Article ID: CJ-21-0315
Article ID: CJ-21-0315
Background: Application of drug-coated balloons (DCBs) is popular for the treatment of percutaneous coronary intervention (PCI). A new DCB has been designed as ultrasound-controlled paclitaxel releasing. This study was conducted to determine whether a DCB-only strategy has a similar safety profile and equal angiographic and clinical outcomes to DES implantation in primary ST-elevation myocardial infarction (STEMI) patients, as well as determine the efficiency and safety of this new DCB.
Methods and Results: Overall, 184 pretreated STEMI patients were randomized into DCB and DES groups with a 1:1 allocation. The main study end-point was late lumen loss (LLL) during the 9 months after PCI. Late lumen loss was reported to be 0.24±0.39 mm in the DCB group and 0.31±0.38 mm in the DES group (P=0.215). Diameter stenosis was 28.27±15.35% in the DCB group and 25.73±15.41% in the DES group (P=0.312). Major adverse cardiovascular events (MACEs) were reported in 3 patients (3.4%) in the DCB group and 4 patients (4.7%) in the DES group (P=0.718). TLR and TVR in the DCB group was 2.3%, 3.4% and 2.4%, 3.5% in the DES group (P=1.000), respectively. No cardiac death and stent thrombosis (ST) was found in the DCB group at 12 months clinical follow up.
Conclusions: The DCB-only strategy showed good angiographic and clinical outcomes in the 9- and 12-month follow-up periods, respectively. The VasoguardTM DCB is safe and feasible to treat STEMI patients.
Since the 1970s, the invention of balloon angioplasty and percutaneous coronary intervention (PCI) have made rapid progress in the treatment of occlusive coronary artery disease (CAD).1,2 In particular, drug-eluting stents (DES) have become an optimal strategy for the benefit of quickly recovering coronary artery blood flow, inhibiting restenosis. However, endothelial dysfunction, neo-atherosclerosis and thrombosis followed by DES implantation greatly affect the efficacy of clinical prognosis.3–5 The permanent existence of stent platforms is considered as the main reason.6–8 Drug-coated balloons (DCBs) are semi-compliant angioplasty balloons covered with an antiproliferative lipophilic agent. When DCB is inflated, the agent is rapidly released locally into the vessel wall.9 This technology allows a broader area of surface contact and more homogenous drug-tissue transfer compared to stent-based local drug delivery.10 CAD patients treated with DCB angioplasty may benefit from the absence of a permanent stent and shortened dual antiplatelet therapy.11–13 DCB application was first recommended by the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) guidelines for the treatment of in-stent restenosis (ISR) after prior bare-metal stent (BMS) (Class IIA, Level B) implantation in 2010.14 Thereafter, DCBs were recommended by the ESC/EACTS guidelines for the treatment of ISR (within BMS or DES, Class IA) in 201415 and 2018.16 Based on the therapeutic principle, the feasibility of DCBs in bifurcation, small vessel coronary diseases, and other de novo CADs has been suggested in several pilot studies.17–21 Nonetheless, there was a paucity of clinical feasibility of DCB treatment for ST-elevation myocardial infarction (STEMI) patients. In addition, STEMI patients are becoming younger and younger. Application of DCBs to perform angioplasty without permanent stent placement may become a more attractive treatment strategy for young and middle-aged adults with STEMI.
Recently, an ultrasound-controlled paclitaxel releasing balloon (VasoguardTM; Rientech, China) was developed and it was found to effectively inhibit restenosis in the treatment of the porcine restenosis model.22 The current study aimed to test the efficiency and safety of this new DCB vs. DES in the treatment of primary STEMI patients using a 9-month angiographic and a 12-month clinical follow up.
This study is a prospective randomized controlled trial comparing VasoguardTM ultrasound-controlled DCB and CordimaxTM stents (Rientech, China). It was designed as a non-inferiority trial for late lumen loss (LLL) at 9 months. The published angiographic results for the CordimaxTM stent showed an in-stent LLL of 0.25±0.47 mm at 9 months.23 Assuming that the anticipated LLL of the VasoguardTM DCB is also 0.25 mm at 9 months and it has a non-inferiority margin of 0.235 mm, a 2-sided alpha of 0.05 and 80% statistical power would require a minimum number of 144 subjects (72 subjects per group). Assuming a 20% loss to angiographic follow up, at minimum of 180 enrolled patients was required.
Primary STEMI patients were eligible for enrollment if they were aged between 18 and 70 years and intended to undergo percutaneous coronary intervention (PCI) treatment of de-novo native coronary artery lesions with heavy thrombus load, had a diameter stenosis ≥50% and a reference vessel diameter between 2.0 and 4.0 mm by visual estimation. In addition, successful predilatation (residual stenosis ≤30% and no limited flow dissection or thrombus) is necessary. The major exclusion criteria included >3 coronary diseases, left main coronary artery lesions, and severely distorted or calcified or angulated vessels. Patients who meet the above conditions were randomly assigned to either the VasoguardTM DCB group or the CordimaxTM stent group in a 1 : 1 allocation. Random assignment was performed by a statistician from Shandong University. A site-stratified block randomization was performed with randomly varying block sizes of 4 and 6. Sequences were concealed from patients and clinical staff until assignment. Angiographic follow up was performed at 9 months after PCI. Clinical follow up was conducted at 1, 6, 9 and 12 months. This study protocol was approved by the independent Medical Ethics Committee (IEC) of Inner Mongolia People’s Hospital with registration number of ChiCTR1800015900. It was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All participating patients or their immediate family provided informed consent. Participants or investigators were not masked to the treatment.
Devices and ProceduresDCBs VasoguardTM DCB is designed as an ultrasound-controlled paclitaxel releasing system. Paclitaxel is encapsulated in an ultrasound-responsive biodegradable PLGA (poly lactic-co-glycolic acid) microcapsules (diameter <8 μm). The drug carrier was coated on a semi-compliant coronary balloon with a paclitaxel dose of 3 μg/mm2. With the acoustic energy (1–10 Hz ultrasound), microcapsules are pushed into the membrane, whose permeability has been changed, and encapsulated paclitaxel is released.22 The DCBs are available in diameters from 2.0 to 4.0 mm and lengths from 15 to 35 mm. The schematic diagram of drug release by ultrasonic is shown in Figure 1.

Schematic diagram of drug release by ultrasonic. (A) When the VasoguardTM DCB is inflated, ultrasound is applied at the body surface (above the lesion) for 60 s. (B) The drug is distributed at the surface and interior of polylactic-co-glyrolic acid (PLGA) microcapsules. The drug was released in 3 patterns: (a) surface drug release by passive diffusion and ultrasonic energy; (b) microcapsules are pushed into the cell membrane and encapsulated paclitaxel is released through ultrasonic; and (c) a small portion of microcapsules degrade and release the drug.
Stents The Cordimax stent has a 316-L stainless steel platform coated on an abluminal surface with a PLGA polymer mixed with sirolimus (1.4 μg/mm2).24,25 The device is available in diameters from 2.25 to 4.00 mm and lengths from 16 to 33 mm.
Procedures Patients were treated strictly according to current standard international guidelines.16,26 Thrombolysis and thrombus aspiration are necessary for patients with heavy thrombotic load. Pre-dilation balloons or cutting balloons were used according to lesions, at the discretion of the surgeon. In cases with flow-limiting dissections or residual angiographically significant stenosis (i.e., >30% stenosis) after pre-dilation, stenting is necessary and the patient would not be enrolled to any group. The DCB, which had to be 2–3 mm longer on each side than the predilatation balloon, was slowly inflated at nominal pressure for 60 s. Meanwhile, a diagnostic ultrasound (10 Hz) was performed when the DCB was fully inflated. The DES was performed conventionally. In cases with flow-limiting dissections or residual angiographically significant stenosis (i.e., >30% stenosis) after DCB treatment, additional spot stenting avoiding geographical mismatch should be conducted immediately. PCI was conducted under dual antiplatelet therapy with acetylsalicylic acid (100 mg per day) and clopidogrel (75 mg per day). Anticoagulation with heparin during the procedure was administered according to the protocol recommendations. After PCI, according to the guidelines, dual antiplatelet therapy with aspirin (100 mg/day) and clopidogrel (75 mg/day) was continued in DCB patients for 3 months and 12 months in DES patients. A case example of the DCB application is shown in Figure 2.

A case example of VasoguardTM drug-coated balloon (DCB) application. (A) Initial angiogram. (B) Result after thrombolysis and thrombus aspiration. (C) Pre-dilation with a 3.5×15 mm semi-compliance balloon. (D) Result after dilation with a semi-compliance balloon. (E) Treatment with a 4.0×18-mm DCB. (F) Treatment with ultrasonic when the DCB inflation. (G) Immediate result after DCB treatment. (H) 9-month result after DCB treatment.
In-segment LLL at 9 months from quantitative coronary angiography (QCA) analysis is the primary endpoint. It was calculated as the difference in minimal lumen diameter (MLD) immediately after PCI (MLD0) and at 9-months follow up (MLD9). Secondary endpoints include: (1) in-segment diameter stenosis (%); (2) major adverse cardiovascular events (MACE), defined as the composite of cardiac death, non-fatal myocardial infarction (MI), and target vessel revascularization (TVR) and stent thrombosis (ST). Cardiac death was defined as any death that was not clearly of extracardiac origin, and myocardial infarction. Definite and probable ST were defined according to the Academic Research Consortium (ARC), classified as early, late, and very late ST; and (3) major bleeding, defined as bleeding ARC type 3 to 5 bleeding. Clinical follow up was conducted at 1, 6, 9 and 12 months. Angiograms were evaluated by an independent specialist in the core laboratory (Zhongshan Hospital Fudan University, Shanghai) using the QAngio XA Version 7.2 Analysis Software (Medis Medical Imaging System Inc., Leiden, The Netherlands). Device success was defined as the studied DCB/DES being successfully expanded and placed at the target lesion. Clinical success was defined as attainment of <50% residual stenosis by visual estimate, TIMI 3 flow, and no in-hospital major adverse cardiac event.
Statistical AnalysisGroup comparisons were performed using the 2-sample t-test for normal distribution continuous variables, and the Chi-squared test or Fisher’s exact test for categorical variables, if using non-parametric tests. Non-inferiority was defined as the upper limit of the 95% confidence interval for the difference between DCB and DES groups if it was not greater than the predetermined non-inferiority margin of 0.235. All analyses were performed with the SAS 9.13 software (SAS Institute Inc., Cary, NC, USA).
Between October 2017 and August 2019, a total of 193 primary STEMI patients with heavy thrombotic load were screened for enrollment. With the pretreatment of thrombus aspiration and prourokinase thrombolysis, followed by pre-dilation using PTCA balloon or cutting balloon, 184 (95.3%) patients received successful predilatation (residual stenosis ≤30% and no limited flow dissection or thrombus) and were randomly assigned either to the DCB group (n=92) or to the DES group (n=92), in a 1 : 1 allocation. The flowchart of patient enrollment is shown in Figure 3.

Flowchart of patient enrollment.
Baseline patient demographics and basic lesion characteristics details are summarized in Table 1. There was no significant difference between the 2 groups in terms of age, gender, diabetes mellitus, hypertension, hyperlipidemia, smoking history, and drinking history. There was also no statistical difference between the 2 groups in terms of symptom onset to hospital arrival time, door-to-balloon time, total ischemic time and chest pain time. However, the groups were balanced in terms of lesion sites and length, as well as TIMI flow grade before surgery.
| DCB group (n=92) |
DES group (n=92) |
P value | |
|---|---|---|---|
| Age (years) | 49.20±10.59 | 49.60±8.82 | 0.780 |
| Male | 88 (95.7) | 84 (91.3) | 0.232 |
| Diabetes mellitus | 71 (77.2) | 79 (85.9) | 0.129 |
| Hypertension | 67 (72.8) | 65 (70.7) | 0.743 |
| Hyperlipidemia | 61 (66.3) | 58 (63.0) | 0.644 |
| Smoking history | 71 (77.2) | 78 (84.8) | 0.189 |
| Drinking history | 82 (89.1) | 77 (83.7) | 0.282 |
| Symptom onset to hospital arrival (h) | 5.34±5.32 | 4.49±3.83 | 0.211 |
| Door-to-balloon time (h) | 0.52±0.15 | 0.50±0.13 | 0.335 |
| Total ischemic time (h) | 5.87±5.47 | 4.94±3.89 | 0.183 |
| Chest pain (h) | 6.61±6.27 | 5.38±4.21 | 0.119 |
| Position | 0.765 | ||
| Anterior MI | 52 (58.7) | 54 (56.5) | |
| Inferior MI | 40 (41.3) | 38 (43.5) | |
| Lesion length (mm) | 30.62±10.01 | 33.51±13.13 | 0.095 |
| Lesion sites | 117 | 113 | 0.836 |
| RCA | 29 (25.0) | 30 (26.5) | |
| LCX | 24 (20.5) | 19 (16.8) | |
| LAD | 64 (55.2) | 64 (56.6) | |
| TIMI flow grade (%) | 0.758 | ||
| 0 | 68 | 72 | |
| 1 | 13 | 10 | |
| 2 | 11 | 10 |
Data are presented as mean±SD or n (%). DCB, drug-coated balloon; DES, drug-eluting stent; MI, myocardial infarction; RCA, right coronary artery; LCX, left circumflex artery; LAD, left anterior descending branch; TIMI, Thrombolysis in Myocardial Infarction.
Detailed angiographic characteristics and procedural data are summarized in Table 2. The mean reference vessel diameter (RVD) of the DCB group was 3.31±0.56 mm, and 3.43±0.48 mm for the DES group (P>0.05). The initial minimal lumen diameter (MLDi) and diameter stenosis (DSi) were 0.53±0.28 mm (DCB) vs. 0.50±0.26 mm (DES) and 83.56±8.55% (DCB) vs. 85.56±7.01% (DES) respectively. For the lesions, 37% of lesions in the DCB group used cutting balloons, and this it was 33.7% for the DES group (P>0.05). There was also no difference in MLDp, acute gainp and DSp between the 2 groups after predilation. However, the MLD immediately after PCI (MLD0), acute gain0 and DS0 showed statistical difference in the 2 groups (P<0.01). Both the DCB group or DES group had 100% lesion success of the target lesion and no in-hospital adverse cardiac events. The ST segment resolution at 90 min (%) was 73.04±14.66% and 75.5±15.99% in the DCB group and DES group, respectively (P>0.05).
| DCB group (n=92) |
DES group (n=92) |
P value | |
|---|---|---|---|
| Proximal RVD (mm) | 3.39±0.56 | 3.52±0.49 | 0.096 |
| Distal RVD (mm) | 3.22±0.56 | 3.34±0.49 | 0.124 |
| Mean RVD (mm) | 3.31±0.56 | 3.43±0.48 | 0.120 |
| MLDi | 0.53±0.28 | 0.50±0.26 | 0.298 |
| Diameter stenosis (DS1, %) | 83.56±8.55 | 85.56±7.01 | 0.229 |
| Predilation balloon (%) | 82 (70.7) | 82 (72.6) | 0.753 |
| PB diameter | 2.69±0.62 | 2.70±0.50 | 0.892 |
| PB length | 16.92±3.31 | 16.97±2.76 | 0.841 |
| Dilation atmosphere (atm) | 8.77±1.07 | 8.66±1.25 | 0.602 |
| Cutting balloon (CB, %) | 34 (37.0) | 31 (33.7) | 0.886 |
| CB diameter | 2.85±0.46 | 2.87±0.41 | 0.815 |
| CB length | 14.12±1.93 | 14.35±1.70 | 0.603 |
| Dilation atmosphere (atm) | 8.18±1.59 | 8.26±1.34 | 0.824 |
| Prourokinase (mg, %) | 0.690 | ||
| 10 | 78 (84.8) | 76 (82.6) | |
| 20 | 14 (15.2) | 16 (17.4) | |
| QCA data after predilation | |||
| MLDp | 2.69±0.53 | 2.76±0.41 | 0.840 |
| Acute gainp | 2.15±0.56 | 2.26±0.38 | 0.482 |
| DS | 18.78±5.88 | 19.12±5.13 | 0.229 |
| Device diameter | 3.19±0.57 | 3.34±0.49 | 0.983 |
| Device number/patient | 1.27±0.45 | 1.23±0.42 | 0.499 |
| QCA data after stent/DCB | |||
| MLD0 | 2.71±0.53 | 2.92±0.44 | 0.001 |
| Acute gain0 | 2.15±0.60 | 2.42±0.40 | <0.01 |
| DS0 (%) | 18.12±5.91 | 14.61±5.01 | <0.01 |
| Device success (%) | 100 | 100 | – |
| Procedural success (%) | 100 | 100 | – |
| ST segment resolution at 90 min (%) | 73.04±14.66 | 75.5±15.99 | 0.270 |
Data are presented as mean±SD or n (%), unless otherwise stated. RVD, reference vessel diameter; MLDi, initial minimal lumen diameter; PB, predilation balloon; QCA, quantitative coronary angiography; MLDp, predilation minimal lumen diameter; DS, diameter stenosis; MLD0, minimal lumen diameter immediately after operation. Other abbreviations as in Table 1.
Patients medications are shown in Table 3. There was no significant difference in the use of aspirin, clopidogrel, β-blocker, calcium channel blocker, RAS inhibitor, statin, ezetimibe and nitrate.
| DCB group (n=92) |
DES group (n=92) |
P value | |
|---|---|---|---|
| Aspirin | 92 (100) | 92 (100) | – |
| Clopidogrel | 92 (100) | 92 (100) | – |
| β-blocker | 63 (68.5) | 69 (75.0) | 0.326 |
| Calcium channel blocker | 10 (10.9) | 5 (5.4) | 0.178 |
| RAS inhibitor | 16 (17.4) | 21 (22.8) | 0.358 |
| Statin | 92 (100) | 92 (100) | – |
| Ezetimibe | 11 (12.0) | 8 (8.7) | 0.467 |
| Nitrate | 21 (22.8) | 15 (16.3) | 0.265 |
RAS, renin and angiotensin aldosterone. Other abbreviations as in Table 1.
Angiographic follow up at 9 months after PCI was completed in 151 (82.1%) patients (Table 4). A total of 77 patients (83.7%) allocated to the DCB group and 74 patients (80.4%) allocated to the DES group underwent QCA. Reference vessel diameter at 9 months follow up (RVD9) was 3.35±0.53 mm in the DCB group and 3.46±0.50 mm in the DES group (P>0.05). Minimum lumen diameter at 9 months follow up (MLD9) was 2.40±0.64 mm in the DCB group and 2.59±0.67 mm in the DES group (P>0.05). Late lumen loss at 9 months follow up was 0.24±0.39 mm in the DCB group and 0.31±0.38 mm in the DES group (P>0.05). According to the pre-specified non-inferiority margin, VasoguardTM DCB was non-inferior to Cordimax DES for the primary angiographic endpoint of in-segment LLL at 9 months (0.24±0.39 mm vs. 0.31±0.38 mm; difference=−0.07±0.06 mm; 95% CI: −0.18 to 0.04). There was also no significant differences between the 2 groups for percentage of diameter stenosis (28.27±15.35% vs. 25.73±15.41%, P>0.05; Table 4). Interestingly, there were 11 patients (14.3%) whose lesion vessels performed positive reconstruction. The increased vessel diameter (MLD9–MLD0) was 0.15±0.16 mm, which also meant that the LLL was −0.15±0.16 mm. Changes in MLD at initial (MLDi), immediate post PCI (MLD0) and 9-months follow up (MLD9), as well as the cumulative distribution of LLL at 9 months follow up are shown in Figure 4.
| Outcomes | DCB group (n=77) |
DES group (n=74) |
P value |
|---|---|---|---|
| 9-month QCA follow up (%) | 83.7 | 80.4 | 0.564 |
| RVD9 (mm) | 3.35±0.53 | 3.46±0.50 | 0.192 |
| Positive reconstruction (%) | 11 (14.3) | 0 (0.0) | 0.001 |
| Increased diameter | 0.15±0.16 | – | – |
| MLD9 (mm) | 2.40±0.64 | 2.59±0.67 | 0.077 |
| LLL (mm) | 0.24±0.39 | 0.31±0.38 | 0.266 |
| Diameter stenosis9 (%) | 28.27±15.35 | 25.73±15.41 | 0.312 |
| 12-month clinical follow up (%) | 94.6 | 92.4 | 0.550 |
| MACE | 3 (3.4) | 4 (4.7) | 0.718 |
| Cardiac death | 0 (0) | 1 (1.2) | 0.494 |
| Non-fatal MI | 3 (3.4) | 3 (3.5) | 1.000 |
| TLR | 2 (2.3) | 2 (2.4) | 1.000 |
| TVR | 3 (3.4) | 3 (3.5) | 1.000 |
| ST | 0 (0) | 0 (0) | – |
| All cause death | 1 (1.1) | 1 (1.2) | 1.000 |
| Major bleeding | 1 (1.1) | 2 (2.4) | 0.618 |
Data are presented as mean±SD or n (%), unless otherwise stated. LLL, late lumen loss; MACE, major adverse cardiovascular events; TLR, target lesion revascularization; TVR, target vessel revascularization. Other abbreviations as in Tables 1,2.
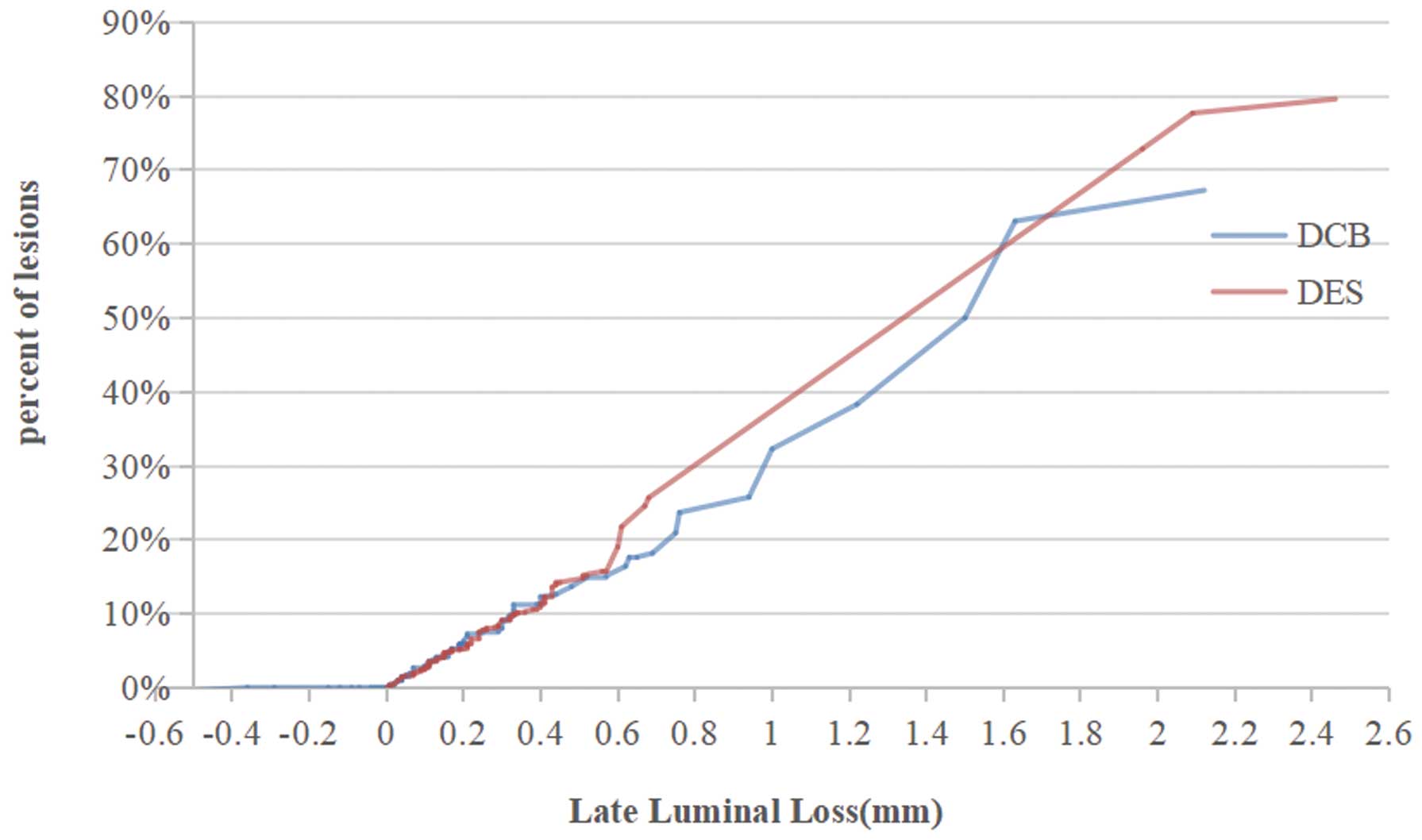
Line graph of the distribution of late lumen loss (LLL). DCB, drug-coated balloon; DES, drug-eluting stent.
Clinical event rates at 12-months follow up are also summarized in Table 4. The rate of cardiac death, non-fatal MI, TLR, TVR and ST were comparable for patients in the DCB group and the DES group (P>0.05). The incidence of MACE and major bleeding in the DCB group demonstrated a slight but not significant decrease as compared to the DES group (P>0.05).
The application of DCBs has been recommended by the ESC/EACTS guidelines for the treatment of ISR (Class IA). Many researchers also support the application of DCBs in bifurcation, small vessel coronary diseases (SVD), and other de novo CADs;17–21 however, a comparison of DCB vs. DES in STEMI patients is rare. Most STEMI patients have heavy thrombus load. The residual thrombus may absorb the antiproliferative drug released by DCBs, decreasing the bioavailability of the drug. In addition, concerns of acute recoil, coronary dissections, and acute vessel closure also restrict the use of the DCB-only strategy in STEMI patients.27,28 A multicenter randomized comparison of DCB plus bare-metal stent (BMS) vs. BMS/DES in primary STEMI patients found that DEB followed by BMS implantation showed similar angiographic results to BMS-only and angiographic inferiority to DES (LLL, BMS+DCB: 0.64±0.56 mm vs. BMS: 0.74±0.57 mm vs. DES: 0.21±0.32 mm).29
This prospective randomized study reports the feasibility, safety and efficacy of DCB-only angioplasty for STEMI patients with de novo lesions at 12-month follow up. The 2 groups were balanced in terms of patient demographics and lesion characteristics. Both the DCB group and the DES group had 100% lesion success of the target lesion and no in-hospital adverse cardiac events. It is interesting that LLL and rate of segment restenosis in the DCB group demonstrated a slight but not a significant decrease as compared to the DES group at 9-month angiographic follow up (P>0.05). No patient suffered cardiac death or ST in the DCB group during 12 month clinical follow up. They were balanced in both groups including the rate of non-fatal MI, TLR and TVR (P>0.05). The incidence of MACE and major bleeding in the DCB group demonstrated a slight but not significant decrease as compared to the DES group (P>0.05). The above results agreed with those from previous reported studies.30–32 The REVELATION trial was also conducted with STEMI patients, comparing DCB angioplasty (Pantera Lux balloon; Biotronik AG, Buelach, Switzerland) with sirolimus or everolimus DES in a single center.30,31 The DCB showed no statistical difference for LLL (0.05±0.13 mm vs. 0.00±0.05 mm, P>0.05) and clinical outcomes (MACE 3% vs. 2%, P>0.05) at 9-month follow up. Similar results at the 6-month follow up in STEMI patients were found by Gobić et al.32 This randomized controlled clinical trial enrolled 37 patients with a cobalt-chromium (CoCr) DES (Biomime; Meril Life Sciences, Vapi, India) and 38 patients with a DCB alone (Sequent Please balloon, B. Braun, Germany). The DCB-alone strategy resulted in a significant decrease in LLL (−0.09±0.09 mm vs. 0.10±0.19 mm, P<0.05) at 6-month follow up. The DCB-alone strategy resulted in no significant difference compared to the DES strategy in terms of reinfarction (5.3% vs. 5.4%, P>0.05) at 1-month follow up and MACE (0% vs. 5.4%, P>0.05) at 6-month follow up. Consistent with previous trials, positive reconstruction of lesion vessels was observed in 11 (14.3 %) STEMI patients using the DCB-only strategy. In addition, LLL and rate of segment restenosis in the DCB group demonstrated a slight but not significant decrease as compared to the DES group. There are several potential reasons. Lesion pretreatment is critical for the success of the DCB-only strategy. Successful predilatation (residual stenosis ≤30% and no limited flow dissection or thrombus) is necessary. Aggressive predilation may result in severe dissection or acute vessel closure, whereas conservative lesion preparation is associated with reduced minimal luminal areas and an over-residual plaque area, which emerged as predictors of restenosis.33 In the present study, all patients accepted thrombolysis and thrombus aspiration treatment. Prourokinase (Tasly Pharmaceutical, China; 10 mg dissolved in 10 mL of saline) was gradually injected through the thrombus aspiration catheter within 10 min. After aspiration, another prourokinase was used to dissolve the residual thrombus. Prourokinase is a specific plasminogen activator and can selectively activate fibrinogen in the thrombus with a fibrinolytic enzyme, which has less effect on systemic fibrinolysis and therefore reduces the risk of bleeding.34,35 The application of a cutting balloon can decrease the damage to the blood vessel, severe dissection and acute vessel closure.36,37 Cutting balloon angioplasty has been proven to be a safe and effective treatment of lesion restenosis, with significantly higher patency than that of conventional balloon angioplasty.38,39 In addition, the first application of VasoguardTM DCB is an important guarantee. Clinical performances of different DCBs are determined by crystal state and dose of paclitaxel, excipient and coating properties. An intact arterial wall poses a significant barrier to drug penetration. The drug bioavailability of current DCBs is only 8.7±4.9%; 80–90% the unabsorbed drug washed off to the downstream systemic circulation.40,41 How to increase the drug bioavailability is an important subject. Researchers have found that the drugs released can be “pushed” into the skin, blood clots, or other tissues by the acoustic force, and ultrasound also has a direct effect on the membrane as it changes the cell membrane permeability.42,43 Based on the principles, ultrasound-responsive biodegradable PLGA microcapsules were designed and paclitaxel was encapsulated in the microcapsules. It has been reported that diagnostic ultrasound can significantly stimulate the rupture of microcapsules to release paclitaxel at a fixed position, thereby achieving the targeted release of paclitaxel.22 The availability of the drug reaches 27% under ultrasound. The unruptured microcapsules will release paclitaxel through the degradation of PLGA shells, inhibiting smooth muscle cell proliferation and vascular restenosis when lesion vessels are repaired.
Study LimitationsThere are several limitations to our study that need to be considered. First, the time of local thrombolysis varied due to personality and chest pain time. The specific dosage and local thrombolysis time required the expertise of experienced doctors. Therefore, extensive clinical research should be performed to accumulate evidence to determine the operation standard in the future. Second, this was a single-center study; a prospective randomized multicentre trial with a large number of participants will be needed to confirm the value of this new ultrasound-responsive DCB in young and middle-aged STEMI patients. Third, we used a wide non-inferiority margin in this trial (94% of the expected LLL in the control group). A large non-inferiority margin may result in products whose efficacy is far inferior to the control group being judged as non-inferior to the control group. Fortunately, the efficacy of DCB shown in this trial is quite good. In the future, we will use more reasonable boundary values to verify the efficacy of DCB. Finally, the ultrasound machine used in the study is specifically designed for the ultrasound-responsive DCB. Four models energy (25%, 50%, 75% and 100%) make different volumes of drug released, which can accommodate different conditions of patients; however, we only used the 100% energy in this study. Personalized treatment of patients with different energy models requires more exploration.
The present study demonstrated the feasibility of DCB-only angioplasty for STEMI patients with de novo lesions, and also verifies the safety and efficacy of this new DCB, an ultrasound-responsive balloon. The LLL at 9 months angiographic follow up and incidences of adverse clinical events were very low. The incidence of acute MI in young and middle-aged adults is increasing due to eating habits, social factors, etc. These patients have the features of high thrombus burden and inflammatory state, as well as milder calcification. Compared with DES, the DCB demonstrates a more homogeneous and concentrated administration of the drug, less malapposition, respection of the vessel anatomy, and reservation of future treatment, thus better preservation of endothelial function. Application of DCB to perform angioplasty without stent placement may become an attractive treatment strategy for STEMI of young and middle-aged adults.
This study was supported by the National Twelfth Five-Year Plan for Science and Technology Support of China (grant no. 2014BAI11B04).
Haijun Zhang holds the intellectual property rights on the DES and DCB technology described in the paper, licensed to Rientech Inc. The other authors have no conflicts of interest to disclose.
This study was approved by the independent Medical Ethics Committee (IEC) of Inner Mongolia People’s Hospital with registration number of ChiCTR1800015900.
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.