Article ID: CJ-22-0308
Article ID: CJ-22-0308
Background: The relationship between venous congestion and acute kidney injury (AKI) in cardiac surgery after cardiopulmonary bypass has not thoroughly investigated. Vacuum-assisted venous drainage (VAVD) reduces venous congestion, so we hypothesized that it would reduce the incidence of AKI in cardiovascular surgery.
Methods and Results: We used a retrospective propensity score-matched analysis to evaluate the effect of VAVD on AKI in adult patients undergoing cardiac surgery. The primary outcomes were AKI and renal replacement therapy (RRT). Multivariable logistic regression was used to explore the association between VAVD exposure and adverse kidney outcomes. Of 15,387 eligible subjects, 13,480 and 1,907 had gravity drainage (GD) or VAVD, respectively, during cardiopulmonary bypass. On the basis of propensity scores, there were 1,468 matched patient pairs for GD and VAVD. The average central venous pressure (CVP) in the GD group was higher than in the VAVD group (4.43±1.23 mmHg vs. 2.30±0.98 mmHg, P<0.001). The occurrence of AKI and RRT was statistically significantly different in the 2 groups [(600/1,468, 40.87%) vs. (445/1,468, 30.31%), P<0.001; (36/1,468, 2.45% vs. 8/1,468; 0.54%), P<0.001, respectively)]. Multivariate logistic regression analysis revealed that VAVD was effective in protecting kidney function.
Conclusions: VAVD was associated with a lower CVP and lower incidence of AKI, suggesting it protects adult cardiac patients from adverse renal outcomes.
As a result of continuous advancements in medical technology, many adverse events associated with cardiac surgery, including death and in-hospital deaths, have greatly decreased,1,2 but acute kidney injury (AKI) remains a widespread and serious complication. The incidence of cardiac procedure-associated AKI (CPA-AKI) fluctuates from 10% to 40% depending on the definition of AKI.3,4 The mechanism of perioperative AKI is multifactorial and complex. Hemodynamics, inflammation, exposure to nephrotoxin, the type of surgery, prolonged cardiopulmonary bypass (CPB), and other risk factors are closely connected with AKI.5,6 New research has shown that renal venous congestion is important in the development of cardiac surgery-associated adverse kidney events; the retrospective analysis demonstrated that increased central venous pressure (CVP), rather than cardiac index or systolic blood pressure, is associated with decreased estimated glomerular filtration rate (eGFR) in patients undergoing cardiovascular surgery.7
Cardiac surgery with CPB involves venous drainage and thus affects renal venous congestion. In the conventional procedure, an obstacle to venous return is the principal problem during CPB in on-pump cardiac surgery.8 Venous drainage volume varies according to CVP, venous cannula size, resistance to the pump line, and the height difference between the patient entrance and the venous line in the venous reservoir.9 This standard gravitational drainage (GD) technique is satisfactory in the vast majority of adult cardiac surgery patients. However, if venous drainage is grossly inadequate CVP will rise and induce venous congestion. To deal with this, vacuum-assisted venous drainage (VAVD) has become widely used in cardiac centers, as it increases the outflow of blood from the patient by extra negative pressure through a vacuum regulator in the hard-shell venous reservoir in CPB.10 Consequently, venous congestion is alleviated.
Studies of the relationship between VAVD and renal function protection during CPB are limited. We hypothesized that the application of VAVD would reduce venous congestion and thus the incidence of acute adverse kidney events and renal replacement therapy (RRT) after cardiac surgery. We conducted the present propensity score-matched cohort study to verify or disqualify our claim.
This retrospective study enrolled 15,387 patients aged ≥18 years who were scheduled for cardiac procedures with CPB at the Nanjing First Hospital, Huai’an First People’s Hospital, Yancheng City No.1 People’s Hospital and Jiangsu Province Hospital on Integration of Chinese and Western Medicine between January 2011 and December 2021 Patients were divided into 2 groups based on whether they received VAVD in CPB (Figure 1).

Flow chart of study enrolment. DHCA, deep hypothermic circulatory arrest; GD, gravity drainage; HT, heart transplantation; RRT, renal replacement therapy; VAVD, vacuum-assisted venous drainage.
Routine intravenous-inhalation combined anesthesia was performed, with monitoring of left radial arterial blood pressure and venous oxygen saturation. Throughout each operation, esophageal ultrasound and bispectral index were used to evaluate cardiac function and depth of intraoperative anesthesia. The core body temperature was monitored by Foley catheter with a temperature probe in the bladder.
CPB ManagementArterial Cannula In most cases, the aortic arterial cannula was the primary choice for perfusion. A small number of cases with other specific cannulas was excluded.
Venous Drainage CPB was launched by draining the venous blood of the patient into a venous reservoir (maximum volume 4.5 L) and finishing oxygenation via a hollow fiber membrane oxygenator (Terumo, Japan). Arterial blood was pumped to the vascular system through a Stockert S5 roller pump and body temperature was regulated by heat exchanger. The extracorporeal circuit was primed using Plasma-Lyte A (500 mL), and hydroxyethyl starch 130/0.4 (1 L), maintaining hematocrit (Hct) ≥25% with red blood cells (RBCs), 20% albumin, plasma products, mannitol, and low-molecular-weight heparin. CPB was managed with goal-directed perfusion (oxygen delivery index (IDO2) ≥280 mL/min/m2). Blood gases were sustained at target PCO2 of 40–45 mmHg with an α-stat strategy.
During CPB, a mean perfusion pressure of 40–60 mmHg was sustained.
Venous Cannula The Cannulation site was determined by the type of operation in all patients. For no-right atrium incision surgery, such as isolated coronary artery bypass grafting (CABG), isolated aortic valve replacement (AVR), AVR+CABG and operations involving the ascending aorta, a 2-stage venous cannula (28/32# for ≤80 kg, 32/40# for >80 kg; Wei Gao, Shan Dong, China) was used. For patients who underwent other cardiovascular operations, 2 venous cannulas with 28-Fr and 32-Fr (inner diameters of 7.33 mm and 8 mm; Wei Gao) for the superior and inferior venae cava, respectively, were chosen. The body mass index (BMI) of the patient was factored into the choice of cannula size.
VAVD ManagementIt is essential to monitor the pressure of the venous reservoir to ensure the security of surgery [maximum negative (−30 to −60 mmHg) and positive pressure alarms (<30 mmHg)]. Before starting CPB, all connections in the venous reservoir and the Y ventilation port inspected twice. AVD (Medtronic, USA) was utilized during CPB at the targeted negative pressure of −30 mmHg to −60 mmHg when appropriate blood flow velocity was obtained (Figure 2).
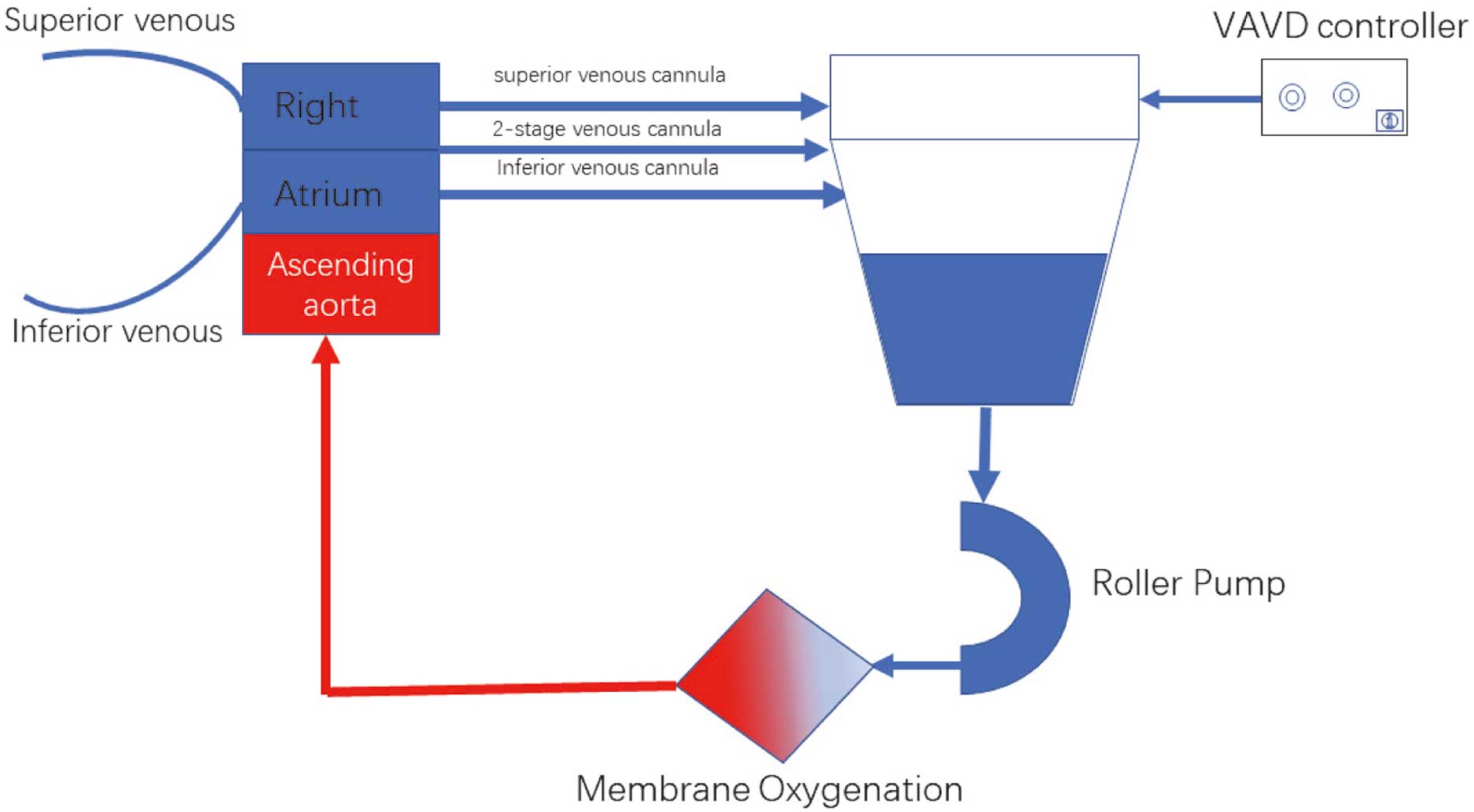
Schematic of in vitro circuit. VAVD, vacuum-assisted venous drainage.
Primary The incidence of acute kidney dysfunction, including AKI, was the cardinal outcome. AKI was defined using the Kidney Disease, Improving Global Outcomes (KDIGO) standard: (1) increase in creatinine ≥0.3 mg/dL or ≥26.5 μmol/L within 48 h after operation, and (2) increase in creatinine to ≥1.5-fold of baseline known or assumed to have emerged within the previous week.11 In addition, the mean CVP and the need for RRT were evaluated.
Secondary Death, cerebrovascular accident (postoperative cognitive dysfunction, transient ischemic attack, cerebral hemorrhage, stroke), duration of intensive care unit (ICU) stay and inhospital stay were recorded.
Statistical AnalysisSPSS Statistics version 24.0 (SPSS, Chicago, IL, USA) was utilized for data analysis. The characteristics of the patient are given as the median with interquartile range (IQR). Differences between groups and relationships between different indices were analyzed using non-parametric tests. The incidence of variables was compared using Fisher’s exact test or χ2 test in contingency tables. Variables with statistically significant differences and prior research recommendations were chosen through univariate analysis screening. The indicators related to the occurrence of AKI were included in the logistic multivariate regression analysis, the risk factors and their coefficients were obtained, and an AKI prediction model was established. Multiple regression logistic regression was performed to evaluate the risk relationship between VAVD management and AKI. For all analyses, P<0.05 was considered statistically significant.
Propensity Score MatchingPropensity scores for each subject were generated using a multivariable logistic regression analysis model to compute the probability of VAVD administration based on the following covariates: age, sex, BMI, diabetes, body weight, hypertension, smoking history, chronic obstructive pulmonary disease, cerebrovascular disease, ejection fraction, myocardial infarction and the results from the main laboratory tests (serum creatinine (sCr), blood urea nitrogen, eGFR, alanine aminotransferase, aspartic transaminase, B-type natriuretic peptide). Propensity scores were then used to create 1 : 1 matched pairs (matching the VAVD users to non-VAVD users) using a nearest neighbor matching algorithm without a caliper method.
Our selection criteria were met by 15,387 patients from the 4 hospitals who underwent a cardiac operation with CPB. Baseline characteristics of the study subjects before and after propensity score-matching are given in Table 1. No statistically significant differences were observed in baseline characteristics. The patients were divided into a GD group (n=13,480) and supplementary VAVD group (n=1,907). A total of 1,468 patient pairs were enrolled after 1 : 1 propensity score matching.
| Variable | Before matching | Propensity score matched | ||||
|---|---|---|---|---|---|---|
| GD group (n=13,480) |
VAVD group (n=1,907) |
P value | GD group (n=1,468) |
VAVD group (n=1,468) |
P value | |
| Age (years) | 56.5 (17) | 58.2 (16) | 0.047 | 56.8 (18.5) | 56.7 (19) | 0.655 |
| Male/female | 7,319/6,161 | 984/923 | 0.308 | 746/722 | 745/723 | 0.985 |
| Body weight (kg) | 65.2 (12) | 66.3 (13) | 0.430 | 65.7 (14) | 66.0 (13) | 0.779 |
| BMI (kg/m2) | 23.8 (4.8) | 24.2 (5.0) | 0.043 | 23.7 (4.7) | 24.1 (4.9) | 0.762 |
| Hypertension | 987 (7.3) | 159 (8.3) | 0.125 | 148 (10.0) | 143 (9.7) | 0.805 |
| Diabetes | 1,411 (10.5) | 190 (10.0) | 0.526 | 36 (2.5) | 22 (1.5) | 0.085 |
| Smoking history | 2,898 (21.5) | 387 (20.3) | 0.241 | 234 (15.9) | 236 (16.0) | 0.960 |
| COPD | 378 (2.8) | 47 (2.5) | 0.440 | 37 (2.5) | 39 (2.7) | 0.907 |
| Cerebrovascular disease history | 147 (1.1) | 19 (1.0) | 0.799 | 14 (1.0) | 12 (0.8) | 0.841 |
| EF (%) | 59 (8) | 59 (7) | 0.346 | 60 (7.0) | 60 (6.5) | 0.737 |
| Creatinine (mg/dL) | 0.84 (0.3) | 0.86 (0.4) | 0.042 | 0.85 (0.5) | 0.84 (0.5) | 0.940 |
| BUN (mg/dL) | 87 (24) | 88 (21) | 0.058 | 74 (25) | 74 (24) | 0.843 |
| BNP (pg/mL) | 516 (48) | 519 (49) | 0.058 | 518 (47) | 518 (48) | 0.713 |
| eGFR (mL/min/1.73 m2) | 113 (14) | 115 (15) | 0.071 | 112 (12) | 114 (13) | 0.815 |
| ALT (U/L) | 22 (18) | 23 (19) | 0.041 | 22 (17) | 21 (18) | 0.334 |
| AST (U/L) | 21 (17) | 21 (18) | 0.354 | 22 (19) | 22 (18) | 0.345 |
| MI | 346 (2.6) | 61 (3.2) | 0.268 | 123 (8.4) | 120 (8.2) | 0.893 |
| UA | 191 (1.4) | 34 (1.8) | 0.935 | 32 (2.2) | 30 (2.0) | 0.898 |
| AF | 665 (4.9) | 80 (4.2) | 0.177 | 77 (5.3) | 75 (5.1) | 0.934 |
Continuous variables are presented as mean±standard deviation or median (interquartile range). Categorical variables are given as n (%). AF, atrial fibrillation; ALT, alanine aminotransferase; AST, aspartic transaminase; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; MI, myocardial infraction; UA, unstable angina.
Operation choice and intraoperative data are shown in Table 2A. There were no significant differences for mean arterial pressure (MAP) in CPB, urinary output, CPB duration, aortic cross-clamp time, first 24-hour vasoactive inotropic score and surgical type but the average CVP was significantly different between the GD and VAVD groups (P<0.001). The postoperative anesthetic recovery, intubation time, ICU and hospital stays were similar in the 2 groups. There were no substantial differences in the incidence of postoperative complications or inhospital deaths between groups (Table 2B).
| (A) Characteristic | Propensity score-matched group | ||
|---|---|---|---|
| GD group (n=1,468) | VAVD group (n=1,468) | P value | |
| Surgical procedure | |||
| Isolated CABG surgery | 556 (37.87) | 562 (38.28) | 0.849 |
| Isolated valve surgery | 405 (27.59) | 401 (27.32) | 0.903 |
| CABG and valve surgery | 152 (10.35) | 150 (10.22) | 0.952 |
| CHD repair | |||
| VSD | 68 (4.63) | 65 (4.43) | 0.859 |
| ASD | 67 (4.56) | 61 (4.16) | 0.651 |
| Vascular surgery | |||
| AAR | 39 (2.66) | 37 (2.52) | 0.907 |
| Wheat procedure | 55 (3.75) | 63 (4.30) | 0.750 |
| Bentall procedure | 56 (3.81) | 59 (4.00) | 0.849 |
| Cardiovascular tumor | |||
| Benign | 51 (3.47) | 45 (3.07) | 0.604 |
| Cancer | 9 (0.61) | 11 (0.75) | 0.904 |
| Other | 10 (0.70) | 14 (0.95) | 0.539 |
| MAP during CPB (mmHg) | 56 (5) | 55 (7) | 0.255 |
| Average CVP (mmHg) | 4 (2) | 2 (1) | <0.001 |
| Aortic cross-clamp time (min) | 82 (23) | 82 (24) | 0.526 |
| CPB time (min) | 118 (32) | 117 (33) | 0.836 |
| Mean pump flow (L/min/m2) | 2.5 (0.4) | 2.5 (0.3) | 0.559 |
| Hct (%) | 25 (3) | 25 (2) | 0.548 |
| Post CPB-6th hours HPT (mg/dL) | 1.25 (0.3) | 1.26 (0.2) | 0.647 |
| RBCs transfused | 178 (12.13) | 156 (10.63) | 0.222 |
| Defibrillation | 178 (12.13) | 193 (13.15) | 0.177 |
| VIS (points) | 22.5 (2.5) | 22.6 (2.4) | 0.386 |
| Urinary volume (mL) | 218 (46) | 219 (45) | 0.237 |
| Furosemide prescribed | 210 (14.31) | 176 (11.99) | 0.071 |
| Ultrafiltrate output (mL) | 1,689 (398) | 1,704 (376) | 0.447 |
| (B) Characteristic | GD group (n=1,468) | VAVD group (n=1,468) | P value |
| Postoperative awaken time (h) | 9.3±2.4 | 9.4±2.5 | 0.269 |
| Intubation time (h) | 8.4±2.4 | 8.3±2.3 | 0.249 |
| Mechanical ventilation time (h) | 8.2±2.0 | 8.2±1.8 | 0.311 |
| Length of stay in the ICU (h) | 12.5±4.5 | 12.8±4.1 | 0.051 |
| Length of stay in hospital (days) | 15.5±3.1 | 15.9±3.8 | 0.390 |
| Cerebrovascular accident | |||
| Temporary (POCD/TIA) | 72/5 | 73/9 | 0.668 |
| Permanent (cerebral hemorrhage/stroke) | 3/4 | 1/5 | 0.676 |
| Early cardiac shock | 10 (0.7) | 12 (0.8) | 0.831 |
| ECMO insertion | 4 (0.3) | 3 (0.2) | 1.000 |
| IABP insertion | 18 (1.2) | 22 (1.5) | 0.633 |
| Hepatic failure | 7 (0.5) | 5 (0.3) | 0.772 |
| Re-exploration | 12 (0.8) | 9 (0.6) | 0.987 |
| Inhospital death | 17 (1.2) | 16 (1.1) | 0.861 |
Continuous variables are presented as mean±standard deviation or median (interquartile range). Categorical variables are given as n (%). AAR, aortic ascending replacement; ASD, atrial septal defect; Bentall, aortic root replacement; CABG, coronary artery bypass grafting; CHD, congenital heart disease repair; CPB, cardiopulmonary bypass; CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; Hct, hematocrit; HPT, haptoglobin; IABP, intra-aortic balloon pump; ICU, intensive care unit; MAP, mean arterial pressure; POCD, postoperative cognitive dysfunction; RBC, red blood cells; TIA, transient ischemic attack; VIS, vasoactive inotropic score; VSD, ventricular septal defect; Wheat, aortic valve & ascending replacement.
Overall, 1,045 patients of 2,936 (35.60%) were confirmed to have developed AKI. Incidence of both AKI and RRT in the GD group was higher than in the VAVD group [(600/1,468, 40.87%) vs. (445/1,468, 30.31%), P<0.001; (36/1,468, 2.45% vs. 8/1,468; 0.54%), P<0.001, respectively) (Figure 3).

Incidence of AKI and RRT between GD and VAVD groups. AKI, acute kidney injury; GD, gravity drainage; RRT, renal replacement therapy; VAVD, vacuum-assisted venous drainage.
Univariate analysis revealed statistically significant differences in age, sex, BMI, history of hypertension, CPB duration, postoperative mechanical ventilation time, intraoperative blood product infusion, furosemide use and VAVD between the AKI and No-AKI groups (P<0.001). Nonetheless, there were no distinct changes between groups for surgical indicators (hemoglobin, Hct, sCr, eGFR), intraoperative MAP, and other indices (Tables 3,4).
| Characteristic | AKI group (n=1,045) |
No-AKI group (n=1,891) |
P value |
|---|---|---|---|
| Age (years) | 59 (5) | 53 (6) | 0.027 |
| Male | 648 (62.00) | 410 (21.68) | <0.001 |
| BMI (kg/m2) | 27.8 (2.5) | 24.5 (2.3) | 0.011 |
| Hypertension | 151 (52.73) | 140 (14.94) | <0.001 |
| Diabetes | 22 (2.10) | 30 (1.59) | 0.382 |
| Creatinine (mg/dL) | 0.83 (0.5) | 0.84 (0.4) | 0.731 |
| BUN (mg/dL) | 86 (25) | 85 (26) | 0.846 |
| eGFR (mL/min/1.73 m2) | 115 (3) | 116 (4) | 0.649 |
| CPB time (min) | 127 (5) | 118 (4) | 0.024 |
| MAP during CPB (mmHg) | 52 (4) | 51 (5) | 0.593 |
| Average CVP (mmHg) | 4 (1) | 2 (1) | 0.015 |
| VAVD used | 468 (44.78) | 1,000 (52.88) | 0.010 |
| Aortic cross-clamp time (min) | 85 (23) | 86 (24) | 0.427 |
| Hct (%) | 24 (4) | 25 (3) | 0.657 |
| RBCs transfused | 174 (16.66) | 160 (8.46) | <0.001 |
| Urinary volume (mL) | 220 (44) | 218 (42) | 0.148 |
| Furosemide prescribed | 216 (20.67) | 170 (8.99) | <0.001 |
| Ultrafiltrate output (mL) | 1,700 (399) | 1,703 (386) | 0.569 |
| Mechanical ventilation time (h) | 8 (2) | 6 (1) | 0.024 |
Continuous variables are presented as mean±standard deviation or median (interquartile range). Categorical variables are given as n (%). AKI, acute kidney injury; VAVD, vacuum-assisted venous drainage. Other abbreviations as in Tables 1,2.
| Characteristic | AKI (+) (n=445) |
AKI (−) (n=1,023) |
P value |
|---|---|---|---|
| Age (years) | 60 (4) | 55 (5) | 0.013 |
| Male | 345 (77.53) | 400 (39.10) | 0.003 |
| BMI (kg/m2) | 27.5 (1.0) | 25.5 (2.0) | 0.001 |
| Hypertension | 83 (18.65) | 60 (5.87) | <0.001 |
| CPB time (min) | 128 (8) | 121 (9) | 0.001 |
| Average CVP (mmHg) | 4 (1) | 4 (1) | 0.761 |
| RBCs transfused | 58 (13.03) | 120 (11.73) | 0.538 |
| Furosemide prescribed | 76 (17.08) | 100 (9.78) | <0.001 |
| Mechanical ventilation time (h) | 9 (3) | 7 (2) | 0.019 |
Continuous variables are presented as mean±standard deviation or median (interquartile range). Categorical variables are given as n (%). Abbreviations as in Tables 1–3.
Multivariate logistic regression analysis was used to detect the potential protective and risk factors in the progress of AKI. Table 5A,B shows that VAVD was protective against CSA-AKI. Advanced age, high BMI, prolonged CPB and prolonged postoperative mechanical ventilation time, furosemide prescribed, RBC transfusion and high CVP independently correlated with CSA-AKI. Advanced age, high BMI, prolonged CPB and prolonged postoperative mechanical ventilation time are also independent risk factors for CSA-AKI in the VAVD group. Notwithstanding, CVP and transfused RBCs were not potential causes of CSA-AKI, and the odds ratio (OR) values of the aforementioned indicators in the VAVD group were lower than the corresponding ORs in the analysis of the entire population (Table 5).
| Variable | Standardized β | OR | 95% CI | P value |
|---|---|---|---|---|
| (A) All eligible subjects (n=2,936) | ||||
| Sex/male | 0.519 | 1.68 | 1.36–2.59 | 0.037 |
| BMI | 0.351 | 1.42 | 1.04–2.27 | 0.041 |
| Age | 0.620 | 1.86 | 1.34–2.67 | 0.021 |
| Hypertension | 0.489 | 1.63 | 1.21–1.98 | 0.001 |
| Blood product transfused | 0.565 | 1.76 | 1.07–2.03 | 0.001 |
| CPB duration | 0.647 | 1.91 | 1.56–2.98 | <0.001 |
| Mechanical ventilation time | 0.113 | 1.12 | 1.03–1.84 | 0.034 |
| VAVD used | −4.308 | 0.65 | 0.39–0.87 | 0.004 |
| Furosemide prescribed | 0.224 | 1.46 | 1.17–1.90 | 0.039 |
| CVP | 0.688 | 1.99 | 1.37–3.22 | 0.001 |
| (B) Propensity score-matched VAVD group (n=1,468) | ||||
| Sex/male | 0.464 | 1.59 | 1.32–2.61 | 0.150 |
| BMI | 0.322 | 1.38 | 1.03–1.99 | 0.045 |
| Age | 0.571 | 1.77 | 1.43–3.02 | 0.002 |
| Hypertension | 0.419 | 1.52 | 1.19–2.30 | 0.027 |
| Blood product transfused | 0.270 | 1.31 | 0.96–1.77 | 0.395 |
| CPB duration | 0.560 | 1.75 | 1.20–2.48 | 0.011 |
| Mechanical ventilation time | 0.077 | 1.08 | 1.01–1.92 | 0.034 |
| Furosemide prescribed | 0.285 | 1.33 | 1.17–2.05 | 0.033 |
| CVP | 0.191 | 1.21 | 0.89–2.34 | 0.052 |
CI, confidence interval; OR, odds ratio. Other abbreviations as in Tables 1–3.
AKI, which is frequent in patients undergoing cardiovascular surgery, is a separate risk factor for postoperative morbidity and mortality.12 The second most influential factor contributing to AKI after sepsis is cardiac surgery. The reports on the incidence of CSA-AKI in diverse centers are currently quite dissimilar, which may be related to the various diagnostic criteria for AKI used in each study as well as the variety of procedures patients have undergone. According to the outcomes of our research based on the 2012 KDIGO diagnostic criteria for AKI, the incidence rate of CAS-AKI was 35.60%, which is consistent with previous findings.13
The pathophysiological mechanism of CSA-AKI is not entirely clear, and currently principally includes hemodynamic disturbance, inflammatory response, CPB and neuroendocrine activation.14 Research has confirmed that the risk factors for CSA-AKI include advanced age, male sex, hypertension, cardiac insufficiency, intraoperative MAP and the type of surgery.15 In addition to these, we also included biological and surgery-related variables in our study. Notably, our results showed that advanced age, high BMI, prolonged CPB, intraoperative RBC transfusion, administration of furosemide, high CVP and prolonged postoperative mechanical ventilation were independent risk factors for CSA-AKI whereas VAVD was beneficial for protecting against CSA-AKI.
Elderly patients often have decreased renal reserve capacity, and are predisposed to other organ insufficiency, and their susceptibility to factors such as ischemia and hypoxia increases.16 High BMI is associated with the occurrence of AKI after cardiac surgery, which may be caused by obesity. The perfusion hyperfiltration state makes the kidney more prone to injury, and oxidative stress and inflammatory response are also involved.17 Male sex is commonly associated with androgens and a high BMI. Androgens significantly raise the receptivity of blood vessels to vasoactive substances, decrease the level of prostacyclin and stimulate the production of thromboxane. Furthermore, they directly increase platelet aggregation and induce microthrombosis, thereby affecting renal blood flow and causing renal injury.18
As an indispensable component of cardiac surgery, CPB may have a connection with CSA-AKI. High CVP is simply a reflection of venous congestion, which can lead to AKI. The following factors may contribute to this phenomenon. First of all, decreased renal blood flow can induce accumulation of neutrophils within the peritubular capillaries.19,20 Secondly, reduced renal blood flow triggers the inflammatory process, signaling upregulation (e.g., NF-κB), which increases neutrophil adhesion on activated endothelial cells.21 Moreover, research has demonstrated that pharmacologic inhibition of inflammatory signaling ameliorates congestion-mediated deterioration of renal injury.22 Our findings showed that VAVD can decrease CVP by increasing venous drainage, thereby protecting renal function during cardiac surgery. For the first time use of furosemide found to be an independent risk factor for CSA-AKI. Possible mechanisms are that intensified diuresis causes an effective blood volume insufficiency, directly activates the renin-angiotensin-aldosterone system (RAAS), enhances renal tubular reabsorption, and causes renal tubular reabsorption. In addition, it also changes the distribution of renal blood flow by reducing the resistance of renal medullary blood vessels to distributing renal blood flow in the renal cortex leading to relative ischemia and hypoxia in the outer medulla, which in turn leads to AKI.23 Furthermore, another report concluded that the use of furosemide in order to restore urine output not only indicates the severity of AKI and does not ameliorate functional outcome.24 Intraoperative RBC transfusion can promote renal injury due to hemolysis, iron load, pro-inflammatory and other factors. Patients with prolonged mechanical ventilation frequently have pulmonary complications. On the one hand, it may induce an abnormality in oxygenation of tissues and organs such as the kidneys, while on the other, it may make pulmonary infections more common, required antibacterial treatment, adding to the burden of renal drug metabolism, and ultimately causing kidney damage. We analyzed the influence of VAVD on these risk factors and the results showed that intraoperative RBC transfusion and CVP were no longer independent risk factors for AKI. The reason may be that the application of VAVD increased venous drainage and reduced CVP. More venous reflux also increased the Hct and decreased the need for intraoperative RBC transfusion via the blood concentrator, both of which are greatly facilitated by VAVD. Other positive factors were still risk factors for AKI, but the OR-values of all positive indicators decreased, which may be due to the fact that VAVD increases venous drainage, improves systemic blood flow, reduces the systemic inflammatory response, and protects renal function.25 The results of our study suggest that VAVD can reduce the incidence of CSA-AKI. However, Gao et al showed that the use of VAVD during cardiac surgery did not reduce the incidence of CAS-AKI, which may be related to the diagnostic criteria of AKI they adopted (AKI was defined as a postoperative increase in sCr to more than twice the baseline within 1 month or to ≥354 mmol/L postoperatively).26 We used the KDIGO standard (details are in the Methods section) for the early identification of AKI during cardiac surgery. Therefore, our findings and that of Gao et al diverge due to the varying diagnostic criteria.
Disadvantages of VAVDTwo of the serious morbidities of CPB are hemolysis and micro-emboli, which relate to mechanical circulation and its components (pump type, oxygenator, sheer forces, foreign surfaces, blood suction recovery in operation filed and left atrium, etc.) and VAVD has a potential risk of air entrapment and systemic micro-embolism.27 Although there is no consensus on the adverse effect of VAVD on hemolysis and micro-emboli, we did not observe a significant difference in the incidence of complications of hemolysis and micro-emboli, such as haptoglobin level and cerebral morbidity, often expressed as postoperative cognitive defects or stroke.
Negative Pressure of VAVDThe negative pressure of VAVD has been in debate for years. After they testing the Hct and plasma free hemoglobin, it was reported by Gao et al that negative pressure up to −80 mmHg may induce hemolysis and in light of this, they advised against preferring higher vacuum pressure.26 Others have reported the safety of negative pressure values as high as −90 mmHg, with an average of 76.4 mmHg.28 In the 4 centers involved in the current study, a negative pressure of −30 to −60 mmHg was chosen, based on a conservative protocol. Compared with the GD group, the patients in the VAVD group did not exhibit obviously increasing levels of haptoglobin associated with hemolysis. In order to maintain the equilibrium between hemolysis and venous drainage, more sensitive indicators and additional study are needed to calculate the appropriate negative pressure of VAVD.
Study LimitationsFirst of all, our study was retrospective, undoubtedly bringing a tendency to bias. Moreover, plasma creatinine was the only marker used to define AKI.
VAVD was valuable for renal protection by reducing venous congestion without a discernible difference in hemolysis or perfusion flow during CPB in cardiac operations.
In a future prospective study, the negative side effects of VAVD will be examined in greater detail and depth.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors declare that there are no conflicts of interest.
The present study was approved by the Regional Human Research Ethics Committee of Nanjing First Hospital. Reference number: ktsb20190817-01-01.