Article ID: CJ-20-0713
Article ID: CJ-20-0713
Background: Because anticoagulant drugs for ambulatory patients with cancer-associated venous thromboembolism (CAT) are limited to warfarin and direct oral anticoagulants (DOACs) in Japan, it is important to assess the outcomes of both drugs.
Methods and Results: We retrospectively assessed the outcomes of CAT patients who were treated with warfarin or edoxaban between 2011 and 2017. The assessment was limited to the duration of anticoagulant administration. CAT patients who did not receive anticoagulation therapy were also compared with the warfarin and edoxaban groups. We enrolled 111 CAT patients treated with warfarin (n=58, mean age 62.6 years, mean time in therapeutic range [TTR] % 61.1) or edoxaban (n=53, mean age 64.6 years). Although venous thromboembolism (VTE) recurred in 2 warfarin-treated patients, the 2 treatment groups were not significantly different (P=0.18). Bleeding during anticoagulation therapy occurred in 6 warfarin-treated patients (2 with major bleeding) and in 5 edoxaban-treated patients (no major bleeding) (P=1.0). The non-anticoagulation group (n=37) showed a high recurrence rate (P<0.01) compared with the anticoagulant group.
Conclusions: This study showed that warfarin and edoxaban are equally effective in preventing VTE recurrence and bleeding. However, warfarin control in CAT patients presented some difficulties. This study also demonstrated the efficacy of anticoagulant drugs, compared with no anticoagulation, for CAT patients to prevent VTE recurrence.
Cancer-associated thromboembolism (CAT) is a frequent complication in cancer patients, which is clinically important because of the high rates of recurrence and hemorrhage-related complications observed during anticoagulant therapy and the risk of death.1 CAT patients treated with conventional anticoagulation therapy reportedly have a 2-fold higher risk of thrombosis recurrence and a 3-fold higher risk of major bleeding compared with venous thromboembolism (VTE) patients without cancer.2 Thus, a narrow therapeutic range is a significant problem related to anticoagulation for CAT.
Several randomized controlled trials (RCTs) have shown the superiority of low-molecular-weight heparin (LMWH) for CAT over vitamin K antagonists (VKA), which used to be the conventional anticoagulant therapy.3–7 Based on this, evidence-based guidelines recommend LMWH for the anticoagulant treatment of patients in Western countries.8 However, LMWH is not approved for use as an anticoagulant to treat VTE in Japan. Thus, the only option before the development of direct oral anticoagulants (DOACs) was conventional anticoagulation, which consists of unfractionated heparin or warfarin.
Recently, large RCTs showed the advantage of DOACs over conventional VKAs for VTE treatment. As a result, DOACs have become the principal oral anticoagulant drugs used in VTE therapy.9–11 Furthermore, DOACs show non-inferiority compared with LMWH for treating CAT.12–14 Based on these results, the number of DOACs used in CAT patients is increasing. As mentioned, LMWH is not available to treat VTE in Japan, and thus, we have only 2 choices, warfarin and DOACs, for ambulatory CAT patients. Because most of the recent CAT treatment studies only compare DOACs and LMWH, we have less evidence of DOACs’ efficacy in direct comparison with conventional VKAs.
Edoxaban was the first DOAC approved for VTE treatment in Japan, and after reports of its efficacy for CAT treatment,12 we shifted to edoxaban from warfarin for several CAT cases. Therefore, we believe that it is important to compare the outcomes of treatment with edoxaban and warfarin for patients with CAT and this study aimed to compare the results of edoxaban and warfarin treatment in patients with CAT.
This was a single-center retrospective study that was approved by the Tohoku University Ethics Committee (approval no. 2018-1-469).
Of newly diagnosed VTE patients with an active malignancy who were administered anticoagulant drugs between 2011 and 2017, those who were administrated warfarin, for which the time in therapeutic range (TTR) % was >30%, or edoxaban were enrolled in this study. Patient information, including sex, age, laboratory data, diagnostic imaging data, VTE type, cancer type, and the anticoagulant and concomitant anticancer drugs, were collected from the medical records. The study period was established so that the administration periods of the warfarin and edoxaban groups were matched. As a reference sample, newly diagnosed patients with CAT who were not treated with anticoagulants for any reason during the same period were enrolled.
The exacerbation or recurrence of VTE, any type of bleeding, and any cause of death were assessed as endpoints. Exacerbation, recurrence, and bleeding were assessed when the anticoagulants were administered. The selection of warfarin or edoxaban depended on each physician’s decision. Follow-up ultrasonography, computed tomography (CT), and blood tests were performed according to each physician’s decision. VTE exacerbation or recurrence was evaluated by ultrasonography or enhanced CT regardless of symptoms. Bleeding was assessed according to the patient’s complaint or laboratory test results.
For this study, active cancer was defined as cancer diagnosed within the previous 3 months, recurrent, regionally advanced, or metastatic cancer, cancer for which treatment was being administered or planned, or hematological cancer that was not in complete remission. Major bleeding was defined as overt bleeding associated with a decreased hemoglobin level of ≥2 g/dL that resulted in a blood transfusion, occurred at a critical site or contributed to death in accordance with the International Society on Thrombosis and Haemostasis criteria.15 Minor bleeding was defined as noticeably less than major bleeding.
Warfarin’s optimal target anticoagulation level was decided as a prothrombin time-international normalized ratio (PT-INR) ≥1.5 but ≤2.5, according to the 2017 Guidelines for diagnosis, treatment, and prevention of pulmonary thromboembolism (PTE) and deep vein thrombosis (DVT) of the Japanese Circulation Society. The therapeutic quality was evaluated according to TTR%, as calculated by the Rosendaal method.16 A TTR% ≥60% is recommended to ensure the therapeutic effect of warfarin.16,17 Therefore, the cutoff value of TTR% was set to ensure that the average TTR% of the warfarin group exceeded 60%. Consequently, the cutoff value of TTR% was set to 30%.
Statistical AnalysisCategorical variables are presented as numbers (%), and continuous variables are presented as mean (±SD) or median (interquartile range [IQR]) according to the data distribution. The chi-square test was used to compare categorical variables. In contrast, the unpaired t-test or Mann-Whitney U test was used to compare continuous variables according to the type of data distribution. Differences were considered significant at P<0.05. Time-to-event curves were calculated using the Kaplan-Meier method, and the survival curves were compared using the log-rank test.
Between 2011 and 2017, 193 patients with active cancer were initially diagnosed with VTE and of them 144 were treated with anticoagulant drugs, comprising 79 patients who received warfarin and 65 patients who received DOACs. Of the 79 patients treated with warfarin, 21 poorly controlled patients whose TTR% was <30% were excluded from the study so that the average TTR% of the warfarin group was ≥60%. The remaining 58 patients were defined as the warfarin group (mean age 62.6 years, male/female 14/44, mean TTR% 61.1%). Of the 65 patients treated with DOACs, 53 who were treated with edoxaban were defined as the edoxaban group (mean age 64.6 years, male/female 12/41). Excluded DOACs other than edoxaban included 6 rivaroxaban, 5 apixaban, and 1 dabigatran cases. Table 1 shows the baseline characteristics of the warfarin and edoxaban groups.
| Warfarin | Edoxaban | P value | |
|---|---|---|---|
| N | 58 | 53 | |
| Male/female | 14/44 | 12/41 | 0.85 |
| Age (years, mean) | 62.6±15.5 | 64.6±12.1 | 0.45 |
| DVT | 53 | 51 | 0.3 |
| Proximal | 32 | 22 | 0.08 |
| Distal | 21 | 29 | |
| PTE | 26 | 17 | 0.17 |
| Massive | 1 | 1 | 0.19 |
| Submassive | 0 | 2 | |
| Non-massive | 25 | 14 | |
| D-dimer at diagnosis (μg/mL, median) | 6.8 (IQR 3.5–14.7) | 8.3 (IQR 4.1–17.0) | 0.35 |
| Inpatient/outpatient | 54/4 | 32/21 | <0.01 |
| TTR% | 61.1±16.9 | – | – |
| Concomitant chemotherapy (%) | 35 (60) | 36 (67.9) | 0.41 |
| Platinum compounds | 25 | 27 | 0.51 |
| Taxanes | 20 | 27 | 0.08 |
| Topoisomerase inhibitors | 8 | 6 | 0.70 |
| Antimetabolites | 7 | 6 | 0.90 |
| Molecular-targeted agents | 3 | 6 | 0.24 |
| Vinca alkaloids | 2 | 4 | 0.34 |
| Antitumor antibiotics | 3 | 9 | 0.05 |
| Alkylating agents | 2 | 4 | 0.34 |
| Hormonal agents | 1 | 0 | 0.34 |
| Administration duration (days, median) | 203 (IQR 63–384) | 192 (IQR 63–378) | 0.57 |
| Follow-up duration (days, median) | 602 (IQR 262–1,629) | 459 (IQR 153–610) | <0.01 |
| Recurrent VTE (%) | 2 (3.4) | 0 (0) | 0.18 |
| Bleeding (%) | 6 (10.3) | 5 (9.4) | 1.0 |
| Major bleeding (%) | 2 (3.4) | 0 (0) | 0.26 |
| Intracerebral hemorrhage | 1 | 0 | – |
| Bleeding from tumor | 1 | 0 | – |
| Minor bleeding (%) | 4 (6.9) | 5 (9.4) | 0.61 |
| Genital bleeding | 1 | 3 | – |
| Urinary bleeding | 2 | 2 | – |
| Other | 1 | 0 | – |
DVT, deep vein thrombosis; IQR, interquartile range; PTE, pulmonary thromboembolism; TTR, time in therapeutic range; VTE, venous thromboembolism.
At the point of analysis, baseline age, sex, site of DVT, PTE, and the D-dimer level at diagnosis and during the administration period were not significantly different between the 2 groups. Ambulatory treatment without admission occurred more often in the edoxaban group. Because warfarin accounted for most treatments during the period before edoxaban was approved in 2014, the total follow-up period was longer in the warfarin group. However, the evaluation was performed only during the administration period, which was not significantly different between groups. Concomitant anticancer drug administration during anticoagulation is detailed in Table 1. There were no intergroup differences with regard to the proportion of chemotherapy; platinum compounds and taxanes were most frequently used in both groups.
The type of underlying malignancy was similar in both groups, with gynecological malignancies most frequent, followed by digestive tract malignancies. Table 2 gives details of each malignancy. In the warfarin group, 50 patients were heparin lead-in, and 2 patients were fondaparinux lead-in, while the other 6 patients were administrated only warfarin from the beginning. In the edoxaban group, 29 patients were heparin lead-in, while the remaining 24 patients received edoxaban alone from the beginning. The types of anticoagulants and parenteral lead-in were determined by each physician.
| Type of cancer | Warfarin | Edoxaban |
|---|---|---|
| Solid (%) | 57 (98.3) | 49 (92.5) |
| Gynecological (%) | 31 (53.4) | 31 (58.5) |
| Uterus | 15 | 11 |
| Ovary | 16 | 20 |
| Upper gastrointestinal (%) | 5 (8.6) | 2 (3.8) |
| Colorectal (%) | 3 (5.2) | 1 (1.9) |
| Pancreatic or hepatobiliary (%) | 1 (1.7) | 3 (5.7) |
| Lung (%) | 4 (6.9) | 3 (5.7) |
| Genitourinary (%) | 3 (5.2) | 4 (7.5) |
| Skin (%) | 5 (8.6) | 2 (3.8) |
| Other (%) | 5 (8.6) | 3 (5.7) |
| Hematological malignancy (%) | 1 (1.7) | 4 (7.5) |
Figure 1 shows exacerbation or recurrence of VTE. Although VTE recurred in 2 patients in the warfarin group, the treatment groups were not significantly different (P=0.18). Of the 2 patients in the warfarin group, 1 had colon cancer with a thrombus that extended proximally on the 84th day, and the other patient, who had ovarian cancer, was initially diagnosed with a proximal DVT and developed new PTE on the 231st day after the initial diagnosis. Both patients experienced exacerbation or recurrence during warfarin administration.

Accumulation curves of exacerbation and recurrence of venous thromboembolism in patients treated with warfarin or edoxaban.
Bleeding during anticoagulation occurred in 6 patients in the warfarin group (2 with major bleeding) and in 5 patients in the edoxaban group (no major bleeding) (Table 1). Figure 2 shows the time from the start of anticoagulation to bleeding in the 2 groups. There were 2 cases of major bleeding in the warfarin group: 1 intracerebral hemorrhage, and 1 uncontrollable bleeding from a recurrent uterine tumor. Cases of minor bleeding included hematuria in 4 patients, atypical genital bleeding in 4 patients, rectal bleeding in 1 patient, and bloody sputum in 1 patient. No patient showed upper gastrointestinal bleeding. Of the 6 patients with bleeding-related complications in the warfarin group, 4 showed a rapid increase in PT-INR >4.

Accumulation curves of all patients with hemorrhage who were treated with warfarin or edoxaban.
In the warfarin group, including the non-bleeding patients, 20 patients (35%) experienced poor warfarin control (PT-INR >4), even temporarily. Of these 20 patients, 9 (45%) were on chemotherapy, and 1 was on hormone therapy (medroxyprogesterone acetate). The breakdown of the 9 chemotherapeutic drugs was 4 platinum compounds (cisplatin 2, carboplatin 2), 3 topoisomerase inhibitors (topotecan 2, etoposide 1), 2 taxanes (docetaxel and paclitaxel), 2 antimetabolites (fluorouracil 2), and 1 molecular-targeted agent (denosumab). These compounds were used in combination.
The overall survival curve during follow-up is shown in Figure 3. The follow-up period included the time after cessation of anticoagulant treatment. In total, 54 subjects died during the observation period: 49 were diagnosed as cancer deaths and 5 were causes other than cancer. The cause of death was judged from descriptions provided in the medical records and examinations during the terminal stage. Of the 54 deaths in our study, 44 patients died in hospital, and the others died during home care. A detailed description of the final period was available for 52 of the 54 cases; 5 patients who died from causes other than cancer included 1 case each of sepsis, cardiac tamponade, stroke, bleeding from tumor, and injury. The remaining patients were diagnosed as cancer death, including 2 patients who exhibited unexpected dyspnea (1 case of massive pleural effusion thought to have been the cause of dyspnea, 1 case of unknown etiology). No patient died of an apparent diagnosed PTE. However, because thorough examinations were not conducted given that patients were in palliative care, it may have been difficult to make an accurate final diagnosis.
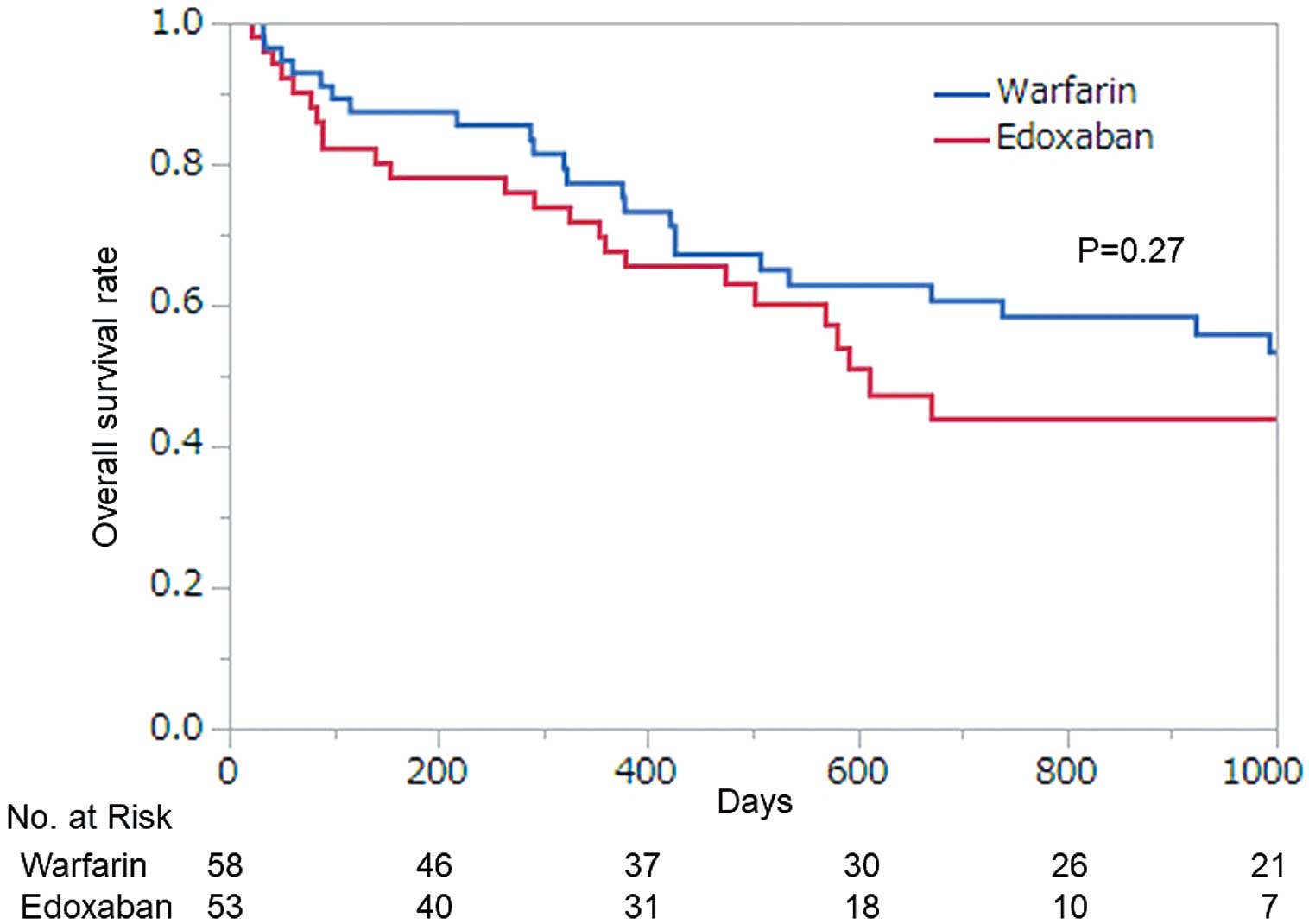
Overall survival curves of patients in the warfarin and edoxaban groups.
Finally, the efficacy of anticoagulant therapy for CAT was verified. During this period, 37 patients with CAT did not receive anticoagulant treatment for reasons that included bleeding disorders, rejection by the patient, and distal site of the thrombus. The decision was made according to each physician’s judgment. The non-anticoagulation group was compared with the warfarin and edoxaban groups (defined together as the anticoagulant group). Table 3 shows the baseline characteristics of the anticoagulation and non-anticoagulation groups. Although age and sex were similar in both groups, proximal DVT and PTE were less frequent in the non-anticoagulation group, which might have been one reason that physicians did not prescribe an anticoagulant. Figure 4 shows the time to recurrence in both groups and shows a higher recurrence ratio in the non-anticoagulation group (P<0.01).
| Anticoagulant group |
Non-anticoagulation group |
P value | |
|---|---|---|---|
| N | 111 | 37 | |
| Male/female | 26/85 | 11/26 | 0.5 |
| Age (mean) | 63.5±13.9 | 68.3±11.3 | 0.08 |
| DVT | 104 | 37 | |
| Proximal | 54 | 9 | <0.01 |
| Distal | 50 | 28 | |
| PTE | 43 | 3 | <0.01 |
| D-dimer at diagnosis (μg/mL, median) | 7.7 (IQR 3.9–16.3) | 4.1 (IQR 2.6–7.2) | <0.01 |
| Follow-up duration (days, median) | 201 (IQR 62–382) | 453 (IQR 167–998) | <0.01 |
| Cancer type | |||
| Solid (%) | 106 (95.5) | 35 (94.6) | |
| Gynecological (%) | 62 (55.9) | 16 (43.2) | |
| Uterus | 26 | 9 | |
| Ovary | 36 | 7 | |
| Upper gastrointestinal (%) | 7 (6.3) | 1 (2.7) | |
| Colorectal (%) | 4 (3.6) | 3 (8.1) | |
| Pancreatic or hepatobiliary (%) | 4 (3.6) | 1 (2.7) | |
| Lung (%) | 7 (6.3) | 2 (5.4) | |
| Genitourinary (%) | 7 (6.3) | 5 (13.5) | |
| Skin (%) | 7 (6.3) | 4 (10.8) | |
| Other (%) | 8 (7.2) | 3 (8.1) | |
| Hematological malignancy (%) | 5 (4.5) | 2 (5.4) | |
| Chemotherapy at onset (%) | 22 (19.8) | 11 (29.7) | 0.21 |
| Recurrent VTE | 2 | 9 | <0.01 |
Abbreviations as in Table 1.

Accumulation curves of exacerbation and recurrence of venous thromboembolism in patients in the anticoagulation and non-anticoagulation groups. The anticoagulant group includes both the warfarin and edoxaban groups.
This retrospective study revealed no significant difference in VTE recurrence and bleeding complications between warfarin and edoxaban treatment for CAT in the patient cohort. There were many cases of low TTR% in the warfarin group, which indicated difficulty in warfarin control for CAT patients. Chemotherapy was an important factor for poor control of warfarin. Poor temporary warfarin control caused major bleeding in 2 cases. This study also showed the anticoagulant treatment’s efficacy compared with no anticoagulation for patients with CAT in terms of preventing recurrence.
LMWH has been the first-line anticoagulant therapy for CAT since major RCTs showed its advantage compared with conventional treatment by VKAs.3–7 Recently, after several large RCTs compared DOACs and LMWH and reported the non-inferiority of DOACs for VTE in patients with CAT,12–14 DOACs have become an alternative to LMWH.8,18,19 Thus, LMWH and DOACs are the 2 major anticoagulants currently used for ambulatory patients with CAT in Western countries, and warfarin may not be used any more. In contrast, anticoagulation, especially for ambulatory patients with CAT, is still limited to warfarin and DOACs in Japan. A recent study showed that warfarin is still used for patients with CAT even after approval for DOACs,20 and we believe that warfarin is still used for some ambulatory patients with CAT (e.g., those who do not receive chemotherapy). There are few studies that directly compare warfarin and DOACs for patients with CAT, especially in Japan. Hence, we believed that it was important to compare the outcome of these 2 drugs, which have different anticoagulation mechanisms, between 2 groups of patients with similar characteristics, and discuss the drugs’ efficacy and adverse effects.
In Japan, edoxaban was approved as an anticoagulant for VTE in 2014, followed by approval for rivaroxaban and apixaban. Though edoxaban’s efficacy for CAT was unknown in the early period, it gradually began to be used for this purpose. Rivaroxaban and apixaban were also used to treat CAT during this period, but because there were fewer treated patients with those drugs, we selected only edoxaban for our comparative analysis. Further assessment including all DOACs showed similar outcomes (data not shown).
This was a retrospective study, and the main period of each drug administration was different, which means that patients in each group possibly had different characteristics. However, an assessment of the baseline data revealed no significant differences in age, sex, DVT location, PTE, or D-dimer value between groups. The most common cancer was gynecological, followed by gastroenterological cancer and lung cancer in both groups. As for cancer staging, the type of malignancy varied so widely, it was difficult to determine whether the cancer stage was the same in patients in the 2 groups. However, because there was no difference in overall survival between the 2 groups, the patients were considered to be at the same stage of cancer progression. Accordingly, the comparison between groups was considered appropriate.
There were no significant differences in recurrence of VTE between the groups. Although the number of recurrences was not high in the anticoagulant group in this study, it has not been investigated whether the anticoagulant is effective. Therefore, we compared the exacerbations and recurrences in CAT patients who did not receive anticoagulant drugs for any reason with those of the anticoagulant group. A simple comparison was difficult because of the different baseline characteristics, but the non-anticoagulation group has less proximal DVT, less PTE, and lower D-dimer values, which could mean this group had milder cases of VTE. Despite this, there were more exacerbations and recurrences of CAT in the non-anticoagulation group during the time course, indicating that anticoagulants had a secondary preventive effect on CAT.
In contrast, bleeding as an adverse effect occurred to some extent in both treatment groups, but there was no significant difference between them. In the warfarin group, several episodes of bleeding, including 2 cases of major bleeding, were due to poor control of PT-INR. Our analysis excluded cases with ≤30% TTR% so that the average of the remainder in the group exceeded 60%. Nevertheless, there were cases of poor PT-INR control in the warfarin group, one of the reasons being interactions with concomitant anticancer drugs. In effect, even after exclusion of lower TTR% cases, 35% of patients experienced poor warfarin control (PT-INR >4) even temporarily. Approximately half of these patients were on chemotherapy, which showed the importance of drug interactions.
Many anticancer drugs interact with warfarin, and care must be taken when they are used together.21 Chemotherapy is often performed in combination with multiple drugs, and the drugs used change as the clinical course progresses, such as second and third line choices. Table 1 shows all anticancer drugs used during anticoagulation, and more than half of both the warfarin and edoxaban groups received chemotherapy during the clinical course. Of these, 10 patients in the warfarin group experienced poor control with PT-INR ≥4 during concomitant anticancer drug therapy, such as platinum compounds, taxanes, topoisomerase inhibitors, antimetabolites, molecular-targeted therapeutic agents, antiestrogens, and hormones. Warfarin’s effect is enhanced by interactions with drugs that inhibit the hepatic drug-metabolizing enzyme CYP2C9, and there have been reports regarding excessive prolongation of PT-INR when warfarin is used in combination with anticancer drugs such as isotopomerase inhibitors, antimetabolic agents and molecular-targeted agents.22–24 Although paclitaxel is reported to have different metabolic pathway to warfarin, it is thought to increase PT-INR by displacing warfarin from its protein binding sites and increasing the blood warfarin concentration.25 Although the interaction between platinum compounds and warfarin is still unknown, it has been reported that PT-INR was excessively prolonged while using platinum compounds,26 and caution should be taken with concomitant use of warfarin. Thus, for CAT during chemotherapy, it is difficult to use warfarin properly. Because physicians have been forced to manage CAT under conditions that make it difficult to use warfarin properly, it is surprising that the warfarin group in this study showed comparable outcomes to the edoxaban group.
In this study, gynecological cancers accounted for more than half of the underlying cancers in both groups, followed by gastrointestinal and lung cancer. This proportion is believed to be a characteristic of our patient cohort. According to the subanalysis targeting CAT of the COMMAND VTE registry,27 which was a multicenter retrospective study, gastrointestinal cancer was the major underlying cancer of CAT and gynecological cancer accounted for 17.3% of CAT cases. According to the Cancer-VTE registry,28 another prospective multicenter study, gastrointestinal cancer was most frequent, followed by lung cancer. Conversely, in many studies in Western countries, gastrointestinal cancer, breast cancer, and lung cancer are the major underlying diseases of CAT.12–14 It is unclear whether the underlying cancer types differ by geographic area. In future, it will be necessary to focus on each cancer type. Although many studies in Western countries report a higher rate of gastrointestinal bleeding, especially upper gastrointestinal bleeding, as an adverse effect, in this study, urinary and genital bleeding were more frequent, a difference that may be caused by the differences in cancer type, race, or frequency of proton pump inhibitor administration.
Khorana et al reported that thrombosis should not be underestimated as a cause of death in patients with cancer.1 In our study, follow-up was performed even after anticoagulant therapy was discontinued, and 54 people died during the observation period. Among them apparent PTE was not included in the causes of death. Khorana et al reported that thrombosis accounted for 9.2% (13 patients) of all-cause deaths in 141 patients with cancer.1 Among those 13 patients, 8 had arterial thrombosis, such as stroke or myocardial infarction, and 5 (3.5%) had PTE. Sakamoto et al reported that among 464 deaths in active cancer patients with CAT, 20 (4.3%) died of fatal PTE.28 Among our cases, adopting the proportion suggested by these studies, 2 patients may have possibly died from PTE. We diagnosed the cause of death from the medical records and examinations that were available. Among the patients whose cause of death was diagnosed as cancer death, 1patient had dyspnea of unknown etiology. However, thorough examinations were not conducted because the patient was in palliative care. We cannot deny the possibility that PTE may have occurred in this patient. Because thorough examinations are typically avoided in terminal cases, precise diagnosis of the cause of death may often not be possible. Recently, because anticoagulation is a more popular option for patients with CAT, and because additional evidence has accumulated, the number of deaths of CAT patients due to PTE may be decreasing. However, because the number of cancer survivors is increasing, VTE is still an important issue.
Further accumulation of this type of data will help in deciding the selection of treatments. We believe that it is important to use DOACs and warfarin properly while understanding that DOACs have a shorter half-life and a fundamental action point different from that of warfarin.
Study LimitationsThis was a single-center retrospective study with a small number of cases and included more patients treated with warfarin in the former period and more patients treated with edoxaban in the latter period. In addition, there was the possibility of change in therapeutic concept for CAT during the study period. Moreover, the distribution of underlying cancer was different from other studies. Hence, there is a possibility of bias in the results. Moreover, the present study could not prove the superiority or non-inferiority of any one drug.
This retrospective single-center study showed that warfarin and edoxaban are equally effective in treating CAT in terms of preventing recurrence and bleeding. However, warfarin therapy is associated with a probable sudden control failure, and the low TTR% ratio showed difficulty in warfarin control. This study showed the difficulty in properly controlling warfarin with concomitant anticancer drug administration, and also showed the effect of anticoagulation in terms of recurrence prevention.
The deidentified patients’ data will be shared on a request basis. Please directly contact the corresponding author for data sharing request. All datasets in this study and additional related documents will be available. Data will be available after publishing until the end of March, 2022. Access criteria for the data are limited to any researchers who study in the same field. Data will be shared in the Excel format via E-mail.
All authors report no disclosures.
This study was approved by the Tohoku University Ethics Committee, approval no. 2018-1-469.