Abstract
Background:
The arctic front cryoballoon (AF-CB) provides effective and durable pulmonary vein isolation (PVI) associated with encouraging clinical outcome. The POLARx cryoballoon incorporates unique features and design changes that may translate into improved efficacy, safety and further simplified balloon-based procedures. Efficacy and safety of the novel POLARx cryoballoon was compared to the fourth generation AF-CB (AF-CB4).
Methods and Results:
Twenty-five consecutive patients with paroxysmal or persistent atrial fibrillation were prospectively enrolled, underwent POLARx-based PVI (POLARx group) and were compared to 25 consecutive patients treated with the AF-CB4 (AF-CB4 group). All PVs were successfully isolated utilizing the POLARx and AF-CB4. A significant difference regarding the mean minimal cryoballoon temperatures reached using the AF-CB4 and POLARx (−50±6℃ vs. −57±7℃, P=0.004) was observed. Real-time PVI was visualized in 81% of POLARx patients and 42% of AF-CB4 patients (P<0.001). Utilizing the POLARx, a trend towards shorter median procedure time (POLARx: 45 [39, 53] min vs. AF-CB4: 55 [50, 60] min; P=0.062) was found. No differences were observed between AF-CB4 and POLARx concerning catheter maneuverability, catheter stability and periprocedural complications.
Conclusions:
The novel POLARx showed similar safety and efficacy compared to the AF-CB4. A higher rate of real-time PV recordings and significantly lower minimal balloon temperatures were observed using the POLARx.
Use of the second- and fourth-generation arctic front advance (pro) cryoballoon (AF-, CB2/CB4; Medtronic, Inc., Minneapolis, MN, USA) for pulmonary vein isolation (PVI) has demonstrated high procedural success rates and encouraging 1-year to 5-year clinical outcomes for patients with paroxysmal (PAF) and persistent atrial fibrillation (PersAF).1–9
The Fire-and-Ice trial showed non-inferiority of cryoballoon vs. radiofrequency-based PVI.10
The clinical outcome is based on a high rate of durable PVI ranging between 69% and 77% for patients undergoing repeat procedures due to AF recurrence.11–13
The recently introduced POLARx cardiac cryoablation system (Boston Scientific, St. Paul, MN, USA) offers unique design changes and features to possibly improve efficacy and safety, as well as to further simplify balloon-based PVI procedures. Cryoballoon dislodgement from the PV ostium (pop-out phenomenon) is frequently observed after initializing the refrigerant injection and results in increased pressure and expansion of the AF-CB. The POLARx offers a stable size of 28 mm and equal balloon pressure during the inflation as well as the ablation period, and therefore possibly prevents the pop-out phenomenon, as well as slightly shifting and causing movements of the balloon after initializing the freezing cycle. Furthermore, the POLARx system offers the control of inflation, ablation, deflation, double stop and documenting the time to effect (TTE) via a foot pedal and a slider switch for increasing the comfort for the operator. A novel diaphragm movement sensor (DMS) is visualizing the phrenic nerve (PN) pacing during the ablation procedures. Whether these features maintain the favorable freezing characteristics seen with the recent version of the AF-CB (AF-CB4) demands evaluation. The present study reports the first experience and acute procedural efficacy using the novel POLARx cryoballoon in comparison to the AF-CB4.
Methods
Inclusion and Exclusion Criteria
Consecutive patients with symptomatic, drug-refractory PAF or short standing PersAF (duration ≤3 months) were recruited for CB-based PVI (Figure 1). From 5 August to 12 October 2020, 25 consecutive patients were treated with the POLARx cryoballoon (POLARx group,
Figure 2). A total of 25 consecutive previous patients (from 22 May to 27 July 2020) treated with the AF-CB4 (AF-CB4 group) served as a control group. The patients were not randomized. The procedures were performed by operators with high experience in CB procedures (n=4) only. Exclusion criteria were prior left atrial (LA) ablation attempts, a LA diameter >60 mm, severe valvular heart disease or contraindications to postinterventional oral anticoagulation. Transesophageal echocardiography was performed in all patients prior to PVI to rule out intracardiac thrombi and to assess the LA diameter. No further pre-procedural imaging was performed. In patients on vitamin K antagonists, the procedure was performed under therapeutic international normalized ratio (INR) values of 2–3. In patients on new oral anticoagulants, the morning dose on the day of the procedure was omitted. All patients gave written informed consent and all patient information was anonymized. The ongoing ICE-AGE-X registry study was approved by the local ethic’s board (Luebeck Ablation Registry Ethical Review Board number: WF-028/15) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The detailed intraprocedural management has been described in previous studies from our center.13,14
In brief, the procedure was performed in patients under deep sedation using midazolam, sufentanyl and propofol. Two ultrasound-guided right femoral vein punctures were performed and 2x 8F short sheaths were inserted. Prior to transseptal puncture, 1 diagnostic catheter was introduced via the right femoral vein and positioned within the coronary sinus (7F; Biosense Webster, Inc. Diamond Bar, CA, USA). Single transseptal puncture was performed under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5F transseptal sheath (AF-CB4 group: SL1; St. Jude Medical, Inc.; POLARx group: TSX transseptal delivery system and TSX transseptal needle; Boston Scientific). After transeptal puncture via the needle injection of contrast medium was performed to confirm LA access, selective PV angiography was performed to identify the pulmonary vein (PV) ostia utilizing a 7F multipurpose catheter or was performed directly via the transseptal sheath. The transseptal sheath was exchanged over a guidewire for the 15.9 F POLARSHEATH (POLARx group) or the 15 F Flexcath Advance sheath (AF-CB4 group). Both sheaths were continuously flushed with heparinized saline (20 mL/h). After transseptal puncture, heparin boluses were administered targeting an activated clotting time of >300 s.
Cryoballoon-Based PVI: General
Figure 3
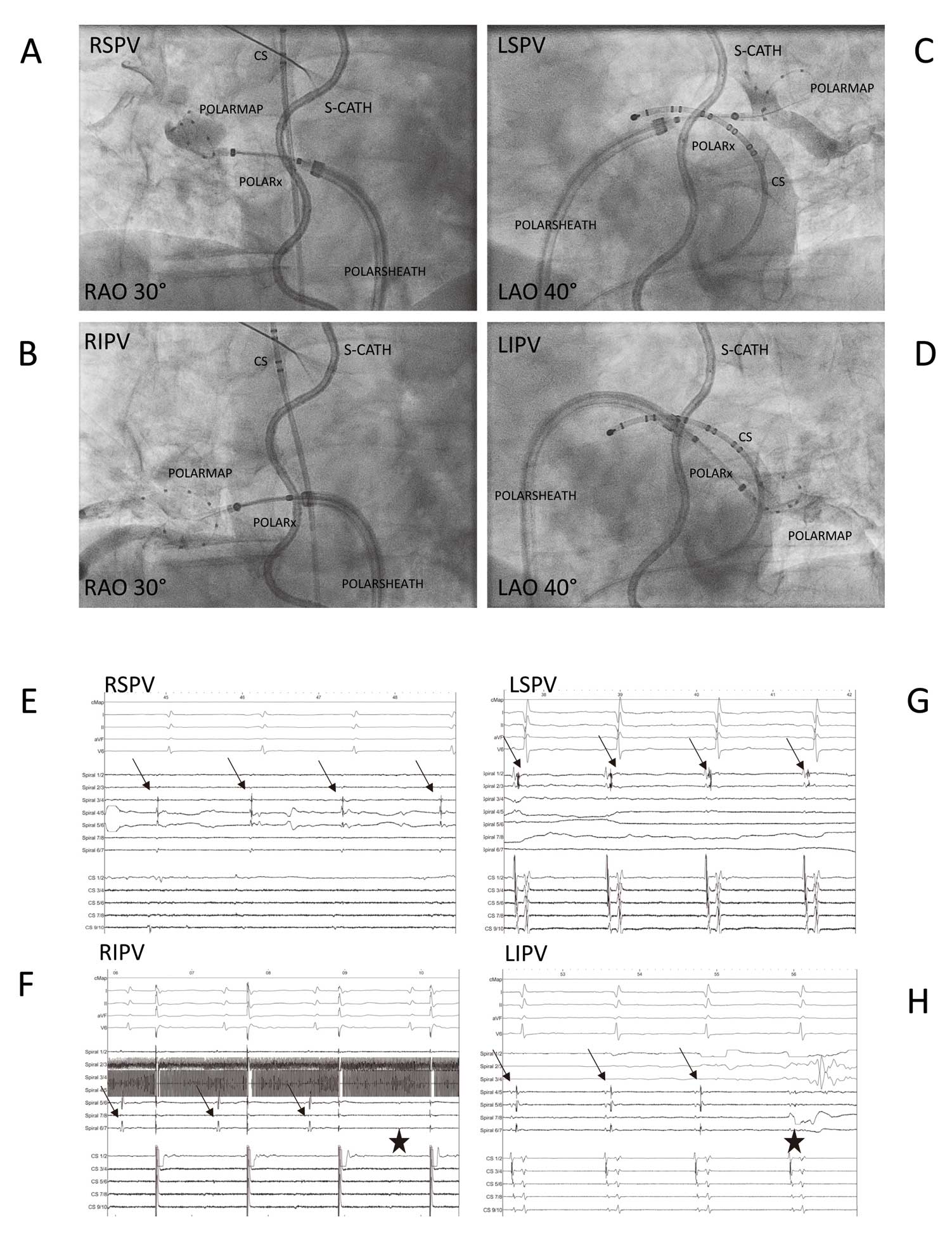
is showing periprocedural images of both cryoablation systems. The PVs were treated following a clockwise sequence (left superior, left inferior, right inferior and right superior pulmonary vein [LSPV, LIPV, RIPV, RSPV]). A gentle pull-down maneuver was performed for LIPV and RIPV after 70 s of freezing time. A TTE-based ablation protocol was utilized for both cryoballoon systems. The standard freeze-cycle duration was 180 s. If the TTE could be visualized and was measured for <60 s, the freeze-cycle duration was 180 s and no further bonus-freeze application was performed. If TTE was measured ≥60 s, the freeze-cycle duration was 180 s and a bonus-freeze application of 180 s was performed. The procedural endpoint was disappearance of PV recordings verified via the circular mapping catheter after the freeze cycle (entrance block). No additional pacing of adenosine testing has been performed. The occlusion of the PV ostium was verified by contrast dye injections. After 5–10 s of freezing, a second contrast dye injection was administered to verify stable occlusion. If stable occlusion was verified, the freeze cycle was continued; if the occlusion was not perfect, the balloon was slightly repositioned and a third contrast dye injection was utilized to verify occlusion or the freeze cycle was stopped and a further try was conducted. The minimal cryoballoon cut-off was set at −60℃ for the AF-CB4 and −70° for the POLARx. A spiral temperature probe (CIRCA S-CATH; Circa Scientific, Englewood, CO, USA) was advanced into the esophagus to monitor esophageal temperatures during each energy application. The intraluminal esophageal temperature cut-off was set at 15℃.15,16
During energy delivery along the septal PVs, continuous PN pacing was performed using a diagnostic catheter introduced into the superior vena cava (7F; Webster TM, Biosense Webster, Inc.). Pacing was set at maximum output and pulse width (12 mA, 2.9 ms) and a cycle length of 1,200 ms. PN capture was monitored by tactile feedback of diaphragmatic contraction and assessment of the right diaphragmatic compound motor action potential (CMAP). Energy delivery was interrupted immediately if weakening or loss of diaphragmatic contraction was noted or a decrease of the CMAP amplitude of ≥30% was seen. In case of persistent PN palsy, no further cryoenergy was delivered to the septal PVs.17,18
The detailed intraprocedural management utilizing AF-CB2 and AF-CB4 has been described in previous studies.9,13
The pop-out phenomenon was defined by the observation of a balloon dislodgement from the PV ostium after initializing the freezing process. This was evaluated by a second injection of contrast medium and fluoroscopy 5–10 s after initializing the freezing process.
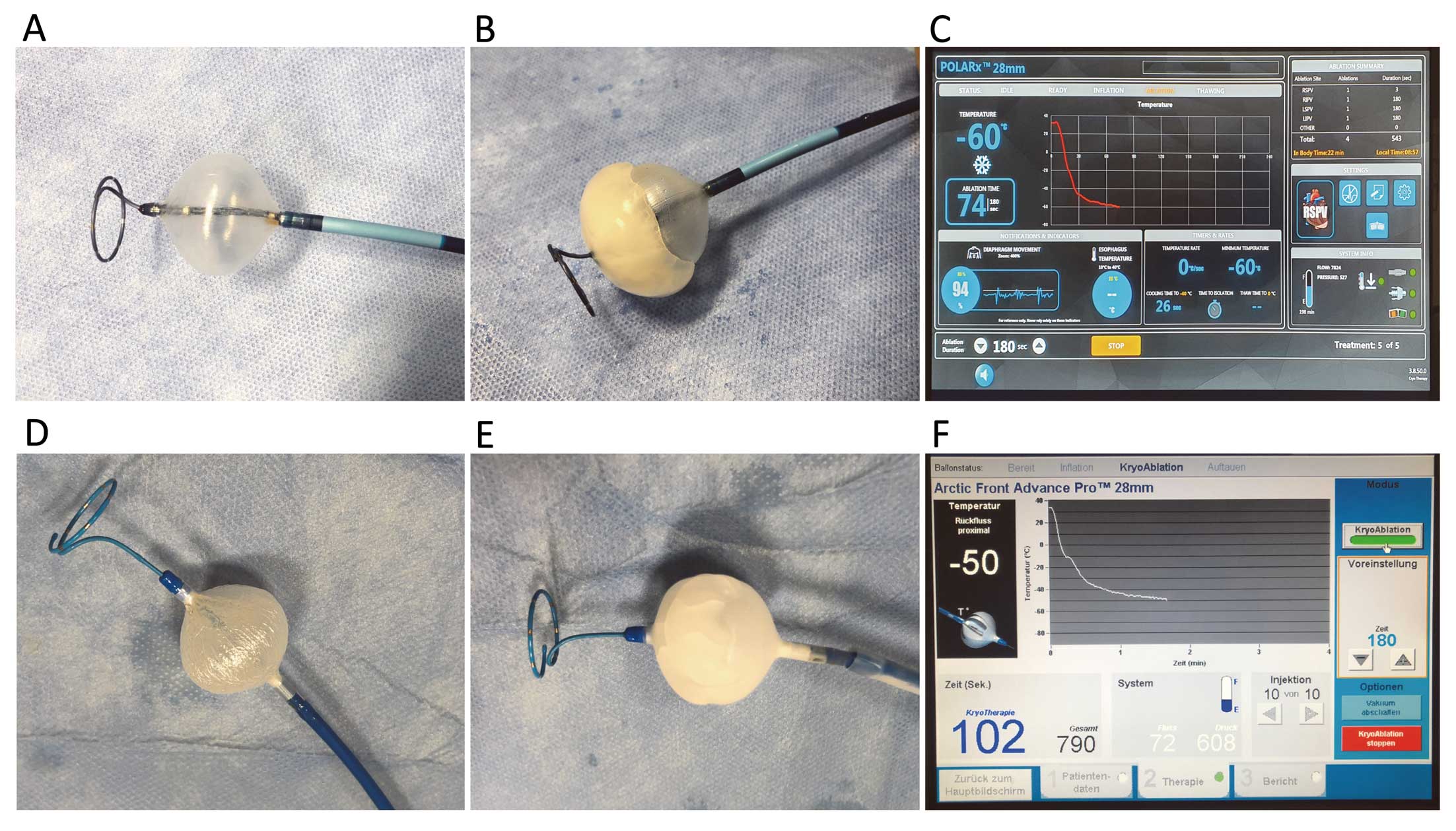
The POLARx cryoballoon is available in 2 versions with different lengths of the distal tip. The short-tip version provides a 5-mm distal tip whereas the long-tip version provides a 12-mm tip. The POLARMAP catheter (Boston) is available in 20 mm diameter only. It provides a total of 8 electrodes (each 1 mm in diameter) with 6-mm spacing.
Figure 4
shows representative periprocedural images. The POLARx was advanced into the LA via the 15.9F steerable POLARSHEATH using the POLARMAP catheter for guidance. For superior PVs, a superior branch and for inferior PVs, the most inferior branch was targeted by the POLARMAP. The POLARx was inflated via the green foot pedal proximal to the PV ostium followed by a gentle push, aiming for complete PV sealing. The POLARMAP was positioned at a proximal position to achieve live PV recordings and measurements of TTE. The occlusion of the PV ostium was verified by contrast dye injections and the freeze cycle was started by the green foot pedal. During the freeze cycle, the TTE was documented via the green foot pedal (press and hold for 3 s). The procedural endpoint was defined as persistent PVI verified by POLARMAP catheter recordings after the individual application. After each application, the POLARx was inflated and deflated via the slider-switch. The minimal cryoballoon cut-off was set at −70℃. Apart from the above-mentioned safety maneuvers for PN palsy prevention, the novel DMS was utilized to monitor PN function. The DMS sensor is based on an accelerometer technology and is placed on a disposable electrode below the right-sided costal cartilage. Baseline DMS is automatically assessed when general PN pacing is started. The DMS cut-off was set at 60% of diaphragm movement. The freeze cycle was terminated by double stop if the cut-off was reached and no PN capture was detected immediately. The double stop was conducted via the orange foot pedal.
A figure-of-eight suture and a pressure bandage were used to prevent femoral bleeding. The pressure bandage was removed after 4 h and the figure-of-eight suture was removed the next day. Following ablation, all patients underwent transthoracic echocardiography immediately, after 2 h, and at day 1 to rule out a pericardial effusion. Low molecular-weight heparin was administered in patients on vitamin K antagonists and with an INR <2.0 until a therapeutic INR of 2–3 was achieved. New oral anticoagulants were re-initiated 6 h post ablation. Anticoagulation was continued for at least 3 months and continued thereafter based on the individual CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs or a new antiarrhythmic drug was prescribed and continued for 3 months post ablation. All patients were treated with proton-pump inhibitors for 6 weeks.
Statistical Analysis
Differences of metric variables between the 2 groups were analyzed with a t-test if the data were normally distributed, and with the Wilcoxon-Mann-Whitney U-test otherwise. Differences between categorical variables were evaluated using the chi-squared test or Fisher’s exact test in case of small expected cell frequencies. PVI data were analyzed using mixed models: linear mixed models were used for continuous data and generalized linear mixed models were used for binary or count data. A hierarchical logistic regression model was applied for binary data. A Poisson distribution was assumed for count data. All P values are 2-sided and a P value <0.05 was considered significant. All calculations were performed with the statistical analysis software, SAS (SAS Institute Inc., version 9.3, Cary, NC, USA).2
Results
Patient Characteristics
A total of 50 consecutive patients underwent CB-based PVI utilizing either the AF-CB4 (1st
n=25 cases) or the POLARx (2nd
n=25 cases). Patient baseline characteristics are shown in
Table 1. No imbalances were apparent between the groups.
Table 1.
Patient Baseline Characteristics
| Variable |
POLARx |
AF-CB4 |
P value |
| Patients, n |
25 |
25 |
|
| Age (years) |
68 (62, 73) |
69 (59, 75) |
0.976 |
| Female gender |
12 (48) |
8 (32) |
0.248 |
| Paroxysmal AF |
12 (48) |
9 (36) |
0.391 |
| LA-size (mL/m2) |
25 (25, 30) |
29 (25, 35) |
0.548 |
| Congestive heart failure |
2 (8) |
4 (16) |
0.384 |
| Arterial hypertension |
20 (80) |
18 (72) |
0.508 |
| Diabetes mellitus type 2 |
3 (12) |
3 (12) |
0.999 |
| Coronary artery disease |
4 (16) |
6 (24) |
0.478 |
| CHA2DS2-VASc-Score 0 |
4 (16) |
1 (4) |
0.157 |
| CHA2DS2-VASc-Score 1 |
2 (8) |
5 (20) |
0.221 |
| CHA2DS2-VASc-Score 2 |
6 (24) |
5 (20) |
0.733 |
| CHA2DS2-VASc-Score 3 |
8 (32) |
9 (36) |
0.765 |
| CHA2DS2-VASc-Score 4 |
2 (8) |
4 (16) |
0.384 |
| CHA2DS2-VASc-Score 5 |
2 (8) |
1 (4) |
0.552 |
| CHA2DS2-VASc-Score 6 |
1 (4) |
0 (0) |
0.322 |
Values expressed as n (%) or median (range). AF, atrial fibrillation; LA, left atrium.
In 50 patients, a total of 197 PVs were identified and targeted for ablation (50 RSPV, 50 RIPV, 47 LSPV, 47 LIPV and 3 left common PVs [LCPV]). All PVs were successfully isolated using either the AF-CB4 (n=97) or the POLARx (n=100). To achieve the highest comparability to the AF-CB4, which is providing a short distal tip, unless the first case used the long-tip POLARx, only the short-tip POLARx was utilized. There was a significant difference with regard to the mean minimal CB temperatures reached using the AF-CB4 and POLARx (−50±6℃ vs. −57±7℃, P=0.004). However, no difference was found for the mean total number of freeze cycles per PV until isolation, the mean total number of freeze cycles per PV until PVI, the mean TTE, the total freeze time applied or the mean minimal esophageal temperature (Table 2). Real-time PVI was visualized in 81% of patients using the POLARX and 42% of patients using the AF-CB4 (P<0.001). A statistical trend was observed towards shorter median of procedures time utilizing the POLARx: (45 [39, 53] min vs. AF-CB4: 55 [50, 60] min; P=0.062). No difference was observed for the median fluoroscopy time and median of amount of contrast dye utilized. No differences have been observed between AF-CB4 and POLARx concerning catheter maneuverability and catheter stability along the targeted PVs. After discharge, all patients received previously ineffective antiarrhythmic drugs or new antiarrhythmic drugs post ablation. The recommended duration was 3 months.
Table 2.
Procedural Details
| Variable |
POLARx |
AF-CB4 |
P value |
| Number of patients, n |
25 |
25 |
|
| Number of PVs, n |
100 |
97 |
|
| Total number of isolated PVs |
100 (100) |
97 (100) |
0.999 |
| Total CB cycles until PVI |
1.1±0.4 |
1.2±0.5 |
0.978 |
| Total CB cycles |
1.2±0.5 |
1.2±0.5 |
0.999 |
| FAAVI |
17 (68) |
11 (44) |
0.087 |
| Minimal CB temperature (℃) |
−57±7 |
−50±6 |
0.004* |
| Minimal esophageal-temperature (℃) |
31.4±6.4 |
31.6±6.1 |
0.846 |
| Time to PVI, s |
48±32 |
41±23 |
0.213 |
| Rate of TTI recordings |
81 (81) |
41 (42) |
<0.001* |
| Duration of total freezing time, s |
211±70 |
208±81 |
0.445 |
| Total procedure time, min |
45 (39, 53) |
55 (50, 60) |
0.062 |
| Total fluoroscopy time, min |
8 (6, 12) |
12 (9, 15) |
0.133 |
| Total amount of contrast, mL |
60 (50, 80) |
70 (50, 83) |
0.847 |
| Periprocedural complications n (%) |
| Major complications |
1 (4) |
1 (4) |
0.999 |
| Cardiac tamponade |
0 |
0 |
0.999 |
| Severe bleeding |
0 |
0 |
0.999 |
| Nervus phrenicus injury |
1 (4) |
1 (4) |
0.999 |
| Stroke / TIA |
0 |
0 |
0.999 |
| Severe bleeding |
0 |
0 |
0.999 |
| Minor complications |
1 (4) |
0 |
0.322 |
| Minor bleeding |
0 |
0 |
0.999 |
| Pericardial effusion |
0 |
0 |
0.999 |
| Transient air embolism |
1 (4) |
0 |
0.322 |
Values expressed as n (%), mean±standard deviation or median (range) as appropriate. *P<0.05. CB, cryoballoon; FAAVI, first attempt all veins isolated; PV(s), pulmonary vein(s); PVI, pulmonary vein isolation; TIA, transient ischemic attack; TTI, time to isolation.
Ablation data per individual PV is summarized in
Table 3. For LSPV, LIPV, RSPV and RIPV, a significant difference was detected concerning minimal cryoballoon temperature, as well as rate of TTE recordings. No differences were observed for the minimal esophagus temperature, time to PVI, rate of pop-out phenomenon and total freezing duration.
Table 3.
Procedural Details for Individual PVs
| Variable |
POLARx |
AF-CB4 |
P value |
| LSPV: |
25 |
22 |
|
| Total cycles until PVI |
1 (1, 1) |
1 (1, 1) |
0.835 |
| Total cycles |
1 (1, 1) |
1 (1, 1) |
0.970 |
| FAAVI |
20 (80) |
17 (77) |
0.333 |
| Bonus Freeze cycles |
2 (8) |
0 (0) |
0.155 |
| Minimal temperature (℃) |
−61 (−65, −56) |
−49 (−54, −49) |
<0.001* |
| Minimal esophageal-temperature (℃) |
34 (29, 35) |
35 (29, 35) |
0.874 |
| Time to PVI, s |
37 (30, 41) |
50 (38, 58) |
0.232 |
| Time to −40℃, s |
30 (27, 30) |
− |
|
| Thawing to 0℃, s |
19 (18, 20) |
– |
|
| Rate of TTI recordings |
22 (88) |
12 (55) |
0.011* |
| Duration of total freezing time, s |
180 (180, 360) |
180 (180, 180) |
0.776 |
| Pop-out phenomenon |
0 (0) |
1 (5) |
0.322 |
| LIPV: |
25 |
22 |
|
| Total cycles until PVI |
1 (1, 1) |
1 (1, 1) |
0.328 |
| Total cycles |
1 (1, 1) |
1 (1, 1) |
0.808 |
| FAAVI |
23 (92) |
17 (77) |
0.157 |
| Bonus Freeze cycles |
2 (8) |
0 (0) |
0.155 |
| Minimal temperature (℃) |
−55 (−58, −53) |
−48 (−53, −44) |
<0.001* |
| Minimal esophageal-temperature (℃) |
33 (25, 35) |
33 (15, 35) |
0.544 |
| Time to PVI, s |
35 (23, 52) |
25 (21, 32) |
0.399 |
| Time to −40℃, s |
32 (28, 35) |
– |
|
| Thawing to 0℃, s |
18 (16, 20) |
– |
|
| Rate of TTI recordings |
21 (84) |
10 (45) |
<0.01* |
| Duration of total freezing time, s |
180 (180, 180) |
180 (180, 180) |
0.999 |
| Pop-out phenomenon |
0 (0) |
0 (0) |
0.999 |
| LCPV: |
0 |
3 |
|
| Total cycles until PVI |
– |
2 (2, 2) |
|
| Total cycles |
– |
2 (2, 2) |
|
| FAAVI |
– |
1 (33) |
|
| Bonus Freeze cycles |
– |
0 (0) |
|
| Minimal temperature (℃) |
– |
−48 (−55, −48) |
|
| Minimal esophageal-temperature (℃) |
– |
35 (33, 36) |
|
| Time to PVI, s |
– |
56 (56, 56) |
|
| Rate of TTI recordings |
– |
1 (33) |
|
| Duration of total freezing time, s |
– |
360 (255, 360) |
|
| RSPV: |
25 |
25 |
|
| Total cycles until PVI |
1 (1, 1) |
1 (1, 1) |
0.959 |
| Total cycles |
1 (1, 1) |
1 (1, 1) |
0.957 |
| FAAVI |
22 (88) |
21 (84) |
0.634 |
| Bonus Freeze cycles |
1 (4) |
1 (4) |
0.999 |
| Minimal temperature (℃) |
−55 (−62, −52) |
−53 (−54, −47) |
0.016* |
| Minimal esophageal-temperature (℃) |
35 (34, 35) |
35 (34, 35) |
0.957 |
| Time to PVI, s |
40 (35, 55) |
30 (15, 40) |
0.101 |
| Time to −40℃, s |
32 (27, 35) |
– |
|
| Thawing to 0℃, s |
18 (15, 21) |
– |
|
| Rate of TTI recordings |
17 (68) |
9 (36) |
0.024* |
| Duration of total freezing time, s |
180 (180, 180) |
180 (180, 180) |
0.405 |
| Pop-out phenomenon |
0 (0) |
0 (0) |
0.999 |
| RIPV: |
25 |
25 |
|
| Total cycles until PVI |
1 (1, 1) |
1 (1, 1) |
0.839 |
| Total cycles |
1 (1, 1) |
1 (1, 1) |
0.679 |
| FAAVI |
22 (88) |
21 (84) |
0.684 |
| Bonus Freeze cycles |
2 (8) |
1 (4) |
0.561 |
| Minimal temperature (℃) |
−56 (−59, −52) |
−48 (−52, −44) |
<0.001* |
| Minimal esophageal-temperature (℃) |
35 (30, 35) |
35 (32, 35) |
0.900 |
| Time to PVI, s |
51 (44, 64) |
40 (40, 45) |
0.474 |
| Time to −40℃, s |
34 (29, 39) |
– |
|
| Thawing to 0℃, s |
18 (15, 19) |
– |
|
| Rate of TTI recordings |
21 (84) |
9 (36) |
<0.001* |
| Duration of total freezing time, s |
180 (180, 180) |
180 (180, 180) |
0.558 |
| Pop-out phenomenon |
0 (0) |
3 (12) |
0.077 |
Values expressed as n (%), mean±standard deviation or median (range) as appropriate. *P<0.05. LCPV, left common pulmonary vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein. Other abbreviations as in Table 2.
Transient PN palsy occurred in 1 of 25 (4%) patients of the AF-CB4 group and 1 of 25 (4%) patients of the POLARx group (both during energy delivery targeting the RSPV after 63 s [AF-CB4] and after 62 s [POLARx], P=0.999). Both RSPV were already isolated when PN palsy was detected. No further cryoballoon application was performed after PN palsy. Fluoroscopy on the next day showed persistent PN palsy for both patients. No further follow up was available to date. Concerning the PN palsy during PVI utilizing the POLARx, the DMS showed a minimal diaphragm movement of 40%.
A transient ST-elevation for <10 min was observed during the procedure in the POLARx group (patient #11: 1 of 25, 4%). Due to the shortness of the ST-elevation, an air embolism was presumed. The patient did not complain about any symptoms of postprocedural chest pain. No pericardial effusion, pericardial tamponade, TIA/stroke, or atrioesophageal fistula was observed either for the AF-CB4 or the POLARx.
Discussion
The current ICE-AGE-X study set out to compare the procedural efficacy and ablation characteristics of the novel POLARx to the AF-CB4 for PVI. The major findings are: (1) the POLARx provides an identical rate of acute PVI as the AF-CB4; (2) the rate of real-time PV-recordings was significantly higher in the POLARx group; (3) the minimal CB temperature was significantly lower in the POLARx group; (4) the trend towards shorter procedure time was observed for the POLARx; and (5) no differences were observed between AF-CB4 and POLARx concerning catheter maneuverability, catheter stability and periprocedural complications.
Up-to-date PVI forms the cornerstone of invasive AF therapy since the Fire-and-Ice trial showed non-inferiority of cryoballoon-based PVI compared to radiofrequency balloon-based PVI is increasingly performed.10
Due to the fact that balloon-based PVI provides shorter learning curves, shorter procedures times as well as high rates of safety and efficacy, various catheter ablation systems utilizing different energy sources are currently under investigation. However, randomized controlled data are only available for the AF-CB2 and the laserballoon (Heartlight, Cardiofocus).10
Although the AF-CB2 and AF-CB4 provides excellent clinical data and performance, the POLARx balloon offers some unique tools that possibly improve patient safety, efficacy and the operators’ convenience.
The present ICE-AGE X study shows that the POLARx provides equal acute success and equal rate of periprocedural complications despite the operators having to pass a certain learning curve. The observed median procedure time showed a trend towards shorter values for the POLARx, which is most likely conducted by the combination of the foot pedal, slider switch, the POLARSHEATH, as well as the stable balloon pressure when initializing the freeze cycle. These features are improving and reducing the workflow, and are providing more direct control to the operator and possibly reducing the procedure time.
The mean minimal balloon temperature reached with the POLARx was significantly lower than that with the AF-CB4; however, this was not translated to lower minimal intraluminal esophagus temperatures, and the acute efficacy was similar for both cryoballoon systems. This observation might be explained by different balloon material properties, differences in expansion pressure or a slightly different position of the temperature probe within the POLARx system compared to the AF-CB4.
Characteristic complications of cryoballoon-based PVI such as PN palsy could be significantly reduced by utilizing recent strategies like CMAP, PN pacing and tactile feedback. The new DMS sensor offers an additional tool to further improve safety of cryoballoon-based procedures. Nevertheless, 1 patient of the POLARx group experienced PN injury despite utilizing the DMS. Therefore, the DMS along with double stop deflation was not able to avoid persistent PN injury, and the usability and the benefit of this additional tool might be shown in future upcoming studies. We strongly suggest to use predefined thresholds of the minimal cryoballoon temperature (here −70℃) and minimal DMS (here 60%) to avoid PN injury. Furthermore, freezing inside the right-sided PVs should be avoided and should be limited to the PV ostia.
Cryoballoon dislodgement from the PV ostium (pop-out phenomenon) is occasionally observed after initializing the refrigerant injection and resulted in increased pressure and expansion of the cryoballoon. Although no significant differences were observed between the groups, the POLARx never showed a pop-out phenomenon in this study. Although we only observed 25 cases, this observation is remarkable.
The POLARMAP catheter provides a high rate of online visualization of PV signals (81%) by mainly using the short-tip POLARx. Here, the AF-CB4 only showed real-time PV isolations in 42% of PVs. Recent observation of real-time isolations utilizing the AF-CB4 showed 78–84.8%, which is similar to the observed rate for the POLARx found in our analysis.9
A potential reason for the observed higher rate of real-time visualizations utilizing the POLARMAP is the fact that the POLARMAP is manufactured with one continuous length of nitinol wire from the connector to the distal hoop. The Achieve catheter used in the AF-CB4 use mechanical joints with stainless steel core wire. Furthermore, the POLARMAP insulated both the electrode signal wires and the core wire, whereas the AF-CB4 only insulates the electrode signal wires. These facts might improve the signal quality and might be responsible for the observed differences. A further reason for higher rates might be the fact that the operators were influenced by a certain bias because the POLARx represents a new device.
As the POLARSHEATH provides a slightly larger diameter of 15.9F compared to the 15F Flexcath Advance sheath, groin complications and air embolism might occur more often utilizing the POLARx. Although 1 air embolism was observed, our data set is to small to draw final conclusions.
The foot pedal, the slider switch and the steerable sheath are valuable innovations to further increase the comfort for the operator and reducing the procedure. As the procedure time showed a trend towards shorter times in the POLARx group, all of the innovations together might provide a measurable procedure rapidity with the same efficacy and safety of the well-established AF-CB4 system. Therefore, the POLARx cryoablation system seems to be at least evenly matched to the AF-CB4.
Study Limitations
The current study is based on a single-center experience involving a limited number of patients. Additionally, no randomization has been performed. Yet, consecutive patients were prospectively evaluated in this study. One further limitation might be different operator experience in both groups, which possibly might influence the periprocedural data, especially the rate of real-time isolations as well as the first attempt all veins isolated (FAAVI). However, only operators with high experience in CB usage performed the procedures, and utilized techniques were similar between both CB systems. Due to the recent launch of the POLARx cryoballoon, only acute efficacy and safety data are provided, whereas long-term clinical outcomes will need future assessment.
Conclusions
To the best of our knowledge, this is the first study reporting on the acute efficacy and safety of POLARx-based PVI as compared to AF-CB4-based PVI. While demonstrating an identical acute efficacy for PVI. Additionally, the POLARx showed significantly lower cryoballoon temperatures and a trend towards shorter procedure times compared to the AF-CB4.
Sources of Funding
This study received no specific funding.
Disclosures
C.-H.H. received travel grants and research grants from Boston Scientific, Biosense Webster and Cardiofocus and Speaker´s Honoraria from Boston Scientific, Biosense Webster and Cardiofocus. R.R.T. is a consultant for Boston Scientific, Biotronik and Biosense Webster and received Speaker’s Honoraria from Biosense Webster, Medtronic, Boston Scientific and Abbot Medical. K.-H.K. reports grants and personal fees from Abbott Vascular, Medtronic, Biosense Webster outside submitted work. All other authors have no relevant disclosures to declare.
IRB Information
The present study was approved by the University of Luebeck IRB (reference number: WF-028/15).
References
- 1.
Metzner A, Reissmann B, Rausch P, Mathew S, Wohlmuth P, Tilz R, et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2014; 7: 288–292.
- 2.
Wissner E, Heeger CH, Grahn H, Reissmann B, Wohlmuth P, Lemes C, et al. One-year clinical success of a ‘no-bonus’ freeze protocol using the second-generation 28 mm cryoballoon for pulmonary vein isolation. Europace 2015; 17: 1236–1240.
- 3.
Metzner A, Heeger CH, Wohlmuth P, Reissmann B, Rillig A, Tilz RR, et al. Two-year outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon: Lessons from the bonus freeze protocol. Clin Res Cardiol 2016; 105: 72–78.
- 4.
Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, et al. Single 3-minute freeze for second-generation cryoballoon ablation: One-year follow-up after pulmonary vein isolation. Heart Rhythm 2015; 12: 673–680.
- 5.
Furnkranz A, Bordignon S, Dugo D, Perotta L, Gunawardene M, Schulte-Hahn B, et al. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2014; 25: 840–844.
- 6.
Lemes C, Wissner E, Lin T, Mathew S, Deiss S, Rillig A, et al. One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: A retrospective analysis. Europace 2016; 18: 201–205.
- 7.
Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira J, et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: One-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 2015; 12: 60–66.
- 8.
Heeger CH, Subin B, Wissner E, Fink T, Mathew S, Maurer M, et al. Second-generation cryoballoon-based pulmonary vein isolation: Lessons from a five-year follow-up. Int J Cardiol 2020; 312: 73–80.
- 9.
Straube F, Dorwarth U, Pongratz J,Brück B, Wankerl M, Hartl S, et al. The fourth cryoballoon generation with a shorter tip to facilitate real-time pulmonary vein potential recording: Feasibility and safety results. J Cardiovasc Electrophysiol 2019; 30: 918–925.
- 10.
Kuck KH, Albenque JP, Chun KJ, Fürnkranz A, Busch M, Elvan A, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016; 37: 2858–2865.
- 11.
Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A, et al. Once isolated, always isolated?: Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol 2015; 8: 1088–1094.
- 12.
Bordignon S, Furnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak B, et al. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: Analysis of repeat procedures. Europace 2015; 17: 725–731.
- 13.
Heeger CH, Rexha E, Maack S, Rottner L, Fink T, Mathew S, et al. Reconduction after second-generation cryoballoon-based pulmonary vein isolation: Impact of different ablation strategies. Circ J 2020; 84: 902–910.
- 14.
Heeger CH, Abdin A, Mathew S, Reissmann B, Yalin K, Liosis S, et al. Efficacy and safety of cryoballoon ablation in patients with heart failure and reduced left ventricular ejection fraction: A multicenter study. Circ J 2019; 83: 1653–1659.
- 15.
Metzner A, Burchard A, Wohlmuth P, Rausch P, Bardyszewski A, Gienapp C, et al. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2013; 6: 769–775.
- 16.
Furnkranz A, Bordignon S, Bohmig M, Konstantinou A, Dugo D, Perrotta L, et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015; 12: 268–274.
- 17.
Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz R et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 2014; 25: 466–470.
- 18.
Casado-Arroyo R, Chierchia GB, Conte G, Levinstein M, Sieira J, Rodriguez-Mañero M, et al. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: A comparison between the first- and second-generation balloon. Heart Rhythm 2013; 10: 1318–1324.