Abstract
Background: The incidence of venous thromboembolism (VTE; pulmonary embolism [PE] and/or deep vein thrombosis [DVT]) in Japan is increasing, but relatively small numbers of patients from Japan have been included in studies investigating rivaroxaban (a direct factor Xa inhibitor) for the treatment of VTE and preventing its recurrence.
Methods and Results: An open-label, prospective, observational study (XASSENT [NCT02558465]) investigated the safety profile and effectiveness of rivaroxaban for ≤2 years in the treatment of VTE and prevention of its recurrence in Japanese clinical practice. Primary outcomes were major bleeding and symptomatic recurrent VTE. Statistical analyses were exploratory and descriptive. Overall, 2,540 patients were enrolled (safety analysis population [SAP], n=2,387; effectiveness analysis population [EAP], n=2,386). In the SAP, >80% of patients received the approved rivaroxaban dose, the mean (standard deviation) age was 66.6 (15.0) years, ≈74% were >50 kg, and 43% had a creatinine clearance ≥80 mL/min. PE+DVT, PE only, and DVT only were reported in 42%, 8%, and 50% of patients, respectively, and active cancer in 17% of patients. Major bleeding was reported in 69 patients (2.89%; 3.60%/patient-year; SAP) and symptomatic PE/DVT recurrence in 26 patients (1.09%; 1.36%/patient-year; EAP) during the treatment period.
Conclusions: XASSENT provided information on the expected proportions of bleeding and VTE recurrence during rivaroxaban treatment in Japanese clinical practice; no new concerns of safety or effectiveness were found.
The worldwide burden of venous thromboembolism (VTE; comprising pulmonary embolism [PE] and deep vein thrombosis [DVT]) is substantial.1 Most studies into VTE, the results from which have informed treatment recommendations, have focused on Western populations,2 but the specific requirements of patients in Japan with VTE have not been studied in such detail. Owing to the adoption of Western lifestyle practices and an aging population, the number and prevalence of risk factors for VTE have increased in Japan, so more specific information is therefore needed to inform treatment strategies for patients in Japan.
The direct oral anticoagulant (DOAC) rivaroxaban is approved in Japan for the treatment of VTE and prevention of its recurrence. The approved dosage is 15 mg twice daily for the first 3 weeks after the onset of the event followed by 15 mg once daily,3 based on results from phase 3 trials, including the open-label, randomized multinational EINSTEIN PE/DVT (n=8,282) and Japanese J-EINSTEIN PE/DVT (n=100) trials, which demonstrated the efficacy and safety profile of rivaroxaban for the treatment of acute VTE using a single-drug approach.4–7 However, there were observable differences in the demographic characteristics of patients with VTE at treatment initiation between those who were treated in Japanese clinical practice and the multinational population assessed in the EINSTEIN PE/DVT studies; for example, with respect to age, body weight, renal function, and the proportion of patients with cancer.6,8 Observational studies can bridge the gap between randomized controlled trials and the real world, and provide more accurate insights into the safety profile and effectiveness of rivaroxaban in clinical practice, but such data are limited. The prospective, observational cohort study J’xactly (NCT03091621) was conducted in 1,039 patients with acute symptomatic/asymptomatic DVT or PE with or without DVT who were being treated in Japanese clinical practice. Results from patients who received rivaroxaban and who were followed up for 18–36 months demonstrated that independent risk factors for the composite of clinically relevant safety and effectiveness outcomes included male sex, being underweight, having chronic heart and lung disease, active cancer, and previous stroke.9 More data from Japanese clinical practice would therefore be beneficial for improved outcomes for patients with VTE in Japan.
Here, we report safety and effectiveness data from XASSENT, a study designed to evaluate rivaroxaban in the treatment of VTE and prevention of its recurrence in Japanese routine clinical practice.
Methods
Details of the study design have been published elsewhere.10 In brief, XASSENT (NCT02558465) was an open-label, prospective, observational, post-marketing surveillance cohort study in patients who were receiving rivaroxaban treatment for VTE. The sample size was set to at least 1,250 based on estimated rates of PE/DVT incidence and recruitment and enrollment periods. Patients who started rivaroxaban for the treatment of PE/DVT and prevention of its recurrence were enrolled between November 2015 and March 2018, and were to be followed for ≤2 years (1-year standard observation period and 1-year follow-up period). Primary outcome variables were major bleeding (as defined by International Society on Thrombosis and Haemostasis criteria11) and symptomatic recurrent VTE. Outcome variables were adjudicated by attending physicians and reported using case report forms. Statistical analyses were exploratory and descriptive only. Missing data were aggregated as a separate category, “unknown,” in frequency tables. Outcome variables were reported as incidence proportions (patients with events per patients in each analysis set) or incidence rates (patients with events per 100 patient-years) with corresponding 95% confidence intervals (CIs). Kaplan-Meier plots were used to show the time course up to the first event of major bleeding and symptomatic recurrent VTE. The safety analysis population (SAP) consisted of all patients who received ≥1 dose of rivaroxaban and attended ≥1 visit afterward. The effectiveness analysis population (EAP) included patients who had a PE/DVT diagnosis and were naive to rivaroxaban at baseline in the SAP.
The initial treatment group was defined as patients who started rivaroxaban for initial treatment of PE/DVT. The maintenance treatment group was defined as patients who started rivaroxaban for maintenance treatment (e.g., switching from other anticoagulants). Other subgroup analyses were also conducted, according to age (<75 years or ≥75 years), body weight (≤50 kg or >50 kg), renal function (creatinine clearance <50 mL/min or ≥50 mL/min), active cancer (present or absent), VTE diagnosis (PE with or without DVT, DVT only, or isolated distal DVT [IDDVT] or proximal DVT in patients with DVT only), rivaroxaban dose, and concomitant therapy (e.g., antiplatelet use).
Daily doses of rivaroxaban were aggregated for data analysis. Safety outcomes were reported based on adverse events (not adverse drug reactions). Wherever possible, all adverse events (only bleeding events and fatal adverse events during the follow-up period) and PE/DVT recurrence were recorded for 3 months after discontinuation or completion of rivaroxaban treatment.
Results
Patients’ Characteristics
In total, 2,540 patients were enrolled in 357 sites across Japan. Of these, 144 patients without data fixed were excluded from analysis, and 9 patients who did not return after the first visit were excluded from the SAP (Figure 1). Patient characteristics for the SAP (n=2,387), for those prescribed rivaroxaban for initial treatment (initial treatment group; n=2,025) in the SAP, and for those prescribed the drug for maintenance treatment (maintenance treatment group; n=362) are presented in Table 1. One patient in the SAP received rivaroxaban off label and was excluded from the EAP (n=2,386). The mean age of patients in the SAP was 66.6 years, with the majority being <75 years of age (65%), female (58%), and weighing >50 kg (74%). Approximately 43% of patients in the SAP had a creatinine clearance ≥80 mL/min, and half had a diagnosis of DVT only, with the remainder having PE with DVT (42%) or PE only (8%), while ≈68% of patients had a history of risk factors for PE/DVT. Active cancer was reported as a risk factor for PE/DVT in ≈17% of patients at baseline. Primary sites of cancer at baseline are summarized in Supplementary Table 1. Prior cardiopulmonary disease was reported as a risk factor for VTE in 143 patients (5.99%). In the SAP, 643 patients were blood type A, 316 were B, 144 were AB, 291 were O, and 993 were unknown. In XASSENT, the proportion of the O blood type (291/1,394 [21%]) was lower than in the general Japanese population (29%)12 (comparison between the two studies for the proportions of O and non-O blood types, P<0.0001 [chi-squared test]). In the initial treatment group, 120 patients (5.93%) had an inferior vena cava filter; thrombolysis, thrombectomy, and catheter-assisted thrombus fragmentation/aspiration were used as a treatment for 88 (4.35%), 2 (0.10%), and 15 (0.74%) patients, respectively.

Table 1. Patients’ Characteristics (Safety Analysis Population, n=2,387)
| |
Total
(N=2,387) |
Purpose of rivaroxaban administration |
Initial treatment
(n=2,025) |
Maintenance treatment
(n=362) |
| Age, years |
| Mean (SD) |
66.6 (15.0) |
66.5 (15.2) |
67.7 (14.0) |
| <75 |
1,548 (64.85) |
1,323 (65.33) |
225 (62.15) |
| ≥75 |
839 (35.15) |
702 (34.67) |
137 (37.85) |
| Sex |
| Male |
1,004 (42.06) |
869 (42.91) |
135 (37.29) |
| Female |
1,383 (57.94) |
1,156 (57.09) |
227 (62.71) |
| Type of patient |
| Inpatient |
1,646 (68.96) |
1,483 (73.23) |
163 (45.03) |
| Outpatient |
741 (31.04) |
542 (26.77) |
199 (54.97) |
| Body weight, kg |
| Mean (SD) |
60.90 (13.87) |
61.28 (13.87) |
58.78 (13.72) |
| ≤50 |
526 (22.04) |
433 (21.38) |
93 (25.69) |
| >50 |
1,777 (74.44) |
1,520 (75.06) |
257 (70.99) |
| Unknown |
84 (3.52) |
72 (3.56) |
12 (3.31) |
| BMI, kg/m2 |
| Mean (SD) |
23.94 (4.20) |
24.03 (4.16) |
23.48 (4.38) |
| Unknown |
200 (8.38) |
171 (8.44) |
29 (8.01) |
| History of PE/DVT |
| No |
2,101 (88.02) |
1,838 (90.77) |
263 (72.65) |
| Yes |
285 (11.94) |
186 (9.19) |
99 (27.35) |
| Unknown |
1 (0.04) |
1 (0.05) |
0 (0.00) |
| History of risk factors for PE/DVT |
| Yes |
1,613 (67.57) |
1,375 (67.90) |
238 (65.75) |
| Risk factors for PE/DVTa |
| Immobilization within 3 months before PE/DVT |
| Yes |
444 (18.60) |
380 (18.77) |
64 (17.68) |
| Obesity |
| Yes |
410 (17.18) |
365 (18.02) |
45 (12.43) |
| Surgery/injury within 3 months before PE/DVT |
| Yes |
455 (19.06) |
391 (19.31) |
64 (17.68) |
| Active cancer |
| Yes |
406 (17.01) |
330 (16.30) |
76 (20.99) |
| Comorbidities other than risk factors for PE/DVT |
| Renal disease |
| No |
2,148 (89.99) |
1,831 (90.42) |
317 (87.57) |
| Yes |
221 (9.26) |
181 (8.94) |
40 (11.05) |
| Unknown |
18 (0.75) |
13 (0.64) |
5 (1.38) |
| CrCl, mL/min |
| Mean (SD) |
83.0 (36.7) |
83.8 (37.0) |
78.9 (34.8) |
| <15 |
0 (0.00) |
0 (0.00) |
0 (0.00) |
| 15 to <30 |
11 (0.46) |
9 (0.44) |
2 (0.55) |
| 30 to <50 |
340 (14.24) |
279 (13.78) |
61 (16.85) |
| 50 to <80 |
916 (38.37) |
771 (38.07) |
145 (40.06) |
| ≥80 |
1,028 (43.07) |
888 (43.85) |
140 (38.67) |
| Unknown |
92 (3.85) |
78 (3.85) |
14 (3.87) |
| Liver disease |
| No |
2,209 (92.54) |
1,887 (93.19) |
322 (88.95) |
| Yes |
173 (7.25) |
135 (6.67) |
38 (10.50) |
| Unknown |
5 (0.21) |
3 (0.15) |
2 (0.55) |
| Cardiovascular disease |
| No |
1,282 (53.71) |
1,097 (54.17) |
185 (51.10) |
| Yes |
1,101 (46.12) |
925 (45.68) |
176 (48.62) |
| Hypertension |
941 (39.42) |
806 (39.80) |
135 (37.29) |
| Atrial fibrillation |
95 (3.98) |
78 (3.85) |
17 (4.70) |
| Unknown |
4 (0.17) |
3 (0.15) |
1 (0.28) |
| Lung disease |
| No |
2,091 (87.60) |
1,778 (87.80) |
313 (86.46) |
| Yes |
286 (11.98) |
240 (11.85) |
46 (12.71) |
| Unknown |
10 (0.42) |
7 (0.35) |
3 (0.83) |
| Diseases or conditions with high risk of bleedingb |
| No |
1,997 (83.66) |
1,698 (83.85) |
299 (82.60) |
| Yes |
390 (16.34) |
327 (16.15) |
63 (17.40) |
| VTE diagnosis |
| PE with DVT |
993 (41.60) |
880 (43.46) |
113 (31.22) |
| PE only |
194 (8.13) |
156 (7.70) |
38 (10.50) |
| DVT only |
1,199 (50.23) |
988 (48.79) |
211 (58.29) |
| Other |
1 (0.04) |
1 (0.05) |
0 (0.00) |
| VTE symptoms |
| Symptomatic PE/DVT |
1,821 (76.29) |
1,597 (78.86) |
224 (61.88) |
| Asymptomatic PE/DVT |
565 (23.67) |
427 (21.09) |
138 (38.12) |
| Clinical severity of PE |
| Cardiac arrest collapse |
14 (0.59) |
9 (0.44) |
5 (1.38) |
| Massive |
71 (2.97) |
63 (3.11) |
8 (2.21) |
| Submassive |
360 (15.08) |
322 (15.90) |
38 (10.50) |
| Nonmassive |
686 (28.74) |
609 (30.07) |
77 (21.27) |
| Unknown |
56 (2.35) |
33 (1.63) |
23 (6.35) |
| Site of DVT in patients with diagnosed DVT-onlyc |
| IDDVT |
409 (17.13) |
338 (16.69) |
71 (19.61) |
| DVT other than IDDVT |
726 (30.41) |
623 (30.77) |
103 (28.45) |
| Proximal DVTd |
694 (29.07) |
594 (29.33) |
100 (27.62) |
| Unidentifiable |
13 (0.54) |
3 (0.15) |
10 (2.76) |
| Unknown |
51 (2.14) |
24 (1.19) |
27 (7.46) |
| Anticoagulation other than rivaroxaban for the index PE/DVT (initial treatment only) |
| No |
– |
1,563 (77.19) |
– |
| Yes |
– |
462 (22.81) |
– |
| Anticoagulation in the 3 months before initiation of rivaroxaban (maintenance treatment only) |
| No |
– |
– |
126 (34.81) |
| Yes |
– |
– |
236 (65.19) |
| Other anticoagulants used |
| Unfractionated heparin |
– |
396 (19.56) |
102 (28.18) |
| Fondaparinux |
– |
2 (0.10) |
0 (0.00) |
| Warfarin |
– |
44 (2.17) |
111 (30.66) |
| Other |
– |
134 (6.62) |
67 (18.51) |
| Use of antiplatelet drugse |
| No |
2,180 (91.33) |
1,861 (91.90) |
319 (88.12) |
| Yes |
207 (8.67) |
164 (8.10) |
43 (11.88) |
Data are presented as n (%) unless otherwise stated. Percentages are based on the number of patients in the safety analysis population (N=2,387), initial treatment group (n=2,025), or maintenance treatment group (n=362). “−”=not applicable or not recorded. aRisk factors reported in ≥10% of total patients are listed. bDiseases or conditions such as hemostasis or coagulation disorders, congenital or acquired hemorrhagic disorders, uncontrollable severe hypertension, vascular retinopathy, active cancer, active ulcerative gastrointestinal disorders, shortly after the onset of gastrointestinal ulcers, shortly after the onset of intracranial hemorrhage, vascular abnormalities in the spinal cord or brain, shortly after cerebral spinal cord or eye surgery, and history of bronchiectasis or pulmonary hemorrhage. cClassified on the basis of information from lower extremity venous ultrasonography and computer tomography (lower extremity). dThrombus in iliac, femoral, or popliteal veins. eDuring the observation periods. BMI, body mass index; CrCl, creatinine clearance; DVT, deep vein thrombosis; IDDVT, isolated distal deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation; VTE, venous thromboembolism.
The duration in days of rivaroxaban treatment, including periods of treatment interruption, is summarized in Table 2. More than 80% of patients were prescribed the approved dosage of rivaroxaban (30 mg/day for initial treatment and 15 mg/day for maintenance treatment) (Supplementary Table 2). The most frequent reason given for not prescribing 30 mg/day for initial treatment or 15 mg/day for maintenance treatment was being judged as “elderly” by the attending physician (Supplementary Table 2).
Table 2. Rivaroxaban Treatment Periods (Safety Analysis Population, N=2,387)
| Treatment duration, days |
Total
(N=2,387) |
Purpose of rivaroxaban administration |
Initial treatment
(n=2,025) |
Maintenance treatment
(n=362) |
| Mean (SD) |
295.5 (292.6) |
285.5 (288.1) |
351.4 (311.1) |
| Median (25th–75th percentile) |
168.0 (68.0–482.0) |
159.0 (66.0–434.0) |
247.0 (73.0–714.0) |
The rivaroxaban treatment period included periods of treatment interruption. SD, standard deviation.
The duration in days of rivaroxaban 30 mg/day treatment in the initial treatment group is summarized in Supplementary Table 2. At the end of the observation period, in the SAP 802 patients (33.60%) had continued rivaroxaban treatment, 472 (19.77%) did not return to the study site (e.g., transferred to another hospital), and 1,113 patients (46.63%) had discontinued or completed rivaroxaban treatment. The reasons for discontinuation or termination of rivaroxaban treatment included completion of treatment (706 patients [29.58%]), adverse events (including death; 266 patients [11.14%]), and patient request (52 patients [2.18%]); more than one reason per patient could be selected in the survey.
Safety and Effectiveness Outcomes
Safety (treatment duration and bleeding events) and effectiveness (treatment duration and recurrence of symptomatic PE/DVT or PE/DVT) outcomes for the overall SAP and EAP, and for patients receiving initial or maintenance treatment are presented in Table 3. Major bleeding during rivaroxaban treatment period was reported in 69 patients (2.89%; 3.60%/patient-year [95% CI, 2.75–4.45%]), 62 patients (3.06%; 3.95%/patient-year [95% CI, 2.97–4.94%]), and 7 patients (1.93%; 2.02%/patient-year [95% CI, 0.52–3.52%]) in the overall SAP, the initial treatment group, and the maintenance treatment group, respectively. Recurrence of symptomatic PE and/or DVT during rivaroxaban treatment period was reported in 26 patients (1.09%; 1.36%/patient-year [95% CI, 0.84–1.89%]) in the overall EAP, 24 patients (1.19%; 1.54%/patient-year [95% CI, 0.92–2.15%]) in the initial treatment group, and 2 patients (0.55%; 0.57%/patient-year [95% CI, 0.00–1.37%]) in the maintenance treatment group.
Table 3. Safety and Effectiveness Outcomes During Rivaroxaban Treatment Periods
Safety outcomes
(safety analysis population, N=2,387)a |
Total
(N=2,387) |
Purpose of rivaroxaban administration |
Initial treatment
(n=2,025) |
Maintenance treatment
(n=362) |
| Treatment duration, days, mean (SD) |
295.5 (292.6) |
285.5 (288.1) |
351.4 (311.1) |
| Any bleeding |
199 (8.34) |
176 (8.69) |
23 (6.35) |
| Major bleeding |
69 (2.89) |
62 (3.06) |
7 (1.93) |
| Intracranial hemorrhage |
13 (0.54) |
12 (0.59) |
1 (0.28) |
| CRNM bleeding |
83 (3.48) |
71 (3.51) |
12 (3.31) |
| Minor bleeding |
64 (2.68) |
58 (2.86) |
6 (1.66) |
Effectiveness outcomes
(effectiveness analysis population, N=2,386)b |
Total
(N=2,386) |
Purpose of rivaroxaban administration |
Initial treatment
(n=2,024) |
Maintenance treatment
(n=362) |
| Treatment duration, days, mean (SD) |
295.6 (292.6) |
285.6 (288.1) |
351.4 (311.1) |
| Recurrence of PE/DVT |
53 (2.22) |
49 (2.42) |
4 (1.10) |
| Recurrence of symptomatic PE/DVT |
26 (1.09) |
24 (1.19) |
2 (0.55) |
Data are presented as n (%) unless otherwise stated. aPercentages are based on the number of patients in the safety analysis population (N=2,387), initial treatment group (n=2,025), or maintenance treatment group (n=362). bPercentages are based on the number of patients in the effectiveness analysis population (N=2,386), initial treatment group (n=2,024), or maintenance treatment group (n=362). CRNM, clinically relevant nonmajor; DVT, deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation.
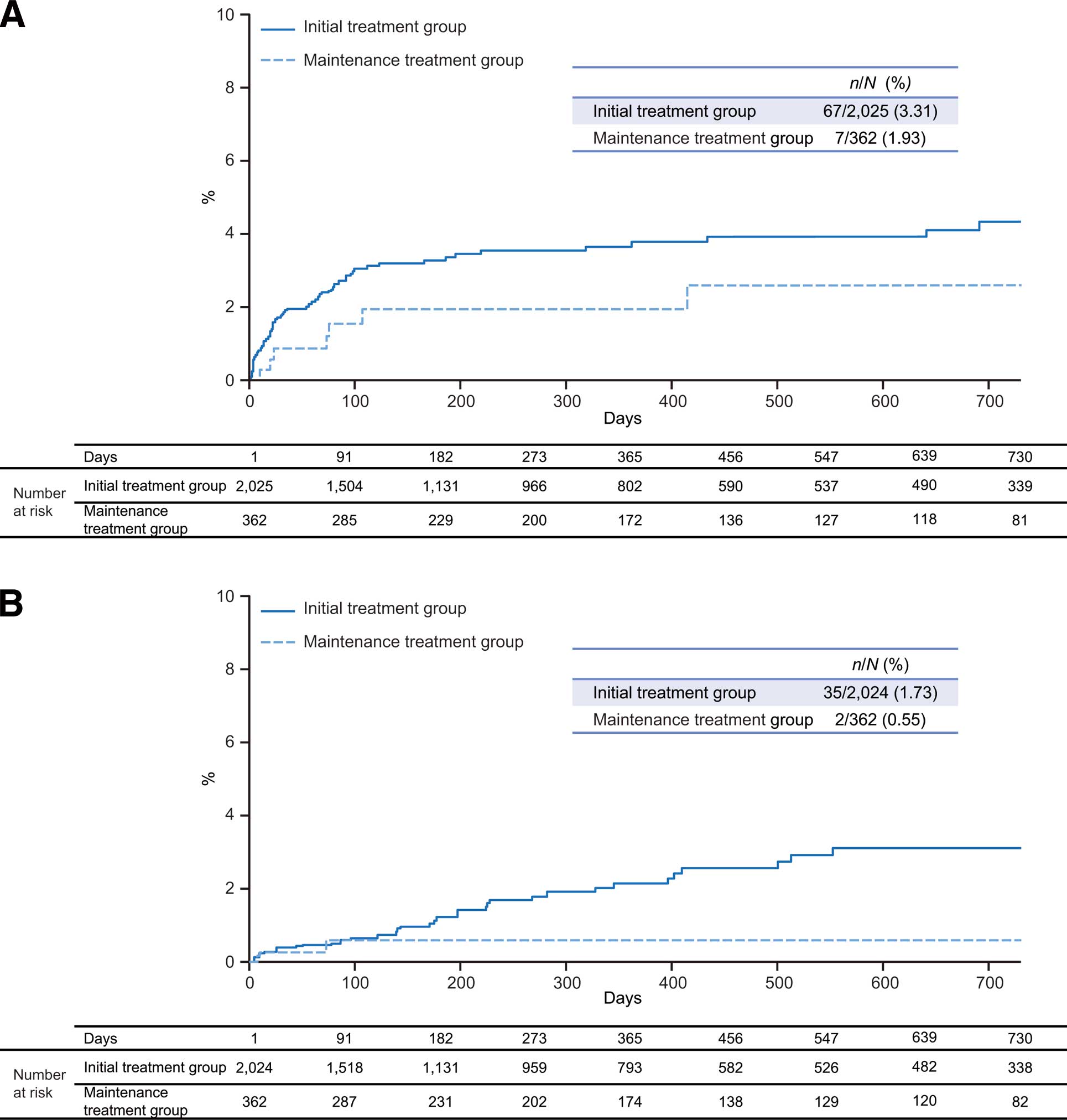
In total, 1,113 patients in the SAP and 1,112 patients in the EAP discontinued or completed rivaroxaban treatment. Of the 1,113 patients in the SAP who discontinued or completed rivaroxaban treatment, major bleeding and any bleeding occurred in 5 patients (0.45%) and 10 patients (0.90%), respectively, during a mean (standard deviation [SD]) observation duration of 89.7 (154.1) days after discontinuation/completion of rivaroxaban treatment. Of the 1,112 patients in the EAP who discontinued or completed treatment with rivaroxaban, recurrence of symptomatic PE/DVT and recurrence of PE/DVT occurred in 10 patients (0.90%) and 21 patients (1.89%), respectively, during a mean (SD) of 89.8 (154.2) days after discontinuation/completion of rivaroxaban. Kaplan-Meier curves for major bleeding and symptomatic PE/DVT recurrence during the study period are shown in Figure 2A and Figure 2B, respectively. In the initial treatment group, 67 patients (3.31%) in the SAP experienced a major bleeding event and 35 patients (1.73%) in the EAP experienced symptomatic PE/DVT recurrence during the study period. Major bleeding was reported in 7 patients (1.93%) and symptomatic PE/DVT recurrence in 2 patients (0.55%) during the study period in the maintenance treatment groups of the SAP and EAP, respectively.
In total, 131 patients (5.49%) died during the study. The most frequent cause of death (75 patients [57.25%]) was “Neoplasms benign, malignant, and unspecified (including cysts and polyps)”. Deaths due to adverse drug reactions (hemorrhagic cerebral infarction and intraperitoneal bleeding) were reported in 2 patients, and PE or suspicious PE was reported as the cause of death in 3 patients.
Safety and effectiveness outcomes stratified by VTE diagnosis, age, body weight, creatinine clearance, presence or absence of active cancer, and antiplatelet use are presented in Table 4; there were increased bleeding events and numerically similar VTE recurrence events in patients with cancer compared with patients without cancer.
Table 4. Safety and Effectiveness Outcomes During Rivaroxaban Treatment Periods in Subgroups
Safety outcomes
(safety analysis population, N=2,387)a |
|
n |
Major bleeding,
n (%) |
Any bleeding,
n (%) |
| VTE diagnosisb |
PE±DVT |
1,187 |
35 (2.95) |
100 (8.42) |
| DVT only |
1,199 |
33 (2.75) |
98 (8.17) |
| IDDVTc |
409 |
10 (2.44) |
32 (7.82) |
| Proximal DVTc,d |
694 |
20 (2.88) |
55 (7.93) |
| Age, years |
<75 |
1,548 |
46 (2.97) |
125 (8.07) |
| ≥75 |
839 |
23 (2.74) |
74 (8.82) |
| Body weight, kg |
>50 |
1,777 |
51 (2.87) |
149 (8.38) |
| ≤50 |
526 |
17 (3.23) |
43 (8.17) |
| CrCl, mL/min |
≥50 |
1,944 |
57 (2.93) |
156 (8.02) |
| <50 |
351 |
11 (3.13) |
34 (9.69) |
| Active cancer |
No |
1,981 |
46 (2.32) |
146 (7.37) |
| Yes |
406 |
23 (5.67) |
53 (13.05) |
| Antiplatelet usee |
No |
2,180 |
64 (2.94) |
177 (8.12) |
| Yes |
207 |
5 (2.42) |
22 (10.63) |
Effectiveness outcomes
(effectiveness analysis population, N=2,386)a |
|
n |
Recurrence of
symptomatic
PE/DVT, n (%) |
Recurrence of
PE/DVT, n (%) |
| VTE diagnosis |
PE±DVT |
1,187 |
13 (1.10) |
29 (2.44) |
| DVT only |
1,199 |
13 (1.08) |
24 (2.00) |
| IDDVTc |
409 |
4 (0.98) |
6 (1.47) |
| Proximal DVTc,d |
694 |
8 (1.15) |
16 (2.31) |
| Age, years |
<75 |
1,547 |
22 (1.42) |
36 (2.33) |
| ≥75 |
839 |
4 (0.48) |
17 (2.03) |
| Body weight, kg |
>50 |
1,777 |
20 (1.13) |
39 (2.19) |
| ≤50 |
525 |
4 (0.76) |
11 (2.10) |
| CrCl, mL/min |
≥50 |
1,943 |
22 (1.13) |
45 (2.32) |
| <50 |
351 |
2 (0.57) |
5 (1.42) |
| Active cancer |
No |
1,980 |
23 (1.16) |
44 (2.22) |
| Yes |
406 |
3 (0.74) |
9 (2.22) |
| Antiplatelet usee |
No |
2,179 |
22 (1.01) |
47 (2.16) |
| Yes |
207 |
4 (1.93) |
6 (2.90) |
Data are presented as n (%). aPercentages are based on the number of patients in each specified category. bOne patient in the safety analysis population did not have a diagnosis of PE/DVT at baseline. cClassified on the basis of information from lower extremity venous ultrasonography and computer tomography (lower extremity) in patients with diagnosed DVT only. dThrombus in iliac, femoral, or popliteal veins. eDuring the observation period. Abbreviations as in Table 1.
Safety and effectiveness outcomes in patients who were prescribed an initial dosage of rivaroxaban 30 mg/day in the initial treatment group are presented in Supplementary Table 3.
Discussion
XASSENT enrolled >2,500 patients with VTE being treated with rivaroxaban in real-world settings in Japan, and our analysis demonstrated the patient characteristics and the safety profile and effectiveness of rivaroxaban for the treatment of VTE in both the overall and in various patient subgroups. A relatively large number of patients was included in XASSENT, which would enable more reliable estimation of incidences of infrequent clinical events such as major bleeding and symptomatic PE/DVT; therefore, it is expected that the results provided by the present study will be important for informing decisions on rivaroxaban treatment in groups of patients highlighted in the study. Overall, no new safety or effectiveness concerns for rivaroxaban were found in XASSENT.
Similar to in other Japanese registries, such as the COMMAND VTE Registry,8 the patient population in XASSENT was older, had lower body weight, and had a higher proportion of patients with impaired renal function compared with those in the global trials or registries such as EINSTEIN PE/DVT or XALIA.6,13 As such, the XASSENT patient population was highly representative of the general Japanese population of patients with VTE, thereby ensuring the applicability of the results to routine clinical practice.
The lower proportion of patients with blood type O that was observed in XASSENT compared with the general Japanese population is consistent with previous studies in which the O blood type is associated with a lower risk of VTE.14,15 Owing to the difficulty in performing multivariate analysis because of the large number of patients with unknown blood type, it was not possible to determine if the ABO blood type affected clinical outcomes such as bleeding and VTE recurrence. Further studies will be needed to clarify the association of ABO blood type and the risk of bleeding and/or recurrent VTE in patients with VTE in Japan.
In XASSENT, the proportion of patients who were prescribed the approved dose of rivaroxaban for patients in Japan (>80%) was higher than that reported in the J’xactly study, in which the proportion of patients administered the initial dosage of 30 mg/day was 65.6%.9 For those patients in XASSENT not prescribed 30 mg/day for initial treatment or 15 mg/day for maintenance treatment, the most frequent reason provided in the survey was being “elderly”.
As shown by the Kaplan-Meier curves, there was a tendency for major bleeding to occur towards the start of rivaroxaban treatment, and there was a constant increase in symptomatic VTE recurrence throughout the study periods in the initial treatment group.
Compared with results from EINSTEIN PE/DVT, in XASSENT the proportion of clinically relevant nonmajor bleeding during the treatment period was numerically lower (3.5% vs. 8.6%) and that of major bleeding during the treatment period was numerically higher (2.9% vs. 1.0%),6 while the proportion of patients experiencing major bleeding was numerically lower than that reported in the COMMAND VTE Registry (≈6% [187/3,027] at 1 year; 187 represents the sum of the number of patients with event in the transient risk, unprovoked, and cancer groups at 1 year).8 Overall, in the XASSENT effectiveness analysis, recurrent symptomatic VTE during the treatment period was reported in 1.1% of patients compared with 2.1% in the pooled results from the EINSTEIN PE/DVT studies, 4.2% (2.6%/patient-year) in J’xactly, and 4.8% (144/3,027; 144 represents the sum of the number of patients with event in the transient risk, unprovoked, and cancer groups at 1 year) in COMMAND VTE.6,8,9 However, comparisons of data between trials are difficult because of differences in factors such as study design, patient backgrounds, and observation duration, and should therefore be made with caution.
Although careful interpretation is needed because of confounding variables such as dose selection by attending physicians, the proportions of bleeding and PE/DVT recurrence in patients aged ≥75 years, patients with a body weight of ≤50 kg, or patients with creatinine clearance <50 mL/min were highlighted in the subgroup analyses in XASSENT. The pooled EINSTEIN PE/DVT analysis demonstrated that, compared with standard therapy, rivaroxaban had similar efficacy and was associated with a significantly lower rate of major bleeding in patients aged >75 years, patients with a body weight ≤50 kg, or patients with a creatinine clearance <50 mL/min.6 The real-world evidence provided by XASSENT and the EINSTEIN PE/DVT analyses add to the information supporting the use of rivaroxaban as an option for VTE treatment in patients who are elderly, of low body weight, or with impaired renal function.
XASSENT also included patients with IDDVT (including asymptomatic IDDVT), a condition that has not been well represented to date in phase 3 trials. Although the number of patients with IDDVT who undergo anticoagulation therapy in clinical practice is moderately high (17% in XASSENT), evidence for the treatment of IDDVT is limited compared with that for proximal DVT, and individualized anticoagulation for patients with IDDVT is recommended in guidelines.16 The results from XASSENT show rivaroxaban treatment patterns and associated outcomes for patients with IDDVT in Japan during the study period. Although low, the incidence of symptomatic VTE recurrence in patients with IDDVT indicated a non-negligible risk of VTE in these patients. These results support the use of anticoagulation therapy for some patients with IDDVT; it is anticipated that further evidence from future, well-controlled studies will help to inform the guideline treatment strategy for this population.
At 17%, the proportion of patients with active cancer at baseline in XASSENT was much higher than in global trials,17 and broad range of cancer types were included. Consistent with results from previous studies, including Japanese real-world evidence,8,17,18 a numerically higher proportion of bleeding events was observed in XASSENT in patients with active cancer than in those without it, indicating that careful treatment is needed for patients with cancer-associated VTE. In contrast, although previous studies have shown an increase in VTE recurrence in patients with cancer,8,17,18 in XASSENT the incidence of VTE recurrence was numerically similar between patients with and without cancer. Taken together with the results from SELECTD, which showed a relatively low rate of VTE recurrence in patients with cancer who were treated with rivaroxaban compared with that in patients with cancer treated with dalteparin,19 the results from XASSENT suggest that rivaroxaban may be a valuable addition to the treatment options for the prevention of VTE recurrence in patients with cancer.
Study Limitations
There were some limitations to this study,10 such as the possibility of selection bias, confounding variables (e.g., dose selection by attending physicians), and loss of patients to follow-up that may have led to underestimation of incidence of clinical events, and a lack of mandatory laboratory tests. Although guidelines provided recommendations for PE/DVT diagnosis methods20 and the diagnosis methods used and clinical presentations (e.g., symptoms, site of occurrence) were recorded on case report forms, there were no formal diagnostic methods or criteria for PE/DVT in the study and central outcome adjudication was not performed. In addition, source data verification was not performed in the study, because it is not required under the Good Post-marketing Study Practice in Japan. The study did not have sufficient power to evaluate rare events. Furthermore, comparison of clinical outcomes in XASSENT that resulted from rivaroxaban treatment with those resulting from other treatments is difficult and should therefore be made with caution owing to the single-arm design.
Conclusions
The incidence of VTE in Japan has been increasing in recent years, and there are some differences in the patients’ characteristics between Japanese clinical practice and global trials. XASSENT provides additional valuable information on treatment patterns in patients prescribed rivaroxaban for the treatment of VTE and prevention of its recurrence in Japanese clinical practice. No new safety or effectiveness concerns for rivaroxaban were found. It is anticipated that the results of the safety and effectiveness outcomes in XASSENT will help to inform decision-making when considering rivaroxaban treatment in Japanese clinical practice.
Acknowledgments
The authors thank the patients who took part in XASSENT and Dr. Norifumi Nakanishi for his contribution in conducting this study. In addition, the authors thank Fujitsu Japan Ltd., Tokyo, Japan, for the Electronic Data Capture system and electronic Case Report Form setup, and the EPS Corporation, Tokyo, Japan, for data management and analysis. The authors also thank Jim Purvis, PhD, of Oxford PharmaGenesis, Oxford, UK, for providing medical writing support in accordance with Good Publication Practice 3 (GPP3) guidelines (https://www.ismpp.org/gpp3). Editorial support was provided by Oxford PharmaGenesis, Oxford, UK.
Conflicts of Interest
I.F. has received payment or honoraria from Bayer Yakuhin, Ltd., Daiichi Sankyo Co. Ltd., and Pfizer. A.H. has received payment or honoraria from Bayer Yakuhin, Ltd. and Daiichi Sankyo Co. Ltd. K.K., T.K., H.M., M.N., and N.Y. declare no conflicts of interest. T.T., M.T., Y.O., T.S., K.H., and T.H. are employees of Bayer Yakuhin, Ltd.
Funding
XASSENT was funded by Bayer Yakuhin, Ltd., Osaka, Japan. Medical writing and editorial support were funded by Bayer Yakuhin, Ltd., Osaka, Japan.
Ethics
XASSENT met all local legal and regulatory requirements and was conducted in accordance with standards for Good Post-Marketing Study Practice provided by the Ministry of Health, Labour and Welfare in Japan, and was reviewed by Pharmaceuticals and Medical Devices Agency. Separate ethics approval and written informed consent from patients for this study were not required under Japanese regulations, but were obtained when required by a participating center.
Disclosures
A.H. is a member of Circulation Journal’s Editorial Team.
Author Contributions
I.F., A.H., K.K., T.K., H.M., M.N., N.Y., Y.O., and T.S. contributed to developing the protocol, study execution, data analysis, interpretation of results, and editing the manuscript. T.T., M.T., K.H., and T.H. contributed to data analysis, interpretation of the results, and drafting/editing the manuscript. The manuscript has been read and approved for submission by all authors.
Data Availability
Deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0104
References
- 1.
Wendelboe AM, Raskob GE. Global burden of thrombosis: Epidemiologic aspects. Circ Res 2016; 118: 1340–1347.
- 2.
Nakamura M, Yamada N, Ito M. Current management of venous thromboembolism in Japan: Current epidemiology and advances in anticoagulant therapy. J Cardiol 2015; 66: 451–459.
- 3.
Bayer Yakuhin, Ltd. Xarelto 10 mg/15 mg package insert. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/630004_3339003F1024_1_21 (accessed April 6, 2022).
- 4.
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510.
- 5.
Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297.
- 6.
Prins MH, Lensing AWA, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J 2013; 11: 21.
- 7.
Yamada N, Hirayama A, Maeda H, Sakagami S, Shikata H, Prins MH, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism: The J-EINSTEIN DVT and PE program. Thromb J 2015; 13: 2.
- 8.
Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, et al. Anticoagulation therapy for venous thromboembolism in the real world: From the COMMAND VTE registry. Circ J 2018; 82: 1262–1270.
- 9.
Okumura Y, Fukuda I, Nakamura M, Yamada N, Takayama M, Maeda H, et al. A multicenter prospective observational cohort study to investigate the effectiveness and safety of rivaroxaban in Japanese venous thromboembolism patients (the J’xactly study). Circ J 2020; 84: 1912–1921.
- 10.
Fukuda I, Hirayama A, Kawasugi K, Kobayashi T, Maeda H, Nakamura M, et al. Design and baseline data for a prospective observational study of rivaroxaban in patients with venous thromboembolism in Japan (XASSENT). TH Open 2021; 5: e521–e532.
- 11.
Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694.
- 12.
Maeda K, Nakamura S, Murakami C, Irie W, Watanabe T, Sasaki C, et al. ABO genotyping by TaqMan assay and allele frequencies in a Japanese population. Leg Med (Tokyo) 2013; 15: 57–60.
- 13.
Haas S, Mantovani LG, Kreutz R, Monje D, Schneider J, Zell ER, et al. Anticoagulant treatment for venous thromboembolism: A pooled analysis and additional results of the XALIA and XALIA-LEA noninterventional studies. Res Pract Thromb Haemost 2021; 5: 426–438.
- 14.
Englisch C, Moik F, Nopp S, Raderer M, Pabinger I, Ay C. ABO blood group type and risk of venous thromboembolism in patients with cancer. Blood Adv 2022; 6: 6274–6281.
- 15.
Sugiura K, Kobayashi T, Ojima T. The epidemiological characteristics of thromboembolism related to oral contraceptives in Japan: Results of a national survey. J Obstet Gynaecol Res 2021; 47: 198–207.
- 16.
Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest 2021; 160: e545–e608.
- 17.
Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): A pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014; 1: e37–e46.
- 18.
Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100: 3484–3488.
- 19.
Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol 2018; 36: 2017–2023.
- 20.
JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J 2011; 75: 1258–1281.



