Article ID: CJ-22-0568
Article ID: CJ-22-0568
Background: This study investigated the association between placental pathology and fetal heart failure.
Methods and Results: Singletons with a congenital heart defect (CHD) and/or arrhythmia (n=168) and gestational age-matched controls (n=52) were included in the study. The associations between macro- and microscopic abnormal findings of the placenta and the severity of fetal heart failure were evaluated using the cardiovascular profile (CVP) score. Nine features were microscopically identified and assessed in sections of the placenta: premature villi, edematous villi, fibrotic villi, chorioamnionitis, chorangiosis, fibrin deposition, subchorionic hematoma, infarcted villi, and nucleated red blood cells in villous vessels. Among singletons with CHD and/or arrhythmia, the final CVP score was ≥8 in 140 cases, 6 or 7 in 15 cases, and ≤5 in 13 cases. Microscopic analysis showed that the frequency and severity of premature and edematous villi and increased nucleated red blood cells in villous vessels were greater in cases of fetal heart failure. These microscopic findings were more common and severe in cases with a final CVP score ≤5 than in gestational age-matched controls. The prevalence of abnormal macroscopic findings of the placenta and umbilical cord was similar regardless of the severity of fetal heart failure.
Conclusions: Premature and edematous villi and increased nucleated red blood cells in villous vessels were correlated with the severity of fetal heart failure in cases of CHD and/or arrhythmia.
Environmental chemicals, drugs, maternal malnutrition, and genetic factors together hinder the development of both the fetal heart and placenta.1 Recent studies showed that abnormal umbilical cord insertion and a single umbilical artery were more prevalent in fetuses with a congenital heart defect (CHD).2–4 Abnormal umbilical cord insertion may disturb the delivery of oxygen and nutrients in the fetal microenvironment. Jones et al showed that placentas of fetuses with hypoplastic left heart syndrome had abnormal parenchymal morphology, which they attributed to vascular and growth abnormalities.5 Although the placenta plays a pivotal role in the maintenance of fetal growth and circulation,2,3,6 the potential contribution of impaired placental structural development and function to fetal heart failure is poorly understood.
Maternal pleural effusion and skin edema in association with severe fetal and placental hydrops is known as mirror syndrome.7 Although mirror syndrome is caused by several diseases, such as rhesus isoimmunization, viral infection, and fetal malformations, its pathophysiology remains unknown. Fetal arrhythmia and CHD are known to be the main causes of fetal hydrops.8 In a recent study, we demonstrated that an increase in central venous pressure secondary to arrhythmia or atrioventricular valve regurgitation due to CHD played pivotal roles in fetal heart failure.9 Therefore, we speculated that elevated central venous pressure increases pressure in the umbilical vein via a ductus venosus shunt, which causes increased hydrostatic pressure in the placental venules, leading to tissue edema. However, little is known about the pathological findings of placentas in the case of fetuses with heart failure. A previous study reported that intravillous edema and intervillous fibrin thrombi were common abnormal microscopic findings of placentas in the case of fetuses with non-immunologic hydrops.10 Because the previous study investigated autopsy cases, thrombotic changes in the placenta may be associated with most severe fetal heart failure, whereas intervillous fibrin thrombi may have been observed as a postmortem change.
In the present study, we investigated the association between microscopic findings in placentas and the severity of fetal heart failure caused by CHD and/or arrhythmia. We also investigated whether abnormal macroscopic findings of the placenta and umbilical cord are associated with fetal heart failure status.
This retrospective study was approved by the Institutional Ethics Board of the National Cerebral and Cardiovascular Center (NCVC) (R20048). Information about the study was published in an opt-out format on the hospital website, providing potential research subjects the opportunity to refuse participation. Consequently, the need for written informed consent was waived by the Institutional Ethics Board. The procedures in this study were performed in accordance with the Declaration of Helsinki and the ethical standards of the institution.
Singletons with fetal CHD and/or arrhythmia treated at the NCVC between April 2011 and March 2016 and for whom the placenta was available were included in the study. Singletons whose placentas were available and who were born at Showa University between January 2019 and December 2020 with no complications, such as structural abnormality, arrhythmia, growth restriction, and heart failure, were included as controls. Controls were gestational age matched in a 1 : 4 ratio with cases of severe heart failure using the nearest-neighbor method and propensity score matching.
Exclusion criteria for both cases and controls included preterm birth before 30 weeks gestation, genetic or chromosomal abnormalities, and critical extracardiac abnormalities that required intervention during the neonatal period. Additional exclusion criteria included maternal and obstetric complications, such as chronic hypertension, diabetes, pre-eclampsia, and gestational diabetes.
Chart reviews were performed to collect clinical data, including maternal age, maternal and obstetric complications, infant sex, mode of delivery, gestational age and weight at birth, small-for-date status (weight <10th percentile for gestational age at birth), the presence of extracardiac anomalies, Apgar score, umbilical cord arterial blood pH, and fetal and neonatal mortality.
Fetuses with CHD were diagnosed prenatally with fetal echocardiography using a Voluson E8 ultrasound system (GE Medical Systems, Zipf, Austria) according to a protocol previously established by the NCVC.9,11,12 The cardiovascular profile (CVP) score was used to characterize fetal heart failure.13 The CVP is a composite scoring system that grades the severity of fetal heart failure using 5 fetal echocardiographic parameters: fetal effusion, venous Doppler findings, heart size, cardiac function, and arterial Doppler findings. Heart failure severity is rated on a 10-point scale, with points deducted for abnormalities in each component marker. CVP scores ≥8 indicate no or mild heart failure, scores of 6 or 7 indicate moderate heart failure, and scores ≤5 indicate severe heart failure.9,12,14 A single individual (T. Miyoshi) evaluated CVP scores for all cases using the image database. The final CVP score was estimated within 1 week before birth in all fetuses with fetal CHD and/or arrhythmia.
All fetal arrhythmias were diagnosed using fetal echocardiography (GE Medical Systems, Zipf, Austria) and magnetocardiography (MC-6400; Hitachi High-Technologies Corporation, Tokyo, Japan).15 Fetal arrhythmias were categorized as tachyarrhythmias, bradyarrhythmias, or non-sustained extrasystoles. Fetal tachyarrhythmias, defined as a ventricular rate of ≥180 beats/min, included supraventricular tachycardia (SVT), atrial flutter (AFL), and ventricular tachycardia (VT). Fetal therapy was administered using digoxin, sotalol, or flecainide, alone or in combination, when fetal SVT or AFL was sustained for ≥50%.16,17 Magnesium sulfate, propranolol, or mexiletine, alone or in combination, were used for VT.15,18 Fetal bradyarrhythmia, defined as a ventricular rate of <100 beats/min, included sinus bradycardia and atrioventricular block (AVB). Beta-sympathomimetics and dexamethasone were used as fetal therapy when fetal bradyarrhythmia was complicated by a fetal ventricular rate of <55 beats/min and myocarditis.19,20 Extrasystole was defined as premature contraction that was independent of the normal rhythm.
Gross placental and umbilical cord evaluation was based on routine clinical pathology reports generated at the time of delivery. The placentas were weighed fresh without umbilical cords and membranes.21 Macroscopic placental abnormalities were defined as placenta circumvallate, placenta previa, or accessory placenta. Macroscopic umbilical cord abnormalities were defined as single umbilical artery, marginal cord insertion, or velamentous cord insertion.22 Tissue sampling and the degree of detail in placental and umbilical cord examinations were similar in cases and controls, based on routine methods.21 At least 4 samples were obtained from each placenta for histopathological examination: 1 near the umbilical cord insertion, 2 from the macroscopically normal-appearing central tissue, and 1 from the umbilical cord and membrane roll. The same pathologist (T. Matsuyama) re-reviewed all placental histology without any knowledge of maternal, fetal, or neonatal backgrounds, including categories of fetal CHD and/or arrhythmia. Sections of the placenta were stained with hematoxylin and eosin and the following 9 items assessed: premature villi (normal villi, intermediate villi in several views [Figure 1A]; intermediate villi in every view, or dysplastic villi [Figure 1B]); edematous villi (none, mild, moderate, or severe; Figure 1C); fibrotic villi (none, rare, several views, or every view); chorioamnionitis (none, Blanc stages 1–3); chorangiosis (none, rare, several views, or every view); fibrin deposition (none, rare, several views, or every view); subchorionic hematoma (none, rare, several views); infarcted villi (none, rare, several views, every view); and nucleated red blood cells in villous vessels (none or rare, several views, or every view; Figure 1D). The diagnosis of chorangiosis was based on the previously defined histologic criterion of ≥10 villi containing ≥10 capillaries in ≥10 microscopic fields.23
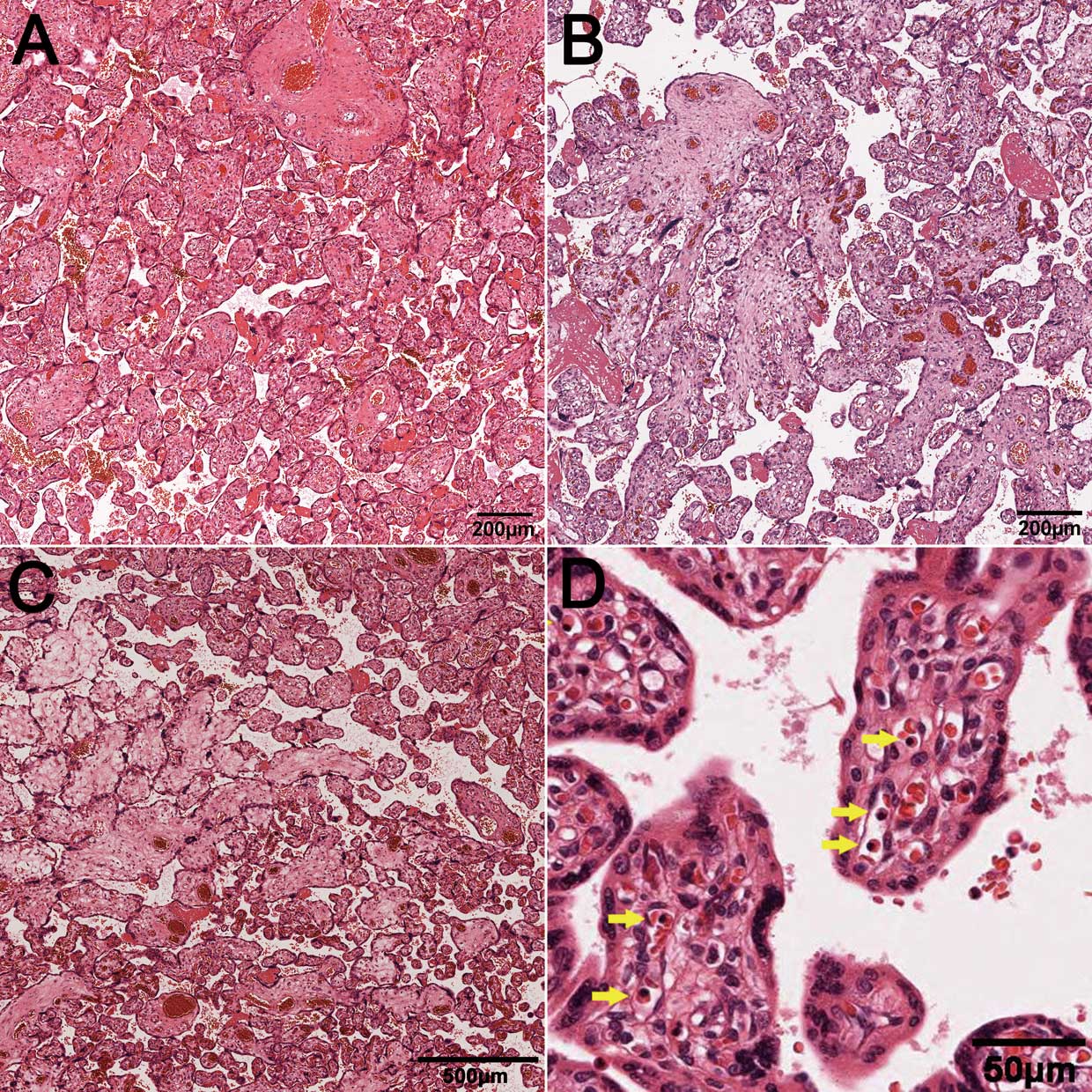
Histopathological findings of the placenta (hematoxylin-eosin staining). (A) Intermediate villi, (B) dysplastic villi, (C) edematous villi, and (D) nucleated red blood cells in villous vessels (arrows).
Data are presented as the mean±SD or number of patients. Missing data were not supplemented. Continuous variables were analyzed using Student’s t-test or the Wilcoxon rank-sum test as appropriate. The Steel-Dwass test was used to compare continuous variables among 3 groups. Categorical variables were evaluated using Fisher’s exact test. Statistical analyses were performed using Stata 14.1 (StataCorp LP, College Station, TX, USA) and JMP 11 (SAS Institute, Cary, NC, USA). In all analyses, 2-sided P<0.05 was considered significant. When comparing 3 groups by the severity of fetal heart failure, Bonferroni’s test was applied with significance of P<0.017 (=0.05/3).
In all, 168 singletons with fetal CHD and/or arrhythmia for whom the placenta was available were enrolled in the present study. The final measured CVP score in these singletons was ≥8 in 140 cases, 6 or 7 in 15 cases, and ≤5 in 13 cases. Baseline perinatal characteristics are presented in Table 1. There were 4 fetal deaths in this cohort: Ebstein’s anomaly (final CVP score 0) and tricuspid valve dysplasia (final CVP score 3) with circular shunt; dilated cardiomyopathy (final CVP score 3); and double outlet right ventricle with severe fetal growth restriction (FGR; final CVP score 8). Gestational age at delivery in cases was significantly lower in those with a final CVP score ≤5 than in those with a final CVP score of ≥8 and 6 or 7 (35.2±1.9 vs. 38.3±1.8 and 38.0±2.2 weeks, respectively; P<0.05). Polyhydramnios, oligohydramnios, fetal and neonatal death, preterm birth, and non-reassuring fetal status were more common in cases with a final CVP score ≤5 than in those with a final CVP score ≥8 (P<0.05). Furthermore, of 157 singletons without fetal CHD and/or arrhythmia, 52 were enrolled in the present study as gestational age-matched controls for cases with severe heart failure (final CVP score ≤5). All controls showed normal growth and no signs of fetal heart failure.
| CHD and/or arrhythmia (n=168) | Controls (n=52) |
|||
|---|---|---|---|---|
| Final CVP score ≥8 (n=140) |
Final CVP score 6 or 7 (n=15) |
Final CVP score ≤5 (n=13) |
||
| Maternal age (years) | 31.1±5.2 | 32.9±5.3 | 29.5±5.6 | 34.3±4.1 |
| Primipara | 78 (55.7) | 7 (46.7) | 9 (69.2) | 28 (53.9) |
| In vitro fertilization | 14 (10.0) | 0 | 0 | 10 (19.2) |
| Gestational age at diagnosis (weeks) | 29.0±4.7 | 27.0±4.2 | 27.0±4.2 | – |
| Male infant | 72 (51.4) | 11 (73.3) | 6 (46.2) | 25 (48.1) |
| Fetal arrhythmia | 40 (28.6) | 3 (20.0) | 10 (35.7) | 0 |
| Extracardiac anomaly | 24 (17.1) | 6 (40.0) | 3 (23.1) | 0 |
| Polyhydramnios | 1 (0.7) | 0 | 2 (15.4)* | 0 |
| Oligohydramnios | 4 (2.9) | 2 (13.3) | 5 (38.5)* | 0 |
| Fetal death | 1 (0.7) | 0 | 3 (23.1)* | 0 |
| Neonatal death | 3 (2.1) | 1 (6.7) | 3 (23.1)* | 0 |
| Gestational age at delivery (weeks) | 38.3±1.8 | 38.0±2.2 | 35.2±1.9* | 35.4±1.9 |
| Preterm birth <37 weeks | 11 (7.9) | 3 (20.0) | 9 (69.2)* | 36 (69.2) |
| Birth weight (g) | 2,768±506 | 2,709±663 | 2,478±350 | 2,359±441 |
| SGA <10th percentile | 29 (20.7) | 5 (33.3) | 6 (46.2) | 0 |
| Cesarean delivery | 44 (31.4) | 8 (53.3) | 9 (69.2) | 18 (34.6) |
| Non-reassuring fetal statusA | 24/130 (18.5) | 7/13 (53.9)* | 5/8 (62.5)* | 9 (17.3) |
| Apgar score <7 at 5 minB | 4/139 (2.9) | 0 | 2/10 (20.0) | 0 |
| Umbilical cord arterial pH <7.15B | 2/139 (1.4) | 0 | 1/10 (10.0) | 2 (3.9) |
Data are presented as the mean±SD or n (%). *P<0.017 (=0.05/3) compared with final CVP score ≥8. AAssessment of non-reassuring fetal status excluded fetuses in whom the cardiotocogram was difficult to interpret due to fetal arrhythmia. BApgar score and umbilical cord arterial pH were not assessed in cases of fetal death. CHD, congenital heart defect; CVP, cardiovascular profile; SGA, small for gestational age.
The CHD and arrhythmia categories in this study cohort are presented in Table 2. The CHDs of all neonates were confirmed after birth by echocardiographic findings, surgical findings, or postmortem examination. Ten fetuses had arrhythmia complicated by CHD: 2 fetuses had SVT, 1 with a cardiac tumor and the other with tricuspid valve dysplasia; 3 fetuses had complete AVB; 2 fetuses with sinus bradycardia had left atrial isomerism; 2 fetuses with atrial extrasystole had an atrioventricular septal defect; and 1 fetus with ventricular extrasystole had a dilated cardiomyopathy. All 14 fetuses with SVT and AFL without CHD, 5 of whom had moderate heart failure at diagnosis (CVP score 6 or 7), were resolved by fetal therapy with digoxin, sotalol, or flecainide, alone or in combination, resulting in sinus rhythm with no or mild heart failure (final CVP score ≥8) at birth. One fetus with VT did not respond to the combination of magnesium sulfate and mexiletine, resulting in severe heart failure, as indicated by a final CVP score of 5 at birth. Complete AVB remained at birth in all 5 fetuses, regardless of fetal therapy with β-sympathomimetics and/or dexamethasone. All fetuses with sinus bradycardia and extrasystole had no indication for fetal therapy or heart failure.
| Category | Final CVP score | Gestational age at birth (weeks) |
Placental weight (g) |
Placental macroscopic abnormality |
Umbilical cord macroscopic abnormality |
||
|---|---|---|---|---|---|---|---|
| ≥8 | 6 or 7 | ≤5 | |||||
| CHD (n=129)A | 106 (82.2) | 14 (10.9) | 9 (7.0) | 38.1±2.1 | 510±143 | 4 (3.1) | 26 (20.2) |
| Isomerism (n=28) | 22 (78.6) | 4 (14.3) | 2 (7.1) | 38.4±1.9 | 477±135 | 1 (3.6) | 5 (17.9) |
| Right atrial isomerism (n=18) |
16 | 2 | 1 | 5 | |||
| Left atrial isomerism (n=10) |
6 | 2 | 2 | ||||
| Hypoplastic left ventricle (n=15) |
13 (86.7) | 1 (6.7) | 1 (6.7) | 38.1±2.3 | 444±100 | 0 | 6 (40.0) |
| Hypoplastic left heart syndrome (n=11) |
11 | 4 | |||||
| Critical aortic stenosis (n=4) |
2 | 1 | 1 | 2 | |||
| Right heart defect (n=26) | 15 (57.7) | 6 (23.1) | 5 (19.2) | 37.3±2.5 | 559±137 | 0 | 6 (23.1) |
| Ebstein’s anomaly or tricuspid valve dysplasia (n=12) |
3 | 4 | 5 | 2 | |||
| Pulmonary atresia with intact ventricular septum (n=5) |
3 | 2 | 2 | ||||
| Tricuspid atresia (n=5) | 5 | 2 | |||||
| Double inlet left ventricle (n=4) |
4 | ||||||
| Cyanotic heart defect with biventricular (n=30) |
29 (96.7) | 1 (3.3) | 0 | 37.8±2.0 | 473±132 | 2 (5.7) | 6 (20.0) |
| Transposition of the great arteries (n=8) |
8 | 2 | |||||
| Double outlet right ventricle (n=6) |
6 | 1 | 2 | ||||
| Tetralogy of Fallot (n=13) | 12 | 1 | 1 | 2 | |||
| Total anomalous pulmonary venous connection (n=3) |
3 | ||||||
| Acyanotic heart defect with biventricular (n=30) |
27 (90.0) | 2 (6.6) | 1 (3.3) | 38.8±1.4 | 568±157 | 1 (3.3) | 3 (10.0) |
| Coarctation of the aorta (n=13) |
13 | 1 | 3 | ||||
| Pulmonary valve stenosis (n=2) |
2 | ||||||
| Atrioventricular septal defect (n=4) |
4 | ||||||
| Cardiac tumor (n=6) | 4 | 2 | |||||
| Other (n=5) | 4 | 1 | |||||
| Arrhythmia (n=39) | 34 (87.2) | 1 (2.6) | 4 (10.3) | 37.7±1.9 | 494±105 | 0 | 5 (12.8) |
| Tachyarrhythmia (n=15) | 14 (93.3)B | 0 | 1 (6.7) | 37.4±0.7 | 476±91 | 0 | 1 (6.7) |
| Supraventricular tachycardia (n=6) |
6 | ||||||
| Atrial flutter (n=8) | 8 | 1 | |||||
| Ventricular tachycardia (n=1) |
1 | ||||||
| Bradyarrhythmia (n=8) | 4 (50.0) | 1 (12.5) | 3 (37.5) | 37.5±1.2 | 512±89 | 0 | 1 (12.5) |
| Sinus bradycardia (n=3) | 3 | ||||||
| Complete atrioventricular block (n=5) |
1 | 1 | 3 | 1 | |||
| Extrasystole (n=16) | 16 (100.0) | 0 | 0 | 38.1±2.8 | 503±127 | 0 | 3 (18.7) |
| Atrial extrasystole (n=14) | 14 | 2 | |||||
| Ventricular extrasystole (n=2) |
2 | 1 | |||||
Data are presented as the mean±SD or n (%). ATen fetuses had arrhythmia complicated by a congenital heart defect (CHD): 2 fetus had supraventricular tachycardia, 1 with a cardiac tumor and the other with tricuspid valve dysplasia; 3 fetuses had complete atrioventricular block; 2 fetuses with sinus bradycardia had left atrial isomerism; 2 fetuses with atrial extrasystole had an atrioventricular septal defect; and 1 fetus with ventricular extrasystole had dilated cardiomyopathy. BAll 14 cases of supraventricular tachycardia and atrial flutter, 5 of which had a cardiovascular profile (CVP) score of 6 or 7 at diagnosis, were resolved by fetal therapy, resulting in sinus rhythm with a final CVP score ≥8 at birth.
No placental macroscopic abnormalities were found in cases of moderate to severe fetal heart failure (Table 3). Placental weight was significantly greater in fetuses with a final CVP score ≤5 than in those with a final CVP score ≥8 and controls (600±149 vs. 498±131 and 490±107 g, respectively; P<0.05), though the size was similar. Umbilical cord abnormalities were more common in fetuses with CHD and/or arrhythmia than in control fetuses (18.5% vs. 5.8%, respectively; P=0.03). The prevalence of abnormal macroscopic findings of the placenta or umbilical cord was not associated with the severity of fetal heart failure.
| CHD and/or arrhythmia (n=168) | Controls (n=52) |
|||
|---|---|---|---|---|
| Final CVP score ≥8 (n=140) |
Final CVP score 6 or 7 (n=15) |
Final CVP score ≤5 (n=13) |
||
| Placenta | ||||
| Weight (g) | 498±131 | 500±137 | 600±149A | 490±107B |
| Long diameter (cm) | 18.4±2.6 | 17.1±2.1 | 19.0±2.7 | 18.6±2.6 |
| Short diameter (cm) | 15.7±2.2 | 15.5±2.7 | 16.6±2.1 | 16.3±2.3 |
| Abnormality | 4 (2.9) | 0 | 0 | 2 (3.9) |
| Circumvallate placenta | 2 (1.4) | 0 | 0 | 0 |
| Placenta previa | 1 (0.7) | 0 | 0 | 2 (3.9) |
| Accessory placenta | 1 (0.7) | 0 | 0 | 0 |
| Umbilical cord | ||||
| Length (cm) | 48.6±18.4 | 45.3±13.0 | 54.5±10.7 | 45.1±9.3 |
| Abnormality | 25 (17.9) | 4 (26.7) | 2 (15.4) | 3 (5.8) |
| Single umbilical artery | 8 (5.7) | 1 (6.7) | 0 | 0 |
| Marginal cord insertion | 13 (9.3) | 3 (20.0) | 2 (15.4) | 0B |
| Velamentous cord insertion | 4 (2.9) | 0 | 0 | 3 (5.8) |
Data are presented as the mean±SD or n (%). AP<0.017 (=0.05/3) compared with a final cardiovascular profile (CVP) score ≥8. BP<0.05 compared with final CVP score ≤5. CHD, congenital heart defect.
Microscopic findings showed that in cases of more severe fetal heart failure, there was a greater frequency and severity of premature and edematous villi and increased nucleated red blood cells in villous vessels (Table 4). Severe edematous villi and increased nucleated red blood cells in villous vessels were found only in cases with a final CVP score ≤5. Both findings were detected in 2 fetal deaths, 1 with Ebstein’s anomaly and the other with tricuspid valve dysplasia with circular shunt. In addition, premature and edematous villi and increased nucleated red blood cells in villous vessels were more common and severe in fetuses with a final CVP score ≤5 than in gestational age-matched controls. Representative histological findings of chorionic villi in fetuses with and without severe heart failure are shown in Figure 2.
| CHD and/or arrhythmia (n=168) | P valueA | Controls (n=52) |
P valueB | |||
|---|---|---|---|---|---|---|
| Final CVP score ≥8 (n=140) |
Final CVP score 6 or 7 (n=15) |
Final CVP score ≤5 (n=13) |
||||
| Prematurity of villi | <0.01 | <0.01 | ||||
| Normal | 18 (12.9) | 0 | 0 | 15 (28.9) | ||
| Intermediate villi in several views |
76 (54.3) | 7 (46.7) | 4 (30.8) | 21 (40.4) | ||
| Intermediate villi in every view |
38 (27.1) | 8 (53.3) | 4 (30.8) | 16 (30.8) | ||
| Dysplastic villi | 8 (5.7) | 0 | 5 (38.5) | 0 | ||
| Edema of villi | <0.01 | <0.01 | ||||
| None | 86 (61.4) | 4 (26.7) | 2 (15.4) | 20 (38.5) | ||
| Mild | 36 (25.7) | 8 (53.3) | 3 (23.1) | 26 (50.0) | ||
| Moderate | 18 (12.9) | 3 (20.0) | 4 (30.8) | 4 (7.7) | ||
| Severe | 0 | 0 | 4 (30.8) | 2 (3.9) | ||
| Fibrosis of villi | 0.74 | 0.10 | ||||
| None | 105 (75.0) | 12 (80.0) | 9 (69.2) | 45 (86.5) | ||
| Rare | 29 (20.7) | 2 (13.3) | 2 (15.4) | 6 (11.5) | ||
| Several views | 5 (3.6) | 1 (6.7) | 2 (15.4) | 1 (1.9) | ||
| Every view | 1 (0.7) | 0 | 0 | 0 | ||
| Chorioamnionitis | 0.83 | 0.51 | ||||
| None | 136 (97.1) | 15 (100.0) | 13 (100.0) | 47 (90.4) | ||
| Blanc stage 1 | 3 (2.1) | 0 | 0 | 4 (7.7) | ||
| Blanc stage 2 | 0 | 0 | 0 | 0 | ||
| Blanc stage 3 | 1 (0.7) | 0 | 0 | 1 (1.9) | ||
| Chorangiosis | 0.64 | 0.88 | ||||
| None | 105 (75.0) | 12 (80.0) | 12 (92.3) | 47 (90.4) | ||
| Rare | 28 (20.0) | 3 (20.0) | 1 (7.7) | 4 (7.7) | ||
| Several views | 6 (4.3) | 0 | 0 | 1 (1.9) | ||
| Every view | 1 (0.7) | 0 | 0 | 0 | ||
| Fibrin deposition | 0.21 | <0.01 | ||||
| None | 74 (52.9) | 8 (53.3) | 7 (53.9) | 6 (11.5) | ||
| Rare | 58 (41.4) | 4 (26.7) | 6 (46.2) | 42 (80.8) | ||
| Several views | 8 (5.7) | 2 (13.3) | 0 | 4 (7.7) | ||
| Every view | 0 | 1 (6.7) | 0 | 0 | ||
| Subchorial hematoma | 0.95 | 0.83 | ||||
| None | 108 (77.1) | 12 (80.0) | 11 (84.6) | 45 (86.5) | ||
| Rare | 31 (22.1) | 3 (20.0) | 2 (15.4) | 6 (11.5) | ||
| Several views | 1 (0.7) | 0 | 0 | 1 (1.9) | ||
| Infarction of villi | 0.35 | 0.20 | ||||
| None | 122 (87.1) | 11 (73.3) | 11 (84.6) | 46 (88.5) | ||
| Rare | 16 (11.4) | 3 (20.0) | 2 (15.4) | 2 (3.9) | ||
| Several views | 0 | 1 (6.7) | 0 | 4 (7.7) | ||
| Every view | 2 (1.4) | 0 | 0 | 0 | ||
| Nucleated red blood cells in villous vessels |
<0.01 | <0.01 | ||||
| None or rare | 140 (100.0) | 15 (100.0) | 10 (76.9) | 52 (100.0) | ||
| Several views | 0 | 0 | 1 (7.7) | 0 | ||
| Every view | 0 | 0 | 2 (15.4) | 0 | ||
Unless indicated otherwise, data are presented as n (%). ACorrelation between the severity of placental microscopic abnormalities and the cardiovascular profile (CVP) score in fetuses with a congenital heart defect (CHD) and/or arrhythmia. BComparison between final CVP score ≤5 and gestational age-matched controls.

Representative histopathological findings of chorionic villi (hematoxylin-eosin staining). (A) Severe irregular-shaped and edematous intermediate villi seen in fetuses with severe heart failure. (B) Small-sized mature terminal villi seen in fetuses without heart failure. Scale bar, 200 μm (A and B).
Of 168 fetuses with CHD and/or arrhythmia, 16 had more than 1 of the following 3 severe placental findings: dysplastic villi, severely edematous villi, and nucleated red blood cells in villi. However, no fetuses had all 3 findings. Four fetuses with 2 severe placental findings were diagnosed with left atrial isomerism (n=1) and either Ebstein’s anomaly or tricuspid valve dysplasia (n=3). Twelve fetuses had 1 severe placental finding. Perinatal mortality in fetuses with 0, 1, or 2 severe placental findings was 3.9% (6/152), 16.7% (2/12), and 100% (4/4), respectively. Of the 5 fetuses with tachyarrhythmia who recovered from heart failure following transplacental treatment and had a CVP score of 10 at birth, 2 had dysplastic villi and 1 had moderate edematous villi.
The present study showed that premature and edematous villi and increased nucleated red blood cells in villous vessels were correlated with the severity of heart failure in fetuses with CHD and/or arrhythmia. Furthermore, these microscopic findings of the placenta were more common and severe in fetuses with severe heart failure than in gestational age-matched controls. Conversely, abnormal macroscopic findings of the placenta and umbilical cord were not correlated with the incidence or severity of fetal heart failure, although umbilical cord abnormalities were more common in fetuses with CHD and/or arrhythmia than in control fetuses.
Premature and edematous villi were found in fetuses with CHD and/or arrhythmia and heart failure. These microscopic findings were more common and severe in fetuses with severe heart failure than in gestational age-matched controls. The placenta physiologically links the fetus and mother.1 In a recent study, we demonstrated that an increase in fetal natriuretic peptide concentrations was correlated with the severity of fetal heart failure and mainly attributable to an increase in central venous pressure.9,24 Abnormal venous Doppler ultrasound findings indicate elevated central venous pressure, which is caused primarily by tachy- or bradyarrhythmia or atrioventricular valve regurgitation due to CHD, such as Ebstein’s anomaly or tricuspid valve dysplasia.9,25 Increased wall stress results in cardiac remodeling and hypertrophy, consequently increasing myocardial oxygen consumption and aggravating myocardial dysfunction. To overcome the reduction in ventricular compliance, end-diastolic filling pressure and hydrostatic central venous pressure increase to maintain cardiac output.25,26 We also showed that maternal serum proinflammatory cytokines and apoptotic and angiogenic factors were associated with fetal heart failure.27 Together, the findings of the present and previous studies suggest that increased central venous pressure in fetal heart failure damages placental trophoblasts and increases the levels of proinflammatory cytokines, resulting in increased villous vasopermeability. Conversely, in the normal placenta, terminal villi form in response to vessel expansion by non-branching angiogenesis, including the longitudinal growth and coiling of fetal capillaries.28,29 These villi develop predominantly in the third trimester to massively increase the placental exchange surface area and capacity to accommodate exponential fetal growth. The combination of arrested trophoblast turnover and failure to evoke an adaptive angiogenic response led us to speculate that hypoxia no longer induces trophoblast proliferation and angiogenesis in these villi.30,31 Fetoplacental circulatory failure may inhibit appropriate gas exchange, thereby resulting in premature and dysplastic villi. These placental findings can further exacerbate the oxygen and nutrient delivery to the fetus with heart failure.
Nucleated red blood cells in villous vessels were detected only in fetuses with severe heart failure. Nucleated red blood cells are present in the placental vessels throughout the first half of pregnancy but are uncommon later in pregnancy.32 Acute asphyxia is known to be the most common cause of increased numbers of nucleated red blood cells.33 Postmortem examinations showed that increased numbers of nucleated red blood cells in placental lesions were associated with brain injury immediately before delivery.34,35 In the present study, nucleated red blood cells in villous vessels were found in 2 of 3 fetuses who died with severe heart failure, whereas they were not detected in fetuses who died without heart failure. Interestingly, the findings in cases of fetal tachyarrhythmia suggest that transplacental antiarrhythmic treatment may cause the disappearance of nucleated red blood cells in villous vessels, but damage to the villi may persist even after recovery from heart failure. Therefore, nucleated red blood cells in villous vessels may be a surrogate marker for acute severe fetal heart failure.
Abnormal macroscopic findings of the placenta and umbilical cord were not associated with fetal heart failure in fetuses with CHD and/or arrhythmia. The fetal heart and placenta develop simultaneously, involving several of the same genes and molecular pathways.1,36 Abnormal umbilical cord insertion may affect the fetal microenvironment and disturb nutrient delivery.2,3 Umbilical cord abnormalities were more common in fetuses with CHD than in normal fetuses, corroborating previous reports.2–4 However, the prevalence of abnormal macroscopic findings in the placenta and umbilical cord did not correlate with the severity of fetal heart failure. This suggests that fetal heart failure is not caused or worsened by macroscopic abnormalities of the placenta and umbilical cord.
Microscopic placental abnormalities related to FGR, such as chorangiosis and fibrin deposition, were not associated with fetal heart failure in fetuses with CHD and/or arrhythmia, although several studies have found a relatively high prevalence of FGR in fetuses with CHD.2,3,6 The pathophysiology of fetal heart failure in FGR differs from that of CHD or arrhythmia. In most cases of FGR, small fetal size is the consequence of placental insufficiency, which has 2 direct effects on fetal cardiovascular development.37 First, reduced oxygen and nutrient supply may disrupt cardiomyocyte growth and fiber architecture. Second, villous hypoplasia and thrombosis lead to increased placental resistance and chronic cardiac afterload. Consequently, to maintain ventricular output, the developing myocardium undergoes cardiac remodeling. In contrast, elevated central venous pressure, which manifests as abnormal venous Doppler findings, is more common in cases of CHD and/or arrhythmia than in those of FGR.11,25 Conversely, abnormal arterial Doppler findings are more common in cases of severe FGR,38 whereas they are rare in fetuses with CHD or arrhythmia,11,39 suggesting differences in the villous vascular resistance.
A previous preliminary study using placental fine needle aspiration biopsy showed that nucleated red blood cell counts were correlated between in vitro placental samples and umbilical cord blood.40 Indeed, placental fine needle aspiration biopsy can be used to assess fetoplacental stress in utero. However, in the clinical setting, this invasive procedure must be replaced with less invasive biomarkers, such as maternal serum proinflammatory cytokines and apoptotic and angiogenic factors that indicate fetal heart failure.27 In future studies, placental microscopic findings should be compared with maternal serum cytokine concentrations to establish biomarkers for fetal heart failure.
The present study had several strengths. First, to the best of our knowledge, this is the first study to microscopically characterize the placenta in fetuses with heart failure. Although umbilical cord abnormalities were more common in fetuses with CHD than in normal fetuses,2,4 they were not associated with the incidence or severity of fetal heart failure. Second, the NCVC is one of the largest tertiary pediatric cardiac centers in Japan, and the study cohort included fetuses with various complex CHDs and rare fetal arrhythmias. All fetuses with CHD and/or arrhythmia were accurately diagnosed prenatally, and CVP scores were assessed within 1 week before birth.
This study also has several limitations. First, preterm birth rate increases with the severity of fetal heart failure, and its effects on microscopic placental findings, particularly intermediate villi and nucleated red blood cells in villous vessels, cannot be completely ignored. Therefore, we excluded fetuses younger than 30 weeks gestation at delivery and compared gestational age-matched controls to fetuses with severe heart failure. Second, the sample size was determined based on feasibility because this study was exploratory, although it was comparable to that of previous reports.3–5 This small sample size made it difficult to evaluate the correlation between placental microscopic abnormalities and different causes of fetal heart failure. Moreover, because the severity of heart failure varies widely from none to severe even in the same type of CHD,14,39 in the present study, to investigate the effect of fetal heart failure on placental pathology, we used the CVP score rather than CHD classification. A larger multicenter study is needed to clarify whether our results are applicable to early gestational age and to each type of CHD and/or arrhythmia.
In conclusion, premature and edematous villi and increased nucleated red blood cells in villous vessels were more common and severe in fetuses with more severe heart failure caused by CHD and/or arrhythmia. Severe edematous villi and increased nucleated red blood cells in villous vessels were found only in fetuses with severe heart failure. This study will guide future work on microscopic placental pathology and its link to various causes of fetal heart failure.
The authors thank Enago (www.enago.jp) for English language review.
This work was supported by JSPS KAKENHI grants (19K08343, 22K07833) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The study was also supported, in part, by grants from the Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics, the Takeda Science Foundation, and the Japan Heart Foundation. These funding sources were not involved in study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
None of the authors has a conflict of interest to disclose.
T. Miyoshi and H.H. conceived and designed the study. T. Miyoshi and T. Matsuyama provided the data. T. Miyoshi diagnosed all fetuses with congenital heart defects and arrhythmias. T. Matsuyama assessed all placentas. M.N. provided statistical support and advice. T. Miyoshi wrote the article and revised it with input from all the authors. All authors discussed the results and implications, and commented on the manuscript at all stages. T. Miyoshi was the grant holder.
This study was approved by the Institutional Ethics Board of the National Cerebral and Cardiovascular Center (Reference no. R20048).