. Symptomatic patients with severe AS require reoperation. In patients with less than severe but moderate or more than moderate recurrent/residual AR, who present with symptoms or progressive dilatation of the LV and/or decline of the LVEF, careful evaluation and consideration of reoperation should be undertaken, because the severity of the AR was possibly underestimated in such cases. Periodic monitoring of BNP is useful for assessing LV function. TEE is reasonable for the patient with TTE imaging that is suboptimal for detailed evaluation of the recurrent/residual AR severity and etiology. When TEE imaging is also suboptimal due to acoustic shadowing resulting from the annuloplasty ring or the vessel graft, cardiac CT is useful for visualizing and evaluating the aortic valve. When there is a discordance between the symptoms and imaging findings, exercise stress echocardiography to observe changes in the symptoms and hemodynamics, is helpful to reveal the indications for reoperation.45
XIV. Others
1. Treatment for Concomitant AF
1.1 Antithrombotic Therapy for AF
1.1.1 Risk Assessment for Cerebral Infarction With AF
Evaluating the risk of cerebral infarction in patients with nonvalvular AF in order to select the appropriate antithrombotic therapy is encouraged. However, evaluation is limited in cases of valvular AF. According to the 2013 JCS guidelines on the pharmacological treatment of AF,524
“valvular” includes both artificial valve replacement (both mechanical and biological) and rheumatic mitral valve disease (primarily stenosis). However, guidelines recently announced in the USA and Europe define “valvular AF” as AF with moderate or severe MS and mechanical valves. AF with native valve diseases and bioprosthetic valves is treated as “nonvalvular AF”. Although it is not based on established evidence, the prospective DOAC registry for AF includes valvular diseases, except moderate or severe MS and mechanical valves, and the efficacy and safety are similar to that in other nonvalvular AF cases. Therefore, the present guidelines also define “valvular AF” as AF with moderate or severe MS and a mechanical valve.
1.1.2 Antithrombotic Therapy for AF
Warfarin is the standard antithrombotic therapy for valvular AF. Warfarin therapy with a PT-INR 2.0–3.0 is recommended, especially given the high risk of embolism (Class I). Note that valvular AF does not apply to DOAC use (Class III). Nonvalvular AF follows the guidelines for the management of AF (except 3 months after bioprosthetic valve replacement – see section
XII.2.2,
Table 47).
Please refer to
CQ5
for the use of DOAC in AF patients who undergo bioprosthetic valve replacement.
CQ5
Can direct oral anticoagulants (DOACs) be used in patients with AF after bioprosthetic valve replacement?
[Conclusion]
DOACs can be used in patients with AF and bioprostheses after the 3rd postoperative month (2C).
[Strength of Recommendation]
2: Weakly recommended (proposed)
[Strength of Evidence]
C (weak): Limited confidence in the estimated effect
Initially, and for many years, warfarin was the only oral anticoagulant. However, since 2011, DOACs that directly and selectively inhibit the thrombin or factor Xa coagulation cascade have become available for nonvalvular AF. Previous RCTs for nonvalvular AF demonstrated that DOACs were comparable to warfarin in terms of preventing embolic events and superior in terms of major bleeding.
When we discuss whether DOACs can be used in patients with AF after bioprosthetic valve replacement, the definition of “valvular AF” becomes an issue.
The European guidelines for the management of AF treat only mitral stenosis and mechanical values as valvular diseases, thus they treat patients with bioprosthetic valves as having “non-valvular” AF.525,526
In the ESC guidelines for the management of VHD,8
it is acceptable to use DOACs with bioprosthetic valves, although clear evidence is not provided.
There are few reports on using DOACs in patients with AF after bioprosthetic valve replacement. In large-scale RCTs comparing warfarin and DOACs in patients with AF, 13–26% of cases were described as having VHD and ≈60% displayed VHD on echocardiography.527–530
The ENGAGE AF-TIMI 48 trial531
included 13% of patients with a history of moderate to severe VHD, most of whom were MR cases, and also included those with a history of valvular surgery. A subanalysis of the same clinical trial was performed with regard to patients after bioprosthetic valve replacement, and it showed no difference in the incidence of embolic events and major bleeding between DOACs and warfarin.532
In the ARISTOTLE study, a subanalysis of patients after bioprosthetic valve replacement and valve repair also revealed that DOACs were not inferior to warfarin.533
A single-center retrospective study534
investigated 73 patients with AF after bioprosthetic valve replacement who were treated with DOACs (44 on dabigatran, 25 on rivaroxaban, 4 on apixaban), and showed the efficacy of DOACs for thromboembolism. The majority (72.6%) of patients were on concurrent aspirin, and 1 transient ischemic attack (1.4%), 5 major episodes of bleeding (6.9%), and 6 minor episodes of bleeding (8.2%) were observed. They concluded that DOACs may be effective for AF in patients with bioprosthetic valves. Japanese data on antithrombotic therapy in patients with AF with bioprosthetic valves are scarce.535
For heart mechanical valves, however, an RCT found the use of dabigatran was associated with increased rates of thromboembolic and bleeding complications, as compared with warfarin.471
Therefore, DOACs should not be used for AF in patients with mechanical valves.
In nonvalvular AF, 90% of intracardiac thrombi forms in the LA appendage,536
whereas bioprosthetic valve thrombus forms at the suture site and is often associated with a failed valve.68,465
Recently, it was reported that valve thrombosis is frequently found by cardiac CT after TAVI. Among 890 cases undergoing cardiac CT following SAVR or TAVI, valve thrombosis was noted in 106 cases (12%), and the prevalence was higher with TAVI than with SAVR (TAVI 13%, SAVR 4%). Anticoagulant therapy decreased the frequency (15% on dual antiplatelet therapy, 4% on warfarin, 3% on DOACs). None of these cases presented clinical manifestations, and thrombosis resolved with anticoagulant therapy in all cases.69
The frequency of valve thrombosis following TAVI was found to be 2.8%, and the use of balloon-expandable valves, valve-in-valve, and antiplatelet therapy alone were all reported to be risk factors.537
It is believed that effectiveness for bioprosthetic valve thrombosis may be equivalent for warfarin and DOACs.
Therefore, DOACs can be used in patients with AF and bioprostheses after the 3rd postoperative month. However, as there is not enough evidence, it is deemed a “weak recommendation”.
1.2 Cox Maze Procedure, LA Appendage Closure, and Resection During Valvular Surgery Concomitant With AF
1.2.1 Overview
Surgical strategies, such as the Cox maze procedure or LA atrial appendage closure/resection, for patients with AF and VHD should be determined based on the potential efficacy to prevent thromboembolic events and the risk of these procedures. Although the Cox maze procedure is one of many surgical rhythm control methods for AF, in this section, “the Cox maze procedure” is used to represent surgical rhythm management for AF in general.
1.2.2 Indications for the Cox Maze Procedure During Mitral Valve Surgery (Table 52)
Table 52.
Concomitant Maze Procedure and LAA Resection/Closure With Valve Surgery
| Recommendations |
COR |
LOE |
Concomitant maze procedure is reasonable for permanent or persistent AF at the time of mitral
valve surgery |
IIa |
B |
Concomitant maze proceudure or pulmonary vein isolation may be considered for paroxysmal
AF at the time of mitral valve surgery |
IIb |
C |
Concomitant maze procedure is reasonable for permanent or persistent AF at the time of aortic
valve surgery |
IIa |
C |
Concomitant LAA resection/closure is reasonable for AF at the time of any valve
surgery |
IIa |
C |
AF, atrial fibrillation; COR, Class of Recommendation; LAA, left atrial appendage; LOE, Level of Evidence.
In an RCT by Gillinov et al,538
260 mitral valve surgery patients with persistent AF were divided into either a surgical ablation (atrial Cox maze or pulmonary venous isolation) or no ablation group, with a comparison of the AF rates at 12 months later. The rate of patients without AF was higher in the ablation group than in the non-ablation group (63% vs. 29%). However, the rate of pacemaker use was significantly higher in the ablation group (21.5% vs. 8.1%). There was no significant difference in the rates of 1-year mortality, cerebral infarction, or re-admission for HF. Other randomized and observational studies have shown that surgical ablation at the time of mitral valve surgery significantly increased the rate of postoperative sinus rhythm compared with mitral valve surgery alone.539–544
In the short term, the Cox maze procedure performed with mitral valve surgery is a treatment that increases the postoperative sinus rhythm rate without increasing the surgical risk. The Cox maze procedure should be considered for chronic/persistent AF during mitral valve surgery (Class IIa). The Cox maze procedure or pulmonary venous isolation may be considered for paroxysmal AF (Class IIb).
Older age, larger LA, and a long history of AF are known predictors of an unsuccessful Cox maze procedure.545,546
However, the cutoff values are unclear, and the Heart Valve Team should thoroughly review the indications for the Cox maze procedure in each case. It remains unclear whether the Cox maze procedure at the time of mitral valve surgery improves long-term survival.547–551
1.2.3 Indications for the Cox Maze Procedure During Aortic Valve Surgery (Table 52)
There are fewer studies on performing the Cox maze procedure during aortic valve surgery than during mitral valve surgery. In 2 single-facility observational studies, the group undergoing the Cox maze procedure at the time of SAVR showed a significantly higher rate of sinus rhythm than the group without the Cox maze procedure; there was no difference in mortality or complication rates.552,553
As in mitral valve surgery, LA size is a predictor of an unsuccessful Cox maze procedure.554,555
In aortic valve surgery, the LA is often not as large as the mitral valve, and that may contribute to the success rate.555
The Cox maze procedure for chronic/persistent AF during aortic valve surgery should be considered (Class IIa). However, the effect on long-term survival remains unclear.
1.2.4 LA Appendage Closure/Resection During Valvular Surgery (Table 52)
LA appendage closure for nonvalvular AF is equivalent to anticoagulation treatment in preventing embolism, with fewer bleeding events and deaths reported than with anticoagulant therapy.556
However, there are no large-scale RCTs to evaluate the effectiveness of LA appendage closure/resection during valvular surgery. According to observational studies, LA appendage closure/resection has contributed to fewer postoperative embolism events, though other studies indicate negative results.557–559
LA appendage closure/resection should be considered during valvular surgery concomitant with AF (Class IIa).
2. Coronary Artery Disease Concomitant With VHD
This section is omitted from the English version.
3. Pregnancy and VHD
This section is omitted from the English version.
4. Evaluation in Noncardiac Surgery
4.1 Preoperative Assessment and Intraoperative Monitoring
Patients with VHD have a high perioperative cardiovascular risk in noncardiac surgery. Careful auscultation is advised to confirm the presence of a murmur, even in patients without a prior history of VHD. In general, functional murmur is rarely above the level of Levine III/VI. Because the magnitude of the murmur can be affected by body size, even a low level of murmur does not completely rule out organic abnormality. Therefore, in patients with VHD, as well as those with a murmur, TTE should be performed prior to surgery to assess the presence and severity of the VHD as well as LV function. It should be considered whether or not intervention for VHD is required prior to noncardiac surgery based on symptoms, severity of VHD, and noncardiac surgical risk (Table 53).8,221,560
Table 53.
Risk Estimate of Noncardiac Surgery According to Type of Surgery or Intervention
| Low risk <1% |
Intermediate risk 1–5% |
High risk >5% |
• Superficial surgery
• Breast
• Dental
• Endocrine: thyroid
• Eye
• Reconstructive
• Carotid asymptomatic (CEA or CAS)
• Gynecologic: minor
• Orthopedic: minor (meniscectomy)
• Urologic: minor (transurethral resection of
the prostate) |
• Intraperitoneal: splenectomy, hiatal hernia
repair, cholecystectomy
• Carotid symptomatic (CEA or CAS)
• Peripheral arterial angioplasty
• Endovascular aneurysm repair
• Head and neck surgery
• Neurologic/orthopedic: major (hip and spine
surgery)
• Urologic or gynecologic: major
• Renal transplant
• Intrathoracic: non-major |
• Aortic and major vascular surgery
• Open lower limb revascularization or
amputation or thromboembolectomy
• Duoneno-pancreatic surgery
• Liver resection, bile duct surgery
• Esophagectomy
• Repair of perforated bowel
• Total cystectomy
• Pneumonoectomy
• Lung or liver transplant |
CAS, carotid artery stent; CEA, cardotid endarterectomy. Kristensen SD, et al. 2014560 Reprinted and translated by permission of Oxford University Press on behalf of the European Society of Cardiology. OUP and the ESC are not responsible or in any way liable for the accuracy of the translation. The Japanese Circulation Society is solely responsible for the translation in this publication/reprint.
4.2 Disease-Specific Considerations
4.2.1 AS
Chronic pressure overload in AS patients reduces LV compliance, which requires enough preload and atrial contraction for LV filling. It is therefore important to maintain normal sinus rhythm. If hypotension is caused by supraventricular tachycardia or AF, electrical defibrillation should be done as soon as possible. Fluid management is important; fluids should be administered so that cardiac output is maintained, but excessive fluids should be avoided.
Emergency noncardiac surgery in patients with severe AS should be performed by a cardiovascular anesthesiologist under hemodynamic or TEE monitoring. The indications for elective noncardiac surgery should be determined by the presence of symptoms and the type of surgery.8
In symptomatic severe AS, SAVR/TAVI is recommended prior to noncardiac surgery.561,562
If SAVR/TAVI is high-risk because of anatomical characteristics and patient background, percutaneous balloon aortic valvuloplasty should be considered (Figure 23). In asymptomatic severe AS, it is possible to safely perform elective noncardiac surgery. For high-risk noncardiac surgery with significant volume overload, SAVR/TAVI prior to noncardiac surgery may be considered in the absence of symptoms.
4.2.2 MS
Management of patients with MS is similar to that of AS; maintaining preload and normal sinus rhythm is important.
Noncardiac surgery can be performed safely in patients with mild MS (valve orifice area >1.5 cm2), asymptomatic patients, and patients with a PASP <50 mmHg. PTMC prior to surgery should be considered in patients with symptoms or a PASP >50 mmHg if planning a high-risk noncardiac surgery.8
4.2.3 AR and MR
In AR and MR, afterload reduction leads to an increase in CO as well as a decrease in regurgitation. In cases of chronic regurgitation, preload should be maintained. Central venous pressure, pulmonary artery pressure, and LV size and function should be monitored using echocardiography and invasive catheterisation.8
Noncardiac surgery can be performed safely in asymptomatic severe AR or MR when LV function is maintained. Valve surgery prior to noncardiac surgery may be considered for symptomatic patients or patients with reduced cardiac function. However, if HF is being managed by medical therapy, valve surgery prior to noncardiac surgery is rarely required. If LV dysfunction is advanced (LVEF <30%), noncardiac surgery should only be performed after optimal medical therapy for HF and only when it is absolutely necessary.
5. Prevention of Infective Endocarditis
This section is omitted from the English version.
6. Prevention of Rheumatic Fever
This section is omitted from the English version.
Appendix 1 Details of Members
Group Leaders
• Kiyoyuki Eishi, Division of Cardiovascular Surgery, Nagasaki University Graduate School of Biomedical Sciences
• Chisato Izumi, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
Members
• Takeshi Arita, Division of Cardiovascular Medicine Heart & Neuro-Vascular Center, Fukuoka Wajiro
• Kyomi Ashihara, Department of Cardiology, Tokyo Women’s Medical University Hospital
• Masao Daimon, Department of Clinical Laboratory/Cardiology, The University of Tokyo Hospital
• Kentaro Hayashida, Department of Cardiology, Keio University School of Medicine
• Tatsuhiko Komiya, Department of Cardiovascular Surgery, Kurashiki Central Hospital
• Takashi Kunihara, Department of Cardiac Surgery, The Jikei University School of Medicine
• Satoshi Nakatani, Division of Health Sciences, Osaka University Graduate School of Medicine
• Hiroshi Ninami, Department of Cardiac Surgery, Tokyo Women’s Medical University
• Hiroyuki Nishi, Department of Cardiovascular Surgery, Osaka General Medical Center
• Yutaka Otsuji, Department of Cardiology, Hospital of University of Occupational and Environmental Health
• Yoshihiro Seo, Department of Cardiology, Nagoya City University Graduate School of Medical Sciences
• Toshihiko Shibata, Department of Cardiovascular Surgery, Osaka City University Postgraduate of Medicine
• Shuichiro Takanashi, Department of Cardiovascular Surgery, Kawasaki Saiwai Hospital
• Hiroyuki Tanaka, Department of Surgery, Kurume University
• Hitoshi Yaku, Department of Cardiovascular Surgery, Kyoto Prefectural University of Medicine
• Junichi Yamaguchi, Department of Cardiology, Tokyo Women’s Medical University
• Kazuhiro Yamamoto, Division of Cardiovascular Medicine, Endocrinology and Metabolism, Faculty of Medicine, Tottori University
• Hiroyuki Watanabe, Department of Cardiology, Tokyo Bay Urayasu Ichikawa Medical Center
Collaborators
• Yukio Abe, Department of Cardiology, Osaka City General Hospital
• Makoto Amaki, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Masashi Amano, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Takashi Miura, Division of Cardiovascular Surgery, Nagasaki University Graduate School of Biomedical Sciences
• Makoto Miyake, Department of Cardiology, Tenri Hospital
• Mitsushige Murata, Department of Laboratory Medicine, Tokai University Hachioji Hospital
• Kikuko Obase, Division of Cardiovascular Surgery, Nagasaki University Graduate School of Biomedical Sciences
• Minoru Tabata, Department of Cardiovascular Surgery, Tokyo Bay Urayasu Ichikawa Medical Center
• Nozomi Watanabe, Department of Cardiology, Miyazaki Medical Association Hospital
External Evaluation Committee Members
• Takashi Akasaka, Department of Cardiovascular Medicine, Wakayama Medical University
• Takeshi Kimura, Department of Cardiology, Kyoto University Graduate School of Medicine
• Yutaka Okita, Department of Cardiovascular Surgery, Takatsuki Hospital
• Yoshiki Sawa, Department of Cardiovascular Surgery, Osaka University Graduate School of Medicine
• Kiyoshi Yoshida, Department of Cardiology, Sakakibara Heart Institute of Okayama
(The affiliations of the members are as of March 2020)
Appendix 2 Disclosure of Potential Conflicts of Interest (COI): JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease
| Author |
Potential COI of the participant |
Potential COI
of the marital
partner, first-
degree family
members, or
those who
share income
and property |
Potential COI of the head of the organization/
department to which the participant belongs
(when the participant is in the position of
cooperative research with the head of the
organization/department) |
Employer/leadership
position
(private company) |
Shareholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research
grant |
Scholarship
(educational)
grant |
Endowed
chair |
Other
rewards |
Research
grant |
Scholarship
(educational)
grant |
Group Leaders:
Chisato Izumi |
|
|
|
Daiichi Sankyo
Company, Limited
Otsuka
Pharmaceutical
Co., Ltd. |
|
Daiichi Sankyo
Company, Limited |
|
|
|
|
Daiichi Sankyo
Company,
Limited |
|
Members:
Takeshi Arita |
Edwards
Lifesciences
Corporation |
|
|
Edwards
Lifesciences
Corporation |
|
|
|
|
|
|
|
|
Members:
Yutaka Otsuji |
|
|
|
|
|
Fukuda Life Tech
Co. Ltd. |
MSD K.K.
Astellas Pharma Inc.
Abbott Vascular
Japan Co., Ltd.
Novartis Pharma
K.K.
Hibiki Clinic
Daiichi Sankyo
Company, Limited
Hagiwara Central
Hospital
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
Members:
Tatsuhiko
Komiya |
|
|
|
Japan Lifeline
Co.,Ltd. |
|
|
|
|
|
|
|
|
Members:
Toshihiko
Shibata |
|
|
|
Daiichi Sankyo
Company, Limited |
|
|
Edwards
Lifesciences
Corporation
St. Jude Medical
Japan Co., Ltd. |
|
|
|
|
|
Members:
Yoshihiro Seo |
|
|
|
Nippon Boehringer
Ingelheim
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma
Corporation
TOSHIBA
MEDICAL
SYSTEMS
CORPORATION |
|
Otsuka
Pharmaceutical
Co., Ltd.
TOSHIBA
MEDICAL
SYSTEMS
CORPORATION |
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
MSD K.K. |
|
|
|
|
|
Members:
Shuichiro
Takanashi |
Edwards
Lifesciences
Corporation
Japan Lifeline
Co.,Ltd.
TERUMO
CORPORATION |
|
|
Abbott Japan LLC
Abbott Medical
Japan L.L.C
Edwards
Lifesciences
Corporation
Medtronic Japan
Co., Ltd.
Japan Lifeline
Co.,Ltd. |
|
|
|
|
|
|
|
|
Members:
Hiroyuki
Tanaka |
|
|
|
Edwards
Lifesciences
Corporation
Century Medical,
Inc.
St. Jude Medical
Japan Co., Ltd.
TERUMO
CORPORATION
KYUSHU
MEDICAL
SERVICE
Co.,Ltd.
Medtronic Japan
Co., Ltd.
Japan Lifeline
Co.,Ltd. |
|
Medtronic Japan
Co., Ltd.
Abbott Japan LLC
Edwards
Lifesciences
Corporation
Japan Lifeline
Co.,Ltd. |
|
|
|
|
|
|
Members:
Satoshi
Nakatani |
|
|
|
Edwards
Lifesciences
Corporation |
|
|
|
|
|
|
|
|
Members:
Hiroshi Ninami |
COSMOTEC
Co., Ltd.
JMS Co., Ltd.
Japan Lifeline
Co.,Ltd. |
|
|
St. Jude Medical
Japan Co., Ltd. |
|
|
|
|
|
|
|
|
Members:
Hiroyuki Nishi |
|
|
|
Otsuka
Pharmaceutical
Co., Ltd. |
|
|
|
|
|
|
|
|
Members:
Kentaro
Hayashida |
Edwards
Lifesciences
Corporation |
|
|
|
|
|
Edwards
Lifesciences
Corporation |
|
|
|
|
|
Members:
Hitoshi Yaku |
|
|
|
Medtronic Japan
Co., Ltd.
Abbott Medical
apan L.L.C |
|
|
COSMOTEC
Co., Ltd.
Daiichi Sankyo
Company, Limited |
|
|
|
|
|
Members:
Junichi
Yamaguchi |
|
|
|
Abbott Japan LLC
Abbott Vascular
Japan Co., Ltd.
TERUMO
CORPORATION
Bayer Yakuhin, Ltd.
Bristol-Myers
Squibb
Boston Scientific
Corporation
Daiichi Sankyo
Company, Limited |
|
|
|
Abbott Japan LLC
Boston Scientific
Corporation
Medtronic Japan
Co., Ltd.
TERUMO
CORPORATION |
|
|
|
|
Members:
Kazuhiro
Yamamoto |
|
|
|
Pfizer Japan Inc.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim
Co ., Ltd. |
|
|
Abbott Medical
Japan L.L.C
Johnson & Johnson
K.K.
Novartis Pharma
K.K.
BIOTRONIK
Japan, Inc.
Fukuda Denshi
Co., Ltd
Boston Scientific
Corporation
LifeScan Japan K.K.
Kowa
Pharmaceutical
Co., Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Teijin Pharma
Limited
Mitsubishi Tanabe
Pharma
Corporation
Japan Lifeline
Co.,Ltd.
Nihon Kohden
Corp.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
Members:
Hiroyuki
Watanabe |
|
|
|
Edwards
Lifesciences
Corporation |
Edwards
Lifesciences
Corporation |
|
Abbott Medical
Japan L.L.C
Boston Scientific
Corporation
Takeda
Pharmaceutical
Company Limited |
Abbott Medical
Japan L.L.C
St. Jude Medical
Japan Co., Ltd.
BIOTRONIK
Japan, Inc. |
|
|
|
|
Collaborators:
Makoto Amaki |
|
|
|
Abbott Vascular
Japan Co., Ltd. |
|
|
|
|
|
|
|
|
Collaborators:
Minoru Tabata |
Edwards
Lifesciences
Corporation
Century Medical,
Inc.
TERUMO
CORPORATION
LivaNova Japan
K.K. |
|
|
Edwards
Lifesciences
Corporation
Abbott Medical
Japan L.L.C
Medtronic Japan
Co., Ltd. |
|
|
|
|
|
|
|
Edwards
Lifesciences
Corporation |
Collaborators:
Mitsushige
Murata |
|
|
|
|
|
Bayer Yakuhin, Ltd. |
|
|
|
|
|
|
Collaborators:
Nozomi
Watanabe |
|
|
|
|
|
|
|
|
|
|
IQVIA Services
Japan K.K. |
|
External
Evaluation
Committee
Members:
Takashi
Akasaka |
|
|
|
Amgen Astellas
BioPharma K.K.
Abbott Vascular
Japan Co., Ltd.
St. Jude Medical
Japan Co., Ltd.
Daiichi Sankyo
Company, Limited
Nippon Boehringer
Ingelheim
Co ., Ltd. |
|
Infraredx, Inc.
Daiichi Sankyo
Company, Limited |
ACIST Medical
Systems
Astellas Pharma Inc.
St. Jude Medical
Japan Co., Ltd.
HeartFlow Japan
G.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Daiichi Sankyo
Company, Limited |
Abbott Vascular
Japan Co., Ltd.
Goodman Co.,LTD.
St. Jude Medical
Japan Co., Ltd.
TERUMO
CORPORATION
Nipro Corporation
Boston Scientific
Corporation |
|
|
The Japan
Research
Foundation for
Healthy Aging
Japan Heart
Foundation |
|
External
Evaluation
Committee
Members:
Yutaka Okita |
|
|
|
|
|
|
JMS Co., Ltd.
Abbott Japan LLC
Edwards
Lifesciences
Corporation
TERUMO
CORPORATION
Medtronic Japan
Co., Ltd.
SENKO MEDICAL
INSTRUMENT
Mfg. Co., Ltd.
Japan Lifeline
Co.,Ltd. |
|
|
|
|
|
External
Evaluation
Committee
Members:
Takeshi Kimura |
|
|
|
Amgen Astellas
BioPharma K.K.
Abbott Vascular
Japan Co., Ltd.
Sanofi K.K.
Bristol-Myers
Squibb
Boston Scientific
Corporation
Kowa
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Nippon Boehringer
Ingelheim
Co ., Ltd. |
|
Nipro Corporation
EP-CRSU Co., Ltd.
Edwards
Lifesciences
Corporation
Daiichi Sankyo
Company, Limited
Pfizer Japan Inc. |
Astellas Pharma Inc.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim
Co., Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
External
Evaluation
Committee
Members:
Yoshiki Sawa |
|
Cuorips
Inc. |
TERUMO
CORPORATION |
Edwards
Lifesciences
Corporation
St. Jude Medical
Japan Co., Ltd.
TERUMO
CORPORATION
Daiichi Sankyo
Company, Limited
Daiichi Sankyo
Healthcare
Co., Ltd.
Medtronic Japan
Co., Ltd. |
|
|
|
|
|
|
Edwards
Lifesciences
Corporation
Nacalai Tesque,
Inc.
Ono
Pharmaceutical
Co., Ltd. |
Edwards
Lifesciences
Corporation
Myoridge
Co., Ltd.
Konishi Medical
Instruments
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Abiomed Japan
K.K.
Medtronic Japan
Co., Ltd. |
Notation of corporation is omitted.
No potential COI for the following members.
Group Leaders: Kiyoyuki Eishi
Members: Kyomi Ashihara
Members: Takashi Kunihara
Members: Masao Daimon
Collaborators: Yukio Abe
Collaborators: Masashi Amano
Collaborators: Kikuko Obase
Collaborators: Takashi Miura
Collaborators: Makoto Miyake
External Evaluation Committee Members: Kiyoshi Yoshida
References
- 1.
Minds Guideline Center, Japan Council for Quality Health Care. Minds handbook for clinical practice guideline development 2014. http://minds4.jcqhc.or.jp/minds/guideline/pdf/MindsHB2014.pdf
- 2.
Yoshikawa J. Physical examination. Bunkodo Co., Ltd., 2005.
- 3.
Steadman CD, Ray S, Ng LL, et al. Natriuretic peptides in common valvular heart disease. J Am Coll Cardiol 2010; 55: 2034–2048. PMID: 20447526
- 4.
Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: Focus on natriuretic peptides. Can J Cardiol 2014; 30: 1027–1034. PMID: 25151285
- 5.
Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol 2009; 54: 1099–1106. PMID: 19744620
- 6.
Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol 2011; 58: 1705–1714. PMID: 21982316
- 7.
Clavel MA, Malouf J, Michelena HI, et al. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J Am Coll Cardiol 2014; 63: 2016–2025. PMID: 24657652
- 8.
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017; 38: 2739–2791. PMID: 28886619
- 9.
Carabello BA, Williams H, Gash AK, et al. Hemodynamic predictors of outcome in patients undergoing valve replacement. Circulation 1986; 74: 1309–1316. PMID: 3779916
- 10.
Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: A simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 1985; 6: 750–756. PMID: 4031289
- 11.
Currie PJ, Seward JB, Reeder GS, et al. Continuous-wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: A simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation 1985; 71: 1162–1169. PMID: 3995710
- 12.
Rokey R, Sterling LL, Zoghbi WA, et al. Determination of regurgitant fraction in isolated mitral or aortic regurgitation by pulsed Doppler two-dimensional echocardiography. J Am Coll Cardiol 1986; 7: 1273–1278. PMID: 3711483
- 13.
Lewis JF, Kuo LC, Nelson JG, et al. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: Clinical validation of two new methods using the apical window. Circulation 1984; 70: 425–431. PMID: 6744546
- 14.
Mascherbauer J, Rosenhek R, Bittner B, et al. Doppler echocardiographic assessment of valvular regurgitation severity by measurement of the vena contracta: An in vitro validation study. J Am Soc Echocardiogr 2005; 18: 999–1006. PMID: 16198875
- 15.
Enriquez-Sarano M, Miller FA Jr, Hayes SN, et al. Effective mitral regurgitant orifice area: Clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol 1995; 25: 703–709. PMID: 7860917
- 16.
Tribouilloy CM, Enriquez-Sarano M, Fett SL, et al. Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol 1998; 32: 1032–1039. PMID: 9768729
- 17.
Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005; 352: 875–883. PMID: 15745978
- 18.
Enriquez-Sarano M, Tajik AJ, Schaff HV, et al. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: Results and clinical implications. J Am Coll Cardiol 1994; 24: 1536–1543. PMID: 7930287
- 19.
Nishimura RA, Rihal CS, Tajik AJ, et al. Accurate measurement of the transmitral gradient in patients with mitral stenosis: A simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol 1994; 24: 152–158. PMID: 8006259
- 20.
Oh JK, Taliercio CP, Holmes DR Jr, et al. Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: Prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol 1988; 11: 1227–1234. PMID: 3366997
- 21.
Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95: 2262–2270. PMID: 9142003
- 22.
Otto CM, Nishimura RA, Davis KB, et al. Doppler echocardiographic findings in adults with severe symptomatic valvular aortic stenosis: Balloon Valvuloplasty Registry Echocardiographers. Am J Cardiol 1991; 68: 1477–1484. PMID: 1746430
- 23.
Otto CM, Pearlman AS, Comess KA, et al. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol 1986; 7: 509–517. PMID: 3950230
- 24.
Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13: 545–550. PMID: 2918158
- 25.
Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005; 111: 3290–3295. PMID: 15956131
- 26.
Zile MR, Gaasch WH, Carroll JD, et al. Chronic mitral regurgitation: Predictive value of preoperative echocardiographic indexes of left ventricular function and wall stress. J Am Coll Cardiol 1984; 3: 235–242. PMID: 6693615
- 27.
Dujardin KS, Enriquez-Sarano M, Schaff HV, et al. Mortality and morbidity of aortic regurgitation in clinical practice: A long-term follow-up study. Circulation 1999; 99: 1851–1857. PMID: 10199882
- 28.
Bonow RO, Lakatos E, Maron BJ, et al. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991; 84: 1625–1635. PMID: 1914102
- 29.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: 2440–2492. PMID: 24589852
- 30.
Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. PMID: 28314623
- 31.
Lancellotti P, Tribouilloy C, Hagendorff A, et al. Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013; 14: 611–644. PMID: 23733442
- 32.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. PMID: 25559473
- 33.
Baumgartner H, Hung J, Bermejo J, et al; EAE/ASE. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009; 10: 1–25. PMID: 19065003
- 34.
Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017; 30: 372–392. PMID: 28385280
- 35.
Malouf JF, Enriquez-Sarano M, Pellikka PA, et al. Severe pulmonary hypertension in patients with severe aortic valve stenosis: Clinical profile and prognostic implications. J Am Coll Cardiol 2002; 40: 789–795. PMID: 12204512
- 36.
Melby SJ, Moon MR, Lindman BR, et al. Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg 2011; 141: 1424–1430. PMID: 21596173
- 37.
Lancellotti P, Magne J, Donal E, et al. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation 2012; 126: 851–859. PMID: 22832784
- 38.
Luçon A, Oger E, Bedossa M, et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Study from the FRANCE 2 Registry. Circ Cardiovasc Interv 2014; 7: 240–247. PMID: 24569597
- 39.
Barbieri A, Bursi F, Grigioni F, et al; Mitral Regurgitation International DAtabase (MIDA) Investigators. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: A multicenter long-term international study. Eur Heart J 2011; 32: 751–759. PMID: 20829213
- 40.
Ghoreishi M, Evans CF, DeFilippi CR, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: Implications for timing of surgery. J Thorac Cardiovasc Surg 2011; 142: 1439–1452. PMID: 21962906
- 41.
Miller WL, Mahoney DW, Enriquez-Sarano M. Quantitative Doppler-echocardiographic imaging and clinical outcomes with left ventricular systolic dysfunction: Independent effect of pulmonary hypertension. Circ Cardiovasc Imaging 2014; 7: 330–336. PMID: 24488981
- 42.
Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. PMID: 20620859
- 43.
Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20: 1021–1041. PMID: 17765820
- 44.
Sicari R, Nihoyannopoulos P, Evangelista A, et al; European Association of Echocardiography. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2008; 9: 415–437. PMID: 18579481
- 45.
Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017; 30: 101–138. PMID: 28164802
- 46.
Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26: 1309–1313. PMID: 15820999
- 47.
Lancellotti P, Lebois F, Simon M, et al. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112 Suppl: I377–I382. PMID: 16159850
- 48.
Otto CM, Pearlman AS, Kraft CD, et al. Physiologic changes with maximal exercise in asymptomatic valvular aortic stenosis assessed by Doppler echocardiography. J Am Coll Cardiol 1992; 20: 1160–1167. PMID: 1401617
- 49.
Rafique AM, Biner S, Ray I, et al. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol 2009; 104: 972–977. PMID: 19766766
- 50.
Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010; 122: 33–41. PMID: 20566950
- 51.
Aviles RJ, Nishimura RA, Pellikka PA, et al. Utility of stress Doppler echocardiography in patients undergoing percutaneous mitral balloon valvotomy. J Am Soc Echocardiogr 2001; 14: 676–681. PMID: 11447412
- 52.
Garbi M, Chambers J, Vannan MA, et al. Valve stress echocardiography: A practical guide for referral, procedure, reporting, and clinical implementation of results from the HAVEC group. JACC Cardiovasc Imaging 2015; 8: 724–736. PMID: 26068289
- 53.
Picano E, Pibarot P, Lancellotti P, et al. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol 2009; 54: 2251–2260. PMID: 19958961
- 54.
Lin SS, Roger VL, Pascoe R, et al. Dobutamine stress Doppler hemodynamics in patients with aortic stenosis: Feasibility, safety, and surgical correlations. Am Heart J 1998; 136: 1010–1016. PMID: 9842014
- 55.
Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J 2016; 37: 2645–2657. PMID: 27190103
- 56.
Monin JL, Quéré JP, Monchi M, et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108: 319–324. PMID: 12835219
- 57.
Levy F, Laurent M, Monin JL, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: A European multicenter study. J Am Coll Cardiol 2008; 51: 1466–1472. PMID: 18402902
- 58.
Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J 2005; 26: 1528–1532. PMID: 15814566
- 59.
Messika-Zeitoun D, Johnson BD, Nkomo V, et al. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: Physiologic and outcome implications. J Am Coll Cardiol 2006; 47: 2521–2527. PMID: 16781383
- 60.
Bertrand PB, Schwammenthal E, Levine RA, et al. Exercise dynamics in secondary mitral regurgitation: Pathophysiology and therapeutic implications. Circulation 2017; 135: 297–314. PMID: 28093494
- 61.
Chan KM, Punjabi PP, Flather M, et al; RIME Investigators. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: Final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012; 126: 2502–2510. PMID: 23136163
- 62.
Clavel MA, Messika-Zeitoun D, Pibarot P, et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol 2013; 62: 2329–2338. PMID: 24076528
- 63.
Hayashida K, Bouvier E, Lefèvre T, et al. Impact of CT-guided valve sizing on post-procedural aortic regurgitation in transcatheter aortic valve implantation. EuroIntervention 2012; 8: 546–555. PMID: 22995080
- 64.
Jilaihawi H, Doctor N, Kashif M, et al. Aortic annular sizing for transcatheter aortic valve replacement using cross-sectional 3-dimensional transesophageal echocardiography. J Am Coll Cardiol 2013; 61: 908–916. PMID: 23449425
- 65.
Hayashida K, Lefèvre T, Chevalier B, et al. Transfemoral aortic valve implantation: New criteria to predict vascular complications. JACC Cardiovasc Interv 2011; 4: 851–858. PMID: 21851897
- 66.
Opolski MP, Staruch AD, Jakubczyk M, et al. CT angiography for the detection of coronary artery stenoses in patients referred for cardiac valve surgery: Systematic review and meta-analysis. JACC Cardiovasc Imaging 2016; 9: 1059–1070. PMID: 27344418
- 67.
Hayashida K, Bouvier E, Lefèvre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circ Cardiovasc Interv 2013; 6: 284–291. PMID: 23756698
- 68.
Makkar RR, Fontana G, Jilaihawi H, et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med 2015; 373: 2015–2024. PMID: 26436963
- 69.
Chakravarty T, Søndergaard L, Friedman J, et al; RESOLVE, SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017; 389: 2383–2392. PMID: 28330690
- 70.
Yanagisawa R, Tanaka M, Yashima F, et al. Early and Late Leaflet Thrombosis After Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv 2019; 12: e007349. PMID: 30732472
- 71.
Gelfand EV, Hughes S, Hauser TH, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: Optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson 2006; 8: 503–507. PMID: 16755839
- 72.
Cawley PJ, Hamilton-Craig C, Owens DS, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 2013; 6: 48–57. PMID: 23212272
- 73.
Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010; 55: 2614–2662. PMID: 20513610
- 74.
Takamoto S, Motomura N, Miyata H, et al. Mission and history of JCVSD. Jpn J Cardiovasc Surg 2017; 46: 187–190.
- 75.
Japan Adult Cardiovascular Surgery Database. Japan SCORE. https://jcvsd.org/JapanSCORE/
- 76.
EuroSCORE Study Group. EuroSCORE II. http://euroscore.org/calc.html
- 77.
The Society of Thoracic Surgeons (STS). Online STS Adult Cardiac Surgery Risk Calculator. http://riskcalc.sts.org/stswebriskcalc/calculate
- 78.
Motomura N, Miyata H, Tsukihara H, et al. Japan Cardiovascular Surgery Database Organization. Risk model of valve surgery in Japan using the Japan Adult Cardiovascular Surgery Database. J Heart Valve Dis 2010; 19: 684–691. PMID: 21214090
- 79.
Kurazumi H, Mikamo A, Fukamitsu G, et al. Validation of the JapanSCORE versus the logistic EuroSCORE for predicting operative mortality of cardiovascular surgery in Yamaguchi University Hospital. Gen Thorac Cardiovasc Surg 2011; 59: 599–604. PMID: 22231786
- 80.
Umehara N, Saito S, Tsukui H, et al. Usefulness of JapanSCORE: Comparative study of the usefulness of JapanSCORE and the logistic EuroSCORE. Jpn J Cardiovasc Surg 2013; 42: 94–102.
- 81.
Geriatric Medicine Research. Dalhousie University. Clinical Frailty Scale: CFS Version 1.2_EN. https://www.dal.ca/sites/gmr/our-tools/clinical-frailty-scale.html
- 82.
Rudolph JL, Marcantonio ER. Postoperative delirium: Acute change with long-term implications. Anesth Analg 2011; 112: 1202–1211. PMID: 21474660
- 83.
Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–922. PMID: 23992774
- 84.
Racine AM, Fong TG, Gou Y, et al. Clinical outcomes in older surgical patients with mild cognitive impairment. Alzheimers Dement 2018; 14: 590–600. PMID: 29190460
- 85.
Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Impact of delirium on postoperative frailty and long term cardiovascular events after cardiac surgery. PLoS One 2017; 12: e0190359. PMID: 29287124
- 86.
Brown CH. Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol 2014; 27: 117–122. PMID: 24514034
- 87.
Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg 2009; 87: 1469–1474. PMID: 19379886
- 88.
Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol 2010; 67: 338–344. PMID: 20373345
- 89.
Kasuga K, Ikeuchi K. Management for dementia. Orthopedics 2014; 65: 895–901.
- 90.
Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87: 663–669. PMID: 19161815
- 91.
Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008; 136: 631–640. PMID: 18805264
- 92.
Jacob KA, Hjortnaes J, Kranenburg G, et al. Mortality after cardiac surgery in patients with liver cirrhosis classified by the Child-Pugh score. Interact Cardiovasc Thorac Surg 2015; 20: 520–530. PMID: 25612743
- 93.
Arif R, Seppelt P, Schwill S, et al. Predictive risk factors for patients with cirrhosis undergoing heart surgery. Ann Thorac Surg 2012; 94: 1947–1952. PMID: 22921237
- 94.
Modi A, Vohra HA, Barlow CW. Do patients with liver cirrhosis undergoing cardiac surgery have acceptable outcomes? Interact Cardiovasc Thorac Surg 2010; 11: 630–634. PMID: 20739405
- 95.
Morimoto N, Okada K, Okita Y. The model for end-stage liver disease (MELD) predicts early and late outcomes of cardiovascular operations in patients with liver cirrhosis. Ann Thorac Surg 2013; 96: 1672–1678. PMID: 23987897
- 96.
Morisaki A, Hosono M, Sasaki Y, et al. Risk factor analysis in patients with liver cirrhosis undergoing cardiovascular operations. Ann Thorac Surg 2010; 89: 811–817. PMID: 20172135
- 97.
Hirata N, Sawa Y, Matsuda H. Predictive value of preoperative serum cholinesterase concentration in patients with liver dysfunction undergoing cardiac surgery. J Card Surg 1999; 14: 172–177. PMID: 10789703
- 98.
Fujimura K, Miyakawa Y, Kurata Y, et al. Reference guide for management of adult idiopathic thrombocytopenic purpura (ITP) 2012 version. [Article in Japanese] Rinsho Ketsueki 2012; 53: 433–442. PMID: 22687977
- 99.
Matsushita T, Amano K, Taki M, et al. Active bleeding in hemophiliac patients without inhibitors: Guideline of clotting factor replacement therapy during medical treatment and surgery. J Jpn Soc Thrombosis Hemostasis 2008; 19: 510–519.
- 100.
Akchurin RS, Davidov MI, Partigulov SA, et al. Cardiopulmonary bypass and cell-saver technique in combined oncologic and cardiovascular surgery. Artif Organs 1997; 21: 763–765. PMID: 9212954
- 101.
Markewitz A, Faist E, Lang S, et al. An imbalance in T-helper cell subsets alters immune response after cardiac surgery. Eur J Cardiothorac Surg 1996; 10: 61–67. PMID: 8776187
- 102.
Sablotzki A, Welters I, Lehmann N, et al. Plasma levels of immunoinhibitory cytokines interleukin-10 and transforming growth factor-β in patients undergoing coronary artery bypass grafting. Eur J Cardiothorac Surg 1997; 11: 763–768. PMID: 9151050
- 103.
Diegeler A, Doll N, Rauch T, et al. Humoral immune response during coronary artery bypass grafting: A comparison of limited approach, “off-pump” technique, and conventional cardiopulmonary bypass. Circulation 2000; 102 Suppl: Iii-95–Iii-100. PMID: 11082370
- 104.
Plumereau F, Pinaud F, Roch A, et al. Do patients with haematological malignancy who need cardiopulmonary bypass have a short-term higher mortality or a higher chance of disease progression? Interact Cardiovasc Thorac Surg 2014; 19: 474–478. PMID: 24920761
- 105.
Carrascal Y, Gualis J, Arévalo A, et al. Cardiac surgery with extracorporeal circulation in cancer patients: Influence on surgical morbidity and mortality and on survival. Rev Esp Cardiol 2008; 61: 369–375. PMID: 18405517
- 106.
Chan J, Rosenfeldt F, Chaudhuri K, et al. Cardiac surgery in patients with a history of malignancy: Increased complication rate but similar mortality. Heart Lung Circ 2012; 21: 255–259. PMID: 22386614
- 107.
Finck SJ, Cockerill KJ, Jeter JE, et al. Coronary artery bypass grafting in patients with chronic lymphocytic leukemia. Ann Thorac Surg 1993; 55: 1192–1196. PMID: 7684218
- 108.
Samuels LE, Kaufman MS, Morris RJ, et al. Open heart surgery in patients with chronic lymphocytic leukemia. Leuk Res 1999; 23: 71–75. PMID: 9933138
- 109.
Fecher AM, Birdas TJ, Haybron D, et al. Cardiac operations in patients with hematologic malignancies. Eur J Cardiothorac Surg 2004; 25: 537–540. PMID: 15037268
- 110.
Ghosh P, Carroll I, Kanhere A, et al. Cardiac operations in patients with low-grade small lymphocytic malignancies. J Thorac Cardiovasc Surg 1999; 118: 1033–1037. PMID: 10595975
- 111.
Makiyama K. Perioperative management for patients treated with glucocorticoids. Jpn J Clin Urol 2012; 66: 316–319.
- 112.
Japanese Circulation Society. Guidelines for management of anticoagulant and antiplatelet therapy in cardiovascular disease (JCS 2009). http://j-circ.or.jp/guideline/pdf/JCS2009_hori_h.pdf
- 113.
Japanese Circulation Society. Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). http://j-circ.or.jp/guideline/pdf/JCS2013_inoue_h.pdf
- 114.
Japanese Circulation Society. Guidelines for perioperative cardiovascular evaluation and management for noncardiac surgery (JCS 2014). http://j-circ.or.jp/guideline/pdf/JCS2014_kyo_h.pdf
- 115.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. PMID: 24685669
- 116.
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med 2015; 373: 823–833. PMID: 26095867
- 117.
Takeda K, Miyata H, Motomura N, et al. Contemporary perioperative results of heart valve replacement in dialysis patients: Analysis of 1,616 patients from the Japan adult cardiovascular surgery database. J Heart Valve Dis 2013; 22: 850–858. PMID: 24597409
- 118.
Klotz S, Vestring T, Rötker J, et al. Diagnosis and treatment of nonocclusive mesenteric ischemia after open heart surgery. Ann Thorac Surg 2001; 72: 1583–1586. PMID: 11722048
- 119.
Groesdonk HV, Klingele M, Schlempp S, et al. Risk factors for nonocclusive mesenteric ischemia after elective cardiac surgery. J Thorac Cardiovasc Surg 2013; 145: 1603–1610. PMID: 23219496
- 120.
Bomberg H, Groesdonk HV, Raffel M, et al. Vasopressin as therapy during nonocclusive mesenteric ischemia. Ann Thorac Surg 2016; 102: 813–819. PMID: 27173069
- 121.
Data Bank of Health, Labour and Welfare Ministry. https://www.mhlw.go.jp/toukei/itiran/
- 122.
Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24: 1231–1243. PMID: 12831818
- 123.
Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: A population-based study. Lancet 2006; 368: 1005–1011. PMID: 16980116
- 124.
Takahashi Y, Abe Y, Sasaki Y, et al. Mitral valve repair for atrial functional mitral regurgitation in patients with chronic atrial fibrillation. Interact Cardiovasc Thorac Surg 2015; 21: 163–168. PMID: 25980774
- 125.
Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Novel mechanistic insights into atrial functional mitral regurgitation: 3-dimensional echocardiographic study. Circ J 2016; 80: 2240–2248. PMID: 27535338
- 126.
Ito K, Abe Y, Takahashi Y, et al. Mechanism of atrial functional mitral regurgitation in patients with atrial fibrillation: A study using three-dimensional transesophageal echocardiography. J Cardiol 2017; 70: 584–590. PMID: 28527865
- 127.
Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: Best practice revolution. Eur Heart J 2010; 31: 1958–1966. PMID: 20624767
- 128.
Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: Direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997; 96: 1999–2008. PMID: 9323092
- 129.
Yiu SF, Enriquez-Sarano M, Tribouilloy C, et al. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000; 102: 1400–1406. PMID: 10993859
- 130.
Otsuji Y, Handschumacher MD, Liel-Cohen N, et al. Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: Three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol 2001; 37: 641–648. PMID: 11216991
- 131.
Otsuji Y, Kumanohoso T, Yoshifuku S, et al. Isolated annular dilation does not usually cause important functional mitral regurgitation: Comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol 2002; 39: 1651–1656. PMID: 12020493
- 132.
Kwan J, Shiota T, Agler DA, et al. Geometric differences of the mitral apparatus between ischemic and dilated cardiomyopathy with significant mitral regurgitation: Real-time three-dimensional echocardiography study. Circulation 2003; 107: 1135–1140. PMID: 12615791
- 133.
Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: Reversal with arrhythmia control. J Am Coll Cardiol 2011; 58: 1474–1481. PMID: 21939832
- 134.
Kagiyama N, Hayashida A, Toki M, et al. Insufficient leaflet remodeling in patients with atrial fibrillation: Association with the severity of mitral regurgitation. Circ Cardiovasc Imaging 2017; 10: e005451. PMID: 28289019
- 135.
Abe Y, Akamatsu K, Ito K, et al. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J 2018; 82: 1451–1458. PMID: 29553091
- 136.
Carpentier A. Cardiac valve surgery: The “French correction”. J Thorac Cardiovasc Surg 1983; 86: 323–337. PMID: 6887954
- 137.
Enriquez-Sarano M, Tajik AJ, Bailey KR, et al. Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: Influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol 1993; 21: 1211–1219. PMID: 8459079
- 138.
Cape EG, Yoganathan AP, Weyman AE, et al. Adjacent solid boundaries alter the size of regurgitant jets on Doppler color flow maps. J Am Coll Cardiol 1991; 17: 1094–1102. PMID: 2007708
- 139.
Matsumura Y, Fukuda S, Tran H, et al. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: Difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J 2008; 155: 231–238. PMID: 18215591
- 140.
Schwammenthal E, Popescu AC, Popescu BA, et al. Mechanism of mitral regurgitation in inferior wall acute myocardial infarction. Am J Cardiol 2002; 90: 306–309. PMID: 12127618
- 141.
Yoshida K, Yoshikawa J, Yamaura Y, et al. Value of acceleration flows and regurgitant jet direction by color Doppler flow mapping in the evaluation of mitral valve prolapse. Circulation 1990; 81: 879–885. PMID: 2306838
- 142.
Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012; 25: 3–46. PMID: 22183020
- 143.
Lancellotti P, Moura L, Pierard LA, et al; European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11: 307–332. PMID: 20435783
- 144.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2438–2488. PMID: 24603192
- 145.
Naji P, Griffin BP, Asfahan F, et al. Predictors of long-term outcomes in patients with significant myxomatous mitral regurgitation undergoing exercise echocardiography. Circulation 2014; 129: 1310–1319. PMID: 24396041
- 146.
Magne J, Lancellotti P, Piérard LA. Exercise-induced changes in degenerative mitral regurgitation. J Am Coll Cardiol 2010; 56: 300–309. PMID: 20633822
- 147.
Myerson SG, Francis JM, Neubauer S. Direct and indirect quantification of mitral regurgitation with cardiovascular magnetic resonance, and the effect of heart rate variability. MAGMA 2010; 23: 243–249. PMID: 20632070
- 148.
Uretsky S, Gillam L, Lang R, et al. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: A prospective multicenter trial. J Am Coll Cardiol 2015; 65: 1078–1088. PMID: 25790878
- 149.
Nishimura RA, Carabello BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation 2012; 125: 2138–2150. PMID: 22547754
- 150.
Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: Rationale for optimizing surgical indications. Circulation 1999; 99: 400–405. PMID: 9918527
- 151.
Gillinov AM, Mihaljevic T, Blackstone EH, et al. Should patients with severe degenerative mitral regurgitation delay surgery until symptoms develop? Ann Thorac Surg 2010; 90: 481–488. PMID: 20667334
- 152.
Kitai T, Okada Y, Shomura Y, et al. Early surgery for asymptomatic mitral regurgitation: Importance of atrial fibrillation. J Heart Valve Dis 2012; 21: 61–70. PMID: 22474744
- 153.
Enriquez-Sarano M, Suri RM, Clavel MA, et al. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg 2015; 150: 50–58. PMID: 25986494
- 154.
Yingchoncharoen T, Negishi T, Stanton T, et al. Incremental value of three-dimensional echocardiography in the evaluation of left ventricular size in mitral regurgitation: A follow-up study after mitral valve surgery. J Am Soc Echocardiogr 2014; 27: 608–615. PMID: 24679742
- 155.
Coutinho GF, Garcia AL, Correia PM, et al. Negative impact of atrial fibrillation and pulmonary hypertension after mitral valve surgery in asymptomatic patients with severe mitral regurgitation: A 20-year follow-up. Eur J Cardiothorac Surg 2015; 48: 548–556. PMID: 25564214
- 156.
Ngaage DL, Schaff HV, Mullany CJ, et al. Influence of preoperative atrial fibrillation on late results of mitral repair: Is concomitant ablation justified? Ann Thorac Surg 2007; 84: 434–443. PMID: 17643612
- 157.
Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 2006; 113: 2238–2244. PMID: 16651470
- 158.
David TE, Ivanov J, Armstrong S, et al. Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg 2003; 125: 1143–1152. PMID: 12771888
- 159.
Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013; 310: 609–616. PMID: 23942679
- 160.
Kang DH, Park SJ, Sun BJ, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: A propensity analysis. J Am Coll Cardiol 2014; 63: 2398–2407. PMID: 24694528
- 161.
De Bonis M, Bolling SF. Mitral valve surgery: Wait and see vs. early operation. Eur Heart J 2013; 34: 13–19. PMID: 22933568
- 162.
Zilberszac R, Heinze G, Binder T, et al. Long-term outcome of active surveillance in severe but asymptomatic primary mitral regurgitation. JACC Cardiovasc Imaging 2018; 11: 1213–1221. PMID: 30031699
- 163.
Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010; 56: 570–578. PMID: 20688212
- 164.
Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996; 335: 1417–1423. PMID: 8875918
- 165.
Rosen SE, Borer JS, Hochreiter C, et al. Natural history of the asymptomatic/minimally symptomatic patient with severe mitral regurgitation secondary to mitral valve prolapse and normal right and left ventricular performance. Am J Cardiol 1994; 74: 374–380. PMID: 8059701
- 166.
Mohty D, Orszulak TA, Schaff HV, et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001; 104 Suppl: I1–I7. PMID: 11568020
- 167.
Suri RM, Schaff HV, Dearani JA, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006; 82: 819–826. PMID: 16928491
- 168.
Daneshmand MA, Milano CA, Rankin JS, et al. Mitral valve repair for degenerative disease: A 20-year experience. Ann Thorac Surg 2009; 88: 1828–1837. PMID: 19932244
- 169.
Lazam S, Vanoverschelde JL, Tribouilloy C, et al; MIDA (Mitral Regurgitation International Database) Investigators. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: Analysis of a large, prospective, multicenter, international registry. Circulation 2017; 135: 410–422. PMID: 27899396
- 170.
Kilic A, Shah AS, Conte JV, et al. Operative outcomes in mitral valve surgery: Combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg 2013; 146: 638–646. PMID: 22914251
- 171.
Bolling SF, Li S, O’Brien SM, et al. Predictors of mitral valve repair: Clinical and surgeon factors. Ann Thorac Surg 2010; 90: 1904–1912. PMID: 21095334
- 172.
Suri RM, Clavel MA, Schaff HV, et al. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: Long-term analysis of competing outcomes. J Am Coll Cardiol 2016; 67: 488–498. PMID: 26846946
- 173.
Rusinaru D, Tribouilloy C, Grigioni F, et al; Mitral Regurgitation International DAtabase (MIDA) Investigators. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: Results from a large international multicenter study. Circ Cardiovasc Imaging 2011; 4: 473–481. PMID: 21737598
- 174.
Arias A, Pizarro R, Oberti P, et al. Prognostic value of left atrial volume in asymptomatic organic mitral regurgitation. J Am Soc Echocardiogr 2013; 26: 699–705. PMID: 23623592
- 175.
Magne J, Donal E, Mahjoub H, et al. Impact of exercise pulmonary hypertension on postoperative outcome in primary mitral regurgitation. Heart 2015; 101: 391–396. PMID: 25326443
- 176.
Naji P, Griffin BP, Barr T, et al. Importance of exercise capacity in predicting outcomes and determining optimal timing of surgery in significant primary mitral regurgitation. J Am Heart Assoc 2014; 3: e001010. PMID: 25213567
- 177.
Witkowski TG, Thomas JD, Debonnaire PJ, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging 2013; 14: 69–76. PMID: 22848021
- 178.
Mentias A, Naji P, Gillinov AM, et al. Strain echocardiography and functional capacity in asymptomatic primary mitral regurgitation with preserved ejection fraction. J Am Coll Cardiol 2016; 68: 1974–1986. PMID: 27591831
- 179.
Lancellotti P, Lebrun F, Piérard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 2003; 42: 1921–1928. PMID: 14662253
- 180.
Kron IL, Hung J, Overbey JR, et al; CTSN Investigators. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015; 149: 752–761. PMID: 25500293
- 181.
Hung J, Mihos C. Toward a better repair for ischemic mitral regurgitation: Thinking outside the ring. J Thorac Cardiovasc Surg 2017; 154: 1256–1257. PMID: 28711330
- 182.
Lee LS, Kwon MH, Cevasco M, et al. Postoperative recurrence of mitral regurgitation after annuloplasty for functional mitral regurgitation. Ann Thorac Surg 2012; 94: 1211–1217. PMID: 22727322
- 183.
Dulgheru R, Magne J, Lancellotti P, et al. Dynamic ischaemic mitral regurgitation and the role of stress echocardiography. J Cardiovasc Echogr 2013; 23: 10–17. PMID: 28465878
- 184.
Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103: 1759–1764. PMID: 11282907
- 185.
Magne J, Pibarot P, Dagenais F, et al. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation 2007; 115: 782–791. PMID: 17283262
- 186.
Gelsomino S, Lorusso R, Caciolli S, et al. Insights on left ventricular and valvular mechanisms of recurrent ischemic mitral regurgitation after restrictive annuloplasty and coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008; 136: 507–518. PMID: 18692665
- 187.
Lee AP, Acker M, Kubo SH, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy: Importance of distal anterior leaflet tethering. Circulation 2009; 119: 2606–2614. PMID: 19414639
- 188.
Roshanali F, Mandegar MH, Yousefnia MA, et al. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg 2007; 84: 745–749. PMID: 17720370
- 189.
McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004; 128: 916–924. PMID: 15573077
- 190.
Gelsomino S, Lorusso R, De Cicco G, et al. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 2008; 29: 231–240. PMID: 17989079
- 191.
Kongsaerepong V, Shiota M, Gillinov AM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol 2006; 98: 504–508. PMID: 16893706
- 192.
Chung H, Amaki M, Takashio S, et al. Effect of mitral valve surgery in patients with dilated cardiomyopathy and severe functional mitral regurgitation. Circ J 2017; 82: 131–140. PMID: 28740056
- 193.
Grayburn PA, Carabello B, Hung J, et al. Defining “severe” secondary mitral regurgitation: Emphasizing an integrated approach. J Am Coll Cardiol 2014; 64: 2792–2801. PMID: 25541133
- 194.
Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005; 45: 381–387. PMID: 15680716
- 195.
Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009; 120: 1474–1481. PMID: 19786637
- 196.
Michler RE, Smith PK, Parides MK, et al. CTSN. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016; 374: 1932–1941. PMID: 27040451
- 197.
Michler RE. Surgical management of moderate ischemic mitral regurgitation at the time of coronary artery bypass grafting remains controversial. J Thorac Cardiovasc Surg 2018; 156: 1498–1500. PMID: 29958669
- 198.
Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation 2012; 125: 2639–2648. PMID: 22553307
- 199.
Kihara T, Gillinov AM, Takasaki K, et al. Mitral regurgitation associated with mitral annular dilation in patients with lone atrial fibrillation: An echocardiographic study. Echocardiography 2009; 26: 885–889. PMID: 19552671
- 200.
Vohra HA, Whistance RN, Magan A, et al. Mitral valve repair for severe mitral regurgitation secondary to lone atrial fibrillation. Eur J Cardiothorac Surg 2012; 42: 634–637. PMID: 22323495
- 201.
Ring L, Dutka DP, Wells FC, et al. Mechanisms of atrial mitral regurgitation: Insights using 3D transoesophageal echo. Eur Heart J Cardiovasc Imaging 2014; 15: 500–508. PMID: 24145456
- 202.
van Rosendael PJ, Katsanos S, Kamperidis V, et al. New insights on Carpentier I mitral regurgitation from multidetector row computed tomography. Am J Cardiol 2014; 114: 763–768. PMID: 25037679
- 203.
Itabashi Y, Mihara H, Berdejo J, et al. Distant position of chordae from coaptation causes mitral regurgitation in patients with atrial fibrillation. J Heart Valve Dis 2016; 25: 323–331. PMID: 27989043
- 204.
Kim DH, Heo R, Handschumacher MD, et al. Mitral valve adaptation to isolated annular dilation: Insights into the mechanism of atrial functional mitral regurgitation. JACC Cardiovasc Imaging 2019; 12: 665–677. PMID: 29248661
- 205.
Cong T, Gu J, Lee AP, et al. Quantitative analysis of mitral valve morphology in atrial functional mitral regurgitation using real-time 3-dimensional echocardiography atrial functional mitral regurgitation. Cardiovasc Ultrasound 2018; 16: 13. PMID: 30126422
- 206.
Saito C, Minami Y, Arai K, et al. Prevalence, clinical characteristics, and outcome of atrial functional mitral regurgitation in hospitalized heart failure patients with atrial fibrillation. J Cardiol 2018; 72: 292–299. PMID: 29752195
- 207.
Ito K, Abe Y, Watanabe H, et al. Prognostic significance of residual functional mitral regurgitation in hospitalized heart failure patients with chronic atrial fibrillation and preserved ejection fraction after medical therapies. J Echocardiogr 2019; 17: 197–205. PMID: 30569445
- 208.
Hashim SW, Youssef SJ, Ayyash B, et al. Pseudoprolapse of the anterior leaflet in chronic ischemic mitral regurgitation: Identification and repair. J Thorac Cardiovasc Surg 2012; 143 Suppl: S33–S37. PMID: 22050989
- 209.
Berdejo J, Shiota M, Mihara H, et al. Vena contracta analysis by color Doppler three-dimensional transesophageal echocardiography shows geometrical differences between prolapse and pseudoprolapse in eccentric mitral regurgitation. Echocardiography 2017; 34: 683–689. PMID: 28317206
- 210.
O’Gara PT, Grayburn PA, Badhwar V, et al. 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: A report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol 2017; 70: 2421–2449. PMID: 29055505
- 211.
Mauri L, Foster E, Glower DD, et al; EVEREST II Investigators. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013; 62: 317–328. PMID: 23665364
- 212.
Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013; 62: 1052–1061. PMID: 23747789
- 213.
Whitlow PL, Feldman T, Pedersen WR, et al; EVEREST II Investigators. Acute and 12-month results with catheter-based mitral valve leaflet repair: The EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012; 59: 130–139. PMID: 22222076
- 214.
Stone GW, Lindenfeld J, Abraham WT, et al; COAPT Investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. PMID: 30280640
- 215.
Obadia JF, Messika-Zeitoun D, Leurent G, et al; MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. PMID: 30145927
- 216.
Hayashida K, Yasuda S, Matsumoto T, et al. AVJ-514 trial: Baseline characteristics and 30-day outcomes following MitraClip® treatment in a Japanese cohort. Circ J 2017; 81: 1116–1122. PMID: 28321004
- 217.
日本心不全学会, 日本心臓病学会, 日本心エコー図学会, 他. 尖間クリッピング式の経皮的僧帽弁接合不全修復システムに関する適正使用指針. http://www.j-circ.or.jp/MitraClip/tekisei_shishin.pdf
- 218.
Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014; 30: 962–970. PMID: 24986049
- 219.
Abramowitz Y, Jilaihawi H, Chakravarty T, et al. Mitral annulus calcification. J Am Coll Cardiol 2015; 66: 1934–1941. PMID: 26493666
- 220.
Akram MR, Chan T, McAuliffe S, et al. Non-rheumatic annular mitral stenosis: Prevalence and characteristics. Eur J Echocardiogr 2009; 10: 103–105. PMID: 18579487
- 221.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: e57–e185. PMID: 24603191
- 222.
Tsang W, Freed BH, Lang RM. Three-dimensional anatomy of the aortic and mitral valves. In: Otto CM, Bonow RO, editors. Valvular heart disease: A companion to Braunwald’s heart disease, 4th edn. Saunders Publishers, 2013: 14–29.
- 223.
Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: An analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60: 299–308. PMID: 3190958
- 224.
Ben Farhat M, Ayari M, Maatouk F, et al. Percutaneous balloon versus surgical closed and open mitral commissurotomy: Seven-year follow-up results of a randomized trial. Circulation 1998; 97: 245–250. PMID: 9462525
- 225.
Turi ZG, Reyes VP, Raju BS, et al. Percutaneous balloon versus surgical closed commissurotomy for mitral stenosis. A prospective, randomized trial. Circulation 1991; 83: 1179–1185. PMID: 2013139
- 226.
Arora R, Nair M, Kalra GS, et al. Immediate and long-term results of balloon and surgical closed mitral valvotomy: A randomized comparative study. Am Heart J 1993; 125: 1091–1094. PMID: 8465732
- 227.
Otto CM, Davis KB, Reid CL, et al. Relation between pulmonary artery pressure and mitral stenosis severity in patients undergoing balloon mitral commissurotomy. Am J Cardiol 1993; 71: 874–878. PMID: 8456774
- 228.
Cheriex EC, Pieters FA, Janssen JH, et al. Value of exercise Doppler-echocardiography in patients with mitral stenosis. Int J Cardiol 1994; 45: 219–226. PMID: 7960267
- 229.
Bouleti C, Iung B, Himbert D, et al. Relationship between valve calcification and long-term results of percutaneous mitral commissurotomy for rheumatic mitral stenosis. Circ Cardiovasc Interv 2014; 7: 381–389. PMID: 24782197
- 230.
Bouleti C, Iung B, Laouénan C, et al. Late results of percutaneous mitral commissurotomy up to 20 years: Development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation 2012; 125: 2119–2127. PMID: 22456478
- 231.
Ishige A, Uejima T, Kanmatsuse K, et al. Giant fenestration and fibrous strand rupture of aortic valve without massive regurgitation. J Cardiol Cases 2012; 5: e163–e165. PMID: 30532930
- 232.
El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005; 20: 115–121. PMID: 15711197
- 233.
Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: Impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009; 137: 286–294. PMID: 19185138
- 234.
Hamirani YS, Dietl CA, Voyles W, et al. Acute aortic regurgitation. Circulation 2012; 126: 1121–1126. PMID: 22927474
- 235.
Borer JS, Hochreiter C, Herrold EM, et al. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation 1998; 97: 525–534. PMID: 9494022
- 236.
Siemienczuk D, Greenberg B, Morris C, et al. Chronic aortic insufficiency: Factors associated with progression to aortic valve replacement. Ann Intern Med 1989; 110: 587–592. PMID: 2930091
- 237.
Wahi S, Haluska B, Pasquet A, et al. Exercise echocardiography predicts development of left ventricular dysfunction in medically and surgically treated patients with asymptomatic severe aortic regurgitation. Heart 2000; 84: 606–614. PMID: 11083736
- 238.
le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation: Accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007; 116 Suppl: I264–I269. PMID: 17846315
- 239.
Schäfers HJ, Schmied W, Marom G, et al. Cusp height in aortic valves. J Thorac Cardiovasc Surg 2013; 146: 269–274. PMID: 22853942
- 240.
Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006; 132: 436–438. PMID: 16872982
- 241.
Komiya T, Shimamoto T, Nonaka M, et al. Is small cusp size a limitation for aortic valve repair?†. Eur J Cardiothorac Surg 2019; 56: 497–502. PMID: 30824918
- 242.
Carr JC, Simonetti O, Bundy J, et al. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology 2001; 219: 828–834. PMID: 11376278
- 243.
Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013; 15: 35. PMID: 23634753
- 244.
Azevedo CF, Nigri M, Higuchi ML, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol 2010; 56: 278–287. PMID: 20633819
- 245.
Klodas E, Enriquez-Sarano M, Tajik AJ, et al. Optimizing timing of surgical correction in patients with severe aortic regurgitation: Role of symptoms. J Am Coll Cardiol 1997; 30: 746–752. PMID: 9283535
- 246.
Bhudia SK, McCarthy PM, Kumpati GS, et al. Improved outcomes after aortic valve surgery for chronic aortic regurgitation with severe left ventricular dysfunction. J Am Coll Cardiol 2007; 49: 1465–1471. PMID: 17397676
- 247.
Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002; 106: 2687–2693. PMID: 12438294
- 248.
Fioretti P, Roelandt J, Bos RJ, et al. Echocardiography in chronic aortic insufficiency: Is valve replacement too late when left ventricular end-systolic dimension reaches 55 mm? Circulation 1983; 67: 216–221. PMID: 6847800
- 249.
Daniel WG, Hood WP Jr, Siart A, et al. Chronic aortic regurgitation: Reassessment of the prognostic value of preoperative left ventricular end-systolic dimension and fractional shortening. Circulation 1985; 71: 669–680. PMID: 3156010
- 250.
Amano M, Izumi C, Imamura S, et al. Pre- and postoperative predictors of long-term prognosis after aortic valve replacement for severe chronic aortic regurgitation. Circ J 2016; 80: 2460–2467. PMID: 27829587
- 251.
Forman R, Firth BG, Barnard MS. Prognostic significance of preoperative left ventricular ejection fraction and valve lesion in patients with aortic valve replacement. Am J Cardiol 1980; 45: 1120–1125. PMID: 7377109
- 252.
Greves J, Rahimtoola SH, McAnulty JH, et al. Preoperative criteria predictive of late survival following valve replacement for severe aortic regurgitation. Am Heart J 1981; 101: 300–308. PMID: 6451163
- 253.
Tornos MP, Olona M, Permanyer-Miralda G, et al. Clinical outcome of severe asymptomatic chronic aortic regurgitation: A long-term prospective follow-up study. Am Heart J 1995; 130: 333–339. PMID: 7631617
- 254.
Turk R, Varadarajan P, Kamath A, et al. Survival benefit of aortic valve replacement in older patients with asymptomatic chronic severe aortic regurgitation. Ann Thorac Surg 2010; 89: 731–737. PMID: 20172118
- 255.
Tornos P, Sambola A, Permanyer-Miralda G, et al. Long-term outcome of surgically treated aortic regurgitation: Influence of guideline adherence toward early surgery. J Am Coll Cardiol 2006; 47: 1012–1017. PMID: 16516086
- 256.
Saisho H, Arinaga K, Kikusaki S, et al. Long term results and predictors of left ventricular function recovery after aortic valve replacement for chronic aortic regurgitation. Ann Thorac Cardiovasc Surg 2015; 21: 388–395. PMID: 25740455
- 257.
Sambola A, Tornos P, Ferreira-Gonzalez I, et al. Prognostic value of preoperative indexed end-systolic left ventricle diameter in the outcome after surgery in patients with chronic aortic regurgitation. Am Heart J 2008; 155: 1114–1120. PMID: 18513527
- 258.
Mentias A, Feng K, Alashi A, et al. Long-term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol 2016; 68: 2144–2153. PMID: 27855803
- 259.
Yang LT, Michelena HI, Scott CG, et al. Outcomes in chronic hemodynamically significant aortic regurgitation and limitations of current guidelines. J Am Coll Cardiol 2019; 73: 1741–1752. PMID: 30846339
- 260.
Wang Y, Jiang W, Liu J, et al. Early surgery versus conventional treatment for asymptomatic severe aortic regurgitation with normal ejection fraction and left ventricular dilatation. Eur J Cardiothorac Surg 2017; 52: 118–124. PMID: 28329360
- 261.
Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2017; 52: 616–664. PMID: 29156023
- 262.
Maeda S, Taniguchi K, Toda K, et al; Osaka Cardiovascular Surgery Research (OSCAR) Group. Outcomes after aortic valve replacement for asymptomatic severe aortic regurgitation and normal ejection fraction. Semin Thorac Cardiovasc Surg 2019; 31: 763–770. PMID: 30731192
- 263.
Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg 2004; 128: 677–683. PMID: 15514594
- 264.
Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg 2008; 85 Suppl: S1–S41. PMID: 18083364
- 265.
Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300: 1317–1325. PMID: 18799444
- 266.
Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: A cohort study. Circulation 2012; 125: 226–232. PMID: 22133496
- 267.
Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet 2016; 9: 548–558. PMID: 27879313
- 268.
Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 2006; 355: 788–798. PMID: 16928994
- 269.
Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis: The Tromsø study. Heart 2013; 99: 396–400. PMID: 22942293
- 270.
Danielsen R, Aspelund T, Harris TB, et al. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: The AGES-Reykjavík study. Int J Cardiol 2014; 176: 916–922. PMID: 25171970
- 271.
Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55: 2789–2800. PMID: 20579534
- 272.
Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111: 920–925. PMID: 15710758
- 273.
Krepp JM, Roman MJ, Devereux RB, et al; GenTAC Investigators. Bicuspid and unicuspid aortic valves: Different phenotypes of the same disease? Insight from the GenTAC Registry. Congenit Heart Dis 2017; 12: 740–745. PMID: 28805011
- 274.
Tsang MY, Abudiab MM, Ammash NM, et al. Quadricuspid aortic valve: Characteristics, associated structural cardiovascular abnormalities, and clinical outcomes. Circulation 2016; 133: 312–319. PMID: 26635401
- 275.
Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000; 343: 611–617. PMID: 10965007
- 276.
Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic stenosis. Circulation 2010; 121: 151–156. PMID: 20026771
- 277.
Lancellotti P, Magne J, Dulgheru R, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol 2018; 3: 1060–1068. PMID: 30285058
- 278.
Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121: 1502–1509. PMID: 20308614
- 279.
Taniguchi T, Morimoto T, Shiomi H, et al. CURRENT AS Registry Investigators. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2015; 66: 2827–2838. PMID: 26477634
- 280.
Thaden JJ, Nkomo VT, Lee KJ, et al. Doppler imaging in aortic stenosis: The importance of the nonapical imaging windows to determine severity in a contemporary cohort. J Am Soc Echocardiogr 2015; 28: 780–785. PMID: 25857547
- 281.
Magne J, Lancellotti P, Piérard LA. Exercise testing in asymptomatic severe aortic stenosis. JACC Cardiovasc Imaging 2014; 7: 188–199. PMID: 24524744
- 282.
Stefanini GG, Stortecky S, Cao D, et al. Coronary artery disease severity and aortic stenosis: Clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur Heart J 2014; 35: 2530–2540. PMID: 24682843
- 283.
Davies JE, Piek JJ. Time for caution interpreting coronary physiology in aortic stenosis? EuroIntervention 2018; 14: 132–134. PMID: 29937425
- 284.
Yamanaka F, Shishido K, Ochiai T, et al. Instantaneous wave-free ratio for the assessment of intermediate coronary artery stenosis in patients with severe aortic valve stenosis: Comparison with myocardial perfusion scintigraphy. JACC Cardiovasc Interv 2018; 11: 2032–2040. PMID: 30154064
- 285.
Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J Am Coll Cardiol 2014; 64: 1202–1213. PMID: 25236511
- 286.
Kakefuda Y, Hayashida K, Yamada Y, et al. Impact of subclinical vascular complications detected by systematic postprocedural multidetector computed tomography after transcatheter aortic valve implantation using balloon-expandable Edwards SAPIEN XT heart valve. Am J Cardiol 2017; 119: 1100–1105. PMID: 28162223
- 287.
Minners J, Allgeier M, Gohlke-Baerwolf C, et al. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J 2008; 29: 1043–1048. PMID: 18156619
- 288.
Nishimura RA, Grantham JA, Connolly HM, et al. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: The clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002; 106: 809–813. PMID: 12176952
- 289.
Cueff C, Serfaty JM, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011; 97: 721–726. PMID: 20720250
- 290.
Ribeiro HB, Lerakis S, Gilard M, et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: The TOPAS-TAVI registry. J Am Coll Cardiol 2018; 71: 1297–1308. PMID: 29566812
- 291.
Hachicha Z, Dumesnil JG, Bogaty P, et al. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115: 2856–2864. PMID: 17533183
- 292.
Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011; 123: 887–895. PMID: 21321152
- 293.
Tribouilloy C, Rusinaru D, Maréchaux S, et al. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: Characteristics, outcome, and implications for surgery. J Am Coll Cardiol 2015; 65: 55–66. PMID: 25572511
- 294.
Yamashita E, Takeuchi M, Seo Y, et al. Prognostic value of paradoxical low-gradient severe aortic stenosis in Japan: Japanese Multicenter Aortic Stenosis Study, Retrospective (JUST-R) Registry. J Cardiol 2015; 65: 360–368. PMID: 25687368
- 295.
Eleid MF, Sorajja P, Michelena HI, et al. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: Clinical characteristics and predictors of survival. Circulation 2013; 128: 1781–1789. PMID: 24048203
- 296.
Mehrotra P, Jansen K, Flynn AW, et al. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J 2013; 34: 1906–1914. PMID: 23533186
- 297.
Turina J, Hess O, Sepulcri F, et al. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8: 471–483. PMID: 3609042
- 298.
Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39: 1356–1363. PMID: 11955855
- 299.
Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015; 99: 831–837. PMID: 25583467
- 300.
Johnson S, Stroud MR, Kratz JM, et al. Thirty-year experience with a bileaflet mechanical valve prosthesis. J Thorac Cardiovasc Surg 2019; 157: 213–222. PMID: 30342758
- 301.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2017; 70: 252–289. PMID: 28315732
- 302.
Leon MB, Smith CR, Mack MJ, et al. PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374: 1609–1620. PMID: 27040324
- 303.
Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016; 387: 2218–2225. PMID: 27053442
- 304.
Reardon MJ, Van Mieghem NM, Popma JJ, et al; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376: 1321–1331. PMID: 28304219
- 305.
Mack MJ, Leon MB, Thourani VH, et al; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695–1705. PMID: 30883058
- 306.
Popma JJ, Deeb GM, Yakubov SJ, et al; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380: 1706–1715. PMID: 30883053
- 307.
Committee for Scientific Affairs, The Japanese Association for Thoracic Surgery. Shimizu H, Endo S, Natsugoe S, et al. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019; 67: 377–411. PMID: 30877649
- 308.
Handa N, Kumamaru H, Torikai K, et al. Japanese TAVR Registry Participants. Learning curve for transcatheter aortic valve implantation under a controlled introduction system: Initial analysis of a Japanese nationwide registry. Circ J 2018; 82: 1951–1958. PMID: 29794375
- 309.
Yamamoto M, Watanabe Y, Tada N, et al; OCEAN-TAVI investigators. Transcatheter aortic valve replacement outcomes in Japan: Optimized CathEter vAlvular iNtervention (OCEAN) Japanese multicenter registry. Cardiovasc Revasc Med 2019; 20: 843–851. PMID: 30553819
- 310.
Shimura T, Yamamoto M, Kano S, et al; OCEAN-TAVI Investigators. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 2017; 135: 2013–2024. PMID: 28302751
- 311.
Yanagisawa R, Tanaka M, Yashima F, et al. Frequency and Consequences of Cognitive Impairmentin Patients Underwent Transcatheter Aortic Valve Implantation. Am J Cardiol 2018; 122: 844–850. PMID: 30072128
- 312.
Pai RG, Kapoor N, Bansal RC, et al. Malignant natural history of asymptomatic severe aortic stenosis: Benefit of aortic valve replacement. Ann Thorac Surg 2006; 82: 2116–2122. PMID: 17126122
- 313.
Pellikka PA, Nishimura RA, Bailey KR, et al. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15: 1012–1017. PMID: 2312954
- 314.
Brown ML, Pellikka PA, Schaff HV, et al. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2008; 135: 308–315. PMID: 18242258
- 315.
Banovic M, Iung B, Bartunek J, et al. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): A randomized multicenter controlled event-driven trial. Am Heart J 2016; 174: 147–153. PMID: 26995381
- 316.
Ledwoch J, Thiele H. Treatment of asymptomatic aortic valve stenosis: Watchful waiting or early intervention? Herz 2017; 42: 528–535. PMID: 28593422
- 317.
Amato MC, Moffa PJ, Werner KE, et al. Treatment decision in asymptomatic aortic valve stenosis: Role of exercise testing. Heart 2001; 86: 381–386. PMID: 11559673
- 318.
Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging 2015; 8: e002917. PMID: 25852129
- 319.
Taniguchi T, Morimoto T, Shiomi H, et al; CURRENT AS Registry Investigators. Prognostic impact of left ventricular ejection fraction in patients with severe aortic stenosis. JACC Cardiovasc Interv 2018; 11: 145–157. PMID: 29289632
- 320.
Ito S, Miranda WR, Nkomo VT, et al. Reduced left ventricular ejection fraction in patients with aortic stenosis. J Am Coll Cardiol 2018; 71: 1313–1321. PMID: 29566814
- 321.
Tastet L, Tribouilloy C, Maréchaux S, et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol 2019; 74: 550–563. PMID: 31345430
- 322.
Kitai T, Honda S, Okada Y, et al. Clinical outcomes in non-surgically managed patients with very severe versus severe aortic stenosis. Heart 2011; 97: 2029–2032. PMID: 21954228
- 323.
Nistri S, Faggiano P, Olivotto I, et al. Hemodynamic progression and outcome of asymptomatic aortic stenosis in primary care. Am J Cardiol 2012; 109: 718–723. PMID: 22154322
- 324.
Bohbot Y, Kowalski C, Rusinaru D, et al. Impact of mean transaortic pressure gradient on long-term outcome in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc 2017; 6: e005850. PMID: 28572283
- 325.
Maréchaux S, Ringle A, Rusinaru D, et al. Prognostic value of aortic valve area by Doppler echocardiography in patients with severe asymptomatic aortic stenosis. J Am Heart Assoc 2016; 5: e003146. PMID: 27143354
- 326.
Pai RG, Varadarajan P, Kapoor N, et al. Aortic valve replacement improves survival in severe aortic stenosis associated with severe pulmonary hypertension. Ann Thorac Surg 2007; 84: 80–85. PMID: 17588389
- 327.
Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109: 2302–2308. PMID: 15117847
- 328.
Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105: 383–388. PMID: 20102953
- 329.
Nakatsuma K, Taniguchi T, Morimoto T, et al; CURRENT AS Registry Investigators. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart 2019; 105: 384–390. PMID: 30530820
- 330.
Henri C, Dulgheru R, Magne J, et al. Impact of serial B-type natriuretic peptide changes for predicting outcome in asymptomatic patients with aortic stenosis. Can J Cardiol 2016; 32: 183–189. PMID: 26371385
- 331.
Smith WT 4th, Ferguson TB Jr, Ryan T, et al. Should coronary artery bypass graft surgery patients with mild or moderate aortic stenosis undergo concomitant aortic valve replacement? A decision analysis approach to the surgical dilemma. J Am Coll Cardiol 2004; 44: 1241–1247. PMID: 15364326
- 332.
Dagenais F, Mathieu P, Doyle D, et al. Moderate aortic stenosis in coronary artery bypass grafting patients more than 70 years of age: To replace or not to replace? Ann Thorac Surg 2010; 90: 1495–1500. PMID: 20971247
- 333.
Bhattacharyya S, Hayward C, Pepper J, et al. Risk stratification in asymptomatic severe aortic stenosis: A critical appraisal. Eur Heart J 2012; 33: 2377–2387. PMID: 22766580
- 334.
Zilberszac R, Gabriel H, Schemper M, et al. Asymptomatic Severe Aortic Stenosis in the Elderly. JACC Cardiovasc Imaging 2017; 10: 43–50. PMID: 27639763
- 335.
Izumi C. Asymptomatic severe aortic stenosis: Challenges in diagnosis and management. Heart 2016; 102: 1168–1176. PMID: 27091844
- 336.
Bohbot Y, Rusinaru D, Delpierre Q, et al. Risk stratification of severe aortic stenosis with preserved left ventricular ejection fraction using peak aortic jet velocity: An outcome study. Circ Cardiovasc Imaging 2017; 10: e006760. PMID: 29021260
- 337.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004; 43: 405–409. PMID: 15013122
- 338.
Behm CZ, Nath J, Foster E. Clinical correlates and mortality of hemodynamically significant tricuspid regurgitation. J Heart Valve Dis 2004; 13: 784–789. PMID: 15473480
- 339.
Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7: 1185–1194. PMID: 25440592
- 340.
Takahashi Y, Izumi C, Miyake M, et al. Actual management and prognosis of severe isolated tricuspid regurgitation associated with atrial fibrillation without structural heart disease. Int J Cardiol 2017; 243: 251–257. PMID: 28536002
- 341.
Lee JW, Song JM, Park JP, et al. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J 2010; 74: 375–380. PMID: 20009355
- 342.
Topilsky Y, Khanna A, Le Tourneau T, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging 2012; 5: 314–323. PMID: 22447806
- 343.
Utsunomiya H, Itabashi Y, Mihara H, et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: A real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging 2017; 10: e004897. PMID: 28073806
- 344.
Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging 2019; 12: 433–442. PMID: 30121261
- 345.
Najib MQ, Vinales KL, Vittala SS, et al. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012; 29: 140–146. PMID: 22067002
- 346.
Yamasaki N, Kondo F, Kubo T, et al. Severe tricuspid regurgitation in the aged: Atrial remodeling associated with long-standing atrial fibrillation. J Cardiol 2006; 48: 315–323. PMID: 17243626
- 347.
Zhou X, Otsuji Y, Yoshifuku S, et al. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J 2002; 66: 913–916. PMID: 12381084
- 348.
Izumi C, Iga K, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis 2002; 11: 353–356. PMID: 12056726
- 349.
Matsuyama K, Matsumoto M, Sugita T, et al. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003; 75: 1826–1828. PMID: 12822623
- 350.
Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: A single-center experience with long-term echocardiographic examinations. Am Heart J 2008; 155: 732–737. PMID: 18371484
- 351.
Izumi C, Miyake M, Takahashi S, et al. Progression of isolated tricuspid regurgitation late after left-sided valve surgery: Clinical features and mechanisms. Circ J 2011; 75: 2902–2907. PMID: 21946358
- 352.
Kammerlander AA, Marzluf BA, Graf A, et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol 2014; 64: 2633–2642. PMID: 25524343
- 353.
Subbotina I, Girdauskas E, Bernhardt AM, et al. Comparison of outcomes of tricuspid valve surgery in patients with reduced and normal right ventricular function. Thorac Cardiovasc Surg 2017; 65: 617–625. PMID: 28841733
- 354.
Song JM, Jang MK, Choi YS, et al. The vena contracta in functional tricuspid regurgitation: A real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr 2011; 24: 663–670. PMID: 21324644
- 355.
Hahn RT, Thomas JD, Khalique OK, et al. Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc Imaging 2019; 12: 469–490. PMID: 30846122
- 356.
Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117: 1436–1448. PMID: 18347220
- 357.
Hsiao SH, Lin SK, Wang WC, et al. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr 2006; 19: 902–910. PMID: 16825000
- 358.
Park JB, Lee SP, Lee JH, et al. Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: A validation study with cardiac magnetic resonance. J Am Soc Echocardiogr 2016; 29: 392–401. PMID: 26969137
- 359.
Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation 2005; 111: 975–979. PMID: 15710756
- 360.
Kühn A, De Pasquale Meyer G, Müller J, et al. Tricuspid valve surgery improves cardiac output and exercise performance in patients with Ebstein‘s anomaly. Int J Cardiol 2013; 166: 494–498. PMID: 22204848
- 361.
Chen Y, Liu YX, Seto WK, et al. Prognostic value of hepatorenal function by modified Model for End-stage Liver Disease (MELD) score in patients undergoing tricuspid annuloplasty. J Am Heart Assoc 2018; 7: e009020. PMID: 30006492
- 362.
Messika-Zeitoun D, Thomson H, Bellamy M, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg 2004; 128: 296–302. PMID: 15282468
- 363.
Kim JH, Kim HK, Lee SP, et al. Right ventricular reverse remodeling, but not subjective clinical amelioration, predicts long-term outcome after surgery for isolated severe tricuspid regurgitation. Circ J 2014; 78: 385–392. PMID: 24225337
- 364.
Dreyfus J, Ghalem N, Garbarz E, et al. Timing of referral of patients with severe isolated tricuspid valve regurgitation to Surgeons (from a French Nationwide Database). Am J Cardiol 2018; 122: 323–326. PMID: 29747858
- 365.
Axtell AL, Bhambhani V, Moonsamy P, et al. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol 2019; 74: 715–725. PMID: 31071413
- 366.
Chikwe J, Itagaki S, Anyanwu A, et al. Impact of concomitant tricuspid annuloplasty on tricuspid regurgitation, right ventricular function, and pulmonary artery hypertension after repair of mitral valve prolapse. J Am Coll Cardiol 2015; 65: 1931–1938. PMID: 25936265
- 367.
Prihadi EA, van der Bijl P, Gursoy E, et al. Development of significant tricuspid regurgitation over time and prognostic implications: New insights into natural history. Eur Heart J 2018; 39: 3574–3581. PMID: 30010848
- 368.
Yajima S, Yoshioka D, Toda K, et al. Definitive determinant of late significant tricuspid regurgitation after aortic valve replacement. Circ J 2018; 82: 886–894. PMID: 29238013
- 369.
Staab ME, Nishimura RA, Dearani JA. Isolated tricuspid valve surgery for severe tricuspid regurgitation following prior left heart valve surgery: Analysis of outcome in 34 patients. J Heart Valve Dis 1999; 8: 567–574. PMID: 10517400
- 370.
Jang JY, Heo R, Lee S, et al. Comparison of results of tricuspid valve repair versus replacement for severe functional tricuspid regurgitation. Am J Cardiol 2017; 119: 905–910. PMID: 28214000
- 371.
Choi JW, Jang MJ, Kim KH, et al. Repair versus replacement for the surgical correction of tricuspid regurgitation: A meta-analysis. Eur J Cardiothorac Surg 2018; 53: 748–755. PMID: 29228165
- 372.
Alkhouli M, Berzingi C, Kowatli A, et al. Comparative early outcomes of tricuspid valve repair versus replacement for secondary tricuspid regurgitation. Open Heart 2018; 5: e000878. PMID: 30228911
- 373.
Park K, Kim HK, Kim YJ, et al. Incremental prognostic value of early postoperative right ventricular systolic function in patients undergoing surgery for isolated severe tricuspid regurgitation. Heart 2011; 97: 1319–1325. PMID: 21628722
- 374.
Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009; 120: 1672–1678. PMID: 19822809
- 375.
Song H, Kim MJ, Chung CH, et al. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009; 95: 931–936. PMID: 19321491
- 376.
Wang J, Han J, Li Y, et al. Preoperative risk factors of medium-term mitral valve repair outcome. Interact Cardiovasc Thorac Surg 2014; 19: 946–954. PMID: 25217622
- 377.
Jeong DS, Shim MS, Sung K, et al. Prophylactic tricuspid annuloplasty in patients undergoing double valve revlacement. J Heart Valve Dis 2015; 24: 508–515. PMID: 26897825
- 378.
Navia JL, Brozzi NA, Klein AL, et al. Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: To repair or not to repair? Ann Thorac Surg 2012; 93: 59–69. PMID: 22093694
- 379.
Lee H, Sung K, Kim WS, et al. Clinical and hemodynamic influences of prophylactic tricuspid annuloplasty in mechanical mitral valve replacement. J Thorac Cardiovasc Surg 2016; 151: 788–795. PMID: 26778212
- 380.
Badhwar V, Rankin JS, He M, et al. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality. Ann Thorac Surg 2017; 103: 587–593. PMID: 27570159
- 381.
Colombo T, Russo C, Ciliberto GR, et al. Tricuspid regurgitation secondary to mitral valve disease: Tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg 2001; 9: 369–377. PMID: 11420162
- 382.
Dreyfus GD, Corbi PJ, Chan KM, et al. Secondary tricuspid regurgitation or dilatation: Which should be the criteria for surgical repair? Ann Thorac Surg 2005; 79: 127–132. PMID: 15620928
- 383.
Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012; 143: 632–638. PMID: 22244561
- 384.
Latib A, Grigioni F, Hahn RT. Tricuspid regurgitation: What is the real clinical impact and how often should it be treated? EuroIntervention 2018; 14 Suppl: AB101–AB111. PMID: 30158090
- 385.
Kwon DA, Park JS, Chang HJ, et al. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol 2006; 98: 659–661. PMID: 16923456
- 386.
Asmarats L, Dagenais F, Bédard E, et al. Transcatheter tricuspid valve replacement for treating severe tricuspid regurgitation: Initial experience with the NaviGate bioprosthesis. Can J Cardiol 2018; 34: 1370.e5–1370.e7. PMID: 30269838
- 387.
Calafiore AM, Iacò AL, Romeo A, et al. Echocardiographic-based treatment of functional tricuspid regurgitation. J Thorac Cardiovasc Surg 2011; 142: 308–313. PMID: 21163499
- 388.
Tager R, Skudicky D, Mueller U, et al. Long-term follow-up of rheumatic patients undergoing left-sided valve replacement with tricuspid annuloplasty: Validity of preoperative echocardiographic criteria in the decision to perform tricuspid annuloplasty. Am J Cardiol 1998; 81: 1013–1016. PMID: 9576162
- 389.
Goldstone AB, Howard JL, Cohen JE, et al. Natural history of coexistent tricuspid regurgitation in patients with degenerative mitral valve disease: Implications for future guidelines. J Thorac Cardiovasc Surg 2014; 148: 2802–2810. PMID: 25218532
- 390.
Pagnesi M, Montalto C, Mangieri A, et al. Tricuspid annuloplasty versus a conservative approach in patients with functional tricuspid regurgitation undergoing left-sided heart valve surgery: A study-level meta-analysis. Int J Cardiol 2017; 240: 138–144. PMID: 28499671
- 391.
Sordelli C, Lancellotti P, Carlomagno G, et al. Tricuspid Annular Size and Regurgitation Progression After Surgical Repair for Degenerative Mitral Regurgitation. Am J Cardiol 2016; 118: 424–431. PMID: 27287061
- 392.
Dreyfus J, Durand-Viel G, Raffoul R, et al. Comparison of 2-dimensional, 3-dimensional, and surgical measurements of the tricuspid annulus size: Clinical implications. Circ Cardiovasc Imaging 2015; 8: e003241. PMID: 26156015
- 393.
Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007; 28: 230–268. PMID: 17259184
- 394.
Shi KH, Xuan HY, Zhang F, et al. Evolution of tricuspid regurgitation after mitral valve surgery for patients with moderate-or-less functional tricuspid regurgitation. Heart Surg Forum 2012; 15: E121–E126. PMID: 22698597
- 395.
Meng H, Pan SW, Hu SS, et al. Surgical treatment of secondary tricuspid regurgitation: Insight derived from annulus sizes and tethering distances. Ann Thorac Surg 2015; 100: 1238–1244. PMID: 26276055
- 396.
Van de Veire NR, Braun J, Delgado V, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011; 141: 1431–1439. PMID: 20832082
- 397.
David TE, David CM, Manlhiot C. Tricuspid annulus diameter does not predict the development of tricuspid regurgitation after mitral valve repair for mitral regurgitation due to degenerative diseases. J Thorac Cardiovasc Surg 2018; 155: 2429–2436. PMID: 29478744
- 398.
Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009; 22: 1–23. PMID: 19130998
- 399.
Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: Not a benign lesion. Eur Heart J 2005; 26: 433–439. PMID: 15640261
- 400.
Takao S, Miyatake K, Izumi S, et al. Clinical implications of pulmonary regurgitation in healthy individuals: Detection by cross sectional pulsed Doppler echocardiography. Br Heart J 1988; 59: 542–550. PMID: 3382565
- 401.
Valente AM, Cook S, Festa P, et al. Multimodality imaging guidelines for patients with repaired tetralogy of fallot: A report from the AmericanSsociety of Echocardiography: Developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J Am Soc Echocardiogr 2014; 27: 111–141. PMID: 24468055
- 402.
Puchalski MD, Askovich B, Sower CT, et al. Pulmonary regurgitation: Determining severity by echocardiography and magnetic resonance imaging. Congenit Heart Dis 2008; 3: 168–175. PMID: 18557879
- 403.
Silversides CK, Veldtman GR, Crossin J, et al. Pressure half-time predicts hemodynamically significant pulmonary regurgitation in adult patients with repaired tetralogy of fallot. J Am Soc Echocardiogr 2003; 16: 1057–1062. PMID: 14566299
- 404.
Mercer-Rosa L, Yang W, Kutty S, et al. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: A comparative analysis of echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging 2012; 5: 637–643. PMID: 22869820
- 405.
Davlouros PA, Kilner PJ, Hornung TS, et al. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: Detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol 2002; 40: 2044–2052. PMID: 12475468
- 406.
van Straten A, Vliegen HW, Hazekamp MG, et al. Right ventricular function late after total repair of tetralogy of Fallot. Eur Radiol 2005; 15: 702–707. PMID: 15726380
- 407.
Oosterhof T, Mulder BJ, Vliegen HW, et al. Cardiovascular magnetic resonance in the follow-up of patients with corrected tetralogy of Fallot: A review. Am Heart J 2006; 151: 265–272. PMID: 16442887
- 408.
Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019; 139: e698–e800. PMID: 30586767
- 409.
Ferraz Cavalcanti PE, Sá MP, Santos CA, et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot: Meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol 2013; 62: 2227–2243. PMID: 24080109
- 410.
Abbas JR, Hoschtitzky JA. Which is the best tissue valve used in the pulmonary position, late after previous repair of tetralogy of Fallot? Interact Cardiovasc Thorac Surg 2013; 17: 854–860. PMID: 23929900
- 411.
van der Hulst AE, Roest AA, Holman ER, et al. Real-time three-dimensional echocardiography: Segmental analysis of the right ventricle in patients with repaired tetralogy of fallot. J Am Soc Echocardiogr 2011; 24: 1183–1190. PMID: 21906911
- 412.
Fukada J, Morishita K, Komatsu K, et al. Influence of pulmonic position on durability of bioprosthetic heart valves. Ann Thorac Surg 1997; 64: 1678–1680. PMID: 9436554
- 413.
Waterbolk TW, Hoendermis ES, den Hamer IJ, et al. Pulmonary valve replacement with a mechanical prosthesis: Promising results of 28 procedures in patients with congenital heart disease. Eur J Cardiothorac Surg 2006; 30: 28–32. PMID: 16730181
- 414.
Kido T, Ueno T, Taira M, et al. Stroke volume ratio predicts redilatation of the right ventricle after pulmonary valve replacement. Ann Thorac Surg 2017; 104: 698–703. PMID: 28347541
- 415.
Warner KG, O’Brien PK, Rhodes J, et al. Expanding the indications for pulmonary valve replacement after repair of tetralogy of fallot. Ann Thorac Surg 2003; 76: 1066–1071. PMID: 14529986
- 416.
Dave HH, Buechel ER, Dodge-Khatami A, et al. Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg 2005; 80: 1615–1621. PMID: 16242426
- 417.
van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J Am Coll Cardiol 2011; 58: 2241–2247. PMID: 22078432
- 418.
Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010): Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2915–2957. PMID: 20801927
- 419.
Pepine CJ, Gessner IH, Feldman RL. Percutaneous balloon valvuloplasty for pulmonic valve stenosis in the adult. Am J Cardiol 1982; 50: 1442–1445. PMID: 6216808
- 420.
Stanger P, Cassidy SC, Girod DA, et al. Balloon pulmonary valvuloplasty: Results of the valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol 1990; 65: 775–783. PMID: 2316460
- 421.
Sievert H, Kober G, Bussman WD, et al. Long-term results of percutaneous pulmonary valvuloplasty in adults. Eur Heart J 1989; 10: 712–717. PMID: 2792113
- 422.
Kaul UA, Singh B, Tyagi S, et al. Long-term results after balloon pulmonary valvuloplasty in adults. Am Heart J 1993; 126: 1152–1155. PMID: 8237759
- 423.
Fawzy ME, Hassan W, Fadel BM, et al. Long-term results (up to 17 years) of pulmonary balloon valvuloplasty in adults and its effects on concomitant severe infundibular stenosis and tricuspid regurgitation. Am Heart J 2007; 153: 433–438. PMID: 17307424
- 424.
Chen PC, Sager MS, Zurakowski D, et al. Younger age and valve oversizing are predictors of structural valve deterioration after pulmonary valve replacement in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg 2012; 143: 352–360. PMID: 22153723
- 425.
Voet A, Rega F, de Bruaene AV, et al. Long-term outcome after treatment of isolated pulmonary valve stenosis. Int J Cardiol 2012; 156: 11–15. PMID: 21078529
- 426.
Unger P, Rosenhek R, Dedobbeleer C, et al. Management of multiple valve disease. Heart 2011; 97: 272–277. PMID: 21156677
- 427.
Unger P, Clavel MA, Lindman BR, et al. Pathophysiology and management of multivalvular disease. Nat Rev Cardiol 2016; 13: 429–440. PMID: 27121305
- 428.
Bissessor N, Shanahan L, Wee YS, et al. The role of natriuretic peptides in patients with chronic complex (mixed or multiple) heart valve disease. Congest Heart Fail 2010; 16: 50–54. PMID: 20412468
- 429.
Bissessor N, Stewart R, Wee YS, et al. Complex valve disease: Pre-surgical functional capacity evaluation using peak oxygen consumption. J Heart Valve Dis 2009; 18: 554–561. PMID: 20099697
- 430.
Zilberszac R, Gabriel H, Schemper M, et al. Outcome of combined stenotic and regurgitant aortic valve disease. J Am Coll Cardiol 2013; 61: 1489–1495. PMID: 23500223
- 431.
Rashedi N, Popović ZB, Stewart WJ, et al. Outcomes of asymptomatic adults with combined aortic stenosis and regurgitation. J Am Soc Echocardiogr 2014; 27: 829–837. PMID: 24874975
- 432.
Egbe AC, Luis SA, Padang R, et al. Outcomes in moderate mixed aortic valve disease: Is it time for a paradigm shift? J Am Coll Cardiol 2016; 67: 2321–2329. PMID: 27199054
- 433.
Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566. PMID: 2139921
- 434.
Haider AW, Larson MG, Benjamin EJ, et al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32: 1454–1459. PMID: 9809962
- 435.
Honda S, Kitai T, Okada Y, et al. Impact of aortic regurgitation on the prognosis of severe aortic stenosis. Heart 2012; 98: 1591–1594. PMID: 22888162
- 436.
Sannino A, Grayburn PA. Mitral regurgitation in patients with severe aortic stenosis: Diagnosis and management. Heart 2018; 104: 16–22. PMID: 28903993
- 437.
Schattenberg TT, Titus JL, Parkin TW. Clinical findings in acquired aortic valve stenosis: Effect of disease of other valves. Am Heart J 1967; 73: 322–325. PMID: 6019193
- 438.
Unger P, Plein D, Van Camp G, et al. Effects of valve replacement for aortic stenosis on mitral regurgitation. Am J Cardiol 2008; 102: 1378–1382. PMID: 18993159
- 439.
Ruel M, Kapila V, Price J, et al. Natural history and predictors of outcome in patients with concomitant functional mitral regurgitation at the time of aortic valve replacement. Circulation 2006; 114 Suppl: I541–I546. PMID: 16820634
- 440.
Toggweiler S, Boone RH, Rodés-Cabau J, et al. Transcatheter aortic valve replacement: Outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol 2012; 59: 2068–2074. PMID: 22483326
- 441.
Cortés C, Amat-Santos IJ, Nombela-Franco L, et al. Mitral regurgitation after transcatheter aortic valve replacement: Prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv 2016; 9: 1603–1614. PMID: 27491611
- 442.
Sannino A, Potluri S, Pollock B, et al. Impact of mitral stenosis on survival in patients undergoing isolated transcatheter aortic valve implantation. Am J Cardiol 2019; 123: 1314–1320. PMID: 30704670
- 443.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2017; 135: e1159–e1195. PMID: 28298458
- 444.
Puskas J, Gerdisch M, Nichols D, et al; PROACT Investigators. Reduced anticoagulation after mechanical aortic valve replacement: Interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014; 147: 1202–1211. PMID: 24512654
- 445.
Puskas JD, Gerdisch M, Nichols D, et al; PROACT Investigators. Anticoagulation and antiplatelet strategies after on-X mechanical aortic valve replacement. J Am Coll Cardiol 2018; 71: 2717–2726. PMID: 29903344
- 446.
Suri RM, Zehr KJ, Sundt TM 3rd, et al. Left ventricular mass regression after porcine versus bovine aortic valve replacement: A randomized comparison. Ann Thorac Surg 2009; 88: 1232–1237. PMID: 19766812
- 447.
Hickey GL, Grant SW, Bridgewater B, et al. A comparison of outcomes between bovine pericardial and porcine valves in 38,040 patients in England and Wales over 10 years. Eur J Cardiothorac Surg 2015; 47: 1067–1074. PMID: 25189704
- 448.
Fouquet O, Flecher E, Nzomvuama A, et al. Haemodynamic performance of the small supra-annular Trifecta bioprosthesis: Results from a French multicentre study. Interact Cardiovasc Thorac Surg 2016; 22: 439–444. PMID: 27002012
- 449.
Cheung AW, Ye J, Dvir D, et al. Aortic Valve-in-valve in externally mounted bioprosthesis: A safe treatment option for bioprosthetic structural valve dysfunction. Innovations (Phila) 2018; 13: 171–176. PMID: 29912738
- 450.
Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg 2015; 149: 340–345. PMID: 25439467
- 451.
Puskas JD, Bavaria JE, Svensson LG, et al; COMMENCE Trial Investigators. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg 2017; 52: 432–439. PMID: 28605428
- 452.
Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J 2016; 37: 2658–2667. PMID: 26559386
- 453.
Zhao DF, Seco M, Wu JJ, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: A systematic review and meta-analysis. Ann Thorac Surg 2016; 102: 315–327. PMID: 26794881
- 454.
Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med 2017; 377: 1847–1857. PMID: 29117490
- 455.
Minakata K, Tanaka S, Tamura N, et al. Comparison of the long-term outcomes of mechanical and bioprosthetic aortic valves: A propensity score analysis. Circ J 2017; 81: 1198–1206. PMID: 28413185
- 456.
Schnittman SR, Adams DH, Itagaki S, et al. Bioprosthetic aortic valve replacement: Revisiting prosthesis choice in patients younger than 50 years old. J Thorac Cardiovasc Surg 2018; 155: 539–547.e9. PMID: 29110948
- 457.
Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017; 38: 2183–2191. PMID: 28444168
- 458.
Nakano K, Hirahara N, Motomura N, et al. Current status of cardiovascular surgery in Japan, 2013 and 2014: A report based on the Japan Cardiovascular Surgery Database. 4. Valvular heart surgery. Gen Thorac Cardiovasc Surg 2018; 66: 13–18. PMID: 29134537
- 459.
Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in collaboration with the Society of Cardiovascular Anesthesiologists: Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114: e84–e231. PMID: 16880336
- 460.
Williams ML, Bavaria JE, Acker MA, et al. Valve selection in end-stage renal disease: Should it always be biological? Ann Thorac Surg 2016; 102: 1531–1535. PMID: 27544289
- 461.
Phan K, Zhao DF, Zhou JJ, et al. Bioprosthetic versus mechanical prostheses for valve replacement in end-stage renal disease patients: Systematic review and meta-analysis. J Thorac Dis 2016; 8: 769–777. PMID: 27162649
- 462.
Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017; 38: 3382–3390. PMID: 29020344
- 463.
Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009; 22: 975–1014. PMID: 19733789
- 464.
Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 589–590. PMID: 27143783
- 465.
Brown ML, Park SJ, Sundt TM, et al. Early thrombosis risk in patients with biologic valves in the aortic position. J Thorac Cardiovasc Surg 2012; 144: 108–111. PMID: 21864857
- 466.
Mylotte D, Andalib A, Thériault-Lauzier P, et al. Transcatheter heart valve failure: A systematic review. Eur Heart J 2015; 36: 1306–1327. PMID: 25265974
- 467.
Pache G, Schoechlin S, Blanke P, et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J 2016; 37: 2263–2271. PMID: 26446193
- 468.
Turpie AG. Safer anticoagulant therapy after heart valve replacement. Recommendations for less intense regimens. Postgrad Med 1997; 101: 85–94. PMID: 9074552
- 469.
Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369: 1206–1214. PMID: 23991661
- 470.
Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA 2012; 308: 2118–2125. PMID: 23188028
- 471.
Heras M, Chesebro JH, Fuster V, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol 1995; 25: 1111–1119. PMID: 7897124
- 472.
Egbe AC, Pislaru SV, Pellikka PA, et al. Bioprosthetic valve thrombosis versus structural failure: Clinical and echocardiographic predictors. J Am Coll Cardiol 2015; 66: 2285–2294. PMID: 26610876
- 473.
Makkar RR, Fontana G, Søndergaard L. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2016; 374: 1591–1592. PMID: 27096589
- 474.
Hansson NC, Grove EL, Andersen HR, et al. Transcatheter aortic valve thrombosis: Incidence, predisposing factors, and clinical implications. J Am Coll Cardiol 2016; 68: 2059–2069. PMID: 27580689
- 475.
Acar J, Iung B, Boissel JP, et al. AREVA: Multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation 1996; 94: 2107–2112. PMID: 8901659
- 476.
Pernod G, Godiér A, Gozalo C, et al. French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb Res 2010; 126: e167–e174. PMID: 20630568
- 477.
Halvorsen S, Storey RF, Rocca B, et al. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: Expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2017; 38: 1455–1462. PMID: 27789570
- 478.
Wahl MJ. Dental surgery in anticoagulated patients. Arch Intern Med 1998; 158: 1610–1616. PMID: 9701094
- 479.
Devani P, Lavery KM, Howell CJ. Dental extractions in patients on warfarin: Is alteration of anticoagulant regime necessary? Br J Oral Maxillofac Surg 1998; 36: 107–111. PMID: 9643595
- 480.
Evans IL, Sayers MS, Gibbons AJ, et al. Can warfarin be continued during dental extraction? Results of a randomized controlled trial. Br J Oral Maxillofac Surg 2002; 40: 248–252. PMID: 12054719
- 481.
Loeliger EA, Broekmans AW. Optimal therapeutic anticoagulation. Haemostasis 1985; 15: 283–292. PMID: 4043830
- 482.
Keuleers S, Herijgers P, Herregods MC, et al. Comparison of thrombolysis versus surgery as a first line therapy for prosthetic heart valve thrombosis. Am J Cardiol 2011; 107: 275–279. PMID: 21211605
- 483.
Deviri E, Sareli P, Wisenbaugh T, et al. Obstruction of mechanical heart valve prostheses: Clinical aspects and surgical management. J Am Coll Cardiol 1991; 17: 646–650. PMID: 1993782
- 484.
Roudaut R, Lafitte S, Roudaut MF, et al. Management of prosthetic heart valve obstruction: Fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis 2009; 102: 269–277. PMID: 19427604
- 485.
Huang G, Schaff HV, Sundt TM, et al. Treatment of obstructive thrombosed prosthetic heart valve. J Am Coll Cardiol 2013; 62: 1731–1736. PMID: 23994405
- 486.
Karthikeyan G, Senguttuvan NB, Joseph J, et al. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: A systematic review and meta-analysis of observational studies. Eur Heart J 2013; 34: 1557–1566. PMID: 23329151
- 487.
Özkan M, Çakal B, Karakoyun S, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation 2013; 128: 532–540. PMID: 23812180
- 488.
Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: The Ultra-slow PROMETEE trial. Am Heart J 2015; 170: 409–418. PMID: 26299240
- 489.
Dvir D, Bourguignon T, Otto CM, et al. VIVID (Valve in Valve International Data) Investigators. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018; 137: 388–399. PMID: 29358344
- 490.
Hamamoto M, Kobayashi T, Ozawa M, et al. Pure cusp tear of trifecta bioprosthesis 2 years after aortic valve replacement. Ann Thorac Cardiovasc Surg 2017; 23: 157–160. PMID: 27980283
- 491.
Kiefer P, Gruenwald F, Kempfert J, et al. Crimping may affect the durability of transcatheter valves: An experimental analysis. Ann Thorac Surg 2011; 92: 155–160. PMID: 21718842
- 492.
Giblett JP, Rana BS, Shapiro LM, et al. Percutaneous management of paravalvular leaks. Nat Rev Cardiol 2019; 16: 275–285. PMID: 30659248
- 493.
Tanis W, Habets J, van den Brink RB, et al. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: A review of the literature. Eur Heart J Cardiovasc Imaging 2014; 15: 119–129. PMID: 23913330
- 494.
Beurskens NEG, Gorter TM, Pieper PG, et al. Diagnostic value of Doppler echocardiography for identifying hemodynamic significant pulmonary valve regurgitation in tetralogy of Fallot: Comparison with cardiac MRI. Int J Cardiovasc Imaging 2017; 33: 1723–1730. PMID: 28567705
- 495.
Shapira Y, Herz I, Sagie A. Fluoroscopy of prosthetic heart valves: Does it have a place in the echocardiography era? J Heart Valve Dis 2000; 9: 594–599. PMID: 10947055
- 496.
Cartlidge TRG, Doris MK, Sellers SL, et al. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol 2019; 73: 1107–1119. PMID: 30871693
- 497.
Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: A report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2019; 32: 431–475. PMID: 30797660
- 498.
Potter DD, Sundt TM 3rd, Zehr KJ, et al. Operative risk of reoperative aortic valve replacement. J Thorac Cardiovasc Surg 2005; 129: 94–103. PMID: 15632830
- 499.
Shaikhrezai K, Tasca G, Amrani M, et al. Third-time aortic valve replacement: Patient characteristics and operative outcome. Ann Thorac Surg 2010; 89: 479–483. PMID: 20103324
- 500.
Kaneko T, Vassileva CM, Englum B, et al. Contemporary outcomes of repeat aortic valve replacement: A benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg 2015; 100: 1298–1304. PMID: 26209480
- 501.
Dvir D, Webb JG, Bleiziffer S, et al; Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014; 312: 162–170. PMID: 25005653
- 502.
Sedeek AF, Greason KL, Sandhu GS, et al. Transcatheter valve-in-valve vs surgical replacement of failing stented aortic biological valves. Ann Thorac Surg 2019; 108: 424–430. PMID: 31055036
- 503.
Gozdek M, Raffa GM, Suwalski P, et al; SIRIO-TAVI group. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: Systematic review and meta-analysis. Eur J Cardiothorac Surg 2018; 53: 495–504. PMID: 29029105
- 504.
Pibarot P, Simonato M, Barbanti M, et al. Impact of pre-existing prosthesis-patient mismatch on survival following aortic valve-in-valve procedures. JACC Cardiovasc Interv 2018; 11: 133–141. PMID: 29348008
- 505.
Kamioka N, Babaliaros V, Morse MA, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in-valve therapy. JACC Cardiovasc Interv 2018; 11: 1131–1138. PMID: 29929633
- 506.
Bouhout I, Mazine A, Ghoneim A, et al. Long-term results after surgical treatment of paravalvular leak in the aortic and mitral position. J Thorac Cardiovasc Surg 2016; 151: 1260–1266.e1. PMID: 26774168
- 507.
Taramasso M, Maisano F, Denti P, et al. Surgical treatment of paravalvular leak: Long-term results in a single-center experience (up to 14 years). J Thorac Cardiovasc Surg 2015; 149: 1270–1275. PMID: 25648478
- 508.
Calvert PA, Northridge DB, Malik IS, et al. Percutaneous device closure of paravalvular leak: Combined experience from the United Kingdom and Ireland. Circulation 2016; 134: 934–944. PMID: 27587432
- 509.
Aoyagi S, Fukunaga S, Tayama E, et al. Benefits of a beta-blocker for intractable hemolysis due to paraprosthetic leakage. Asian Cardiovasc Thorac Ann 2007; 15: 441–443. PMID: 17911077
- 510.
Saiki Y, Kasegawa H, Kawase M, et al. Intraoperative TEE during mitral valve repair: Does it predict early and late postoperative mitral valve dysfunction? Ann Thorac Surg 1998; 66: 1277–1281. PMID: 9800820
- 511.
Sidebotham DA, Allen SJ, Gerber IL, et al. Intraoperative transesophageal echocardiography for surgical repair of mitral regurgitation. J Am Soc Echocardiogr 2014; 27: 345–366. PMID: 24534653
- 512.
Riegel AK, Busch R, Segal S, et al. Evaluation of transmitral pressure gradients in the intraoperative echocardiographic diagnosis of mitral stenosis after mitral valve repair. PLoS One 2011; 6: e26559. PMID: 22087230
- 513.
Crescenzi G, Landoni G, Zangrillo A, et al. Management and decision-making strategy for systolic anterior motion after mitral valve repair. J Thorac Cardiovasc Surg 2009; 137: 320–325. PMID: 19185145
- 514.
Dumont E, Gillinov AM, Blackstone EH, et al. Reoperation after mitral valve repair for degenerative disease. Ann Thorac Surg 2007; 84: 444–450. PMID: 17643613
- 515.
Gillinov AM, Cosgrove DM, Lytle BW, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 1997; 113: 467–475. PMID: 9081091
- 516.
Cerfolio RJ, Orzulak TA, Pluth JR, et al. Reoperation after valve repair for mitral regurgitation: Early and intermediate results. J Thorac Cardiovasc Surg 1996; 111: 1177–1184. PMID: 8642818
- 517.
Nishida H, Fukui T, Kasegawa H, et al. Causes of repair failure for degenerative mitral valve disease and reoperation outcomes. Eur J Cardiothorac Surg 2018; 53: 1244–1250. PMID: 29309559
- 518.
le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: Predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009; 2: 931–939. PMID: 19679280
- 519.
Kunihara T, Aicher D, Rodionycheva S, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg 2012; 143: 1389–1395. PMID: 21855091
- 520.
Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010; 38: 400–406. PMID: 20219388
- 521.
de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011; 142: 1430–1438. PMID: 21955470
- 522.
de Meester C, Pasquet A, Gerber BL, et al. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: A propensity score analysis. J Thorac Cardiovasc Surg 2014; 148: 1913–1920. PMID: 24656668
- 523.
Sharma V, Suri RM, Dearani JA, et al. Expanding relevance of aortic valve repair: Is earlier operation indicated? J Thorac Cardiovasc Surg 2014; 147: 100–107. PMID: 24084289
- 524.
JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version. Circ J 2014; 78: 1997–2021. PMID: 24965079
- 525.
Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17: 1467–1507. PMID: 26324838
- 526.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2893–2962. PMID: 27567408
- 527.
Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. PMID: 21870978
- 528.
Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. PMID: 19717844
- 529.
Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. PMID: 24251359
- 530.
Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. PMID: 21830957
- 531.
De Caterina R, Renda G, Carnicelli AP, et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 2017; 69: 1372–1382. PMID: 28302288
- 532.
Carnicelli AP, De Caterina R, Halperin JL, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation 2017; 135: 1273–1275. PMID: 28209729
- 533.
Guimarães PO, Pokorney SD, Lopes RD, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clin Cardiol 2019; 42: 568–571. PMID: 30907005
- 534.
Yadlapati A, Groh C, Malaisrie SC, et al. Efficacy and safety of novel oral anticoagulants in patients with bioprosthetic valves. Clin Res Cardiol 2016; 105: 268–272. PMID: 26384981
- 535.
Izumi C, Miyake M, Amano M, et al. Registry of antithrombotic therapy in atrial fibrillation patients with bioprosthetic valves: A retrospective observational study.
J Cardiol March 07, 2020. DOI: https://doi.org/10.1016/j.jjcc.2020.02.006 PMID: 32156512
- 536.
Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61: 755–759. PMID: 8572814
- 537.
Jose J, Sulimov DS, El-Mawardy M, et al. Clinical bioprosthetic heart valve thrombosis after transcatheter aortic valve replacement: Incidence, characteristics, and treatment outcomes. JACC Cardiovasc Interv 2017; 10: 686–697. PMID: 28385406
- 538.
Gillinov AM, Gelijns AC, Parides MK, et al; CTSN Investigators. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015; 372: 1399–1409. PMID: 25853744
- 539.
Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: A randomized controlled trial. JAMA 2005; 294: 2323–2329. PMID: 16278360
- 540.
Blomström-Lundqvist C, Johansson B, Berglin E, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: The SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J 2007; 28: 2902–2908. PMID: 17984136
- 541.
Jessurun ER, van Hemel NM, Defauw JJ, et al. A randomized study of combining maze surgery for atrial fibrillation with mitral valve surgery. J Cardiovasc Surg (Torino) 2003; 44: 9–18. PMID: 12627066
- 542.
Saint LL, Damiano RJ, Cuculich PS, et al. Incremental risk of the Cox-maze IV procedure for patients with atrial fibrillation undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013; 146: 1072–1077. PMID: 23998785
- 543.
Chevalier P, Leizorovicz A, Maureira P, et al. Left atrial radiofrequency ablation during mitral valve surgery: A prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis 2009; 102: 769–775. PMID: 19944393
- 544.
Abreu Filho CA, Lisboa LA, Dallan LA, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation 2005; 112 Suppl: I20–I25. PMID: 16159816
- 545.
Ad N, Holmes SD, Shuman DJ, et al. Impact of atrial fibrillation duration on the success of first-time concomitant cox maze procedures. Ann Thorac Surg 2015; 100: 1613–1619. PMID: 26212511
- 546.
Ad N, Holmes SD. Prediction of sinus rhythm in patients undergoing concomitant cox maze procedure through a median sternotomy. J Thorac Cardiovasc Surg 2014; 148: 881–887. PMID: 25043863
- 547.
Iribarne A, DiScipio AW, McCullough JN, et al. Northern New England Cardiovascular Disease Study Group. Surgical atrial fibrillation ablation improves long-term survival: A multicenter analysis. Ann Thorac Surg 2019; 107: 135–142. PMID: 30300644
- 548.
Lee R, McCarthy PM, Wang EC, et al. Midterm survival in patients treated for atrial fibrillation: A propensity-matched comparison to patients without a history of atrial fibrillation. J Thorac Cardiovasc Surg 2012; 143: 1341–1351. PMID: 22465031
- 549.
Attaran S, Saleh HZ, Shaw M, et al. Does the outcome improve after radiofrequency ablation for atrial fibrillation in patients undergoing cardiac surgery? A propensity-matched comparison. Eur J Cardiothorac Surg 2012; 41: 806–811. PMID: 22219413
- 550.
Louagie Y, Buche M, Eucher P, et al. Improved patient survival with concomitant Cox Maze III procedure compared with heart surgery alone. Ann Thorac Surg 2009; 87: 440–446. PMID: 19161756
- 551.
McCarthy PM, Manjunath A, Kruse J, et al. Should paroxysmal atrial fibrillation be treated during cardiac surgery? J Thorac Cardiovasc Surg 2013; 146: 810–823. PMID: 23890981
- 552.
Yoo JS, Kim JB, Ro SK, et al. Impact of concomitant surgical atrial fibrillation ablation in patients undergoing aortic valve replacement. Circ J 2014; 78: 1364–1371. PMID: 24670879
- 553.
Malaisrie SC, Lee R, Kruse J, et al. Atrial fibrillation ablation in patients undergoing aortic valve replacement. J Heart Valve Dis 2012; 21: 350–357. PMID: 22808837
- 554.
Kainuma S, Mitsuno M, Toda K, et al; Osaka Cardiovascular Surgery Research (OSCAR) Group. Dilated left atrium as a predictor of late outcome after pulmonary vein isolation concomitant with aortic valve replacement and/or coronary artery bypass grafting. Eur J Cardiothorac Surg 2015; 48: 765–777. PMID: 25612746
- 555.
Geidel S, Ostermeyer J, Lass M, et al. Permanent atrial fibrillation ablation surgery in CABG and aortic valve patients is at least as effective as in mitral valve disease. Thorac Cardiovasc Surg 2006; 54: 91–95. PMID: 16541348
- 556.
Reddy VY, Doshi SK, Kar S, et al; PREVAIL and PROTECT AF Investigators. 5-Year outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017; 70: 2964–2975. PMID: 29103847
- 557.
Lee CH, Kim JB, Jung SH, et al. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg 2014; 97: 124–132. PMID: 24075500
- 558.
García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: A transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42: 1253–1258. PMID: 14522491
- 559.
Melduni RM, Schaff HV, Lee HC, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: A propensity score-matched analysis of 10 633 patients. Circulation 2017; 135: 366–378. PMID: 27903589
- 560.
Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383–2431. PMID: 25086026
- 561.
Calleja AM, Dommaraju S, Gaddam R, et al. Cardiac risk in patients aged >75 years with asymptomatic, severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol 2010; 105: 1159–1163. PMID: 20381670
- 562.
Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: A reappraisal in contemporary practice. Eur Heart J 2014; 35: 2372–2381. PMID: 24553722