Article ID: CJ-20-0345
Article ID: CJ-20-0345
Background: The dysfunction of vascular smooth muscle cells (VSMCs) contributes to the development of atherosclerosis. This study aimed to investigate the role of circular RNA-0010283 (circ_0010283) in oxidized low-density lipoprotein (ox-LDL)-treated VSMCs and the associated action mechanism.
Methods and Results: The expression of circ_0010283 was investigated using quantitative real-time polymerase chain reaction (qRT-PCR). Cell proliferation was monitored by using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cell apoptosis was detected by using flow cytometry assay. A transwell assay was performed to observe migration and invasion, and a scratch assay was implemented to test migration. The expression of proliferation, apoptosis and migration/invasion-related proteins was measured by using a western blot. The targeted relationship was predicted by using a bioinformatics tool (Starbase) and verified by using a dual-luciferase reporter assay, a RNA immunoprecipitation (RIP) assay and a RNA pull-down assay. circ_0010283 was highly expressed in serum samples from atherosclerosis patients and ox-LDL-treated human VSMCs (HVSMCs). circ_0010283 knockdown suppressed ox-LDL-induced proliferation, migration and invasion in HVSMCs. MicroRNA-133a-3p (miR-133a-3p) was confirmed as a target of circ_0010283, and miR-133a-3p deficiency reversed the effects of circ_0010283 knockdown. Moreover, pregnancy-associated plasma protein A (PAPPA) was targeted by miR-133a-3p, and PAPPA overexpression reversed the effects of miR-133a-3p restoration. Interestingly, circ_0010283 could regulate PAPPA expression by mediating miR-133a-3p.
Conclusions: circ_0010283 participated in ox-LDL-induced dysfunctions of HVSMCs by modulating the miR-133a-3p/PAPPA pathway, suggesting that circ_0010283 might be associated with atherosclerosis pathogenesis.
Atherosclerosis is a chronic vascular inflammatory disease that causes the development of lesions on the arterial wall, characterized by lipid deposition and plaque fibrosis in the arterial wall.1,2 Vascular smooth muscle cells (VSMCs) are a significant component of the vascular wall, with their potential for self-renewal and differentiation.3,4 Mechanically, the controlled proliferation of VSMCs is beneficial in atherosclerosis, whereas dysregulated VSMC proliferation contributes to plaque formation and inflammatory responses during the progression of atherosclerosis.3,5,6 Exploring the causes of dysfunctions of VSMCs is a reliable strategy to understand the pathogenesis of atherosclerosis.
Oxidized low-density lipoprotein (ox-LDL) can promote the growth, migration and differentiation of VSMCs, leading to VSMC dysfunction, and is considered a crucial element in atherosclerosis pathogenesis.7 However, the mechanisms about ox-LDL-induced VSMC dysfunction are complex and have not been fully elucidated. Circular RNA (circRNA) is a type of non-coding RNA, well-known by its closed-loop structure,8 and its role in cardiovascular diseases, including atherosclerosis, has been widely reported.9 In addition, several studies asserted that non-coding RNAs were involved in ox-LDL-induced VSMC dysfunction,10–12 hinting a novel approach to the involvement of non-coding RNAs in atherosclerosis pathogenesis. Nevertheless, similar studies of circRNAs in ox-LDL-induced VSMC dysfunction are limited. circ_0010283 was previously documented to be differently expressed in ox-LDL VSMCs compared with controls.13 Given that the function of circ_0010283 has not been investigated for any diseases, we speculate that circ_0010283 plays a substantial role in ox-LDL VSMCs. MicroRNA-133a-3p (miR-133a-3p) was reported to be implicated in numerous cardiovascular diseases,14 including atherosclerosis.15 It is concluded that miR-133a-3p plays an indispensable role in the development of atherosclerosis. However, knowledge about its function in VSMCs remains limited, and the associated mechanism is also poorly understood. Pregnancy-associated plasma protein A (PAPPA) has served as an indicator of cardiovascular events, and PAPPA promoted the expression of inflammatory markers, thus triggering atherosclerosis.16,17 In addition, PAPPA also participated in the regulation of proliferation and apoptosis of ox-LDL-treated VSMCs,10 whereas the mechanism of PAPPA action in VSMCs is not been completely understood.
In this study, we investigated the expression of circ_0010283 in serum from atherosclerosis patients and ox-LDL-treated human VSMCs (HVSMCs), and explored the functions of circ_0010283 in ox-LDL-treated HVSMCs. The mechanism of circ_0010283 in ox-LDL-induced HVSMC dysfunction associated with miR-133a-3p and PAPPA was clarified. Our study provided a molecular basis for the involvement of circ_0010283 in atherosclerosis pathogenesis.
The present study was approved by the ethical review committee of Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology (with the approval No. 201808025).
Blood samples were collected from atherosclerosis patients (n=36) and paired normal volunteers (n=36) recruited from Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology. The sampling was conducted after obtaining informed consent from each subject. Blood samples were placed at room temperature for over 1 h followed by centrifugation to isolate serum samples. Serum samples were placed in liquid nitrogen and stored at −80℃. The procedures followed were in accordance with the Declaration of Helsinki.
Cells and TreatmentHuman VSMCs purchased from Bena Culture Collection (BNCC; Beijing, China) were cultured in 90% Dulbecco’s modified Eagle’s medium (DMEM; BNCC) supplemented with 10% fetal bovine serum (FBS). HVSMCs were administered with ox-LDL (Solarbio, Beijing, China) at a concentration of 100μg/mL, and untreated HVSMCs were used as the controls. HVSMCs in culture medium were maintained in a 37℃ incubator containing 5% CO2.
TransfectionSmall interference RNA-targeting circ_0010283 (si-circ_0010283: 5’-GGUUGUGAGUUCUUUAGAAGC-3’) for circ_0010283 knockdown and its corresponding control (si-NC: 5’-GAGCCCCAGCCTTCTCCATG-3’) were assembled by Ribobio Company (Guangzhou, China). MicroRNA (miRNA) mimics of miR-133a-3p for miR-133a-3p enrichment (miR-133a-3p) or miRNA inhibitors of miR-133a-3p for miR-133a-3p inhibition (anti-miR-133a-3p) and their corresponding control (miR-NC or anti-miR-NC) were purchased from Ribobio Company. circ_0010283 overexpression (circ_0010283) or PAPPA overexpression (PAPPA) were assembled by using a pCD-ciR vector or pcDNA vector, which was constructed by Genepharma Company (Shanghai, China), and an empty vector (pCD-ciR or pcDNA) was used as the corresponding control. All transfection was carried out into ox-LDL-treated HVSMCs using a Lipofectamine 3000 kit (Invitrogen, Carlsbad, CA, USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)For expression detection, RNA was extracted using a Total RNA Extraction Kit (Solarbio) according to the manufacturer’s instructions. Complementary DNA (cDNA) for circ_0010283 and PAPPA was synthesized using a Universal RT-PCR Kit (Solarbio), and cDNA for miR-133a-3p was synthesized using a miRNA cDNA Synthesis Kit (HaiGene, Haerbin, China). Afterwards, SYBR Green Realtime PCR Master Mix (Solarbio) was used for quantitative polymerase chain reaction (qPCR) analysis on a PCR system (Bio-Rad, Hercules, CA, USA). Glyceraldehyde-phosphate dehydrogenase (GAPDH) or U6 was used as the housekeeping gene. The primer sequences were listed as follows: circ_0010283, F: 5’-GGGATATTCCTCCGGGCAAG-3’ and R: 5’-TGACTGCATTTCAGGCTGATG-3’; UBR4, F: 5’-GCAGGAACCCTCTCTGACAC-3’ and R: 5’-AGGTCCGAAGGGTAGAACCA-3’; GAPDH, F: 5’-GAGTCAACGGATTTGGTCGT-3’ and R: 5’-TTGATTTTGGAGGGATCTCG-3’; miR-133a-3p, F: 5’-GGGAGCCAAATGCTTTGCTAG-3’ and R: 5’-CCCTCGGTTTACGAAACGATC-3’; U6, F: 5’-ATTGGAACGATACAGAGAAGATT-3’ and R: 5’-GGAACGCTTCACGAATTTG-3’; PAPPA, 5’-ATATCTCACGTGACCGAGGA-3’ and R: 5’-AGCTGATGGTGCTGGAAGTC-3’. Relative expression was calculated using the 2−∆∆ct method.
RNase R and Actinomycin D TreatmentRNA samples were treated with or without RNase R (3U/μg; Epicentre Technologies, Madison, WI, USA) for 30 min at 37℃, and then circ_0010283 expression and UBR4 (linear molecule) mRNA expression were measured by using qRT-PCR.
HVSMCs were exposed to actinomycin D (2 mg/L; Sigma-Aldrich, St. Louis, MO, USA) and continued to be incubated at 37℃ for different time (4, 8, 12 and 24 h). Cells were harvested at the indicated time points (4, 8, 12 and 24 h) for RNA isolation. Afterwards, the expression of circ_00010283 and UBR4 mRNA was measured by using qRT-PCR.
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) AssayFor proliferation analysis, 100 μL cell suspension was added into 96-well plates and cultured in a 37℃ incubator containing 5% CO2 for 24, 48, 72, 96 and 120 h. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (10 μL; Solarbio) was then pipetted into each well followed by culture for another 4 h. 100 µL formazan-solving liquid was used to dissolve formazan. The absorbance at 490 nm was examined using a microplate reader (Bio-Rad).
Flow Cytometry AssayFor apoptosis analysis, a Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Solarbio) was utilized following the manufacturer’s instructions. Briefly, transfected cells were exposed to serum-deprived culture medium for 6 h, and cells (1×106) were then collected, treated with Trypsin and washed with cold phosphate-buffered saline. Binding buffer (1 mL) was used to resuspend cells. Next, 100μL cell suspension was transferred into a new tube followed by the addition of 5μL Annexin V-FITC, and this was cultured for 10 min in the dark. Then, propidium iodide (PI) was added into the tube before being cultured for another 5 min in the dark. The apoptotic cells were monitored on a Flow cytometer (Beckman; Miami, FL, USA).
Transwell AssayTranswell migration and invasion assays were performed using transwell chambers (Corning Company, Corning, NY, USA). Partial chambers were coated with Matrigel at 4℃ overnight. The HVSMCs in fresh culture medium were transferred into the top of chambers with or without Matrigel for invasion or migration analysis. The bottom of the chambers was supplemented with fresh culture medium. After 24 h, cells that migrated or invaded into the lower surface were fixed with formaldehyde and stained with 0.1% crystal violet. Cells were observed using a microscope (Olympus, Tokyo, Japan).
Scratch AssayThe HVSMCs were plated to 90% confluence on 6-well plates. A scratch was generated using pipette tips. Migration status of cell scratch region was checked under an inverted microscope (Olympus) after scratching for 24 h and photographed.
Western BlotA radioimmunoprecipitation assay (RIPA) kit (Solarbio) was used to extract total proteins. Then, proteins were separated, electro-transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad) and blocked with skim milk. Subsequently, membranes were incubated with the corresponding primary antibodies (Abcam, Cambridge, MA, USA), including an anti-proliferating cell nuclear antigen (anti-PCNA; ab92552), anti-Ki-67 (ab92742), anti-B-cell lymphoma-2 (anti-Bcl-2; ab59348), anti-cleaved caspase 3 (anti-c-caspase 3; ab32042), anti-caspase 3 (ab13847), anti-metalloproteinase-2 (anti-MMP2; ab97779), anti-metalloproteinase-9 (MMP9; ab76003), anti-PAPPA (ab174314) and anti-GAPDH (ab9485), at 4℃overnight. Next, the secondary antibody (goat anti-rabbit; ab205718) was added for incubation at room temperature for 1.5 h. Finally, an enhanced chemiluminescence (ECL) reagent (Solarbio) was used to visualize the protein blots.
Bioinformatics AnalysisFor the prediction of assumed targets, the Starbase website (http://starbase.sysu.edu.cn/) was utilized to analyze the targets of circ_0010283 and miR-133a-3p.
Dual-Luciferase Reporter AssayA dual-luciferase reporter assay was conducted to verify the interaction between miR-133a-3p and circ_0010283 or PAPPA. At first, target wildtype sequence and mutation sequence of circ_0010283 and PAPPA were created according to the binding sites between miR-133a-3p and circ_0010283 or PAPPA 3’ untranslated region (3’UTR). Then, these sequences were amplified and separately cloned into a pmirGLO reporter plasmid (Promega, Madison, WI, USA) to generate fusion plasmids, named as wt-circ_0010283, mut-circ_0010283, wt-PAPPA 3’UTR and mut-PAPPA 3’UTR. Afterwards, wt-circ_0010283, mut-circ_0010283, wt-PAPPA 3’UTR or mut-PAPPA 3’UTR and miR-133a-3p or miR-NC were cotransfected into HVSMCs. After a 48-h transfection, luciferase activity in cells was determined using a dual-luciferase reporter system (Promega).
RNA Binding Protein Immunoprecipitation (RIP) AssayThe RIP assay was performed to confirm the interaction between miR-133a-3p and circ_0010283 or PAPPA using a Magna RIP Kit (Millipore, Bedford, MA, USA) according to the manufacturer’s instructions. Antibodies against Argonaute 2 (Ago2) or Immunoglobulin G (IgG; control) were purchased from Abcam. The immunoprecipitated RNA was extracted and used for qRT-PCR analysis.
RNA Pull-Down AssayThe RIP assay was performed to confirm the interaction between miR-133a-3p and circ_0010283 or PAPPA using the Magnetic RNA-Protein Pull-Down Kit (Pierce; Rockford, IL, USA). In brief, biotin-labeled circ_0010283, PAPPA and negative controls (Bio-circ_0010283, Bio-PAPPA and Bio-NC) were obtained from RiboBio Company and introduced into HVSMCs. At 48 h post-transfection, cells were collected and subjected to lysis buffer. Cell lysate was incubated with streptavidin magnetic beads at 4℃ overnight. The bound RNA was purified and subjected to qRT-PCR to detect the expression of miR-133a-3p.
Statistical AnalysisAll experiments contained at least 3 repetitions. Statistical analyses were implemented using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Data were presented as the mean±standard deviation. All data met normality conditions, which was examined using the Shapiro-Wilk test. The significance of differences between the 2 groups was evaluated by using the Student’s t-test (unpaired), and the significance of differences among multiple groups was evaluated by using analyses of variance (1-way ANOVA and 2-way ANOVA), followed by Tukey’s post-hoc test. Pearson product-moment correlation coefficient was used to assess expression correlation. A P value <0.05 was considered to be statistically significant.
To test whether circ_0010283 was dysregulated in atherosclerosis, the expression of circ_0010283 was checked in clinical samples and cell models. As shown in Figure 1A, the expression of circ_0010283 was relatively higher in serum samples of atherosclerosis patients (n=36) than that in serum samples of normal volunteers (n=36). The expression of circ_0010283 was also elevated in HVSMCs treated with ox-LDL in a dose-dependent manner (Figure 1B). We next used RNase R and Actinomycin D to detect the stability of circ_0010283. The data showed that the addition of RNase R significantly diminished the expression level of linear UBR4 mRNA, but hardly affected the expression of circ_0010283 (Figure 1C). Besides, Actinomycin D treatment led to a notable decrease of UBR4 mRNA expression compared with circ_0010283 (Figure 1D). These data suggested that the expression of circ_0010283 was aberrantly enhanced in atherosclerosis, and the stability of circ_0010283 was higher than UBR4.
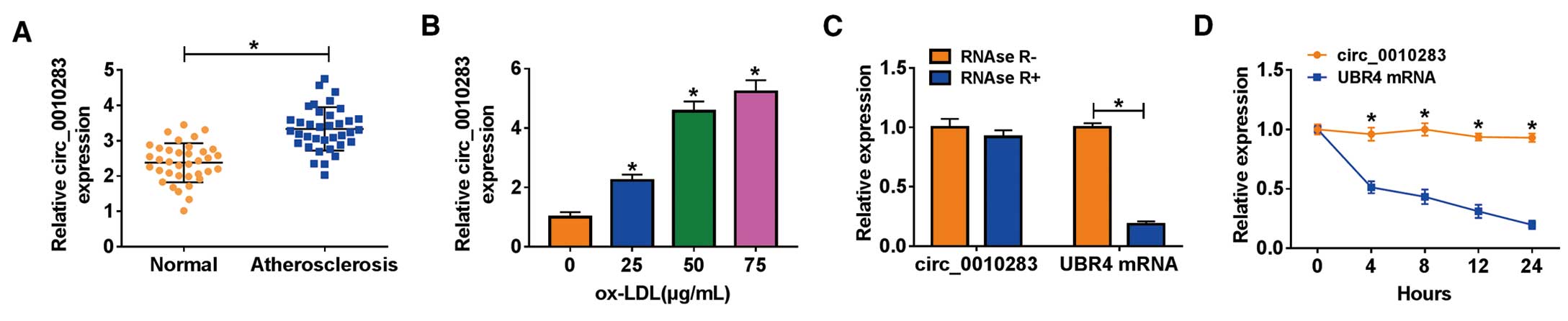
circ_0010283 was upregulated in atherosclerosis serum samples and ox-LDL-treated HVSMCs. (A) The expression of circ_0010283 in clinical samples (n=36) was detected by using qRT-PCR. (B) The expression of circ_0010283 in ox-LDL-treated HVSMCs was detected by using qRT-PCR. (C,D) RNase R and Actinomycin D were used to test the stability of circ_0010283 according to its expression. *P<0.05. circ_0010283, circular RNA_0010283; HVSMCs, human vascular smooth muscle cells; ox-LDL, oxidized low-density lipoprotein; qRT-PCR, quantitative real-time polymerase chain reaction.
HVSMCs treated with ox-LDL were transfected with si-circ_0010283 to explore the potential role of circ_0010283. The expression of circ_0010283 was increased in ox-LDL-treated HVSMCs, but significantly declined in ox-LDL-treated HVSMCs transfected with si-circ_0010283 (Figure 2A). In terms of function, ox-LDL promoted HVSMC proliferation, whereas si-circ_0010283 transfection weakened proliferation capacity (Figure 2B). In addition, the expression of proliferation-related proteins confirmed this result, which found that the expression of PCNA and Ki-67 was elevated in ox-LDL-treated HVSMCs but reduced in ox-LDL-treated HVSMCs transfected with si-circ_0010283 (Figure 2C). ox-LDL treatment diminished the number of apoptotic cells, whereas the transfection of si-circ_0010283 partly increased the number of apoptotic cells (Figure 2D). Likewise, this result was verified by the expression of apoptosis-related proteins. The data showed that the expression of Bcl-2 was strengthened in ox-LDL-treated HVSMCs but lessened in ox-LDL-treated HVSMCs transfected with si-circ_0010283, whereas the expression of c-caspase 3 was the opposite to Bcl-2 expression (Figure 2E). Next, transwell migration and an invasion assay showed that HVSMC migration and invasion were induced by ox-LDL treatment, but partially impaired by the transfection of si-circ_0010283 (Figure 2F–H). A scratch assay was also carried out to determine the effect of circ_0010283 silence on migration, and showed that the migration ratio was promoted in cells treated with ox-LDL but repressed in cells treated with ox-LDL as well as being transfected with si-circ_0010283 (Figure 2I). These results were validated by the expression of MMP2 and MMP9, migration and invasion-related proteins. We discovered that the expression of MMP2 and MMP9 was reinforced in ox-LDL-treated HCSMCs, but notably decreased in ox-LDL-treated HVSMCs also transfected with si-circ_0010283 (Figure 2J,K). As a result of validation, we constructed a circ_0010283 overexpression vector and examined the efficiency of circ_0010283 overexpression. The data showed that the expression of circ_0010283 was strikingly enhanced in ox-LDL-treated HCSMCs transfected with circ_0010283 relative to pCD-ciR (Supplementary Figure A). In terms of function, circ_0010283 overexpression strengthened the effects of ox-LDL, thus aggravating HVSMC proliferation, migration and invasion, but lessening the apoptotic rate (Supplementary Figure B–E). These data indicated that ox-LDL treatment promoted proliferation, migration and invasion but restrained apoptosis in HVSMCs, whereas these effects could be partly reversed by circ_0010283 knockdown but strengthened by circ_0010283 overexpression.
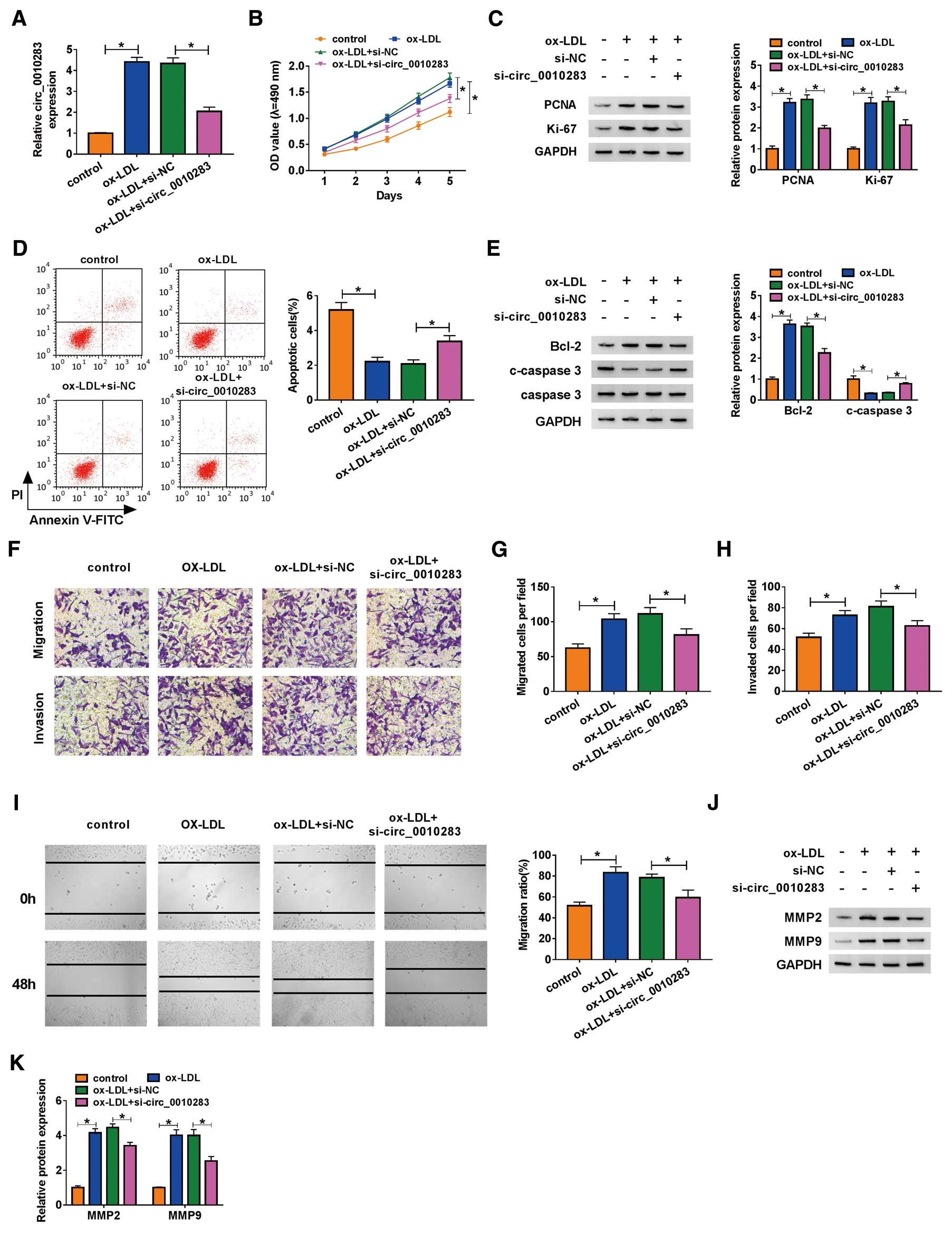
circ_0010283 knockdown inhibited ox-LDL-induced proliferation, migration and invasion of HVSMCs. (A) Silencing efficiency was tested by circ_0010283 expression using qRT-PCR. (B) Cell proliferation was assessed using a MTT assay. (C) The expression of proliferation-related proteins (PCNA and Ki-67) was measured by using a western blot. (D) Cell apoptosis was determined by using a flow cytometry assay. (E) The expression of apoptosis-related proteins (Bcl-2 and c-caspase 3) was measured by using a western blot. (F–H) Cell migration and invasion were monitored by using a transwell assay; and (I) cell migration was also monitored by using a scratch assay. (J,K) The expression of migration/invasion-related proteins (MMP2 and MMP9) was measured by using a western blot. *P<0.05. circ_0010283, circular RNA_0010283; HVSMCs, human vascular smooth muscle cells; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ox-LDL, oxidized low-density lipoprotein; qRT-PCR, quantitative real-time polymerase chain reaction.
Considering the potential mechanism of circ_0010283, we speculated that circ_0010283 might serve as molecular sponges to mediate the expression of miRNAs. Bioinformatics analysis (Starbase) predicted potential interaction between circ_0010283 and miR-133a-3p, and there were binding sites between their sequence fragments (Figure 3A). Further analyses found that miR-133a-3p reintroduction remarkably weakened the luciferase activity in HVSMCs transfected with wt-circ_0010283 (Figure 3B), and both circ_0010283 and miR-133a-3p were abundantly expressed in the Ago2 RIP reaction relative to the control (IgG RIP) (Figure 3C). Meanwhile, a RNA pull-down assay was conducted using biotin-coupled circ_0010283 (Bio-circ_0010283) and control (Bio-NC), and the data showed that Bio-circ_0010283 could capture more miR-133a-3p compared with Bio-NC (Figure 3D). All these analyses indicated that miR-133a-3p was actually a target of circ_0010283. In addition, we found that the expression of miR-133a-3p was decreased in serum samples from atherosclerosis patients relative to normal volunteers (Figure 3E), and miR-133a-3p expression was negatively correlated with circ_0010283 expression in atherosclerosis serum samples (Figure 3F). The expression of miR-133a-3p was also decreased in HVSMCs treated with ox-LDL (Figure 3G). Moreover, miR-133a-3p expression was enhanced in HVSMCs after circ_0010283 knockdown, but repressed in HVSMCs after circ_0010283 overexpression (Figure 3H). All data demonstrated that miR-133a-3p was a target of circ_0010283, and circ_0010283 negatively regulated miR-133a-3p expression.
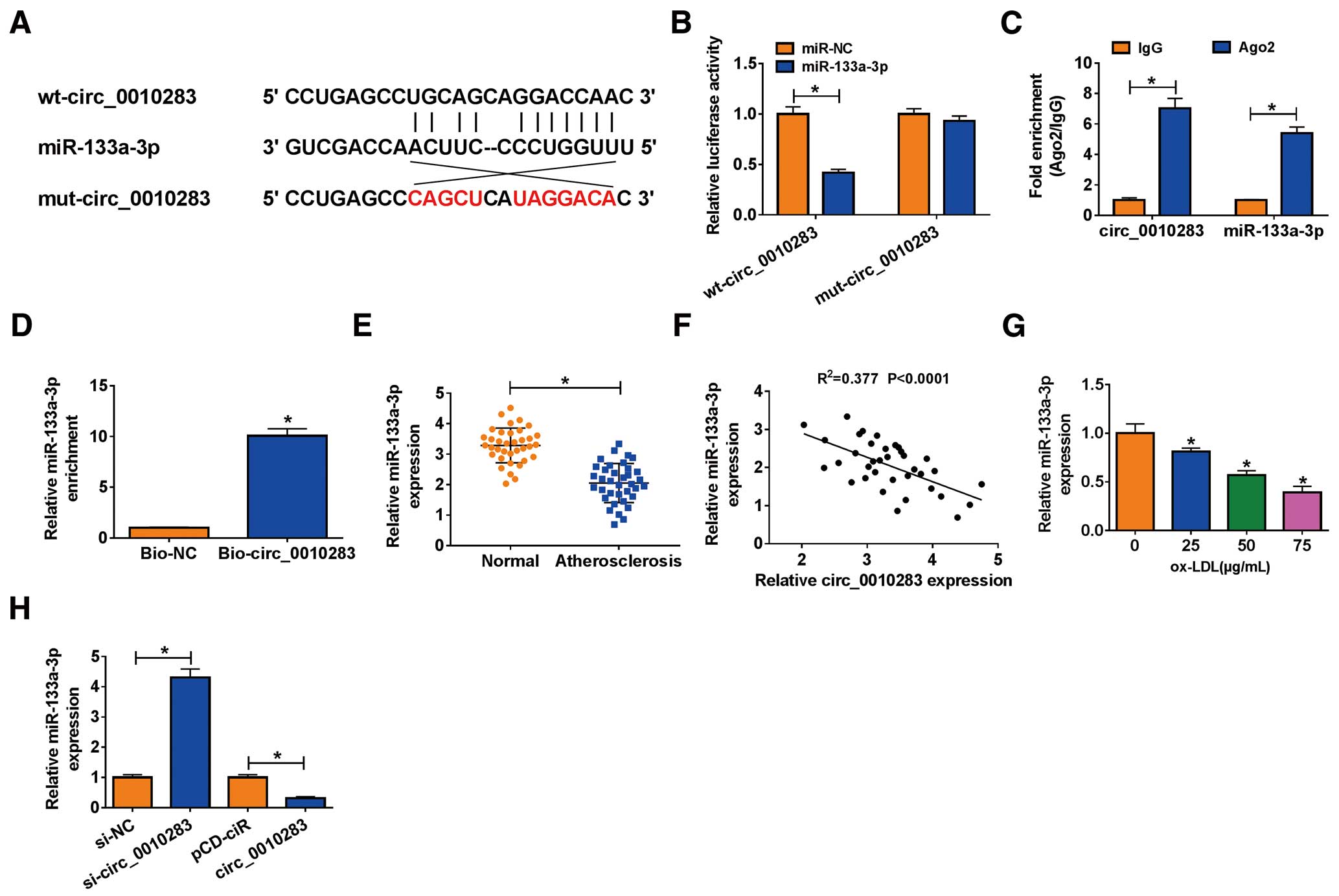
miR-133a-3p was a target of circ_0010283. (A) The binding sites between miR-133a-3p and circ_0010283 were analyzed by using a bioinformatics tool (Starbase). The interaction between miR-133a-3p and circ_0010283 was validated by (B) a dual-luciferase reporter assay, (C) a RIP assay and (D) a RNA pull-down assay. (E) The expression of miR-133a-3p in clinical serum samples was detected by using qRT-PCR. (F) The correlation between miR-133a-3p and circ_0010283 expression in atherosclerosis serum samples was analyzed. (G) The expression of miR-133a-3p in ox-LDL-treated HVSMCs was detected by using qRT-PCR. (H) The expression of miR-133a-3p in HVSMCs after circ_0010283 knockdown or overexpression was detected by using qRT-PCR. *P<0.05. circ_0010283, circular RNA_0010283; HVSMCs, human vascular smooth muscle cells; ox-LDL, oxidized low-density lipoprotein; qRT-PCR, quantitative real-time polymerase chain reaction; RIP, RNA Binding Protein immunoprecipitation.
To test whether circ_0010283 negatively regulated the expression of miR-133a-3p and might affect HVSMCs cellular activities, circ_0010283 and miR-133a-3p were knocked down alone or together in ox-LDL-treated HVSMCs. miR-133a-3p expression in the si-circ_0010283 transfection group was enhanced, but was lessened in the si-circ_0010283+anti-miR-133a-3p group (Figure 4A). In terms of function, cell proliferation suppressed in the si-circ_0010283 transfection group was partly recovered in the si-circ_0010283+anti-miR-133a-3p group (Figure 4B), and the expression of PCNA and Ki-67 decreased in the si-circ_0010283 transfection group was partly increased in the si-circ_0010283+anti-miR-133a-3p group (Figure 4C). In addition, cell apoptosis inhibited by ox-LDL treatment was promoted by si-circ_0010283 transfection alone, but partially suppressed by simultaneous si-circ_0010283 and anti-miR-133a-3p transfection (Figure 4D), and the expression of Bcl-2 induced by ox-LDL treatment was weakened by si-circ_0010283 transfection alone, but partially promoted by si-circ_0010283 and anti-miR-133a-3p transfection together, whereas the expression tendency of c-caspase3 was opposite to Bcl-2 in these transfected cells (Figure 4E). The number of migrated and invaded cells detected by the transwell assay was decreased in the si-circ_0010283 group but partly elevated in the si-circ_0010283+anti-miR-133a-3p group (Figure 4F,G). The migration ratio detected by using the scratch assay was also repressed in the si-circ_0010283 group, but partly reinforced in the si-circ_0010283+anti-miR-133a-3p group (Figure 4H). The expression of MMP2 and MMP9 was reduced in the si-circ_0010283 transfection group, but recovered in the si-circ_0010283+anti-miR-133a-3p group (Figure 4I). Combining with the above data, it is concluded that the effects of circ_0010283 knockdown on proliferation, migration/invasion and apoptosis were reversed by miR-133a-3p deficiency, hinting that circ_0010283 played functions by suppressing miR-133a-3p.

circ_0010283 regulated cell growth and metastasis by targeting miR-133a-3p. HVSMCs treated with ox-LDL were transfected with si-circ_0010283 or si-circ_0010283+anti-miR-133a-3p, and si-NC or si-circ_0010283+anti-miR-NC serving as a corresponding control. (A) The expression of miR-133a-3p in these transfected cells was examined by using qRT-PCR. (B) A MTT assay was performed to test cell proliferation. (C) The expression of proliferation-related proteins (PCNA and Ki-67) was measured by using a western blot. (D) Cell apoptosis was investigated by flow cytometry assay. (E) The expression of apoptosis-related proteins (Bcl-2 and c-caspase 3) was checked by using western blot. (F,G) Cell migration and invasion were evaluated by using a transwell assay; and (H) cell migration was also assessed by using a scratch assay. (I) The expression of migration/invasion-related proteins (MMP2 and MMP9) was measured by using a western blot. *P<0.05. circ_0010283, circular RNA_0010283; HVSMCs, human vascular smooth muscle cells; miR-133a-3p, microRNA-133a-3p; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ox-LDL, oxidized low-density lipoprotein; qRT-PCR, quantitative real-time polymerase chain reaction.
Given that the posttranscriptional regulation of miRNAs was conducted generally by targeting mRNAs,18 we further determined the target mRNAs of miR-133a-3p. Upon analysis of the bioinformatics tool (Starbase), PAPPA was found to be a putative target of miR-133a-3p, with several binding sites between its PAPPA 3’UTR and miR-133a-3p fragments (Figure 5A). Similarly, the interaction between miR-133a-3p and PAPPA was verified by using a dual-luciferase reporter assay (Figure 5B), a RIP assay (Figure 5C) and a RNA pull-down assay (Figure 5D). Moreover, we found that the expression of PAPPA was significantly increased in serum samples from atherosclerosis patients relative to normal volunteers (Figure 5E), and PAPPA expression was negatively correlated with miR-133a-3p expression in atherosclerosis serum samples (Figure 5F). In addition, the data from both qRT-PCR and western blot suggested that the expression of PAPPA was also enhanced in HVSMCs treated with ox-LDL in a dose-independent manner (Figure 5G,H), and its expression was suppressed in HVSMCs with miR-133a-3p restoration, but promoted in HVSMCs with miR-133a-3p deficiency (Figure 5I,J). We could hypothesize that PAPPA was a target of miR-133a-3p.
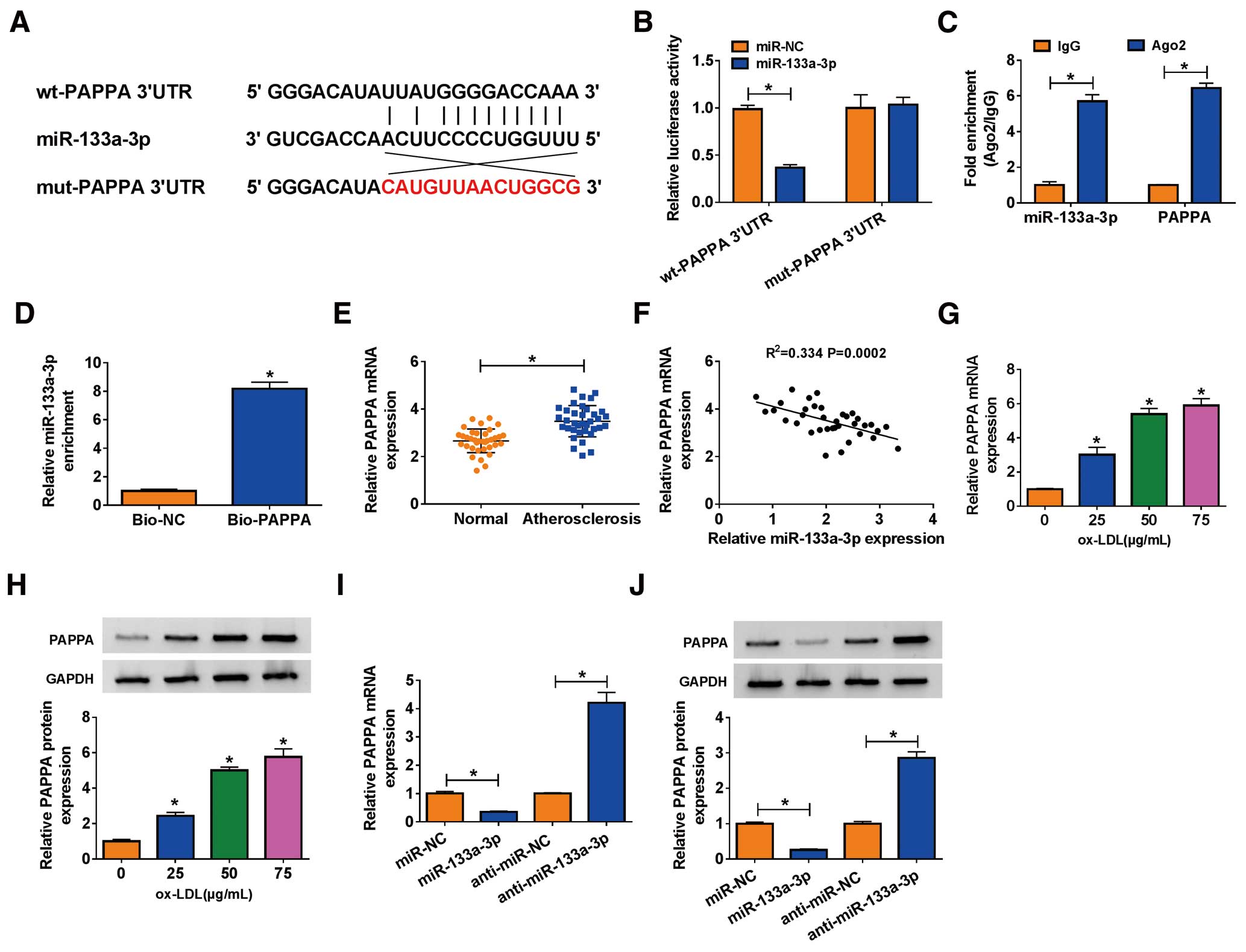
PAPPA was targeted by miR-133a-3p. (A) The binding sites between miR-133a-3p and PAPPA 3’UTR were analyzed by using a bioinformatics tool (Starbase). The relationship between miR-133a-3p and PAPPA was validated by using a (B) dual-luciferase reporter assay; (C) RIP assay and (D) a RNA pull-down assay. (E) The expression of PAPPA in clinical serum samples (n=36) was examined by using qRT-PCR. (F) The correlation between miR-133a-3p and PAPPA expression in atherosclerosis serum samples was analyzed. (G,H) The expression of PAPPA in ox-LDL-treated HVSMCs was detected by using qRT-PCR and western blot. (I,J) The expression of PAPPA in HVSMCs after miR-133a-3p knockdown or overexpression was detected by using qRT-PCR and western blot. *P<0.05. HVSMCs, human vascular smooth muscle cells; miR-133a-3p, microRNA-133a-3p; ox-LDL, oxidized low-density lipoprotein; PAPPA, pregnancy-associated plasma protein A; qRT-PCR, quantitative real-time polymerase chain reaction; RIP, RNA Binding Protein immunoprecipitation.
We next used miR-133a-3p or miR-133a-3p+PAPPA to transfect ox-LDL-treated HVSMCs to explore the function of miR-133a-3p and PAPPA. The data from qRT-PCR and western blot showed that the expression of PAPPA was suppressed in ox-LDL-treated HVSMCs with miR-133a-3p transfection, but partly restored in cells with miR-133a-3p+PAPPA transfection compared with their corresponding control (Figure 6A,B). Cell proliferation was observed to be suppressed in the miR-133a-3p transfection group, but facilitated in the miR-133a-3p+PAPPA group (Figure 6C), and the expression of PCNA and Ki-67 declined in ox-LDL-treated HVSMCs with miR-133a-3p restoration alone, but partly recovered in ox-LDL-treated HVSMCs with miR-133a-3p restoration and PAPPA overexpression together (Figure 6D,E). The transfection of miR-133a-3p led to an increasing number of apoptotic cells, whereas the number of apoptotic cells was partly declined in ox-LDL-treated HVSMCs with miR-133a-3p+PAPPA transfection (Figure 6F). The expression of Bcl-2 was degressive in ox-LDL-treated HVSMCs with miR-133a-3p transfection, but partly restored in cells with miR-133a-3p+PAPPA transfection, whereas the expression of c-caspase 3 was opposite to Bcl-2 expression in these transfected cells (Figure 6G,H). The data from the transwell assay found that the capacities of migration and invasion were repressed by miR-133a-3p transfection, but partly recovered by miR-133a-3p+PAPPA transfection in ox-LDL-treated HVSMCs (Figure 6I,J). The data from the scratch assay exhibited the consistent results found with the transwell migration assay (Figure 6K). The expression of MMP2 and MMP9 was decreased by miR-133a-3p transfection, but partly promoted by miR-133a-3p+PAPPA transfection in ox-LDL-treated HVSMCs (Figure 6L,M). Collectively, miR-133a-3p restoration impaired proliferation, migration and invasion, but stimulated apoptosis of ox-LDL-treated HVSMCs, whereas the reintroduction of PAPPA reversed these effects.

miR-133a-3p restoration suppressed growth and metastasis of ox-LDL-treated HVSMCs by mediating PAPPA. HVSMCs treated with ox-LDL were transfected with miR-133a-3p or miR-133a-3p+PAPPA, miR-NC or miR-133a-3p+pcDNA serving as a corresponding control. (A,B) The expression of PAPPA was examined by using qRT-PCR and western blot. (C) A MTT assay was utilized to check cell proliferation. (D,E) The expression of proliferation-related proteins (PCNA and Ki-67) was measured by using western blot. (F) A flow cytometry assay was performed to detect cell apoptosis. (G,H) The expression of apoptosis-related proteins (Bcl-2 and c-caspase 3) was measured by using western blot. (I,J) A transwell assay was adopted to assess cell migration and invasion, and (K) a scratch assay was also utilized to assess cell migration. (L,M) The expression of migration/invasion-related proteins (MMP2 and MMP9) was measured by using western blot. *P<0.05. HVSMCs, human vascular smooth muscle cells; miR-133a-3p, microRNA-133a-3p; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; NC, negative control; ox-LDL, oxidized low-density lipoprotein; PAPPA, pregnancy-associated plasma protein A; qRT-PCR, quantitative real-time polymerase chain reaction.
Interestingly, we discovered that PAPPA expression was positively associated with circ_0010283 expression in atherosclerosis serum samples (Figure 7A). Further, the expression of PAPPA was detected in ox-LDL-treated HVSMCs transfected with si-circ_0010283 or si-circ_0010283+anti-miR-133a-3p, si-NC or si-circ_0010283+anti-miR-NC serving as the corresponding control. The data showed that PAPPA expression was strikingly reduced in cells transfected with si-circ_0010283, but substantially recovered in cells transfected with si-circ_0010283+anti-miR-133a-3p (Figure 7B,C), hinting that PAPPA expression was modulated by circ_0010283 and miR-133a-3p, and circ_0010283 regulated PAPPA expression by targeting miR-133a-3p.
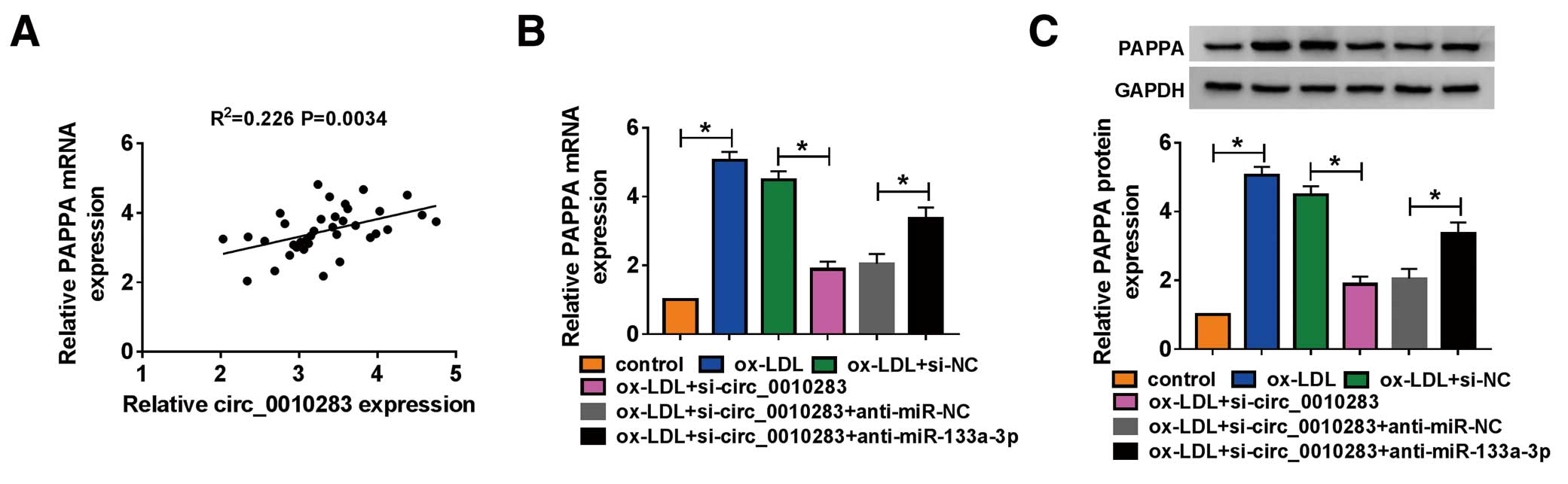
circ_0010283 regulated PAPPA expression by controlling miR-133a-3p. (A) Correlation between PAPPA expression and circ_0010283 expression in atherosclerosis serum samples. (B,C) The expression of PAPPA in HVSMCs transfected with si-circ_0010283, si-NC, si-circ_0010283+anti-miR-133a-3p or si-circ_0010283+anti-miR-NC was detected by using qRT-PCR and western blot. *P<0.05. circ_0010283, circular RNA_0010283; HVSMCs, human vascular smooth muscle cells; miR-133a-3p, microRNA-133a-3p; PAPPA, pregnancy-associated plasma protein A; ox-LDL, oxidized low-density lipoprotein; qRT-PCR, quantitative real-time polymerase chain reaction.
Atherosclerosis is a disease with complex pathogenesis, and may be involved in chemotaxis of macrophages, dysregulation of related genes, dysfunction of VSMCs and other factors.2,19,20 Research has shown that ox-LDL exerts an indispensable role in atherogenesis, and ox-LDL can interact with lectin-like ox-LDL receptor 1 (LOX-1), leading to the aberrant proliferation of VSMCs, macrophage migration and inflammatory responses.21 Recently, numerous studies elucidated that non-coding RNAs are vital elements to regulate ox-LDL-induced dysfunction of VSMC. For example, long non-coding RNA MEG3 was weakly expressed in ox-LDL-treated VSMCs, and its silencing promoted proliferation and impaired apoptosis of VSMCs treated with ox-LDL.22 miR-195 restoration contributed to proliferation, metastasis and an increase of pro-inflammatory factors in ox-LDL-treated VSMCs.23 circRNA-FHFR knockdown also restrained the growth and activities of ox-LDL-treated VSMCs.13 These data hinted that non-coding RNAs are substantial participators in atherogenesis progression. circ_0010283 was addressed to be prominently overexpressed in ox-LDL-administered VSMCs.13 In our study, we further validated its expression, and an increased level of circ_0010283 expression was observed in clinical atherogenesis serum samples and ox-LDL-treated HVSMCs. In terms of its function, circ_0010283 downregulation blocked ox-LDL-induced proliferation, migration and invasion, but stimulated ox-LDL-suppressed apoptosis. Our study was the first to explore the effect of circ_0010283 on growth and migration in ox-LDL-treated HVSMCs.
circRNAs canonically function as competing endogenous RNAs to sponge miRNAs and suppress miRNA functions, thus regulating multiple biological processes.24,25 Following this mechanism, we identified the putative miRNAs targeted by circ_0010283. miR-133a-3p was confirmed to be a target of circ_0010283, which was downregulated in atherosclerosis serum samples and ox-LDL-treated HVSMCs. A previous study reported that the expression of miR-133a-3p was reduced in injured carotid arteries of rats.26 In addition, miR-133a-3p was significantly downregulated in mouse VSMCs, and miR-133a-3p reintroduction reversed the effects of long non-coding RNA taurine-up-regulated gene 1 (TUG1) to block proliferation and inflammatory responses in ox-LDL-treated mouse VSMCs.15 Similar to these findings, this study demonstrated that miR-133a-3p deficiency reversed the effects caused by circ_0010283 knockdown, and miR-133a-3p restoration also weakened ox-LDL-induced proliferation and migration of HVSMCs.
Furthermore, our study found that miR-133a-3p paired with target sequences in the 3’UTR of PAPPA and suppressed PAPPA expression in HVSMCs. Throughout previous research, we discovered that PAPPA was well recognized as a molecular biomarker of diverse cardiovascular diseases, including atherosclerosis.17,27,28 Additionally, PAPPA was also recorded to reverse the function of its upstream miRNAs, thus reinforcing ox-LDL-induced proliferation of VSMCs.10,29 Consistent with these sequences, PAPPA was also monitored to be upregulated in serum samples from atherogenesis patients and ox-LDL-induced HVSMCs, and PAPPA overexpression partly reversed the effects caused by miR-133a-3p enrichment. Innovatively, our study uncovered that circ_0010283 regulated PAPPA expression by sponging miR-133a-3p, which represented a novel mechanism for regulating VSMC dysfunction of PAPPA.
In summary, the present study was the first to determine that circ_0010283 downregulation ameliorated the dysfunctions of HVSMCs induced by ox-LDL, which was attributed to the regulation of the miR-133a-3p/PAPPA pathway. Our study enriched the information known about the role of circ_0010283 in HVSMC dysfunctions, and established a basis of knowledge about the involvement of circ_0010283 in the pathogenesis of atherosclerosis.
The authors declare that they have no competing interests.
No funding was received for this study.
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
All authors made substantial contribution to the study’s conception and design, acquisition of the data, or analysis and interpretation of the data; they took part in drafting the article or revising it critically for important intellectual content; gave final approval of the revision to be published; and agree to be accountable for all aspect of the work.
Not applicable.
1. circ_0010283 is upregulated in serum from atherosclerosis patients and ox-LDL-treated HVSMCs.
2. circ_0010283 downregulation alleviates ox-LDL-induced dysfunctions of HVSMCs.
3. circ_0010283 regulates the expression of PAPPA by targeting miR-133a-3p.
4. circ_0010283 participates in ox-LDL-induced HVSMC dysfunction partly by the miR-133a-3p/PAPPA pathway.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0345