Article ID: CJ-22-0334
Article ID: CJ-22-0334
Background: Three-dimensional aortic root evaluation using virtual reality (VR) techniques for valve-sparing aortic root replacement (VSARR) preparation has not yet been implemented, so we demonstrated VR computed tomography (VR-CT) and assessed its utility for VSARR.
Methods and Results: We enrolled 72 patients who underwent multidetector CT before elective VSARR for annuloaortic ectasia with tricuspid aortic valve. The geometries of their aortic roots were measured with a VR-CT workstation. The mean values of geometric height (GH), free margin length (FML), and commissural height (CH) were 17.2±2.4 mm, 36.0±5.2 mm, and 24.0±4.3 mm, respectively. The right coronary/noncoronary CH was significantly greater than the left coronary/right coronary and left coronary/noncoronary CH. The left coronary cusp had the shortest FML, intercommissural distances (ICD), and smallest central angle. Although the right coronary cusp had the largest values for FML, ICD, and central angle, the right coronary cusp had the lowest GH and EH. The VR-CT measurements strongly correlated with intraoperative alternatives, especially with mean GH (R2=0.75) and left coronary/noncoronary CH (R2=0.79). Furthermore, mean GH was observed to be significantly different among the selected graft size groups; therefore, the preoperative mean GH could play a significant role in graft sizing.
Conclusions: VR-CT evaluation allows a thorough understanding of aortic root anatomy, which could facilitate VSAAR.
Valve-sparing aortic root replacement (VSARR) emerged in 1990,1–3 and recently its surgical indications have been expanding. A thorough understanding of aortic root anatomy as the basis for appropriate graft size selection is important for the success of this procedure,4 but detailed stereoscopic characteristics of pre-VSARR patients have not been reported. Therefore, we aimed to clarify this using preoperative CT datasets of patients who underwent VSARR in Kobe University Hospital. Generally, radiologic measurements of CT data sets are performed using multiplanar reconstruction (MPR) or curved planar reconstruction (CPR);5,6 however, this is a complicated and time-consuming process requiring various measurements, as well as an expert’s interpretation for comprehensive evaluation of the aortic root.7 Thus, we evaluated the aortic root anatomy using a novel virtual reality (VR) workstation, True 3D (EchoPixel, Inc., Mountain View, CA, USA), which displays 3D images as objects that can be directly manipulated in the 3D space using a stylus pen (Figure 1). This new tool has already been successfully used in clinical settings, such as in preoperative planning of transcatheter surgeries8 or congenital cardiac surgeries.9 Although it remains untested for aortic root evaluation, its potential cannot be understated.

Virtual reality computed tomography (VR-CT) evaluation using a digital model on a 3D monitor. All VR-CT measurements were conducted via True3D using a stylus pen.
First, we used the VR workstation to measure the geometries of the aortic root of patients who underwent VSARR and evaluated the anatomical features. Second, to assess the feasibility of the VR-CT measurements, we compared them to the corresponding intraoperative measurements. Lastly, we analyzed the differences among selected graft size groups to determine which value played a significant role in graft sizing.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Kobe University Hospital (approval no. B200328). Informed consent was obtained in the form of an opt-out on the relevant website.
Datasets for this retrospective single-center study were obtained from 103 consecutive patients who underwent multidetector cardiac CT scan before VSARR between September 2008 and May 2021. Cases of indistinct images (n=15), acute aortic dissection (n=4), and bicuspid or quadricuspid (n=12) aortic valves were excluded from the analysis.
Image AcquisitionClinical image acquisition was performed using two-generation dual-energy scanner systems from GE Healthcare (Discovery CT750 HD; GE Healthcare, Waukesha, WI, USA) and Siemens (Somatom Definition Flash; Siemens Medical Solutions, Erlangen, Germany). All images were acquired during a deep inspiratory breath-hold and subsequently processed to obtain imaging of mid-diastole undergoing retrospective ECG-gated scanning.
VR-CT MeasurementsAll images were exported to the VR-CT workstation in the Cartesian Digital Imaging and Communications in Medicine format. The 3D aortic roots were then displayed as true 3D volumetric isolated objects at the front of the 3D monitor when wearing 3D glasses (Figure 1). First, we extracted the aortic root and labeled the anatomical landmarks (Figure 2A–D), including the nadir of each aortic cusp, the commissure posts of each inter-leaflet triangle (ILT), the midpoint of the free margin, and the bases of the ILT. Based on these landmarks, we performed the following VR-CT measurements (Figure 2E,F): the free margin length (FML), geometric height (GH), effective height (EH), root dimensions of the virtual basal ring plane (VBR: defined as the plane passing through the nadir of each aortic cusp), root dimensions of the sinotubular junction plane (STJ) , commissural height (CH: defined as the length between the base of the ILT and the commissure post), and intercommissural distance (ICD: defined as the distance between 2 commissure posts). The key processes of VR-CT measurements are explained in the Supplementary Movie for clarity. In addition, the tilting angles between the VBR and STJ were mathematically calculated based on the positional coordinates of the 3 nadirs and 3 commissures.
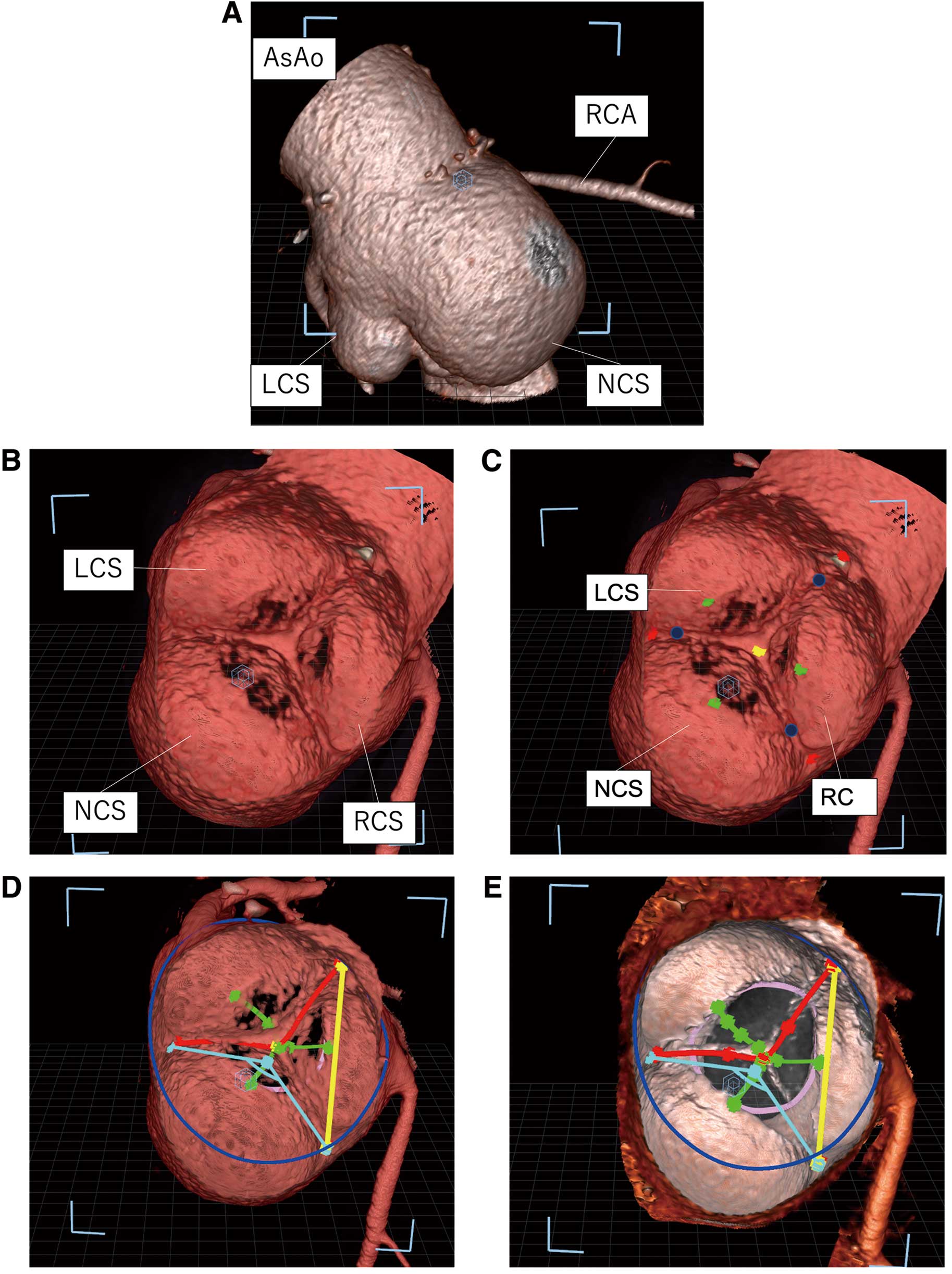
Virtual reality (VR) images on the workstation. (A) Extracted aortic root: AsAo at the top and left ventricle at the bottom of the image. (B) VR image of the aortic valve in diastole in traverse view from ascending aorta. (C) VR image after labeling the anatomical landmarks. Yellow label: midpoint of free margin line; Red labels: commissure posts of each inter-leaflet triangle; Green labels: nadirs of each sinus; Blue labels: points where 2 leaflets attach. (D) VR image after measurements of aortic root geometry. Red line: free margin length; Green lines: geometric height; Yellow line: intercommissural distance; Light blue angle: central angle of NCS; Blue circle: sinotubular junction; Pink circle: virtual basal ring. (E) VR image in which valve leaflets have been erased. AsAo, ascending aorta; LCS, left coronary sinus; NCS, noncoronary sinus; RCA, right coronary artery; RCS, right coronary sinus.
STJ plane: a1x + b1y + c1z + d1 = 0, VBR plane: a2x + b2y + c2z + d2 = 0
Tilting angle = θ,

A research fellow (T. Tsujimoto, 9 years of experience as a cardiovascular surgeon), who completed these measurements with the cooperation of the surgeon (K.O., 33 years of experience as a cardiovascular surgeon) was blinded to the intraoperative measurements.
Surgical Approach, Intraoperative Measurements, and Graft SizingAll patients underwent aortic root reconstruction with the reimplantation technique through a median sternotomy performed by 2 surgeons (Y.O. and K.O.).10 While the patient was in cardiac arrest, the aortic root geometries were directly measured by the surgeon: VBR, GH, FML, EH, and left coronary/noncoronary CH (LN-CH).
Before 2011, graft size selection was based on the measurement of VBR, with grafts being 3–5 mm larger than the VBR.11 Following the findings of de Kerchove et al in 2011,12 the LN-CH became the new standard for graft sizing. Depending on the particularities of each case, GH, FML, VBR, and distal aortic diameter were relevant for selecting a suitable graft size, and therefore they were also considered in this study.
Statistical AnalysisCategorical variables are presented as frequencies (percentage). Continuous variables with normal distribution are presented as mean±standard deviation, and those with non-normal distribution are reported as median. The Shapiro-Wilk test was used to assess the normality of the distribution. Analysis of variance (ANOVA) was used to compare continuous variables related to aortic sinuses, cusps, and commissures. Correlations between continuous variables with normal distribution were evaluated using the coefficient of determination (R2), and Student’s t-test was used to compare measurements with normal distributions. All statistical analyses were performed using commercially available software (JMP 14.3.0; SAS Institute, Cary, NC, USA), and statistical significance was set at P<0.05.
According to the stated criteria, 72 patients were enrolled. Their preoperative characteristics and echocardiographic data are shown in Table 1. The mean age of the included participants was 58.4±17.9 years, and 20.8% were female. Moderate or severe aortic insufficiency (AI) was observed in 53 (73.6%) patients, and 16 (22.2%) had ≥1 prolapsed cusps.
| Variables | All (n=72) |
|---|---|
| Age, years | 58.4±17.9 |
| Female, n (%) | 15 (20.8) |
| Body surface area, m2 | 1.72±0.21 |
| Annuloaortic ectasia, n (%) | 51 (70.8) |
| Marfan syndrome, n (%) | 13 (18.1) |
| Prior ascending aorta replacement, n (%) | 2 (2.8) |
| Chronic type A aortic dissection, n (%) | 2 (2.8) |
| Preoperative echocardiography | |
| Left ventricular ejection fraction, % | 59.9±8.9 |
| Left ventricular dimension in diastole, mm | 55.0±9.7 |
| Aortic root dimension, mm | |
| Ventriculoaortic junction | 24.4±3.1 |
| Sinotubular junction | 38.2±6.5 |
| Aortic regurgitation | |
| Severe | 34 (47.2) |
| Moderate | 19 (26.4) |
| Mild | 10 (13.9) |
| Trace or none | 9 (12.5) |
| Cusp prolapse, n (%) | 16 (22.2) |
VR-CT measurements using preoperative CT are shown in Table 2. The VR-CT measurements showed dilated dimensions of VBR and STJ of 25.9±3.3 and 39.6±7.2 mm, respectively, and a mean tilting angle of 13.0±5.7° between them. The mean values of GH, FML, EH, and CH were 17.2±2.4 mm, 36.0±5.2 mm, 10.5±3.2 mm, and 24.0±4.3 mm, respectively. The left coronary/right coronary CH was similar to the LN-CH (P=0.63); however, the right coronary/noncoronary CH was significantly greater than the aforementioned CH values (P<0.001). The left coronary cusp had the shortest FML, intercommissural distances (ICD), and smallest central angle, and although the right coronary cusp (RCC) had the largest values for FML, ICD, and central angle, it had the lowest GH and EH.
| Variables | n=72 | P value (ANOVA) |
|---|---|---|
| VBR, mm | 25.9±3.3 | |
| STJ, mm | 39.6±7.2 | |
| Tilting angle between VBR and STJ, degrees | 13.0±5.7 | |
| GH, mm | 0.0007 | |
| LCC | 17.1±2.2 | |
| RCC | 16.6±2.7 | |
| NCC | 17.8±2.5 | |
| Mean | 17.2±2.4 | |
| FML, mm | <0.0001 | |
| LCC | 34.0±4.8 | |
| RCC | 37.3±5.6 | |
| NCC | 36.7±5.8 | |
| Mean | 36.0±5.2 | |
| EH, mm | <0.0001 | |
| LCC | 10.9±3.1 | |
| RCC | 9.0±3.9 | |
| NCC | 11.1±2.9 | |
| Mean | 10.5±3.2 | |
| ICD, mm | <0.0001 | |
| LCC | 31.4±5.6 | |
| RCC | 35.8±6.8 | |
| NCC | 34.5±7.2 | |
| Mean | 33.9±6.2 | |
| Central angle, degrees | <0.0001 | |
| LCC | 109.7±7.9 | |
| RCC | 128.3±7.9 | |
| NCC | 122.0±9.1 | |
| CH | <0.0001 | |
| Left coronary/right coronary | 22.4±4.6 | |
| Right coronary/noncoronary | 26.7±5.5 | |
| Left coronary/noncoronary | 22.8±4.1 | |
| Mean | 24.0±4.3 |
ANOVA, analysis of variance; CH, commissural height; EH, effective height; FML, free margin length; GH, geometric height; ICD, intercommissural distance; LCC, left coronary cusp; NCC, noncoronary cusp; RCC, right coronary cusp; STJ, sinotubular junction; VBR, virtual basal ring; VR-CT, virtual reality computed tomography.
The VR-CT measurements and corresponding intraoperative measurements are shown in Table 3. These included VBR in 70 (97.2%) patients, GH in 66 (91.7%) patients, FML in 30 (41.7%) patients, EH in 33 (45.8%) patients, and LN-CH in 55 (76.4%) patients. The VR-CT measurements strongly correlated with the intraoperative values, especially for mean GH (R2=0.75) and left coronary/noncoronary CH (R2=0.79); however, there were significant differences (P<0.05). Compared with the VR-CT measurements, the intraoperative measurements had positive discrepancies in mean GH (+3.2 mm), mean FML (+2.0 mm), and LN-CH (+4.0 mm), and negative discrepancies in VBR (−0.9 mm) and mean EH (−1.3 mm).
| Variables | Intraoperative measurement |
VR-CT measurement |
Discrepancy (Intraoperative–VR-CT) |
Correlation (R2) |
|---|---|---|---|---|
| VBR, mm, n=71 (98.6%) | 25.0±2.4 | 25.9±3.3 | −0.90±1.93 | 0.63 |
| GH, mm, n=66 (91.7%) | ||||
| LCC | 20.3±2.8 | 17.1±2.2 | 3.12±1.54 | 0.73 |
| RCC | 20.0±3.2 | 16.6±2.7 | 3.16±1.76 | 0.70 |
| NCC | 21.0±3.3 | 17.8±2.5 | 3.21±1.37 | 0.76 |
| Mean | 20.4±3.0 | 17.2±2.4 | 3.16±1.06 | 0.76 |
| FML, mm, n=30 (41.7%) | ||||
| LCC | 37.4±4.3 | 34.0±4.8 | 2.56±1.80 | 0.51 |
| RCC | 40.1±5.5 | 37.3±5.6 | 1.61±1.79 | 0.62 |
| NCC | 39.8±5.9 | 36.7±5.8 | 1.95±1.95 | 0.70 |
| Mean | 39.1±4.9 | 36.0±5.2 | 2.02±1.59 | 0.81 |
| EH, mm, n=33 (45.8%) | ||||
| LCC | 10.1±2.3 | 10.9±3.1 | −1.34±1.91 | 0.56 |
| RCC | 8.5±3.0 | 9.0±3.9 | −1.59±1.84 | 0.71 |
| NCC | 10.7±2.3 | 11.1±2.9 | −0.88±2.09 | 0.48 |
| Mean | 9.8±2.3 | 10.5±3.2 | −1.27±1.73 | 0.39 |
| LN-CH, mm, n=55 (76.4%) | 26.5±3.6 | 22.8±4.1 | 4.04±1.63 | 0.79 |
LN-CH, left coronary/noncoronary commissural height; R2, coefficient of determination. Other abbreviations as in Table 2.
Of the 72 patients included in the analysis, 66 had mild or less than mild AI with no evidence of aortic stenosis at discharge. For these 66 patients, the number of patients and the VR-CT measurements of each graft size group are shown in Table 4. When analyzing how the VR-CT measurements differed among the 4 graft size groups (24–30) (The 32 mm-sized group was excluded as it included only a single case.), the mean GH was found to be singular because it was significantly different (P<0.05) among them (Figure 3). The detailed VR-CT measurements of the remaining 6 patients with stronger than mild AI at discharge are shown in the Supplementary Table.
| Graft size group |
n (%) | VR-CT measurements | |||||
|---|---|---|---|---|---|---|---|
| Mean GH | Mean FML | Mean EH | LN-CH | VBR | STJ | ||
| 24-mm | 8 (12.1) | 14.9±1.2 | 32.9±2.5 | 8.7±1.8 | 20.5±2.9 | 24.0±1.8 | 35.4±5.4 |
| 26-mm | 21 (31.8) | 16.6±1.4 | 33.3±3.8 | 9.1±2.8 | 22.1±2.5 | 24.9±2.5 | 36.1±4.4 |
| 28-mm | 27 (40.9) | 18.0±1.5 | 38.5±3.9 | 11.9±2.2 | 26.2±3.5 | 26.1±2.2 | 42.3±5.5 |
| 30-mm | 9 (13.6) | 20.1±3.1 | 42.0±3.7 | 12.8±3.0 | 28.7±2.1 | 31.0±3.9 | 48.3±7.7 |
| 32-mm | 1 (1.5) | 22 | 48.6 | 16.2 | 31.8 | 31.4 | 59.5 |
Abbreviations as in Tables 2,3.

Boxplots of the virtual reality computed tomography (VR-CT) measurements in the 4 graft size groups. Significant differences in the VR-CT measurements between groups were examined using Student’s t-test. EH, effective height; FML, free margin length; GH, geometric height; LN-CH, left coronary/noncoronary commissural height; STJ, sinotubular junction; VBR, virtual basal ring.
This is the first report on how preoperative evaluation using a VR-CT workstation can facilitate better evaluation of the physiological geometries of the aortic root in pre-VSARR patients.
Recent advances in VR that allow 3D reconstruction of CT and other imaging modalities are increasingly being used for diagnosis and preoperative planning in cardiac surgery.8,9,13 Conventional evaluations using MPR or CPR are unsuitable for some measurements of complicated geometries, such as free margin lines of aortic cusps that are not aligned in a single plane, whereas a VR workstation can display CT datasets as stereoscopic objects that have true depth, allowing rapid and precise measurement of all geometries. Each measurement in this study was performed in ≤60 s. In addition, because the visual presentation of the anatomical landmarks of the aortic root is analogous to the surgeon’s experience, CT evaluation seems to be clinically useful for VSARR. For example, on the VR image, labels are put on commissure posts; similarly, the commissure stiches are placed in the same positions during VSARR. Besides, in the MPR evaluation the commissure posts are defined as where the 2 leaflets attach, which is slightly lower than the commissure position in VR (Figure 2C). The VR workstation’s superior interface enables cardiovascular surgeons to bridge the gap between intraoperative evaluation and preoperative CT evaluation.
Anatomical Features of AAE PatientsNormal aortic root sinuses are known for the slightly asymmetric configurations in clinical practice.14,15 Izawa et al,15 also from Kobe University Hospital, demonstrated normative aortic valvar measurements in adults using CT, which showed that the left coronary aortic sinus is the smallest, with the FML mirroring its respective sinus. Contrarily, the GH of the RCC was found to be shorter than that of the other aortic cusps. This mismatch was accentuated in the present study (Table 2). The ICD, central angle, and FML of the right coronary sinus are larger than their noncoronary cusp equivalents, making the left coronary sinus disproportionately smaller. Although the dilated sinus requires a longer GH for sufficient coaptation length, the RCC had the shortest GH, as shown in the normative aortic valve, resulting in the RCC having the shortest EH. This accentuated mismatch is supposedly associated with AI, and RCC deterioration often plays a major role in AI, supporting this hypothesis.16
Regarding CH, De Kerchove et al demonstrated that fresh human normative aortic roots have asymmetric CH, being highest at the left coronary/noncoronary (LN) commissure, followed by the right coronary/noncoronary (RN) commissure, and then the left coronary/right (LR) coronary commissure (LN>RN>LR).8 However, in our study, the RN commissure was higher than the LN and LR commissures (RN>Ln=LR), and this phenomenon was related to the steeper tilt angle (13.0±5.7°) between the STJ and VBR than that of normative aortic roots (5.5°–11°).17,18
Discrepancies Between VR-CT and Intraoperative MeasurementsPrevious studies have reported from CT measurements that the mean GH in normal aortic geometry is 13–16 mm.15,19,20 In contrast, Schäfers et al, using direct measurements during aortic valve repair procedures, reported a mean intraoperative GH of approximately 20.0 mm.21 In many studies, CT measurements generated estimates smaller than the directly measured equivalents, which was also the case in the present study.
In our study, the two measurement methods correlated for all cusps; however, numerical discrepancies between them existed in most cases. It may be postulated that these discrepancies are related to the specificity of each method; the cusp geometry in preoperative CT is measured when the heart is physiologically stable, whereas intraoperatively, surgeons measure the cusp’s geometry by stretching it manually under the condition of cardiac arrest (Figure 4).

Intraoperative measurement of GH; red arrow shows the direction of traction force. GH, geometric height; LCA, left coronary artery; NCC, noncoronary cusp; RCA, right coronary artery.
Regarding FML, CH, and VBR, although the two measurement methods were also statistically correlated, the degree of discrepancy was different. In the intraoperative measurements, the FML was approximately 2 mm longer, and the CH was approximately 4 mm longer; contrarily, the VBR was slightly smaller (Table 3). The FML and CH were also measured when the cusps or aortic walls were stretched, unlike for the VBR; therefore, tissue distensibility is probably involved in these discrepancies.
Moreover, the discrepancy in the EH necessitates a different explanation. EH is defined as the difference between the central free margins and VBR in the diastolic phase of the cardiac cycle. Although EH has been identified as an important parameter in determining the function of repaired aortic valves,22 the accuracy of EH measured intraoperatively using a designated caliper remains uncertain. Notably, EH estimated from the CT datasets of diastole was comparatively more reliable.
Clinical ImplicationsAn appropriate-sized graft is important to achieve a favorable outcome of VSRR. Although various simple approaches to graft size selection have been proposed by de Paulis11 and de Kerchove12 et al, the best approach remains controversial. Moreover, these approaches are based on intraoperative measurements performed by particular surgeons during cardiac arrest; thus, the data have limited scientific use. On the other hand, preoperative measurements are more reproducible because they are obtained under physiological heart conditions, and so can be used to effectively determine the appropriate graft size and aid decision making on the addition of cusp repair.19
We extracted the VR-CT measurements and the corresponding graft sizes from patients whose postoperative echocardiographic data did not reveal greater than mild AI on the same admission (n=66, Table 4). The remaining 6 cases were purposely excluded from this analysis because of the possibility of inappropriate graft size selection. On subsequently analyzing how the VR-CT measurements differed among the selected graft size groups, the mean GH was found to be significantly different among the 6 cases (Figure 3), and the selected graft size was close to the value of the mean GH + 10 mm in all study subjects (Table 4). Regarding the LN-CH of VR-CT, there was no significant difference between the 24-mm graft size group and the 26-mm graft size group and the other study groups.
Although graft size selection was based on the size being equal to that of LN-CH in principle, in reality graft size selection was often modified at the surgeon’s discretion, considering the whole anatomical configuration of the aortic root. In fact, grafts of the same size as that of the LN-CH were selected in only 25/61 (41%) cases since the size of LN-CH became the standard for graft sizing, probably because the surgeons often adjusted the graft size according to the length of the GH.
Based on these findings, we consider the mean GH plays a significant role in graft size selection. A formula (Graft size ≈ mean GH + 10 mm) based on the mean GH of VR-CT did not necessarily apply in all cases and does not mean that intraoperative measurements can be omitted; nevertheless, it can serve as a guide for graft selection in further cases.
Regarding the 6 cases excluded from the analysis due to residual AI (their VR-CT measurements are shown in the Supplementary Table), their GH values were relatively less, except in 1 case. This finding supports the viewpoint that a short GH is likely to be disadvantageous for VSRR. Interestingly, in addition to this, the tilting angle was steep in 4/6 cases, and the central angle displayed strong asymmetry in 5/6 cases, which suggests that horizontal and vertical asymmetries may also affect early postoperative outcomes.
Study LimitationsFirst, this was a single-center retrospective study using CT datasets that were all obtained from pre-VSARR patients without a control group; hence, we could not eliminate selective bias. However, no other study has analyzed the anatomical features of such patients in detail and compared the VR-CT and intraoperative measurements with selected graft sizes; thus, we believe that this study is clinically significant. Second, it is necessary to discuss whether the definition of an appropriate graft size in this study was reasonable. Residual AI is known to be related to many factors other than graft size, such as preoperative severity of AI, cusp deformity, cusp repair, and so on.23 To assess the utility of preoperative VR-CT evaluation to inform appropriate graft size selection for favorable outcomes, further studies are still needed and these should take into consideration cusp repair, postoperative VR-CT measurements, and long-term outcomes. Finally, CT images visualized on any workstation can be affected by the applied window level or width adjustment and can inaccurately displayed if there is poor imaging quality.7
Our VR-CT evaluation enabled a more thorough understanding of aortic root anatomy and revealed that the pre-VSARR patients had an accentuated mismatch between sinus size and GH, which might be associated with AI. The findings of this study could facilitate valve-sparing root procedures and might be helpful for appropriate graft size selection. The results warrant further research to develop a more comprehensive understanding of the technique and its clinical applications.
None.
This study was approved by the Institutional Review Board of Kobe University Hospital (approval no. B200328).
Supplementary Movie. Key process of virtual reality computed tomography (VR-CT) measurements of inter-commissural distance (ICD), free margin length (FML) and geometric height (GH).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-22-0334