Article ID: CJ-20-0883
Article ID: CJ-20-0883
Background: SATAKE HotBalloon® catheter (HBC) is a radiofrequency balloon catheter for the treatment of atrial fibrillation (AF), and was approved for use in Japan to treat drug-resistant paroxysmal AF in 2015. Post-marketing surveillance study was conducted by Toray Industries, Inc. to evaluate the efficacy and safety of HBC treatment in patients with paroxysmal AF in a real-world setting. This study is the first nation-wide survey of HBC treatment for paroxysmal AF in clinical practice in Japan.
Methods and Results: This was a single-arm, multicenter observational study with an observation period of 48 weeks after ablation. Pulmonary vein isolation and AF non-recurrence rates were evaluated and adverse events (AEs) were observed at 46 sites in Japan. An AF event was defined as recurrence of AF or re-ablation from 12 to 48 weeks after ablation. The success rate of pulmonary vein isolation was 99.0% (486/491) for patients with AF. The cumulative AF non-recurrence rate was 94.1% at 24 weeks and 87.8% at 48 weeks. AEs were found to occur 21.5% (114/530), and ablation-related AEs were found to occur 2.6% (14/530) during the study period, with the most common being pericardial effusion (0.8%, 4/530).
Conclusions: This study demonstrates the efficacy and safety of HBC ablation in Japanese patients with recurrent symptomatic paroxysmal AF refractory to antiarrhythmic therapy.
Atrial fibrillation (AF) is an arrhythmia that can cause disease with a high mortality risk such as stroke, heart failure, and sudden death, and it may induce left ventricular dysfunction even in patients without a history of heart disease.1 The prevalence of AF is estimated to be ~3% of adults,2,3 and increases with aging and the presence of hypertension, diabetes or other complications.4–10 According to a survey by the Japanese Circulation Society, the prevalence of AF is estimated to be 3.44% for men and 1.12% for women aged in their 70 s, and 4.43% for men and 2.19% for women aged in their 80 s in Japan.11 In Europe, 120,000–215,000 people are diagnosed with AF annually, and it is estimated that the number of patients with AF will be 14–17 million in 2030.4,12
AF is classified as paroxysmal, persistent or permanent based on duration of the episodes, and the rate at which paroxysmal AF becomes persistent is 5.0–8.6% per year.13 In general, AF initially responds to pharmacotherapy; however, it gradually develops resistance to treatment14 and catheter ablation is considered if pharmacotherapy is ineffective. The pathophysiology of AF is thought to involve the occurrence of abnormal electrical excitation and structural changes that cause reentry in the atrium including the pulmonary veins (PVs).14 In 1998, Haïssaguerre et al reported that 94% of extrasystoles that triggered AF occurred in the PVs and that ablation treatment against the PVs was effective.15 Previous reports suggest that initial ablation reduces 50–80% of paroxysmal AF.14,16–18
SATAKE HotBalloon® catheter (HBC) is a radiofrequency balloon catheter that ablates myocardial tissue through heating of the liquid inside the balloon, and it is possible to ablate the sites all at once by fitting the surface of the balloon to the shape of the PV ostium.19–21 In a pivotal clinical trial comparing PV isolation with HBC and pharmacotherapy in patients with paroxysmal AF who were resistant to drug treatment, the non-recurrence rate of AF was 4.7% in the pharmacotherapy group and 59.0% in the HBC group (P<0.001).22 HBC was approved in Japan in 2015 to treat recurrent symptomatic paroxysmal AF refractory to antiarrhythmic therapy and is now widely used in clinical practice. We conducted post-marketing surveillance study to evaluate the efficacy and safety of HBC under real clinical practice in Japan.
The purpose of this study was to evaluate the efficacy and safety of HBC ablation in patients with recurrent symptomatic paroxysmal AF refractory to antiarrhythmic therapy. This single-arm, multicenter observational study was carried out in compliance with the articles of the revised Declaration of Helsinki October 2013 and the Good Post-marketing Surveillance Practice (GPSP) ordinance from the Ministry of Health, Labor and Welfare in Japan. Consent to data publication was obtained from all the patients. This survey was conducted at 46 sites (Supplementary Table) in Japan, and all physicians participating in the study were required to participate in training programs for HBC conducted by Toray Industries, Inc. The target number of patients was set to be 400. From November 2015 to March 2017, the first 20 cases with HBC ablation at each site were to be enrolled in the study.
EndpointsIn this study, acute and chronic success rates were examined for efficacy endpoints. Acute success was defined as the achievement of electrical PV isolation and was calculated on a patient and a PV basis. Acute success rates were also evaluated by whether touch-up ablation using catheters other than HBC was performed or was not perfomed. Success or failure of PV isolation was determined by a physician according to institutional standards. Chronic success was defined as non-recurrence of AF from 12 to 48 weeks after ablation, and the cumulative AF non-recurrence rate during this period was evaluated. Twelve weeks after ablation was defined to be the blanking period, and recurrence of AF during the blanking period was not considered to be recurrence. Re-ablation between 12 and 48 weeks was counted as an AF event. An AF was determined by a physician based on a 12-lead ECG, 24-h Holter ECG or another ECG. The following procedural data were tabulated: balloon injection volume per PV, ablation time per PV, and number of ablations per PV. The total time from patient entry and exit from a cardiac catheterization (cath) lab, procedure time from transseptal puncture to removal, and fluoroscopy time were also tabulated.
For safety evaluation, adverse events (AEs), serious AEs (SAEs), device-related AEs, and ablation-related AEs were collected. Device-related AEs were defined as AEs for which a causal relationship with HBC cannot be ruled out. Ablation-related AEs were defined as AEs for which a causal relationship with the ablation procedure cannot be ruled out regardless of the causal relationship with HBC. Patient characteristics including age, sex, body weight, duration of AF, type of AF, left atrial diameter, left ventricular ejection fraction, history of PV ablation, underlying disease, CHA2DS2-VASc score, and anatomical characteristics of PVs were collected.
ProceduresHBC ablation was performed according to standard procedures at each site with the aim of electrical PV isolation of 4 (left superior PV [LSPV], left inferior PV [LIPV], right superior PV [RSPV], and right inferior PV [RIPV]) or other PVs (middle PV or common trunk PV) with esophageal cooling for avoiding esophagus injury.23 Transseptal catheterization was performed under fluoroscopic guidance and a navigation tool via the femoral vein and standard transseptal sheath, which was exchanged for a 17F outer diameter steerable sheath (Treswaltz®; Toray Industries, Inc.). Thereafter, HBC was advanced over a guidewire to the left atrial and to each PV ostium. Ablation was performed after confirmation of PV occlusion by inflated balloon for PV ostium, antrum and, subsequently, near the carina. Ablation temperature setting and ablation time were determined by the physician. The site of ablation was decided on by the physician and either PV ostium or antrum was selected in the case report form. Additional ablation was allowed if PV isolation was not achieved by HBC ablation. Pulmonary vein isolation was confirmed after ablation for each PV with the mapping catheter.
Statistical AnalysisA safety analysis set and an efficacy analysis set were determined for analysis. The safety analysis set was defined as the population for which case report forms (CRFs) were collected. The efficacy analysis set was the population excluding the following from the safety analysis set: (1) off label use; (2) cases with no description of efficacy data in the CRF; and (3) cases with pacemaker use other than AF treatment. Descriptive statistics were expressed using n, mean±SD, median (interquartile range), and min/max. AF non-recurrence rate after ablation was estimated using the Kaplan-Meier method; α was set to be 0.05. Statistical analysis was performed using SAS version 9.2 software (SAS Institute Inc., Cary , NC, USA). AEs were coded by the Medical Dictionary for Regulatory Activities (MedDRA), Japanese Version 21.1 and tabulated by System Organ Class (SOC) and Preferred Term (PT).
Five-hundred and forty-six cases were enrolled from 47 sites. Five-hundred and thirty patients with collected CRFs were included in the safety analysis set (Figure 1). The efficacy analysis was performed in 491 patients excluding the following cases from the safety analysis set: non-paroxysmal AF (n=33), unknown ablation details (n=4), pacemaker implantation before ablation (n=2), and unknown success or failure in isolation (n=1) (duplicated). Patient characteristics are shown in Table 1. The mean age was 65.4 years and 71.3% were male. Paroxysmal AF accounted for 93.8% of the patients and the mean left ventricular ejection fraction was 65.4%. Regarding the underlying disease, hypertension was found in 54.0% of the cases and 14.2% had diabetes. The mean CHA2DS2-VASc score was 2.0.
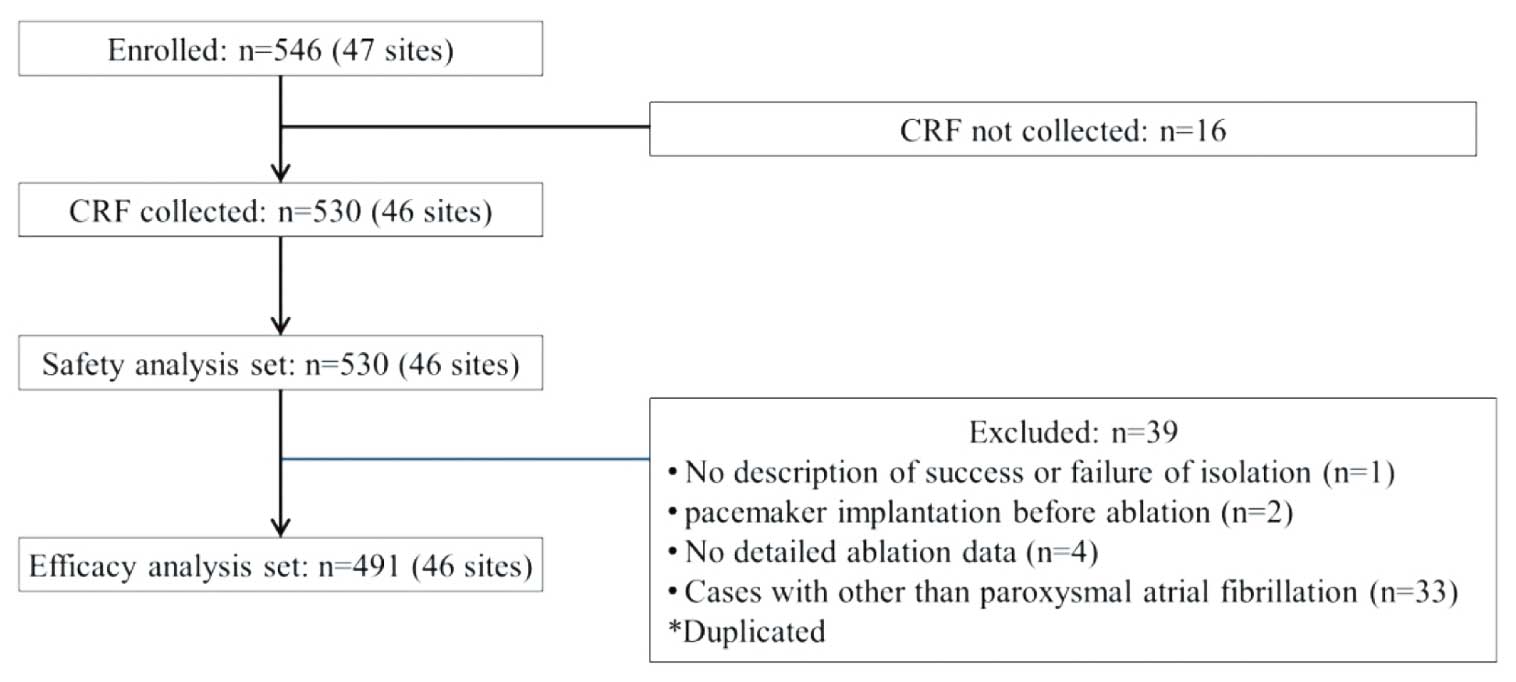
Flowchart detailing patient selection and disposition in the HBC post-marketing surveillance study. CRF, case report form.
| Safety analysis set, n | 530 |
| Age (years), mean±SD | 65.4±10.0 |
| Sex (male), n (%) | 378 (71.3) |
| Body weight (kg), mean±SD | 66.0±12.6 |
| Duration of AF (months), mean±SD | 38.5±51.4 |
| Types of AF, n (%) | |
| Paroxysmal AF | 497 (93.8) |
| Persistent AF | 31 (5.9) |
| Permanent AF | 2 (0.4) |
| Left atrial diameter (mm), mean±SD | 38.8±7.7 |
| Left ventricular ejection fraction (%), mean±SD | 65.4±7.4 |
| History of PV ablation, n (%) | 4 (0.8) |
| Underlying disease, n (%) | |
| Heart failure | 35 (6.6) |
| Hypertension | 286 (54.0) |
| Diabetes | 75 (14.2) |
| Cerebral infarction/TIA | 40 (7.6) |
| CHA2DS2-VASc score, mean±SD | 2.0±1.4 |
| Anatomical characteristics of PV (Paroxysmal AF cases), n | 497 |
| Right PV, n (%) | |
| Superior and inferior PV | 485 (97.6) |
| Common trunk | 0 (0) |
| Middle PV | 11 (2.2) |
| Others | 1 (0.2) |
| Left PV, n (%) | |
| Superior and inferior PV | 475 (95.6) |
| Common trunk | 19 (3.8) |
| Middle PV | 2 (0.4) |
| Others | 1 (0.2) |
AF, atrial fibrillation; PV, pulmonary vein; SD, standard deviation.
In the efficacy analysis set, the mean balloon injection volume was 11.5 mL, and the range of mean volume for the major pulmonary veins (RSPV, RIPV, LSPV and LIPV) was 10.9–12.0 mL (Figure 2A). The range of mean number of ablations for each PV was 1.6–2.6 (Figure 2B). The range of mean total ablation time for each pulmonary vein was 4.0–8.2 min (Figure 2C). The mean time from patient entry to exit was 201.7 min, procedure time was 120.1 min, and fluoroscopy time was 55.0 min. For those institutes with physicians with experience in conducting HBC ablation in the pivotal clinical trial, the mean time from patient entry to exit was 183.4 min, with a mean procedure time of 108.1 min and a mean fluoroscopy time of 47.9 min. Those institutes without physicians with HBC experience had a mean time from patient entry to exit of 212.9 min, a mean procedure time of 128.3 min, and a mean fluoroscopy time of 59.1 min.

(A) Balloon volume per PV (mL). (B) Number of ablations per PV. (C) Ablation time per PV (min). PV, pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein.
One-thousand, nine-hundred and thirty-eight PVs were ablated using HBC, and isolation was achieved in 1,933 PVs; the acute success rate was 99.7% on a pulmonary vein basis and 99.0% on a patient basis (486/491 patients) (Figure 3A). Touch-up ablation was performed in 21.0% (407/1,938) of PVs. The rate of using only HBC for ablation was 77.3% (1,499/1,938) on a PV basis and 48.3% (237/491) on a patient basis. The success rate of PV isolation using only HBC was 85.3% for RSPV, 79.5% for the RIPV, 61.7% for LSPV, 83.3% for LIPV, and 61.9% for other PVs (Figure 3B). The cumulative AF non-recurrence rate (95% CI) was 94.1% (91.5–95.9) at 24 weeks and 87.8% (84.4–90.5) at 48 weeks (Figure 4A). The cumulative AF non-recurrence rate (95% CI) for patients ablated with the HBC alone was 93.9% (89.9–96.4) at 24 weeks and 88.8% (83.8–92.3) at 48 weeks (Figure 4B).
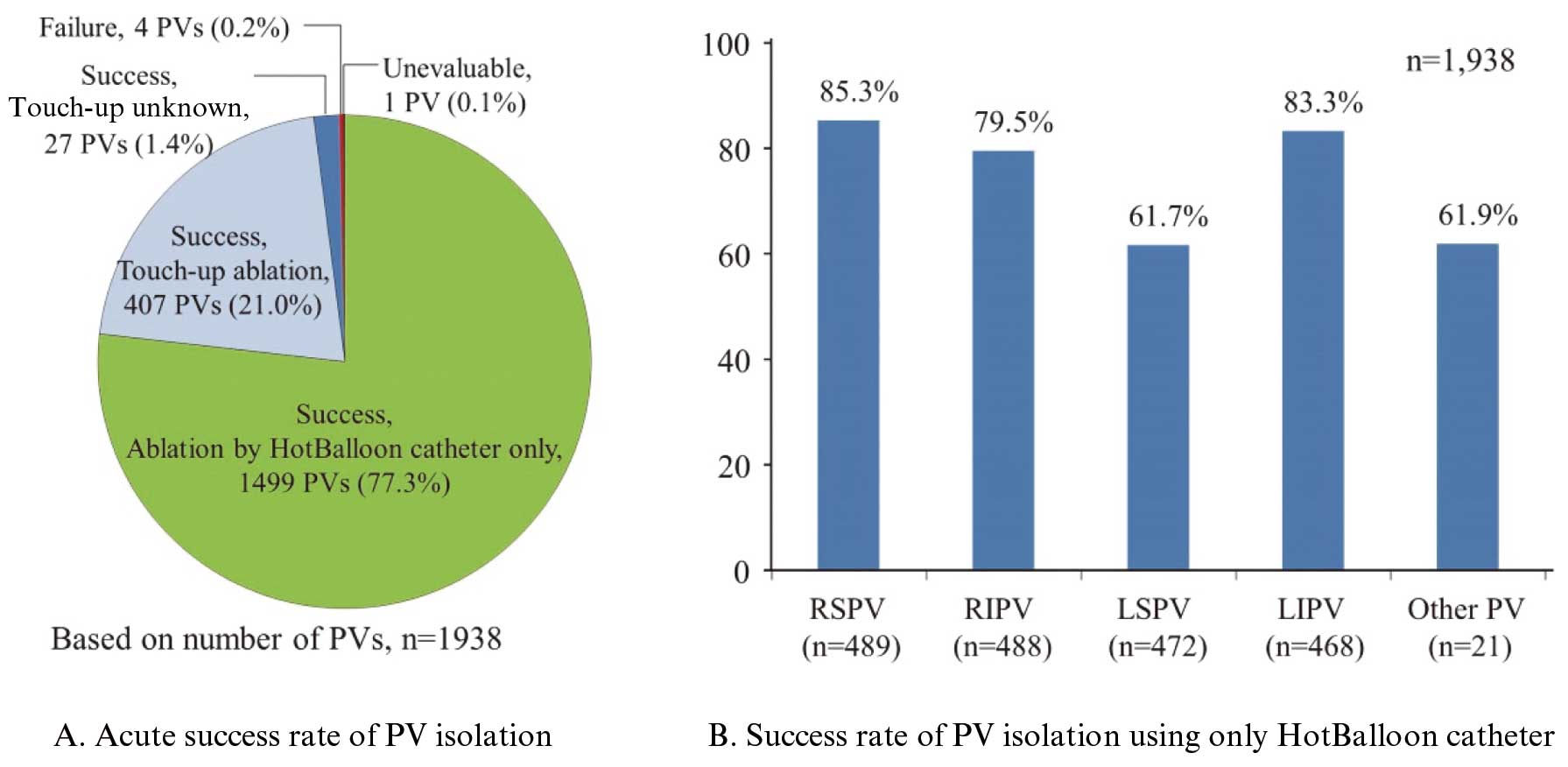
(A) Acute success rate of success rate of PV isolation. (B) Success rate of PV isolation using only HotBalloon catheter (HBC). PV, pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein.

Cumulative AF non-recurrence rate (%). (A) Cumulative AF non-recurrence rate: All cases. (B) Cumulative AF non-recurrence rate: Cases using only HBC. AF, atrial fibrillation; HBC, SATAKE HotBalloon® catheter.
Adverse events occurred in 2 or more patients, and details are shown in Table 2. AEs were found in 21.5% (114/530). The most common AEs were C-reactive protein increase and white blood cell count increase (both 3.4%, 18/530), followed by an aspartate aminotransferase increase (3.0%, 16/530), creatine phosphokinase increase (2.6%, 14/530), and pneumonia aspiration (2.6%, 14/530). PV stenosis was observed in 2 cases (0.4%). The definition of PV stenosis included the following: a PV stenosis rate >70%, a requirement of invasive treatment such as a PV stent, cases with serious clinical symptoms due to PV stenosis, or a physician’s diagnosis based on other symptoms. SAEs were found in 6.5% (35/539) including in one patient who died. The course of the deceased patient was as follows: the ablation procedure was completed without any problems and the patient was discharged from the hospital, but atrio-oesophageal fistula developed 16 days after the ablation. The patient had undergone esophageal fistula obstruction and had recovered, but died of sepsis 5 months after the ablation procedure. Any relationship to the device or procedure is unknown. The most common SAE was atrial fibrillation (1.5%, 8/530), followed by cardiac tamponade (0.9%, 5/530), and pneumonia aspiration (0.6%, 3/530). The onset of all AF as SAEs was not during the ablation procedure and occurred within 12 months after ablation. Device-related AEs were found in 14.0% (74/530), including phrenic nerve palsy (0.9%, 5/530), pericardial effusion (0.8%, 4/530) and cardiac tamponade (0.8%, 4/530). Ablation-related AEs were found in 2.6% (14/530), including pericardial effusion (0.8%, 4/530), cardiac tamponade (0.4%, 2/530), and cerebral infarction (0.4%, 2/530). AEs that were likely to accompany ablation were phrenic nerve paralysis (0.2%, 1/530), air embolism (0.2%, 1/530), and atrio-oesophageal fistula (0.2%, 1/530).
| Safety analysis set, n | 530 |
|---|---|
| AEs, n (%) | |
| Total | 114 (21.5) |
| Nervous system disorders | 7 (1.3) |
| Phrenic nerve paralysis | 5 (0.9) |
| Cerebral infarction | 2 (0.4) |
| Cardiac disorders | 37 (7.0) |
| Atrial fibrillation | 11 (2.1) |
| Atrial tachycardia | 7 (1.3) |
| Cardiac tamponade | 5 (0.9) |
| Atrial flutter | 4 (0.8) |
| Pericardial effusion | 4 (0.8) |
| Cardiac failure | 2 (0.4) |
| Pericarditis | 2 (0.4) |
| Sinus node dysfunction | 2 (0.4) |
| Vascular disorders | 10 (1.9) |
| Arteriovenous fistula | 3 (0.6) |
| Air embolism | 2 (0.4) |
| Vein dissection | 2 (0.4) |
| Respiratory, thoracic and mediastinal disorders | 24 (4.5) |
| Pneumonia aspiration | 14 (2.6) |
| Aspiration | 3 (0.6) |
| Pulmonary alveolar hemorrhage | 2 (0.4) |
| Pulmonary vein stenosis | 2 (0.4) |
| General disorders and administration site conditions | 3 (0.6) |
| Puncture site hemorrhage | 2 (0.4) |
| Investigations | 41 (7.7) |
| C-reactive protein increased | 18 (3.4) |
| White blood cell count increased | 18 (3.4) |
| Aspartate aminotransferase increased | 16 (3.0) |
| Blood creatine phosphokinase increased | 14 (2.6) |
| Blood lactate dehydrogenase increased | 6 (1.1) |
| Blood urea increased | 2 (0.4) |
| Troponin I increased | 2 (0.4) |
| SAEs, n (%) | |
| Total | 35 (6.6) |
| Nervous system disorders | 2 (0.4) |
| Cerebral infarction | 2 (0.4) |
| Cardiac disorders | 23 (4.3) |
| Atrial fibrillation | 8 (1.5) |
| Atrial flutter | 2 (0.4) |
| Atrial tachycardia | 2 (0.4) |
| Cardiac tamponade | 5 (0.9) |
| Sinus node dysfunction | 2 (0.4) |
| Vascular disorders | 6 (1.1) |
| Arteriovenous fistula | 2 (0.4) |
| Vein dissection | 2 (0.4) |
| Respiratory, thoracic and mediastinal disorders | 7 (1.3) |
| Pneumonia aspiration | 3 (0.6) |
| Pulmonary alveolar hemorrhage | 2 (0.4) |
| Ablation-related AEs, n (%) | |
| Total | 14 (2.6) |
| Nervous system disorders | 3 (0.6) |
| Cerebral infarction | 2 (0.4) |
| Cardiac disorders | 7 (1.3) |
| Pericardial effusion | 4 (0.8) |
| Cardiac tamponade | 2 (0.4) |
AE, adverse events; SAEs, serious adverse events.
The success rate of PV isolation in patients with paroxysmal AF was 99.0% on a patient basis, and the cumulative AF non-recurrence rate by 48 weeks after ablation was 87.8%. The rate for ablation-related AEs was 2.6%.
The cumulative AF non-recurrence rate at 48 weeks after ablation was 59.0% in a pivotal clinical trial of HBC reported by Sohara et al22 and was 87.8% in this study, indicating an improved chronic success rate. One reason for this difference could be procedural expertise. Dukkipati et al report that the AF non-recurrence rate was relatively higher when physicians with more ablation experience conducted the procedure.24 In a pivotal study of HBC, 104 drug refractory paroxysmal AF cases were collected from 17 sites, for an average of 6.1 cases per site,22 whereas in this study, 546 cases were collected from 47 sites, for an average of 11.6 cases per site. Considering that the average number of cases per site in this study was almost twice that of the Sohara et al study, the experience of the operating physicians may have contributed to the improved chronic success rate. Okumura et al reported that the cumulative AF non-recurrence rate (95% CI) was 88.4% (84.1–91.6) at 24 weeks after ablation in a post-marketing surveillance study of cryoballoon ablation in patients with paroxysmal AF in Japan.25 In our study, the cumulative AF non-recurrence rate after ablation was 94.1% (91.5–95.9) at 24 weeks and 87.8% (84.4–90.5) at 48 weeks. These results showed sufficient efficacy compared to the results obtained by Okumura et al, indicating that the efficacy of HBC ablation was confirmed in real-world clinical practice.
In this study, the acute success rate was 99.7% on a PV basis and 99.0% on a patient basis. The patient-based acute success rate in our study was equivalent to that of 93.0% in the pivotal study of HBC,22 99.8% in the post-marketing surveillance of cryoballoon ablation,25 and 97.6% in the pivotal study of cryoballoon.26 These results suggest that HBC ablation is also effective in the acute success rate. In a previous study of HBC reported by Sohara et al,22 the PV isolation rate using HBC alone was 93.0% on a patient basis, but it was only 48.3% in this study. The reason for this is that a left atrial ablation with another ablation catheter was prohibited and aimed to isolate PVs using the investigational device in the previous study, and this study did not restrict the use of other catheter. Thus, in this study, other catheters that the physicians were accustomed to use may have been used for touch-up ablations. However, the chronic success rates (95% CI) in patients who used only a HBC in this study were 93.9% (89.9, 96.4) at 24 week and 88.8% (83.8%, 92.3) at 48 weeks, and these results indicate the effectiveness of HBC.
In the safety evaluation, AEs were found in 21.5% (114/530) of enrolled cases. PV stenosis, an AE caused by ablation, was observed in 5.2% (7/134) of enrolled cases in the pivotal study of HBC22 and in 0.4% (2/530) of enrolled cases in this study. AEs related to diaphragm nerve damage were observed in 3.7% (5/134) of enrolled cases in the pivotal study of HBC;22 in this study, the rate for phrenic nerve paralysis was 0.9% (5/530), and all of these cases were non-serious in severity and they all recovered. The reason for the relatively lower rates of AEs related to diaphragm nerve damage compared to that in the pivotal study of HBC is not clear; however, it is possible that physician experience also had an effect on the lower rates of these AEs. The rate of SAEs was 6.6% (35/530), which was relatively lower than that for the pivotal study, which was 10.4% (14/134).22 The rate of ablation-related AEs was 2.6% (14/530), and was mainly pericardial effusion (0.8%), cardiac tamponade (0.4%), and cerebral infarction (0.4%). In the post-marketing surveillance of cryoballoon for paroxysmal AF in Japan, the incidence of ablation-related AEs was 3.9%,25 and the results of our study were similar. We consider our results to have demonstrated the safety of ablation with HBC in clinical practice in Japan.
In this study, the mean total procedure time was 120.1 min and the mean fluoroscopy time was 55.0 min. These times varied from case to case, with a total procedure time of 18.0 min minimum and 385.0 min maximum, and a fluoroscopy time of 15 min minimum and 256 min maximum. Catheter ablation time has been shown to be reduced according to the physician’s experience.22,25 One reason for the wide range of ablation times may be that this study collected the first 20 cases at each site and not all physicians had equal experience. In our study, the ablation time for the institutions with previous HBC experience showed a shorter ablation time than those institutions without HBC experience. Ablation time may be further reduced as experience is gained, and patient burden may be reduced accordingly. In the post-marketing surveillance of Cryoballoon,25 the procedure time and fluoroscopy time was 150.2 min and 53.2 min, respectively. Considering these times, the procedure time and fluoroscopy time for HBC are considered to be acceptable.
Study LimitationsThis was a post-marketing surveillance study. The catheter ablation procedure followed institutional guidelines and was not standardized. The results of this study did not consider the influence of the learning curve of the staff performing the procedure. The results of this study need to also be interpreted taking these into account. Physicians should start to use HBC under the support of experienced operators to achieve the appropriate levels of efficacy and safety.
Post-marketing surveillance in Japanese patients with paroxysmal AF shows sufficient efficacy and safety for HBC ablation.
We are grateful to the HotBalloon PMS sites and staff for their valuable contributions to this surveillance. The authors wish to thank AMY Information Planning LLC for providing medical writing support.
This study was conducted by Toray Industries, Inc.
Y.Y. received remuneration from Medtronic Japan Co., Ltd., BIOTRONIK Japan, Inc., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Company, Limited, Abbott Japan LLC, and Century Medical, Inc. and consulting fee from Toray Industries, Inc. T.Y. received remuneration from Daiichi Sankyo Company, Limited, Nippon Boehringer Ingelheim Co., Ltd., Abbott Japan LLC, Bristol-Myers Squibb K.K., Bayer Yakuhin, Ltd., Medtronic Japan Co., Ltd., and Toray Industries, Inc. S.N., A.K. and H.S. received remuneration from Toray Industries, Inc. K.A. received remuneration from Daiichi Sankyo Company, Limited and Nippon Boehringer Ingelheim Co., Ltd., scholarship funds to the affiliated department from Abbott Medical Japan LLC., ASTEC Co., Ltd., and belongs to an endowed department from Boston Scientific Japan K.K., Japan Lifeline Co., Ltd., NIHON KOHDEN CORPORATION, BIOTRONIK Japan, Inc., Nippon Boehringer Ingelheim Co., Ltd., Toray Industries, Inc., Century Medical, Inc., and Abbott Medical Japan LLC. S.S. holds patents related to the HotBalloon catheter.
This post-marketing surveillance was conducted in compliance with good post-marketing surveillance practices (GPSP) issued by the Ministry of Health, Labor and Welfare in Japan, and approval of the protocol by the Institutional Review Board was not required.
Participant data will not be shared.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0883