Article ID: CJ-22-0764
Article ID: CJ-22-0764
Background: Grafting the right gastroepiploic artery (GEA) to the right coronary artery (RCA) is effective, but preoperative evaluation of arterial conduit availability has not been established. By comparing the midterm graft results, we aimed to assess the efficacy of preoperative evaluation of the GEA using computed tomography (CT).
Methods and Results: We retrospectively examined patients who underwent isolated coronary artery bypass grafting surgery between April 2010 and December 2020, and those whose GEA was grafted to the RCA were selected: 55 patients were included in the study analysis. Postoperative evaluations were performed during the early phase, 1 year postoperatively, and at follow-up evaluations. The outer diameter of the proximal GEA was compared with the midterm graft patency grade on CT and patients were classified as Functional (Grade A) or Dysfunctional (Grades O or B). The proximal GEA outer diameters were significantly different between the Functional and Dysfunctional groups (P<0.001). Furthermore, multivariate Cox regression analysis revealed that this diameter was an independent predictor of graft functionality (P<0.001). Patients with outer proximal diameters larger than the cutoff value had superior graft results at 3 years postoperatively. The rate of freedom from a dysfunctional graft at 3 years postoperatively was 95.5% and 45.5% for the Larger and Smaller diameter subgroups, respectively (P<0.001).
Conclusions: Preoperative evaluation of the outer diameter of the proximal GEA, excluding calcified GEA, using CT is a minimally invasive and useful method, and may improve midterm results of in-situ GEA grafting, even in severe stenotic lesions.
The left internal thoracic artery (ITA) is the first choice for bypass grafts to the left anterior descending artery in coronary aorta bypass grafting (CABG).1 For improved clinical outcomes, the right ITA and radial artery are widely used as better second or third arterial conduits in Western countries. However, gastroepiploic artery (GEA) grafting to the right coronary artery (RCA) has been reported as effective.2–4 Furthermore, a previous meta-analysis5 revealed that the GEA is superior to a saphenous vein graft (SVG) in terms of long-term survival, acting as a third conduit for revascularization of the RCA system among patients in whom both ITAs have been anastomosed to the left coronary artery (LCA). Other studies have reported no significant differences in long-term outcomes between the GEA and SVG in RCA revascularization.6,7 As one reason for this absence of a clear benefit of GEA grafting, competitive flow, a characteristic of arterial grafts, is a well-known risk factor for decreased graft patency attributable to graft spasm.8 If flow competition can be predicted preoperatively, the effectiveness of the GEA should not be impaired. However, few reports have discussed preoperative evaluation methods concerning the use of GEA as an arterial conduit.9,10 Therefore, establishing an optimal method for preoperative assessment of the suitability of the GEA for CABG would be clinically useful. Advancements in computed tomography (CT) have enabled visualization of the GEA with high resolution, even without contrast medium, thus providing more information while being minimally invasive. Furthermore, three-dimensional images reconstructed by coronary CT angiography (CCTA) make it possible to estimate intraluminal properties in more detail, although it remains difficult to use CCTA in patients with severe allergies or renal impairment. Because cardiovascular disease is commonly associated with renal insufficiency, CCTA is not possible for all patients scheduled for cardiovascular surgery.
Therefore, we aimed to assess the accuracy and reliability of preoperative evaluation of the GEA using non-contrast CT and to determine whether such assessment would improve midterm graft patency and functionality.
Of the patients who underwent isolated CABG between April 2010 and December 2020, those for whom RCA system revascularization was performed using in-situ GEA grafts were included in this study. We excluded the following patients: did not undergo preoperative CT, including chest to pelvis imaging or postoperative graft evaluation in the early phase or up to 1 year after surgery; preoperative CT findings showed a calcified lesion in the GEA; in-situ GEA graft was sequentially anastomosed to other territories. Patients with <90% stenotic lesion or lesions ≥1.0 mm in minimum lumen diameter (MLD) were also excluded.11,12 The study protocol was approved by the Institutional Review Board of Musashino Red Cross Hospital, Tokyo, Japan (No. 3044). The requirement for obtaining informed consent was waived by the review board because of the retrospective nature of the study, which was carried out in accordance with the principles embodied in the Declaration of Helsinki and its revisions.
Preoperative Imaging EvaluationAll patients underwent preoperative coronary angiography (CAG) for measurement of the MLD and the reference diameter of the native RCA in 2 projections, as orthogonally as possible, and the stenosis rate was calculated using G-NAVI version 6.1.2 (GOODMAN, Nagoya, Japan). Preoperative CT images were reviewed and magnified for measurement of the GEA diameters. Because only the outer diameter could be measured on non-enhanced CT images, the proximal portion of the GEA near the gastroduodenal artery was measured as the ‘outer diameter’ (Figure 1). Among the study group, only patients who underwent preoperative CT scan using contrast medium were analyzed with SYNAPSE VINCENT software (Fujifilm, Japan) for measurement of the inner GEA diameter. Image processing recreated the GEA between the proximal and distal points as straight lines enabling the inner and outer diameters to be measured as (Figure 2A). Using a previous study as a reference,9 the distal point was defined in each case as the point distal to the crossing of the GEA and vertical midline of the spine (i.e., the CT-middle point) (Figure 2B) and proximal to the branching of ≥2 narrow branches. The minimum inner diameter on this image (GEA inner MLD) and the outer diameter at the same point (GEA outer MLD) were extracted, and the rate of narrowing (GEA MLD narrowing rate [GEA MLD NR]) was calculated for each patient using the formula:

Measuring the right gastroepiploic artery (GEA) on computed tomography (CT). The outer diameter of the proximal portion (yellow arrows) of the GEA (red arrow) near the gastroduodenal artery was measured on CT as GEA proximal diameter.
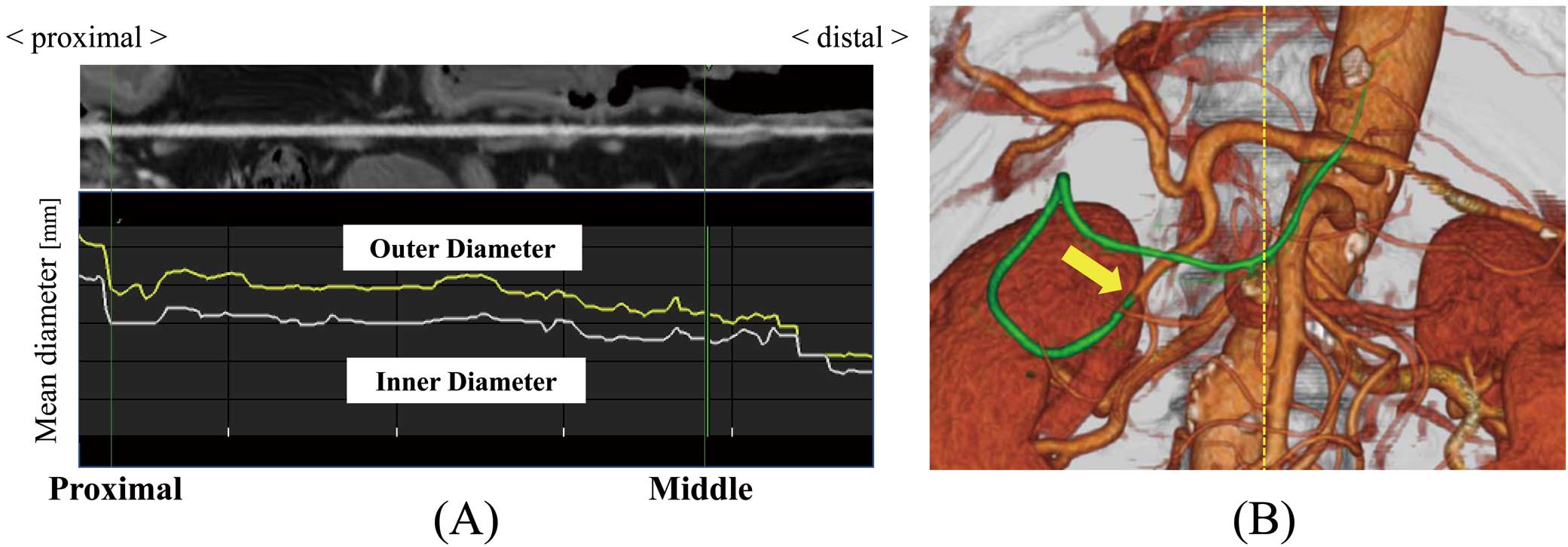
(A) GEA rebuilt by image processing as a straight-line image showing the mean inner and outer diameters at each point from its proximal to distal part. (B) Three-dimensional GEA constructed by CT analytical software. The crossing point of the green curved line representing the GEA and yellow broken line representing the CT-middle line is the CT-middle point. CT, computed tomography; GEA, gastroepiploic artery.
GEA MLD NR [%] = GEA outer MLD − GEA inner MLD / GEA outer MLD × 100.
All measurements were repeated three times to avoid errors, and the mean values were used for statistical analysis.
Surgical ProcedureAll procedures were performed through a median sternotomy. The primary indications for the use of in-situ GEA grafting were stenosis severity (≥90%), and good patient life expectancy without complications (except if cardiovascular-related). Patients with a previous history of upper abdominal surgery, not insignificant degree of narrowing or calcification of the celiac axis or around the abdominal aorta, or hemodynamic instability were not indicated.
After the skeletonized ITA was harvested, a short extension of the median incision to the peritoneal cavity was made, and the GEA was harvested using a Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, OH, USA) in all patients using the technique reported by Asai and Tabata.13 After systemic heparinization, the harvested ITA and GEA were divided distally, and wrapped with milrinone-soaked gauze for pharmacologic dilation before use. Intraluminal injection of vasodilator was not performed. We did not use GEAs that had an imperceptible pulse or showed no dilation after harvesting. The in-situ GEA was routed anterior to the left lobe of the liver to the pericardial cavity, through a perforation in the diaphragm produced by electrocautery. A sequential anastomosis technique was used to complete revascularization in some cases as required. Intraoperative graft evaluation was made with a transit time flow meter (TTFM; Medi-Stim As, Oslo, Norway), and mean graft flow (Qm [mL/min]), pulsatility index (PI), and diastolic filling index (DFI [%]) were measured for all patients.
Intravenous nicorandil and diltiazem were continuously infused postoperatively, and diltiazem or an alternative calcium-channel receptor blocker with nicorandil and aspirin were continued by oral administration for as long as possible. All GEA harvesting and grafting procedures were performed by a single surgeon.
Follow-up and Clinical EvaluationEarly postoperative and subsequent annual examinations of graft patency using CAG or CCTA were routinely performed for all participants. Those who had renal impairment or contrast medium allergy or who refused examinations were excluded from the angiographic follow-up. The latest patient information was acquired from the institution’s medical records. Graft patency was graded on CAG as described by FitzGibbon.14 An occluded graft was classified as Grade O, and a graft was assessed as patent and classified as Grade A when there was complete continuity of the graft lumen from the GEA proximal portion near the gastroduodenal artery to all target RCA branches. Flow competition was defined and classified as Grade B when there was balanced native coronary flow and graft flow in the grafted area or the graft flow was retrospectively reflected by native coronary backflow. For CCTA, complete continuity without interruption at any level between the proximal GEA and all target RCA branches was classified as Grade A; the condition in which the total length of the graft was not enhanced was classified as Grade O; and that in which the graft had a contrast effect only around the anastomotic site or GEA proximal portion, and partial interruption was observed, was considered as Grade B. Graft functionality was stratified based on the last angiographic evaluation, with Grade A patients defined as Functional and Grades B or O patients defined as Dysfunctional.
Statistical AnalysisCategorical variables are presented as frequencies and percentages and continuous variables as mean±SD. Intergroup differences were compared using the chi-square or Fisher’s exact test, t-test or the Mann-Whitney U test. Differences were considered to be significant at P<0.05. Univariate and multivariate Cox proportional hazard regression analyses were performed for assessment of midterm graft outcomes. The multivariate analysis included the factors with P<0.05 set to indicate an important factor in the univariate analysis. The cutoff value of the GEA proximal diameter for graft functionality was calculated by receiver operating characteristic (ROC) curve analysis. Subgroup analysis of midterm results was performed based on the cutoff value. Midterm graft patency and survival were estimated using the Kaplan-Meier method. Statistical analyses were performed using R software, version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria).
One patient whose celiac artery was found to be injured at postoperative early evaluation by CAG was excluded, so 55 of a total of 414 isolated CABG surgeries were finally included in the present study (Figure 3). Preoperative clinical and angiographic characteristics and intraoperative and postoperative data are summarized in Table 1 and Table 2. Off-pump CABG was performed in 53 (96.4%) patients. One of the major reasons for the relatively low use of bilateral ITAs (23.6%) and total arterial reconstruction (41.8%) was our reluctance to use these techniques in diabetic patients. The numbers of distal anastomoses/patient in the overall and RCA systems were 4.4±1.0 and 1.5±0.5, respectively. Sequential grafting in the RCA system was performed in 26 (47.3%) patients and the posterior descending artery was reconstructed in 51 (92.7%) patients. Warfarin was postoperatively administered to more than two-thirds of eligible patients (67.3%), because our previous study showed that its therapeutic dose in combination with aspirin improves long-term venous graft patency.15 All patients underwent early postoperative (13.3±6.3 days) and 1-year (12.8±2.8 months) angiographic evaluations. The GEA anastomotic diameter was 2.70±0.58 mm at the early postoperative evaluation. In the follow-up period (4.6±3.1 years, maximum 10.5 years), only 1 death occurred (cerebral bleeding, 3.0 years). The overall survival rates at 1, 3, and 5 years after surgery were 100%, 99.5%, and 99.5%, respectively.

Flowchart of patient inclusion in the study. CABG, coronary artery bypass grafting; CAG, coronary angiography; CCTA, coronary CT angiography; CT, computed tomography; GEA, gastroepiploic artery; MDCT, multidetector CT; MLD, minimum lumen diameter.
| Variables (n=55) | n (%), value (range) |
|---|---|
| Clinical characteristics | |
| Age | 63.9±10.3 (36–82) |
| Male | 47 (85.5) |
| Body mass index | 23.4±4.2 (20–30.8) |
| Obesity (BMI ≥25) | 18 (32.7) |
| Smoking history | 30 (54.5) |
| Hypertension | 45 (81.8) |
| Hyperlipidemia | 46 (83.6) |
| Diabetes mellitus | 21 (38.2) |
| Insulin-dependent | 11 (20.0) |
| Renal function (eGFR) | 65.3±19.2 (5.3–105) |
| Hemodialysis | 2 (3.6) |
| Left ventricular ejection fraction <40% | 8 (14.5) |
| Angiographic data | |
| RCA | |
| Minimum lumen diameter (mm) | 0.17±0.22 (0–0.70) |
| Stenosis rate (%) | 92.5±9.3 (75.3–100) |
| Total occlusion | 32 (58.2) |
| GEA | |
| Outer proximal diameter (mm) | 3.69±0.92 (1.05–5.55) |
BMI, body mass index; eGFR, estimated glomerular filtration rate; GEA, gastroepiploic artery; RCA, right coronary artery.
| Variables (n=55) | n (%), value |
|---|---|
| Operative data | |
| Operation time (min) | 406.6±86.5 (279–675) |
| Operative procedure | |
| Off-pump | 53 (96.4) |
| On-pump beating | 2 (3.6) |
| Bilateral internal thoracic artery use | 13 (23.6) |
| All arterial graft reconstruction | 23 (41.8) |
| No. of total anastomoses | 4.4±1.0 (2–7) |
| No. of anastomoses for RCA | 1.5±0.5 (1–2) |
| 1 | 29 (52.7) |
| 2 | 26 (47.3) |
| Sequential grafting technique | 26 (47.3) |
| Transit time flow meter data of GEA | |
| Mean graft flow (Qm) (mL/min) | 54.1±44.5 (5–186) |
| Pulsatility index | 4.0±5.4 (0.9–32.4) |
| Diastolic filling index (%) | 65.6±11.9 (3–86) |
| Postoperative data | |
| Mortality | |
| ≤30 days | 0 |
| >30 days | 1 (1.8) |
| Cardiovascular-related death | 0 |
| Medications | |
| Aspirin | 55 (100) |
| Warfarin | 37 (67.3) |
| Diltiazem | 35 (63.6) |
| Other calcium-channel receptor blocker | 40 (72.7) |
| Statin | 45 (81.8) |
GEA, gastroepiploic artery; RCA, right coronary artery.
At the time of their last evaluation (3.9±2.7 years), 46 patients were classified as Grade A, 5 as Grade B, and 4 as Grade O. Therefore, 9 (19.6%) were classified as Dysfunctional (Supplementary Figure 1). All of the Grade O patients showed deterioration in their functional grade from Grade B at ≥1 year after surgery, although there were no patients in Grade O at the early-phase evaluation. The cumulative GEA patency rate was 96.3% at 1 year, and 93.9% at 3 years after surgery. The cumulative rates of freedom from a dysfunctional graft were 85.3% at 1 year, and 85.3% at 3 years. Of all the perioperative clinical and angiographic factors, GEA outer proximal diameter and PI were included in the multivariate analysis, based on the results of the univariate analysis (Table 3). Multivariate Cox proportional hazard regression analysis showed that both were independent predictors for graft functionality.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Univariate model | |||
| Preoperative data | |||
| GEA proximal diameter | 0.146 | 0.0534–0.3984 | <0.001 |
| Intraoperative data | |||
| Pulsatility index | 1.0720 | 1.0030–1.1450 | 0.040 |
| Multivariate model | |||
| Preoperative data | |||
| GEA proximal diameter | 0.1408 | 0.04885–0.4059 | <0.001 |
| Intraoperative data | |||
| Pulsatility index | 1.0740 | 1.0000–1.1530 | 0.049 |
CI, confidence interval; GEA, gastroepiploic artery; HR, hazard ratio.
Of the 55 patients in this study, 29 underwent preoperative CT using contrast medium. The GEA outer proximal diameter and GEA inner MLD were compared in this group. The GEA outer proximal diameter showed a positive correlation with the GEA inner proximal diameter (correlation coefficient=0.508, P=0.009). Furthermore, GEA MLD NR revealed the absence of severe stenotic lesions in this group (18.1±11.0, 3.5–45.5%).
Impact of GEA Proximal Diameter for Graft FunctionalitySupplementary Figure 2A shows the distribution of GEA outer proximal diameters by graft functionality. The GEA outer proximal diameter of the Functional group was significantly larger than that of the Dysfunctional group (3.90±0.80 vs. 2.60±0.73 mm; P<0.001). The cutoff GEA proximal diameter for predicting graft functionality determined by ROC curve analysis was 2.9 mm (area under the curve 0.877; 83.3% sensitivity and 95.7% specificity) (Supplementary Figure 2B).
Comparison of the Midterm Results With Reference to the Cutoff ValueThe 3-year results of graft functionality were compared by dividing the participants based on the cutoff value: Large proximal group (outer proximal diameter >2.9 mm; n=43 [78.2%]); Small proximal group (diameter ≤2.9 mm; n=12 [21.8%]). There was no significant difference in Qm, PI and GEA anastomotic diameter between these groups (Large proximal group vs. Small proximal group=59.4±48.2 vs. 35.1±19.2, P=0.095; 3.41±4.10 vs. 6.26±8.43, P=0.106; 2.72±0.59 vs. 2.46±0.60, P=0.567). The comparison for freedom from Dysfunctional graft at 3 years is shown in Figure 4 (Large proximal vs. Small proximal, 95.5% vs. 45.5%, respectively; P<0.001).
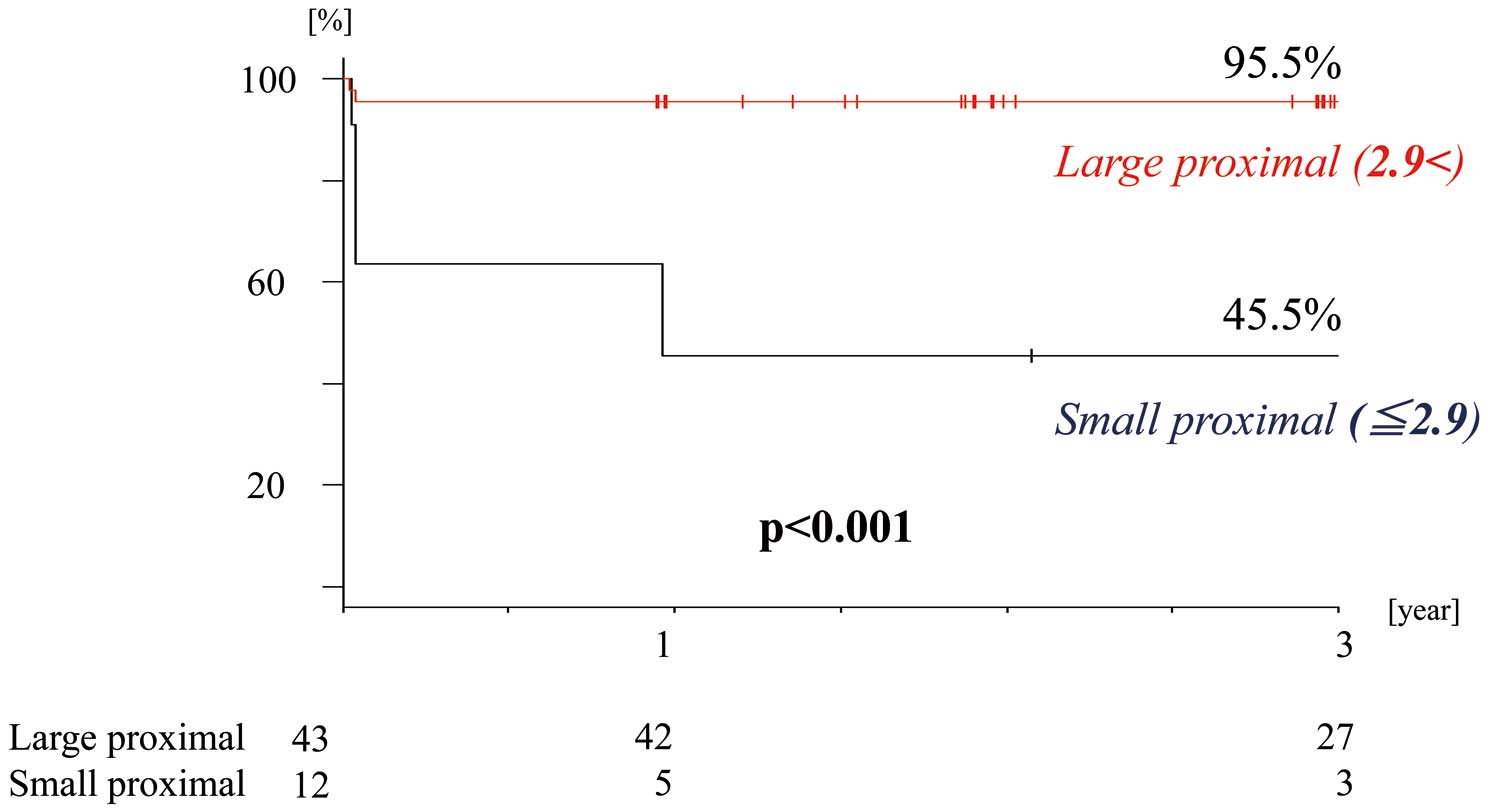
Comparison of postoperative 3-year freedom from a dysfunctional GEA, graft in the 2 proximal diameter groups. GEA gastroepiploic artery.
Table 4 demonstrates the superior midterm graft outcome in the Large proximal group. Of the 43 patients in this cohort, 27 underwent postoperative 3-year angiographic evaluation except for 1 patient, whose graft was recognized to be competitive on early CAG (POD 13) and remained competitive at postoperative 1-year evaluation (POD 363). All other GEA grafts in this group had been Functional at 1 year after surgery and remained Functional at the postoperative 3-year evaluation.
| 1-year evaluation (12.8±2.8 months) |
3-year evaluation (36.8±2.9 months) |
|
|---|---|---|
| Rate of freedom from revascularization of RCA | ||
| Large proximal | 100 (43/43) | 100 (27/27) |
| Small proximal | 100 (12/12) | 80.0 (4/5) |
| GEA patency rate (Grade A + Grade B) | ||
| Large proximal | 100 (43/43) | 100 (27/27) |
| Small proximal | 83.3 (10/12) | 100 (5/5) |
| Rate of freedom from GEA dysfunctional graft (Grade A) | ||
| Large proximal | 97.7 (42/43) | 100 (27/27) |
| Small proximal | 41.7 (5/12) | 60.0 (3/5) |
GEA, gastroepiploic artery; RCA, right coronary artery.
The 2 main findings of this study were: (1) the preoperative proximal outer diameter of the GEA measurable on non-contrast CT in the Functional group was significantly larger than that in the Dysfunctional group, and the cutoff value for graft functionality was calculated to be 2.9 mm and (2) even in coronary lesions with the same severity of stenosis, patients in the Large proximal group had remarkably higher 3-year patency and freedom from a dysfunctional graft rates than the Small proximal group.
One of the important reasons for previous studies emphasizing consideration of the inner diameter when discussing preoperative evaluation methods is that focusing on the outer diameter alone cannot completely exclude severely stenotic lesions or occlusion resulting from noncalcified plaque. Of the total participants in this study, only 29 underwent contrast preoperative CT. Based on the results of this subgroup analysis, after exclusion of patients whose preoperative CT findings revealed the presence of a calcified lesion in GEA, patients with a larger GEA outer proximal diameter also had a larger inner MLD because the 2 diameters showed a positive correlation and the GEA MLD NR was not severe.
Although the use of free GEA grafts for aorto-coronary bypass,16 and Y-composite based on the in-situ left ITA17 were recently reported to have good 10-year patency, in-situ GEA grafting to the RCA system has other clinical utility to those graft designs because it does not require anastomosis to the atherosclerotic ascending aorta, which is not uncommon in multivessel disease patients.18,19 More recently, I-composite grafting using the in-situ GEA and RA has been reported as a safe and effective method of total arterial revascularization in minimally invasive CABG.20 This technique improves the eases of surgery by avoiding harvesting of the right ITA and manipulation of the ascending aorta via a left anterior thoracotomy. However, in contrast to vein grafts, arterial grafts are prone to spasm, are dynamic, and their flow capacity tends to be influenced by their tone and competitive flow. For this reason, we evaluated graft flow and classified grafts into 3 grades of patency, based on past studies.11,12 In the present study, patency deteriorated from Grade B in the early phase to Grade O over the observation period in 4 patients (Supplementary Figure 1). This result was consistent with the conclusions in the previous studies.
Several studies have reported intraoperative evaluations of GEA availability as a conduit. using TTFM, Tokuda et al suggested that cutoff values of mean flow (Qm) for early graft failure were 20 mL/min and 15 mL/min for the RCA and LCA, respectively.21 Shimizu et al recommended that the GEA should not be used as an in-situ graft if the inner diameter at the anastomotic site is <1.5 mm.8 However, intraoperative evaluation methods can result in an additional laparotomy if the graft is found to be unusable only at the time of anastomosis; therefore, they are not considered to be clinically useful. Only a few previous studies in coherent cases have reported preoperative evaluation methods of the GEA itself,9,10,22,23 and an optimal method has not yet been established. Two of those studies implied that the inner proximal diameter of the GEA on enhanced CT could be an important predictor for the vessel’s suitability as an arterial conduit,9,10 and we also focused on the proximal diameter of the GEA as a preoperative evaluation target. However, we believe we are the first to preoperatively evaluate the GEA in 2 other aspects: first, the outer proximal diameter of the GEA, measured without contrast medium, was assessed as a predictor, and second, the midterm results of graft patency were evaluated as a research outcome.
Preoperative evaluation of coronary stenosis has been performed several past studies. When evaluating it by CAG, the MLD was reported to be a more reliable measure than the stenosis diameter percentage.11,12 However, one of our main indications for using in-situ GEA was the severity of stenosis, according to stenosis percent classification of the American Heart Association.24 Therefore, in the present study, we were limited to patients with a severe stenotic lesion corresponding to both criteria. The Society of Thoracic Surgeons specifies that GEA grafting should be limited to severely stenotic lesions, and preferably RCA revascularization;25 however, there is no description of GEA requirements. Considering the superior rate of freedom from a dysfunctional graft at 3 years in the Large proximal group compared with the Small proximal group, not only stenosis severity as a native vessel factor, but also GEA proximal diameter as graft factors should be included in the preoperative evaluation to improve midterm graft patency. In a large study of GEA grafting, Suma et al reported predominant use as a single pedicle in-situ graft in 1,352 patients, with cumulative postoperative patency rates of 92.3%, 82.5%, and 66.5% at 1, 5, and 10 years, respectively.26 Suzuki et al reported late patency rates of 96.0% at 3 years, 94.7% at 5 years, and 90.2% at 8 years after surgery using a skeletonized GEA.27 The 3-year results of the Large proximal group in this study were comparable to those 2 reports. Thus, preoperative selection of patients for in-situ GEA grafting if it has a large proximal outer diameter (i.e., Large proximal group) and without calcified lesions on non-contrast CT could be useful.
Study LimitationThere are several inherent limitations that must be considered when evaluating the results. First, this was a non-randomized, retrospective study, with a small number of subjects in a single institute. Furthermore, some selection bias could have occurred due to our main indicational criterion of grafting the GEA to only severely stenotic lesions. Second, we could not evaluate the inner diameter of the GEA without contrast, but we excluded calcified GEAs on preoperative CT, and did not use any grafts with an imperceptible pulse after harvest, as with other arterial conduits. Third, a sequential grafting technique cohort was included in our study population, but the number of patients was too small to allow a statistical examination of graft functionality for each anastomotic design. Fourth, as one of the causes of competitive graft flow, flow demand should be taken into consideration, but was not assessed in this study. Fifth, some patients were excluded from midterm graft evaluation because CT angiography could not be performed for several reasons, resulting in a selection bias.
In conclusion, preoperative evaluation of the proximal GEA outer diameter after excluding calcified GEA by non-enhanced CT may play a role in improving midterm graft functionality and patency when in-situ GEA grafting to the RCA system is planned, even in severe stenotic lesions. This evaluation method is considered to be minimally invasive and clinically useful.
None.
The authors declare that there are no conflicts of interest and that no funding was received in association with the present study.
The investigation conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Musashino Red Cross Hospital, Tokyo, Japan (No. 3044).
The identified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0764