Article ID: CJ-21-0999
Article ID: CJ-21-0999
Background: The incidence of sudden cardiac death (SCD) after discharge in Japanese acute myocardial infarction (AMI) patients with reduced left ventricular ejection fraction (LVEF) treated with primary percutaneous coronary intervention (PCI) remains unknown.
Methods and Results: The study population included 1,429 AMI patients (199 with LVEF ≤35% and 1,230 with LVEF >35%) admitted to the Hirosaki University Hospital, treated with primary PCI within 12 h after onset, and survived to discharge. LVEF was evaluated in all patients before discharge, and the patients were followed up for a mean of 2.6±0.8 years. The Kaplan-Meier survival curves revealed LVEF ≤35% was associated with all-cause death and SCD. The incidence of SCD was 2.6% at 1 year and 3.1% at 3 years in patients with LVEF ≤35%, whereas it was 0.1% at 1 year and 0.3% at 3 years in patients with LVEF >35%. Sixty-seven percent of SCDs in patients with LVEF ≤35% occurred within 4 months after discharge, and the events became less frequent after this period. A Cox proportional hazard model indicated LVEF ≤35% as an independent predictor for all-cause death and SCD.
Conclusions: The incidence of SCD was relatively low in Japanese AMI patients treated with primary PCI, even in patients with LVEF ≤35% upon discharge. Careful management of patients with reduced LVEF is required to prevent SCD, especially in the early phase after discharge.
The treatment for acute myocardial infarction (AMI) has been progressively developed over the past three decades by reperfusion therapy including primary percutaneous coronary intervention (PCI),1 developments in antiplatelets and antiarrhythmic agents,2 and widespread use of coronary care units for early identification of life-threatening ventricular arrhythmias.3 Although these advancements contributed to the improvement of patient outcome, the morbidity and mortality following AMI are still significant. The in-hospital outcome of AMI patients is predominantly related to low cardiac output syndrome, mechanical complications, and life-threatening ventricular tachyarrhythmias including sustained ventricular tachycardia (VT) or fibrillation (VF).4
Regarding the patient outcome after hospital discharge, it has been widely accepted that left ventricular ejection fraction (LVEF) is the most useful predictor for worse clinical outcome including sudden cardiac death (SCD).5,6 In previous European studies, the incidence of SCD in AMI patients with reduced LVEF after hospital discharge was reported to be 8.5% at 2.5 years,7 10.0% at 2.1 years,8 and 13.2% at 3.1 years.9 In contrast, in the Heart Institute of Japan Acute Myocardial Infarction (HIJAMI)-II study, SCD occurred in 2.9% at 1 year and 5.1% at 3 years after discharge, in AMI patients with LVEF ≤30%.10 Although the inclusion and exclusion criteria of these studies varied, and therefore direct comparisons should be interpreted with caution, the incidence of SCD seems to be lower in Japanese AMI patients than in Caucasian AMI patients. In addition, the rate of SCD was much lower in Japanese patients who met the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II criteria than that reported in MADIT-II.11–13 However, there are only limited data available for the incidence of SCD in Japanese AMI patients with reduced LVEF treated with primary PCI and survived to discharge. In this study, we examined the mid-term clinical outcome after hospital discharge in these Japanese AMI patients.
The study population included 1,536 AMI patients admitted to the Hirosaki University Hospital, treated with primary PCI within 12 h after onset, and survived to hospital discharge from April 2005 to March 2019. LVEF was evaluated before discharge. Among these patients, 1,429 were followed up for a mean of 2.6±0.8 years. Patients were divided into 2 groups: LVEF ≤35% and >35%, which has been used as a cut-off value for class I indication for implantable cardioverter defibrillator (ICD) implantation at least 40 days after MI in the Japanese Circulation Society/Japanese Heart Rhythm Society guidelines.14 We used the Killip classification to assess the severity of AMI upon hospital admission.15 This study was conducted based on the ethical guidelines for medical research on humans in the Declaration of Helsinki, and approved by the ethical committee of the Hirosaki University Graduate School of Medicine (2020-070).
DefinitionsThe clinical definition of AMI denoted the presence of acute myocardial injury detected by the increase in serum creatine phosphokinase (CPK) greater than twice the normal upper limit, with the increase in myocardial-specific isoenzyme of CPK fraction (CPK-MB) in the setting of evident acute myocardial ischemia with at least one of the following: symptoms of myocardial ischemia; new ischemic electrocardiogram changes; development of pathological Q waves; imaging evidence of loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; identification of coronary thrombus; or severe stenosis by angiography.16 Cardiac death was defined as death due to AMI, congestive heart failure, or SCD. SCD was defined as: (1) sudden and unexpected death within 1 h of cardiac symptoms in the absence of progressive cardiac deterioration; (2) unexpected death in bed during sleep; and (3) unexpected death within 24 h after last being seen alive.
Primary PCIAfter oral administration of 200 mg of aspirin and either 200 mg of ticlopidine, 300 mg of clopidogrel, or 20 mg of prasugrel, primary PCI was performed. Only the culprit coronary artery was treated if the patients were not under hemodynamic instability. One hundred IU per kg of unfractionated heparin was injected intravenously at the beginning of PCI, and additional boluses were given if needed to maintain an activated clotting time of over 250s. The coronary blood flow was evaluated with the Thrombolysis in Myocardial Infarction (TIMI) flow grade,17 and dual antiplatelet therapy was continued following stent implantation during hospitalization.
Measurement of LVEFLVEF was assessed by left ventriculography (79.7% of all patients), echocardiography (34.4%), or both (14.1%) before hospital discharge. In patients whose LVEF were evaluated by both left ventriculography and echocardiography, the lower value was used for the analysis. In LVEF measurement by left ventriculography, the ventricular tracings at end-diastole and end-systole in the 30-degree right anterior oblique projection were digitized with a cardiovascular measurement system (Medis Medical Imaging Systems, Nuenen, Netherlands), and LVEF was determined as previously described.18 For echocardiography, standard echocardiographic recordings and calculations were performed, and LVEF was calculated according to the modified Simpson biplane method.19
Data Collection and Follow upAll data, including the baseline and angiographic characteristics, laboratory data, and medications during hospitalization and upon discharge, were collected from the medical records and database of our institution. Clinical events, including all-cause death, cardiac death, SCD, and the occurrence of VT/VF were collected by the medical records, inquiring primary care physicians, patients themselves, or families of the patients up to 3 years after hospital discharge.
Statistical AnalysisThe variables are expressed as mean±one standard error or number (percentages). The differences in the distribution of selected characteristics between the patient groups were analyzed using chi-squared tests for categorical variables (Fisher’s exact test when necessary), and comparisons between 2 media by a Student’s t test. The survival curves after hospital discharge were estimated using the Kaplan-Meier method, and the differences between the groups were compared using the Log rank test. Hazard ratios and 95% confidence intervals for the incidence of all-cause death, cardiac death, SCD, and SCD+VT/VF were calculated using the Cox proportional hazards model after adjustment of covariates for which the P value was <0.10 by univariate analysis. JMP Pro statistical software version 13 (SAS Institute Inc., Cary, NC, USA) was used for all analyses, and a P value <0.05 was considered as significant.
The patients were divided based on their LVEF before hospital discharge; LVEF ≤35% (n=199) and >35% (n=1,230), and their baseline and angiographic characteristics were compared as shown in Table 1. The patients with LVEF ≤35% were characterized by older age, delayed hospital admission from AMI onset, higher proportions of Killip classification III or IV upon admission, requirement of intra-aortic balloon pumping support, atrial fibrillation (AF) during hospitalization, and higher maximum CPK and CPK-MB levels compared with those with LVEF >35%. The proportion of ICD implantation during hospitalization was higher in patients with LVEF ≤35% than in those with LVEF >35%. Among these patients who underwent ICD implantation during hospitalization, 1 of 6 patients with LVEF ≤35% and 1 of 5 patients with LVEF >35% had VT over 48 h after AMI onset. The other 4 patients with LVEF >35% had VT or VF in a very acute phase of AMI and were considered as high-risk for VT and VF by electrophysiological study. Angiographic characteristics showed that patients with LVEF ≤35% had the left anterior descending artery or left main trunk as the culprit coronary artery more than those with LVEF >35%. The incidence of successful reperfusion, indicated as TIMI flow grade 3 after PCI, was lower in patients with LVEF ≤35% than in those with LVEF >35%. The laboratory data at hospital discharge showed that patients with LVEF ≤35% were characterized by lower creatinine clearance and higher B-type natriuretic peptide value compared with those with LVEF >35%. The prescription rate of angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) was lower, and β-blocker, loop diuretics, mineralocorticoid receptor antagonist, warfarin, direct oral anticoagulants, and amiodarone were more often prescribed in patients with LVEF ≤35% than in those with LVEF >35%.
| LVEF ≤35% (n=199) |
LVEF >35% (n=1,230) |
P value | |
|---|---|---|---|
| Age (years) | 67.2±0.8 | 64.8±0.3 | 0.007 |
| Female gender (%) | 37 (18.7) | 288 (23.4) | 0.13 |
| Body mass index (kg/m2) | 23.8±0.3 | 24.4±0.1 | 0.07 |
| The time from onset to hospital admission (min) | 268±11 | 241±4 | 0.02 |
| Prior MI (%) | 13 (6.5) | 47 (3.8) | 0.10 |
| Prior PCI (%) | 12 (6.0) | 57 (4.6) | 0.41 |
| Prior CABG (%) | 3 (1.5) | 9 (0.7) | 0.23 |
| Co-morbidities (%) | |||
| Hypertension | 139 (69.9) | 811 (65.9) | 0.27 |
| Diabetes | 86 (43.2) | 498 (40.5) | 0.47 |
| Dyslipidemia | 111 (55.8) | 703 (57.2) | 0.72 |
| Current smoker | 92 (46.2) | 554 (45.0) | 0.75 |
| Killip classification III or IV on admission (%) | 41 (20.6) | 57 (4.6) | <0.0001 |
| Maintenance hemodialysis (%) | 5 (2.5) | 16 (1.3) | 0.18 |
| Use of VA-ECMO (%) | 3 (1.5) | 5 (0.4) | 0.09 |
| Use of IABP (%) | 95 (47.7) | 222 (18.1) | <0.0001 |
| AF during hospitalization (%) | 46 (23.1) | 147 (12.0) | <0.0001 |
| VT/VF during hospitalization (%) | 14 (7.0) | 72 (5.9) | 0.52 |
| Maximum CPK (U/L) | 5,757±196 | 3,262±79 | <0.0001 |
| Maximum CPK-MB (U/L) | 517±16 | 303±6 | <0.0001 |
| LVEF (%) | |||
| Evaluated by left ventriculography | 30.8±0.6 | 49.8±0.3 | <0.0001 |
| Evaluated by left echocardiography | 33.2±1.1 | 52.8±0.4 | <0.0001 |
| ICD implantation during hospitalization (%) | 6 (3.0) | 5 (0.4) | 0.002 |
| Culprit coronary artery (%) | |||
| RCA | 31 (15.6) | 502 (40.8) | <0.0001 |
| LAD | 141 (70.9) | 545 (44.3) | <0.0001 |
| LMT | 8 (4.0) | 16 (1.3) | 0.02 |
| LCX | 19 (9.6) | 167 (13.6) | 0.10 |
| Number of stenotic artery (%) | |||
| 1 | 87 (43.7) | 622 (50.6) | |
| 2 | 66 (33.2) | 391 (31.8) | 0.11 |
| 3 | 46 (23.1) | 217 (17.6) | |
| TIMI flow grade 0 before PCI (%) | 148 (74.4) | 826 (67.2) | 0.04 |
| TIMI flow grade 3 after PCI (%) | 135 (70.3) | 1,044 (88.0) | <0.0001 |
| Laboratory data | |||
| Total cholesterol (mg/dL) | 158±3 | 163±1 | 0.12 |
| Triglyceride (mg/dL) | 120±4 | 121±2 | 0.85 |
| HDL cholesterol (mg/dL) | 38±1 | 37±0.3 | 0.66 |
| LDL cholesterol (mg/dL) | 97±2 | 101±1 | 0.07 |
| Creatinine clearance (mL/min) | 64±2 | 75±1 | <0.0001 |
| HbA1c (%) | 6.2±0.1 | 6.1±0.04 | 0.18 |
| BNP (pg/mL) | 420±28 | 176±12 | <0.0001 |
| Medications at hospital discharge (%) | |||
| ACE inhibitor / ARB | 175 (87.9) | 1,155 (93.9) | 0.004 |
| β-blocker | 188 (94.5) | 1,055 (85.8) | 0.0002 |
| Statin | 168 (84.4) | 995 (80.9) | 0.23 |
| Loop diuretics | 97 (48.7) | 125 (10.2) | <0.0001 |
| Mineralocorticoid receptor antagonist | 76 (38.2) | 73 (5.9) | <0.0001 |
| Thiazide diuretics | 4 (2.0) | 9 (0.7) | 0.09 |
| Warfarin | 78 (39.2) | 110 (8.9) | <0.0001 |
| DOACs | 9 (4.5) | 23 (1.9) | 0.03 |
| Amiodarone | 14 (7.0) | 21 (1.7) | <0.0001 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CPK, creatinine phosphokinase; CPK-MB, myocardial-specific isoenzyme of CPK; DOAC, direct oral anticoagulant; HbA1c, hemoglobin A1c; IABP, intra-aortic balloon pumping; ICD, implantable cardioverter defibrillator; LAD, left anteriodescending artery; LCX, left circumflex artery; LMT, left main trunk; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; VT/VF, sustained ventricular tachycardia/ventricular fibrillation.
The survival curves after hospital discharge revealed that LVEF ≤35% was significantly associated with all-cause death (P<0.0001), cardiac death (P<0.0001), and SCD (P<0.0001) (Figure A–C). The curves of all-cause death were separated progressively in the first 6 months (Figure A), and this separation was primarily due to the higher incidence of cardiac death in patients with LVEF ≤35% (Figure B). Table 2 shows the causes of death during follow up in patients with LVEF ≤35% and >35%. Fifty-eight percent of deaths in patients with LVEF ≤35% and 22% of deaths in patients with LVEF >35% was due to cardiac causes. SCD accounted for 42.9% of cardiac deaths in patients with LVEF ≤35%, whereas it accounted for 27.3% of cardiac deaths in patients with LVEF >35%. A majority of SCDs occurred in the early phase after hospital discharge, especially within the first 4 months in patients with LVEF ≤35% (Figure C). The all-cause mortality was 6.1% at 1 year and 12.5% at 3 years in patients with LVEF ≤35%, whereas it was 1.4% at 1 year and 4.7% at 3 years in patients with LVEF >35%. The mortality due to SCD was 2.6% at 1 year and 3.1% at 3 years in patients with LVEF ≤35%, whereas it was 0.1% at 1 year and 0.3% at 3 years in patients with LVEF >35%. Among patients who received ICD implantation during hospitalization, appropriate ICD therapy for VT occurred in 1 of 6 patients with LVEF ≤35% at 2 years after hospital discharge, whereas no patients with LVEF >35% received appropriate ICD therapy. After hospital discharge, 4 patients with LVEF ≤35% and 2 patients with LVEF >35% received ICD implantation as primary prevention, and none of these patients received appropriate ICD therapy during the follow-up period. Two patients with LVEF >35% developed VT or VF at 2 or 7 months after hospital discharge and received ICD implantation as secondary prevention. Figure D shows survival curves for SCD or the occurrence of VT/VF in patients with LVEF ≤35% and LVEF >35%.
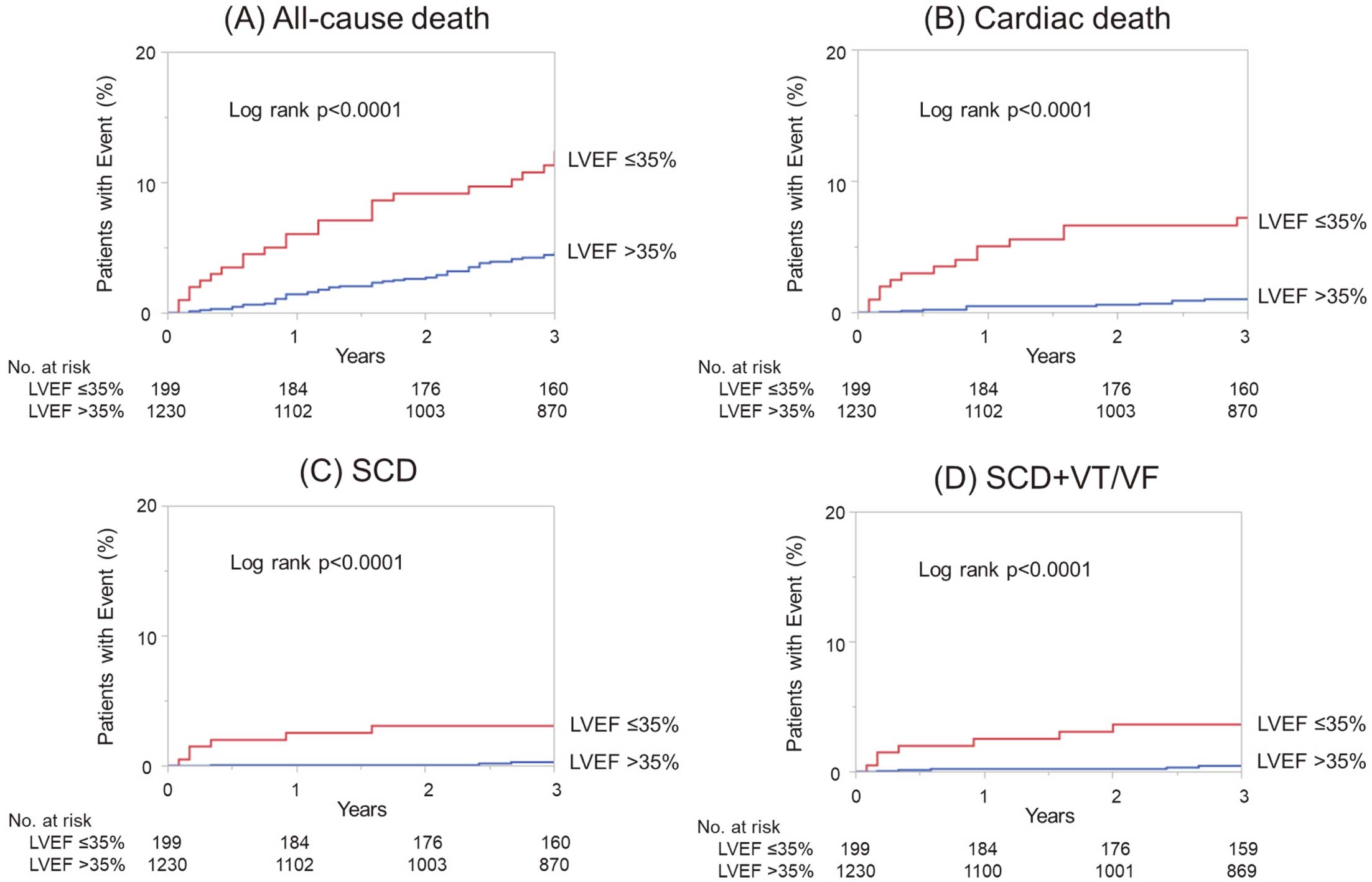
Survival curves after hospital discharge estimated using the Kaplan-Meier method in patients with left ventricular ejection fraction (LVEF) ≤35% and LVEF >35%. (A) All-cause death, (B) cardiac death, (C) sudden cardiac death (SCD), and (D) SCD or sustained ventricular tachycardia or fibrillation (VT/VF).
| LVEF ≤35% | LVEF >35% | |
|---|---|---|
| Cardiac death | n=14 | n=11 |
| SCD | 6 (42.9) | 3 (27.3) |
| Congestive heart failure | 6 (42.9) | 6 (54.5) |
| AMI | 0 (0.0) | 1 (9.1) |
| Not described | 2 (14.3) | 1 (9.1) |
| Non-cardiac death | n=10 | n=39 |
| Cancer | 3 (30.0) | 21 (53.8) |
| Pneumonia | 0 (0.0) | 2 (5.1) |
| Stroke | 1 (10.0) | 2 (5.1) |
| Others | 4 (40.0) | 8 (20.5) |
| Not described | 2 (20.0) | 6 (15.4) |
AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; SCD, sudden cardiac death.
Table 3 shows Cox proportional hazards model for all-cause death (A), cardiac death (B), SCD (C), and SCD+VT/VF (D). LVEF ≤35%, AF during hospitalization, age ≥75 years, and hospital admission over 6 h after AMI onset were considered as independent factors for all-cause death and cardiac death by multivariate analysis. Furthermore, LVEF ≤35% was independently associated with SCD, and LVEF ≤35% and hospital admission over 6 h after AMI onset were independently associated with SCD+VT/VF. We then performed an additional analysis of Cox proportional hazards model to evaluate factors associated with SCD or SCD+VT/VF in patients with LVEF ≤35% (n=199). Table 4 shows Cox proportional hazards model for SCD (A), and SCD+VT/VF (B) in patients with LVEF ≤35%. Hospital admission over 6 h after AMI onset is an independent factor associated with SCD and SCD+VT/VF, and comorbidity with diabetes is an independent factor associated with SCD.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| (A) All-cause death | ||||||
| LVEF ≤35% | 2.83 | 1.11–4.55 | 0.0001 | 2.36 | 1.42–3.85 | 0.001 |
| TIMI flow grade <3 after PCI | 1.19 | 0.61–2.13 | 0.59 | |||
| AF during hospitalization | 2.60 | 1.53–4.24 | 0.0006 | 2.13 | 1.23–3.57 | 0.008 |
| Age ≥75 years | 2.87 | 1.81–4.52 | <0.0001 | 2.45 | 1.50–4.03 | 0.0004 |
| Hospital admission >6 h after onset | 2.11 | 1.28–3.38 | 0.004 | 2.40 | 1.45–3.87 | 0.0009 |
| Hypertension | 1.05 | 0.66–1.74 | 0.84 | |||
| Diabetes | 1.24 | 0.78–1.95 | 0.36 | |||
| Dyslipidemia | 0.67 | 0.42–1.06 | 0.09 | 0.71 | 0.45–1.12 | 0.14 |
| Current smoker on admission | 0.65 | 0.40–1.03 | 0.07 | 0.94 | 0.56–1.56 | 0.81 |
| (B) Cardiac death | ||||||
| LVEF ≤35% | 7.01 | 3.23–15.42 | <0.0001 | 5.77 | 2.47–13.65 | <0.0001 |
| TIMI flow grade <3 after PCI | 2.77 | 1.13–6.22 | 0.03 | 1.00 | 0.37–2.49 | 1.00 |
| AF during hospitalization | 4.07 | 1.78–8.84 | 0.001 | 2.70 | 1.08–6.54 | 0.03 |
| Age ≥75 years | 6.00 | 2.74–14.10 | <0.0001 | 4.78 | 1.98–12.58 | 0.0004 |
| Hospital admission >6 h after onset | 3.01 | 1.35–6.51 | 0.008 | 3.92 | 1.71–8.87 | 0.002 |
| Hypertension | 1.68 | 0.72–4.59 | 0.24 | |||
| Diabetes | 1.25 | 0.57–2.70 | 0.58 | |||
| Dyslipidemia | 0.87 | 0.40–1.92 | 0.73 | |||
| Current smoker on admission | 0.36 | 0.13–0.84 | 0.02 | 0.78 | 0.27–1.98 | 0.61 |
| (C) SCD | ||||||
| LVEF ≤35% | 12.03 | 3.17–57.03 | 0.0003 | 7.13 | 1.70–36.13 | 0.007 |
| TIMI flow grade <3 after PCI | 7.36 | 1.95–29.74 | 0.004 | 3.34 | 0.78–14.91 | 0.10 |
| AF during hospitalization | 5.20 | 1.29–19.65 | 0.02 | 3.06 | 0.71–12.53 | 0.13 |
| Age ≥75 years | 2.54 | 0.63–9.59 | 0.18 | |||
| Hospital admission >6 h after onset | 3.27 | 0.81–12.35 | 0.09 | 3.16 | 0.78–12.07 | 0.10 |
| Hypertension | 1.77 | 0.43–11.86 | 0.46 | |||
| Diabetes | 2.91 | 0.77–13.77 | 0.11 | |||
| Dyslipidemia | 1.50 | 0.40–7.10 | 0.56 | |||
| Current smoker on admission | 0.60 | 0.13–2.28 | 0.46 | |||
| (D) SCD+VT/VF | ||||||
| LVEF ≤35% | 8.44 | 2.69–28.53 | 0.0004 | 6.10 | 1.81–21.74 | 0.004 |
| TIMI flow grade <3 after PCI | 4.21 | 1.25–13.20 | 0.02 | 2.13 | 0.57–7.34 | 0.25 |
| AF during hospitalization | 3.25 | 0.87–10.33 | 0.08 | 2.32 | 0.58–8.03 | 0.22 |
| Age ≥75 years | 1.58 | 0.42–5.02 | 0.47 | |||
| Hospital admission >6 h after onset | 4.10 | 1.28–13.12 | 0.02 | 4.13 | 1.28–13.35 | 0.02 |
| Hypertension | 1.51 | 0.45–6.81 | 0.52 | |||
| Diabetes | 2.04 | 0.65–6.88 | 0.22 | |||
| Dyslipidemia | 1.05 | 0.33–3.54 | 0.94 | |||
| Current smoker on admission | 0.86 | 0.25–2.69 | 0.79 | |||
CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction. Other abbreviations as in Table 1.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| (A) SCD | ||||||
| LVEF ≤30% | 3.45 | 0.67–24.88 | 0.14 | |||
| TIMI flow grade <3 after PCI | 4.87 | 0.95–35.10 | 0.06 | 4.85 | 0.94–35.12 | 0.06 |
| AF during hospitalization | 1.68 | 0.23–8.60 | 0.56 | |||
| Age ≥75 years | 2.49 | 0.46–13.44 | 0.27 | |||
| Hospital admission >6 h after onset | 6.91 | 1.35–49.87 | 0.02 | 8.72 | 1.68–63.34 | 0.01 |
| Hypertension | 0.85 | 0.17–6.16 | 0.86 | |||
| Diabetes | 6.62 | 1.07–126.69 | 0.04 | 7.59 | 1.21–146.01 | 0.03 |
| Dyslipidemia | 0.79 | 0.15–4.24 | 0.77 | |||
| Current smoker on admission | 1.16 | 0.22–6.28 | 0.85 | |||
| (B) SCD+VT/VF | ||||||
| LVEF ≤30% | 4.38 | 0.94–30.57 | 0.06 | 4.59 | 0.99–32.12 | 0.052 |
| TIMI flow grade <3 after PCI | 3.25 | 0.72–16.49 | 0.12 | |||
| AF during hospitalization | 1.34 | 0.19–6.23 | 0.73 | |||
| Age ≥75 years | 1.87 | 0.37–8.50 | 0.42 | |||
| Hospital admission >6 h after onset | 4.73 | 1.04–24.03 | 0.04 | 4.96 | 1.09–25.27 | 0.04 |
| Hypertension | 1.07 | 0.23–7.50 | 0.93 | |||
| Diabetes | 3.30 | 0.71–23.04 | 0.13 | |||
| Dyslipidemia | 1.05 | 0.23–5.33 | 0.95 | |||
| Current smoker on admission | 1.55 | 0.34–7.89 | 0.56 | |||
Abbreviations as in Tables 1,3.
In this study, we performed survival analysis for 2.6±0.8 years after hospital discharge in Japanese AMI patients treated with primary PCI within 12 h after AMI onset. The survival curves revealed that LVEF ≤35% was significantly associated with all-cause death, cardiac death, and SCD. The incidence of SCD was 2.6% at 1 year and 3.1% at 3 years in patients with LVEF ≤35% and a majority of SCDs occurred in the early phase after hospital discharge, especially within the first 4 months. These findings suggest that careful management in the early phase after hospital discharge may be important for AMI patients with LVEF ≤35% to prevent SCD.
Risk for SCD in Japanese AMI Patients With Reduced LVEFCox proportional hazards model revealed that LVEF ≤35% was an independent predictor for SCD, and SCD+VT/VF in patients with AMI who survived to discharge. The MADIT-II study demonstrated the survival benefit of ICD implantation for patients with reduced LVEF due to coronary artery disease; however, 88% of the study population were enrolled >6 months after AMI onset. Similarly, 78% of patients were enrolled >6 months after AMI onset in a study by Tanno et al,12 and half of the patients had no history of MI and only 25% of patients were enrolled at the time of AMI diagnosis in the Japanese Coronary Artery Disease registry,20 indicating that the results of these studies might not reflect the clinical outcome in AMI patients. In the Defibrillator in Acute Myocardial Infarction Trial (DINAMIT), AMI patients at 6–40 days after onset with reduced LVEF ≤35% were examined. The event rate of arrhythmic death was 8.5% during the 30±13 month follow up, which accounted for 50.0% of all-cause deaths.7 In the Valsartan in Acute Myocardial Infarction Trial (VARIANT), SCD occurred in 10.0% of AMI patients with LVEF ≤30% during the 25-month follow up.8 In addition, in the Immediate Risk Stratification Improves Survival (IRIS) trial, SCD occurred in 13.2% of AMI patients with LVEF ≤40% during the 37-month follow up, which accounted for 51.3% of all-cause deaths.9 In contrast, in the HIJAMI-II study, SCD occurred in 2.9% at 1 year and 5.1% at 3 years in AMI patients who were discharged alive with LVEF ≤30%.10 In the present study, SCD occurred in 2.6% and 3.1% of patients with LVEF ≤35% after 1 and 3 years, respectively, and SCD was accounted for 25% of all-cause deaths. These results indicate that the risk of SCD in AMI patients with reduced LVEF seems to be lower in Japan compared with Europe. However, the proportion of primary PCI was much lower in the DINAMIT (36.1%), VARIANT (15.2%), and IRIS trial (54.8%) than in the HIJAMI-II registry (77.8%) and the present study (100%), suggesting that a higher proportion of primary PCI may contribute to the better outcome in Japanese AMI patients. In fact, another study in the Netherlands showed a much lower rate of SCD in patients with LVEF ≤30% treated with primary PCI (2.3% at 1-year follow up).21 Future studies are needed to clarify whether a SCD event in AMI survivors with reduced LVEF would be lower in Japan than in Western countries in the primary PCI era.
The HIJAMI-II study is a multicenter observational prospective registered study including 4,122 AMI patients who were discharged alive.10 Although the survival curve for SCD in patients with reduced LVEF showed a similar trend to the present study results, the incidence of SCD was higher than that in our study. A lower cut-off value of LVEF in the HIJAMI-II study than in the present study (30 vs. 35%), lower rate of primary PCI (77.8 vs. 100%), and lower prescription rates of ACE inhibitor/ARB (60.8 vs. 93.1%), β-blocker (39.7 vs. 87.0%), or statins (27.1 vs. 81.4%) may have affected the difference in the incidence of SCD between the 2 studies. It should be noted that all patients in the present study were treated with primary PCI within 12 h after AMI onset and most of them had optimal medical therapy. These patient profiles may reflect the current clinical practice in Japan, and be one of the most important points in the present study.
Furthermore, some previous studies demonstrated that earlier reperfusion up to 6 h after AMI onset was associated with lower all-cause mortality and an independent predictor for 6-months or 1-year mortality.22–25 Although no data are available regarding time from AMI onset to reperfusion as a risk for SCD, these previous studies may support our finding showing that hospital admission >6 h after AMI onset was an independent risk for SCD in patients with LVEF ≤35%. Moreover, diabetes is reported to be a risk for SCD in post-MI patients, which is consistent with the present study results.26,27 Thus, extra careful attention should be given to patients with LVEF ≤35% who are admitted to hospital >6 h after AMI onset or those with diabetes in order to prevent SCD.
Majority of SCDs Occurs in the Early Phase After Hospital DischargeAnother important finding of the present study is that majority of SCDs occurred in the early phase after hospital discharge, and SCD became less frequent in the later phase if primary PCI was successfully performed within 12 h after AMI onset. In agreement, SCD was relatively frequent in the first year after AMI onset in patients with LVEF ≤30% in the HIJAMI-II study.10 These results suggest that the early phase after hospital discharge in AMI patients with reduced LVEF is a high-risk period for SCD, and provides the hypothesis about whether early ICD implantation could reduce SCD in AMI survivors. To address this issue, 2 randomized trials were previously performed and failed to demonstrate the mortality benefit of early ICD implantation due to an increase of non-cardiac death; however, the risk of SCD was reduced by ICD implantation.7,9 The survival curves for SCD or death from arrhythmia separated progressively in the first year between ICD and the control arm in the 2 studies. More strikingly, in the VARIANT, the risk of SCD or cardiac arrest with resuscitation was greatest in the early period after AMI and declined significantly over time in patients with LVEF ≤30%.8 The current guidelines do not recommend ICD implantation in patients with reduced LVEF within 40 days after AMI onset or 90 days after revascularization in order to give allowance for recovery from myocardial stunning.28,29 Although the reason why non-cardiac death was increased in the ICD arm is unclear, our results may reinforce that the early phase after hospital discharge in AMI patients with reduced LVEF seems to be a high-risk period for SCD, and careful management, such as a wearable cardioverter-defibrillator (WCD; Life Vest 4000, Zoll, Pittsburgh, PA, USA) use, could possibly improve survival.30,31
The WCD has been expected to prevent SCD due to sustained VT/VF as an effective bridge therapy until the necessity of ICD can be determined,31 and the effectiveness of the WCD has been shown in several studies.32–34 In the setting of AMI, 133 of 8,453 (1.6%) recent MI patients with LVEF ≤35% received appropriate shocks and the risk was the highest in the first month of WCD use.35 In the Vest Prevention of Early Sudden Death Trial, 1.3% of patients with the WCD received an appropriate shock and WCD use led to an improvement in total mortality.36 Thus, the risk for life-threatening ventricular tachyarrhythmia is relatively high in the early period after AMI. In contrast, almost half of patients with LVEF ≤35% following MI had an improvement in LVEF to >35% within 3 months,37 and ICD implantation was not required at the end of WCD use.34 In our study, most SCD occurred in an early phase after hospital discharge, and a WCD might be a promising therapeutic choice to prevent SCD during this high-risk period.
Study LimitationsThere are several limitations in the present study. First, this is a retrospective single-center study that covered a period of 15 years. During this period, there were advancements or both PCI devices38–41 and antiplatelets,42 which may influence the patients’ clinical outcome. Second, we analyzed the data at hospital discharge; therefore, subsequent changes in medication and laboratory data were not considered in the present study. Third, although there is a possibility that LVEF could be improved during follow up,43 LVEF measurement was made at only 1 point before hospital discharge; however, the most practical timing to assess LVEF for risk stratification is before discharge, because a substantial proportion of cardiac events occurs during the early period after hospital discharge. Fourth, missed follow-up appointments exists due to this study being retrospective, which may have affected the results. Fifth, stent thrombosis is a serious complication of AMI after PCI and possibly causes SCD. It is difficult to distinguish death due to stent thrombosis from clinical circumstances at the time of death without an autopsy. Although the possibility of stent thrombosis as a cause of SCD cannot be completely excluded in the present study, its incidence is reported to be low in the current clinical practice.44,45 Finally, the number of the study populations may not be large enough, and a longer follow-up period is required to assess long-term outcomes.
We performed a survival analysis of Japanese AMI patients with reduced LVEF treated with primary PCI. The incidence of SCD in Japan was significant but lower than that in Western countries. A majority of SCDs occurred in the early phase after hospital discharge, and the events become less frequent in the later phase. Careful management in the early phase after hospital discharge may be important for AMI patients to prevent SCD.
The authors wish to thank Machiko Kogawa for technical support.
H.T. received research funding from Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Pfizer, and Speakers’ Bureau/Honorarium from Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Bristol-Myers Squibb. K.O. received Speakers’ Bureau/Honorarium from Johnson and Johnson, Medtronic, Daiichi-Sankyo, and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
The ethical committee of the Hirosaki University Graduate School of Medicine (approved number 2020-070) approved this study.
The deidentified participant data will not be shared.