Article ID: CJ-23-0229
Article ID: CJ-23-0229
Background: High shock impedance is associated with conversion failure among patients with subcutaneous implantable cardioverter defibrillators (S-ICD). Currently, there is no preoperative assessment method for predicting high shock impedance. This study examined the efficacy of chest computed tomography (CT) as a preoperative evaluation tool to assess the shock impedance of S-ICDs.
Methods and Results: The amount of adipose tissue adjacent to the device and anteroposterior diameter at the basal heart region were measured preoperatively using chest CT. We examined the correlation between these measurements and shock impedance at the conversion test. We enrolled 43 patients with S-ICDs (mean [±SD] age 54±15 years; body mass index 23±4 kg/m2; PRAETORIAN score 30–270 points; amount of adipose tissue 1,250±716 cm3), who underwent intraoperative conversion tests by inducing ventricular fibrillation, which was terminated with a 65-J shock. A sufficient concordance correlation coefficient was observed between the shock impedance and the amount of adipose tissue (r=0.616, P<0.01) and anteroposterior diameter (r=0.645, P<0.01). In multiple regression analysis, the amount of adipose tissue (β=0.439, P=0.009) and anteroposterior diameter (β=0.344, P=0.038) were identified as independent predictive factors of shock impedance.
Conclusions: The preoperative CT-measured amount of adipose tissue and basal heart anteroposterior diameter are independent predictors of shock impedance. These parameters may be more accurate in identifying higher shock impedance in patients with S-ICDs.
A subcutaneous implantable cardioverter defibrillator (S-ICD) is as effective as a transvenous implantable cardioverter defibrillator (TV-ICD) in preventing sudden cardiac death by terminating life-threatening ventricular arrhythmias without endovascular lead placement.1,2 For patients with S-ICDs, a higher intraoperative shock impedance is associated with a high defibrillation threshold (DFT) and conversion failure.3 There is a risk for lower shock efficacy, specifically when the intraoperative shock impedance exceeds 100 Ω.4 Therefore, periprocedural conversion testing is recommended.4 Previously, a computer modeling study showed that subcoil and subgenerator fat affects shock impedance because adipose tissue increases impedance and reduces the effective current.5 That study also demonstrated that combining the anterior generator position with subcoil fat resulted in a high DFT.5 The PRAETORIAN score was reported to identify patients with high DFT during postoperative assessment using the above findings. The PRAETORIAN score is calculated using body mass index (BMI), the generator position along the anteroposterior axis, and the coil and generator width measurements on postoperative chest radiographs, assuming the amount of adipose tissue around the device.6 Currently, there are no studies for the preoperative assessment of patients with high shock impedance. Since the adipose tissue measurement method on computed tomography (CT) was reported in 1983,7 various studies on metabolic risk factors have successfully used it,8,9 and CT has been able to distinguish visceral fat from subcutaneous fat at the measurement site. Thus, the aim of the present study was to evaluate the efficacy of chest CT as a preoperative tool to assess the shock impedance of an S-ICD.
All consecutive patients undergoing S-ICD implantation (Boston Scientific Inc., Natick, MA, USA) at St. Marianna University Hospital and Yokohama City Seibu Hospital between 2016 and 2021 were identified retrospectively. The medical records of all identified patients were manually reviewed, and patients with preoperative chest CT and intraoperative DFT tests were included in the present study.
The institutional review boards approved the study, which was conducted in accordance with the Declaration of Helsinki.
S-ICD Implantation ProcedureS-ICD implantation was performed on patients under general anesthesia. The lead was deployed using the 2- or 3-incision technique without any preprotocol indications. The device was placed in the intramuscular plane. Before placing the final pocket suture, conversion testing was performed. Ventricular fibrillation (VF) was induced with a 50-Hz transthoracic burst pacing. The first shock energy of 65 J in direct polarity was attempted to terminate the induced VFs. If the defibrillation was unsuccessful, a second shock of 80 J was delivered, followed by external rescue shocks.
Measurement of Adipose Tissue and Basal Heart Diameter on Chest CTPatients’ chest CT scans were obtained with a 64-row spiral CT (Aquillion PRIME; Canon Medical Systems Corporation, Otawara, Japan) in the supine position a mean (±SD) of 27±10 days before S-ICD implantation. All chest CT images were retrospectively reviewed using a postprocessing workstation (Ziostation2, Version 2.4.3.3; Ziosoft Inc., Tokyo, Japan). The amount of adipose tissue was measured from the base of the heart to the most superior portion of the sternum. Adipose tissue was identified as follows: (1) the region was visually identified as surrounded by the most superior portion of the sternum, the base of the heart, and the right sternal border; (2) CT numbers for fat tissue were identified using a window level of −100 HU and a width of 100 HU, and only fat tissue was extracted; and (3) mediastinal fat tissue was manually removed. In addition, the anteroposterior diameter of the basal heart region in the sagittal view and the lateral fat depth, defined as the transverse diameter of adipose tissue at the fifth rib level in the coronal view, were measured (Figure 1).
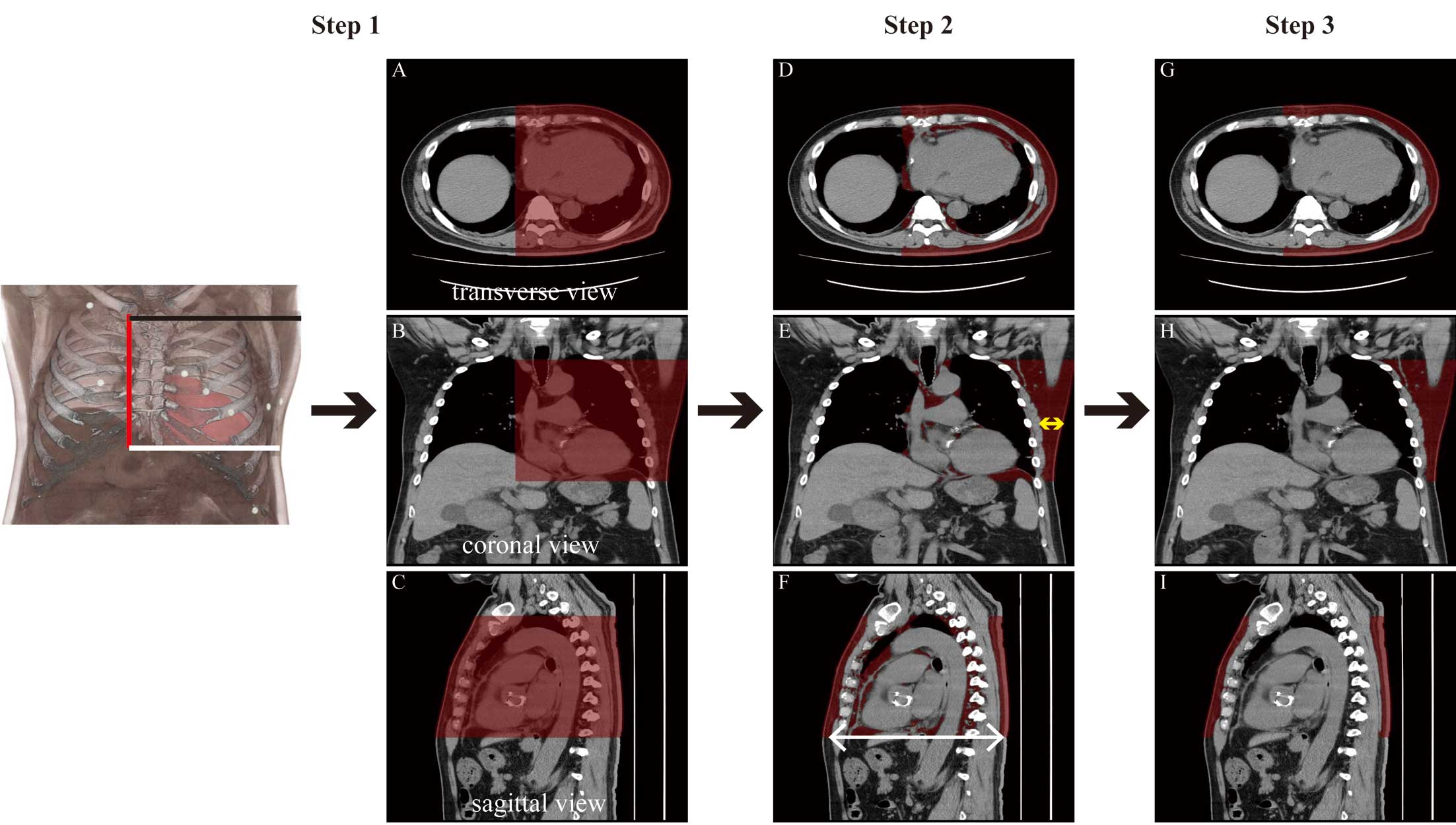
Assessment flow of measurements on chest computed tomography (CT) in a representative patient. First, the amount of adipose tissue is determined. Step 1: areas involved in shock therapy, which are surrounded by the most superior portion of the sternum (black line), the base of the heart region (white line), and the right sternal border (red line), are visually extracted (Left panel). The red shaded area in each CT view (A–C) is the selected area. Step 2: fat tissue on CT was identified as that corresponding to −50 to −150 HU, and only fat tissue was extracted (D–F, red areas). Step 3: Mediastinal fat tissue is manually removed (red area in G–I, red areas). Second, the basal heart anteroposterior diameter is measured in the sagittal view (F, white arrow). Third, lateral fat depth was determined as the measurement of the transverse diameter of the adipose tissue at the level of the 5th rib in the coronal view (E, yellow arrow).
Postoperative Assessment
Postoperative posterior-anterior and lateral chest radiography were analyzed to calculate the PRAETORIAN score, as described previously.6,10 Briefly, the thickness of the adipose tissue between the coil and the sternum was assessed (from 30 to 150 points), and the posterior or anterior positioning of the generator was determined in relation to the mid-line (multiplication factor ranging from ×1 to ×4). The adipose tissue between the generator and the thorax was assessed using the width of the generator as a reference (multiplication factor ranging from ×1 to ×1.5). In patients with a BMI ≤25 kg/m2, 40 points were subtracted in the case of scores of ≥90. The final score indicated the risk of conversion failure according to the following cut-off values: <90 points, low risk; ≥90 to <150 points, intermediate risk; and ≥150 points, high risk.6,10 In addition, electrode coil depth was assessed via lateral images, based on the distance between the lead and the sternum using the width of the coil (3-mm diameter) as 1 U.11
Statistical AnalysisContinuous data are expressed as the mean±SD for a normal distribution or as the median with interquartile range (IQR) for a skewed distribution. Dichotomous data are expressed as numbers and percentages. The Shapiro-Wilk test was used to determine data distribution. Comparisons of continuous unpaired data were made using Student’s t-test. Analysis of variance (ANOVA) was used for comparisons between 3 groups. Correlations between continuous variables were calculated using Pearson’s or Spearman’s correlation coefficient. Subsequently, multiple stepwise regression analyses were performed to assess the relative importance of each independent variable as a predictor of shock impedance. Two-tailed P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 22.0; SPSS, Inc., Chicago, IL, USA).
During the study period, 43 patients with S-ICD (mean age 54±15 years, 88% men) were eligible for inclusion in this study. The baseline characteristics of the patients are summarized in Table 1. Among the 43 patients, 11 had left ventricular systolic dysfunction related to cardiomyopathy. Most patients had a preserved left ventricular ejection fraction (LVEF) and the mean BMI was 22.9±3.9 kg/m2. Thirty percent of patients had an implant placed for primary prevention. The 2-incision technique deployed the lead in 32 patients. Based on postoperative chest radiographs, S-ICD generators were placed on or posterior to the midline in 22 (51.2%) patients and anterior to the midline in 21 (48.8%) patients, and the median PRAETORIAN score was 60 points. Of note, in all patients, the electrode positions were above the xiphoid, and the generator positions were above the 6th intercostal space. The mean amount of adipose tissue measured on the CT scan was 1,250±716 cm3, and the mean anteroposterior diameter at the basal heart region was 22.3±3.0 cm (Table 1).
Baseline Characteristics (n=43)
| Age (years) | 54.0±14.7 |
| Male sex | 39 (88.4) |
| BMI (kg/m2) | 22.9±3.94 |
| Etiology | |
| Idiopathic VF | 10 (23.3) |
| HCM | 1 (2.33) |
| CAD/ICM | 15 (44.1) |
| Others | 17 (39.5) |
| Primary prevention | 13 (30.2) |
| Two-incision technique | 32 (74.4) |
| LVEF (%) | 50.5±17.4 |
| Amiodarone | 4 (9.3) |
| PRAETORIAN score | 60 [30–270] |
| Generator position | |
| On or posterior to the midline | 22 (51.2) |
| Anterior to the midline | 21 (48.8) |
| >1/2 length anterior | 0 (0) |
| CT scan | 36 (83.7) |
| Amount of adipose tissue (cm3) | 1,250±716 |
| Anterior-posterior diameter (cm) | 22.3±3.0 |
| Shock impedance (Ω) | 56.4±14.7 |
Data are given as the mean±SD, median [interquartile range], or n (%). BMI, body mass index; CAD, coronary artery disease; CT, computed tomography; HCM, hypertrophic cardiomyopathy; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; VF, ventricular fibrillation.
Shock Impedance and Clinical Factors
The induced VF in all patients enrolled in this study was successfully terminated with a 65-J shock, including 3 patients with PRAETORIAN scores >150 points. The mean shock impedance was 56.4±14.7 Ω, and there was no difference in shock impedance between men and women (57.0±8.3 vs. 56.5±14.9 Ω, respectively; P=0.076). No patients presented a shock impedance >100 Ω. There was a sufficient concordance correlation coefficient between shock impedance and the amount of adipose tissue and (r=0.616, P<0.01), basal heart anteroposterior diameter (r=0.645, P<0.01), and lateral fat depth (r=0.795, P<0.01; Figure 2). However, no significant correlation was found between shock impedance and the PRAETORIAN score (r=0.1, P=0.95; Figure 3).
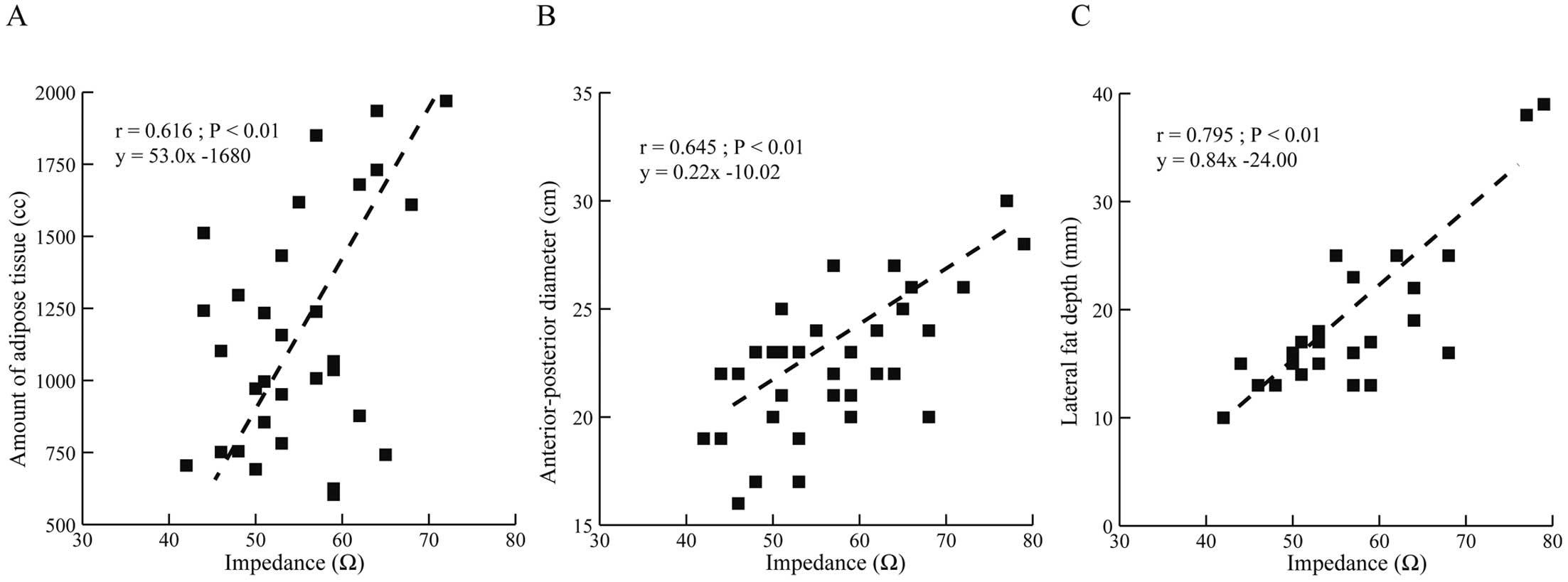
Correlations between computed tomography (CT) measurements and shock impedance: (A) amount of adipose tissue; (B) anterior-posterior diameter; and (C) lateral fat depth. There was a sufficient concordance correlation coefficient between chest CT measurements and shock impedance.
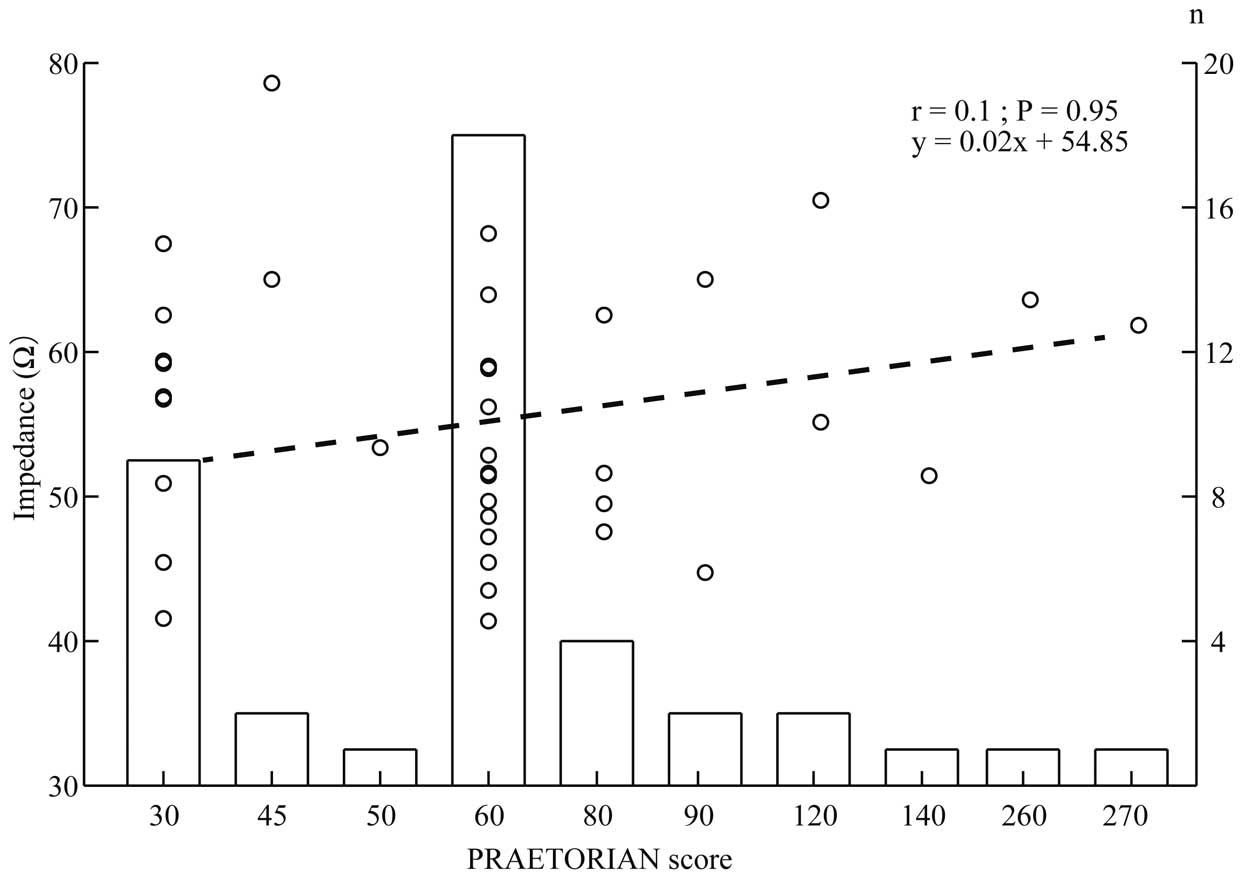
Shock impedance according to the PRAETORIAN score. Open circles show the distribution of shock impedance according to PRAETORIAN score (only 3 patients belonged to the high-risk group [score >150 points], 5 patients were in the intermediate-risk group [score ≥90 to <150 points], and the remaining patients were in the low-risk group [score <90 points]). There was no concordance correlation coefficient between the PRAETORIAN score and shock impedance (dashed line).
We further analyzed the shock impedance according to the postoperative generator position used to calculate the PRAETORIAN score. Compared with the optimal generator position, defined as the generator on or behind the midline, patients with a suboptimal generator position had a similar shock impedance (57.1±8.9 vs. 56.2±9.1 Ω, respectively; P=0.763; Figure 4A). No significant difference in shock impedance was observed with increasing electrode coil depth (0–1 coil, 56.8±9.9 Ω; 2 coils, 53.1±7.6 Ω; >2 coils, 53.0±7.4 Ω; P=0.730; Figure 4B). However, in the case of the amount of fat tissue between the generator and the thorax, there was a significant difference in shock impedance between patients with and without >1 generator width (55.5±81.6 vs. 69.7±8.2 Ω for generator width <1 vs. ≥1, respectively; P<0.006; Figure 4C).
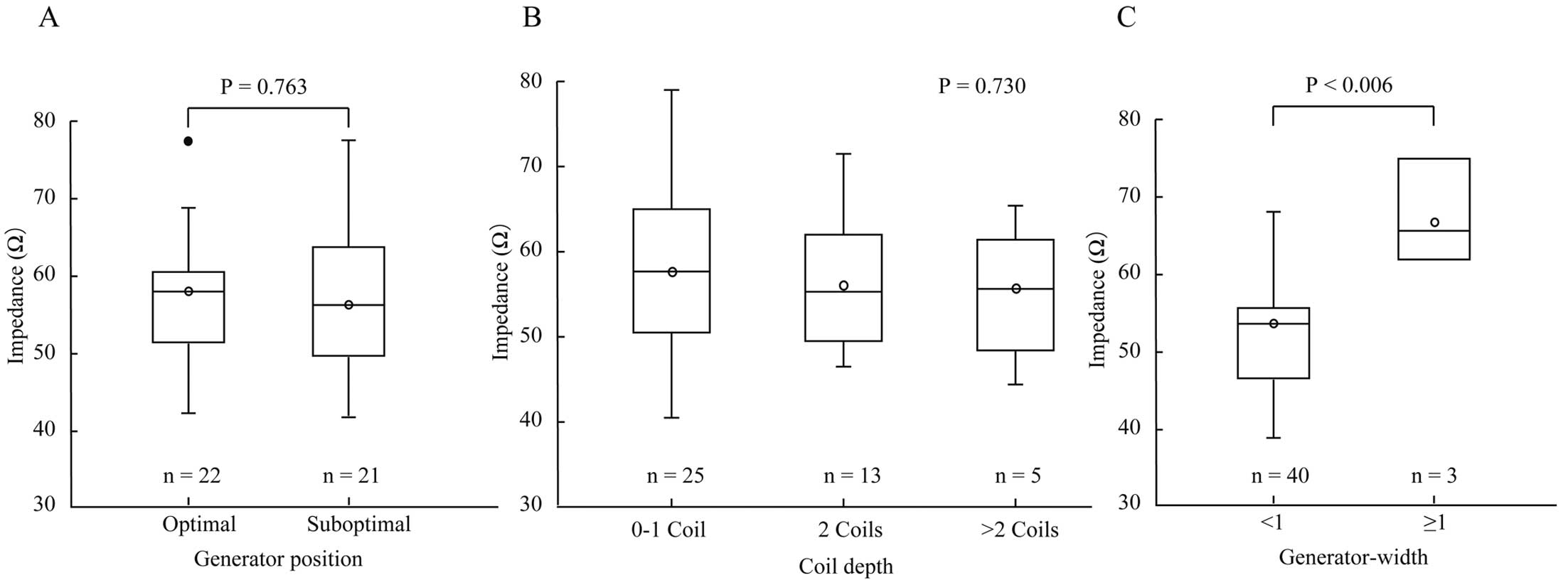
Shock impedance according to findings on postoperative chest radiography: (A) generator position; (B) coil depth, and (C) generator-thorax distance. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. Open circles show mean value, and closed circles show outlier.
With respect to factors related to implant technique, shock impedance was similar between the 2- and the 3-incision techniques (57±9.4 vs. 52±7.0 Ω, respectively; P=0.425). For other clinical factors, BMI showed a moderate correlation with shock impedance (r=0.498, P<0.01; Figure 5). Further detailed analysis revealed a strong concordance correlation coefficient between BMI and the amount of adipose tissue (r=0.735, P<0.01) and basal heart anteroposterior diameter (r=0.606, P<0.01; Figure 6).
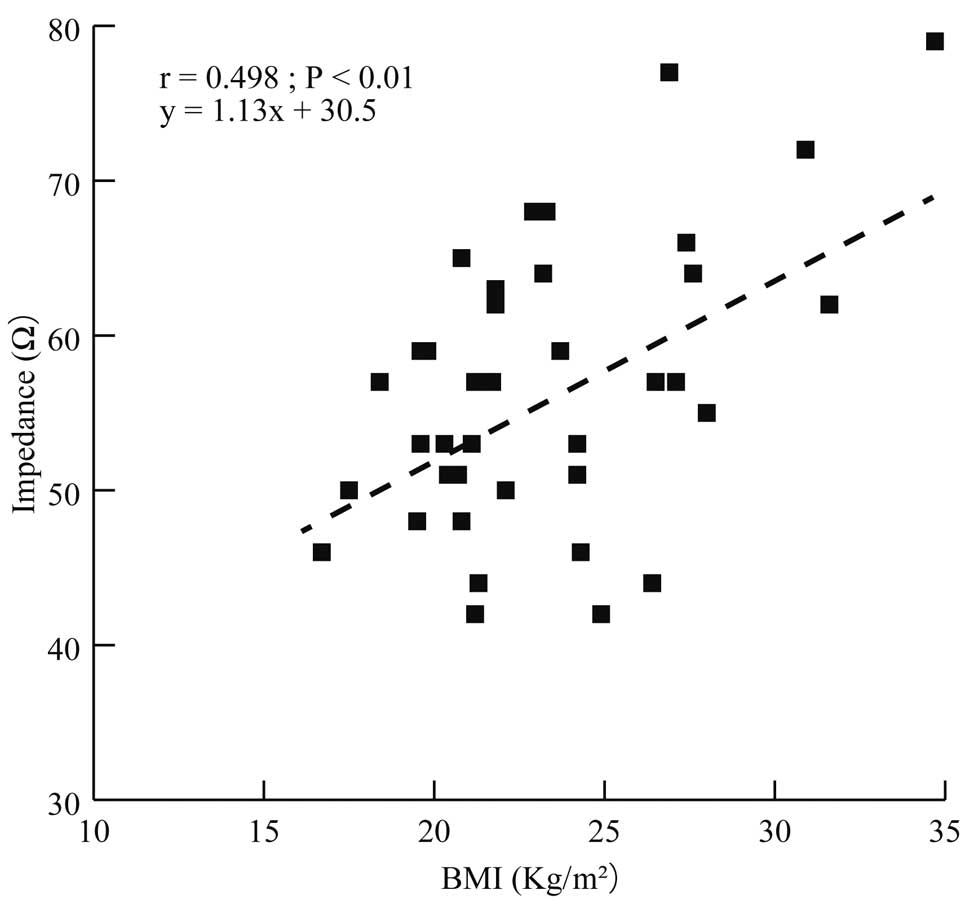
Correlation between shock impedance according to body mass index (BMI). There was a strong concordance correlation coefficient between BMI and shock impedance.
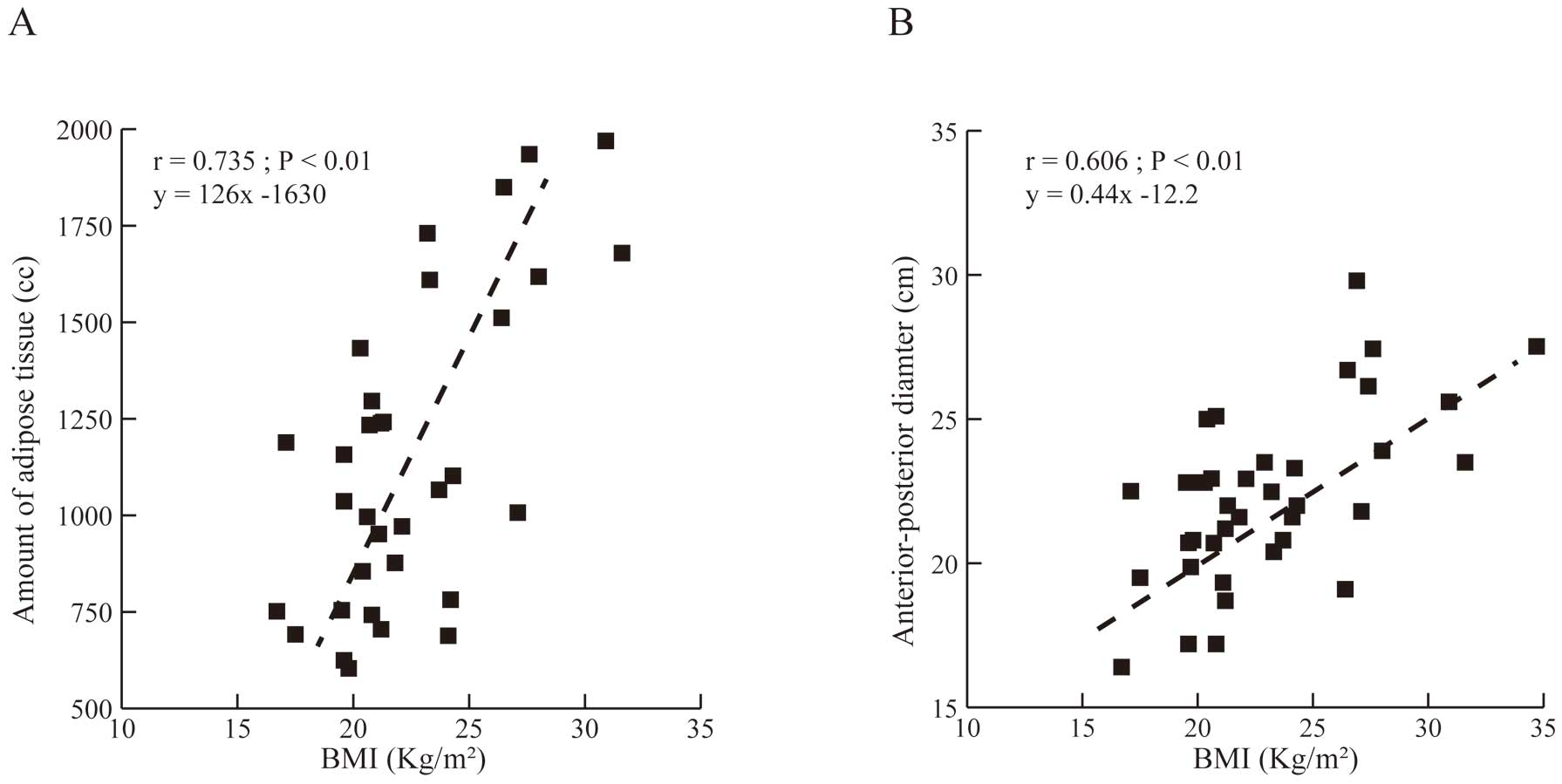
Correlations between body mass index (BMI) and chest computed tomography measurements: (A) amount of adipose tissue and (B) anterior-posterior diameter. There was a strong concordance correlation coefficient between BMI and chest CT measurements.
In multiple stepwise regression analyses to eliminate potential confounders, predictor variables included BMI, CT-determined amount of adipose tissue, basal heart anteroposterior diameter, lateral fat depth, coil depth, suboptimal generator position, and incision technique. In this multivariable modeling, lateral fat depth was no longer significant (β=−0.038, P=0.811), whereas the CT-determined amount of adipose tissue and basal heart anteroposterior diameter remained significant (β=0.439 [P=0.009] and β=0.344 [P=0.038], respectively; Table 2).
Stepwise Regression Analysis of Factors Predicting Shock Impedance in Patients With a Subcutaneous Implantable Cardioverter Defibrillator
| Variables | Unstandardized coefficients | Standardized coefficients | t | P value | 95% CI | ||
|---|---|---|---|---|---|---|---|
| B | SE | β | Lower | Upper | |||
| Constant | 26.299 | 9.609 | 2.737 | 0.01 | |||
| Amount of adipose tissue (cm3) | 0.005 | 0.002 | 0.439 | 2.778 | 0.009 | 0.001 | 0.009 |
| Anterior-posterior diameter (cm) | 1.039 | 0.478 | 0.344 | 2.172 | 0.038 | 0.061 | 2.018 |
| R2 | 0.429 | ||||||
CI, confidence interval; SE, standard error.
System Position and Clinical Factors
Suboptimal device position was defined as coil depth >3 mm, generator width ≥1, the generator anterior to the midline, or coil and generator position being inferior. There was a significant difference in the CT-determined amount of adipose tissue between patients with appropriate device position, defined as the absence of any suboptimal positional variables, and those with suboptimal device position (917±248 vs. 1,345±812 cm3, respectively; P=0.015). However, there were no significant differences between patients with or without appropriate system positioning in basal heart anteroposterior diameter (21.6±1.8 vs. 22.6±3.4 cm, respectively; P=0.14), BMI (21.6±1.6 vs. 23.5±4.3 kg/m2, respectively; P=0.08), or shock impedance (54.5±8.5 vs. 57.3±9.1 Ω, respectively; P=0.19; Supplementary Table).
The main findings of our study on the clinical significance of shock impedance of patients with S-ICD can be summarized as follows: (1) the preoperative CT-measured amount of adipose tissue and basal heart anteroposterior diameter were strongly associated with shock impedance in patients with S-ICDs; (2) the relationship between PRAETORIAN score and shock impedance was not clear; (3) there was a correlation between BMI and shock impedance; and (4) multiple stepwise regression analyses identified the amount of adipose tissue and basal heart anteroposterior diameter as independent predictors of shock impedance. Prior studies have focused primarily on postprocedural chest radiographs to estimate high shock impedance in patients with S-ICDs.6 The present study showed that preoperative CT evaluation could identify patients with higher shock impedance and those with a risk for conversion failure.
Factors Related to Shock ImpedanceIt was reported that shock impedance increases with both subcoil and subgenerator adipose tissue.5 We found that the amount of adipose tissue in the left half of the thorax, including the heart, was strongly related to shock impedance. This study clinically validates such findings. In addition, we showed that the larger the basal heart anteroposterior diameter developed, the greater the shock impedance. This is thought to be due to increased lung volume and subcutaneous adipose tissue, which affects the shock impedance. Based on the findings of our study, we suggest that preoperative direct measurement of the amount of adipose tissue and basal heart anteroposterior diameter may improve the accuracy of identifying higher shock impedance in patients with S-ICDs.
Quast et al reported a positive correlation between BMI and shock impedance and that patients with a failed shock had a significantly higher BMI.6 Our analysis is consistent with that report. The BMI of patients in this study appears to be lower than in previous reports.6 However, there was still a positive correlation between BMI and shock impedance. Moreover, there was a strong concordance correlation coefficient between BMI and measurements made on chest CT. These results suggest that the amount of subcoil/subgenerator adipose tissue or the basal heart anteroposterior diameter may be reflected in BMI. Accordingly, BMI may be a useful clinical predictor of risk for high shock impedance. However, in our analysis, BMI was not an independently associated factor for shock impedance. The amount of subcoil/subgenerator adipose tissue or the basal heart anteroposterior diameter measured on chest CT may be more specific in predicting shock impedance.
The PRAETORIAN score had a limited effect in predicting shock impedance in the present study. The PRAETORIAN score consists of 2 main factors: generator malposition and the amount of subsoil/subgenerator adipose tissue. For generator positioning, an anterior position of the generator would result in the current shunting over the chest wall instead of across the myocardium, increasing defibrillation energy requirements.6 Conversely, as for the shock impedance, when the device is implanted in the anterior position, the coil and generator are physically closer together, which may reduce shock impedance. However, in our analysis, shock impedance was similar between patients with an anterior generator position and those with an optimal generator position. A computer modeling study showed that impedance increased with fat under the coil and/or generator, but was only slightly affected by anterior/posterior generator position.5 For subcoil adipose tissue, Amin et al reported that a greater distance between the lead and sternum was associated with a higher impedance;11 however, our study found no significant association between coil depth and increased impedance. This may be because the electrodes were placed appropriately close to the sternum in most patients, and few patients with greater lead-sternum distances were included. For subgenerator adipose tissue, our study showed that lateral fat depth was significantly correlated with shock impedance, and that greater postoperative generator-thorax distance was associated with higher impedance. These findings suggest that the amount of adipose tissue around the generator may be related to the increase in shock impedance. However, multiple stepwise regression analysis indicated that these factors were possible confounding factors, and CT-measured total fat volume could be a more accurate predictor of shock impedance.
A previous S-ICD study demonstrated that inadequate coil depth and an inferiorly placed pulse generator or electrode system were associated with higher impedance.11 No significant difference in shock impedance was observed in the present study. This may be because our cohort included only a small number of patients with larger lead-to-sternum distances and did not include patients with inferiorly placed S-ICD systems.
Clinical Implications of This StudyThe first clinical implication of this study is that, based on preoperative CT findings, patients with higher shock impedance can be identified before surgery to assist in preoperative strategies, such as appropriate device selection (S-ICD or TV-ICD) or optimal device position. According to recent extensive clinical studies, 90% of patients with S-ICDs could undergo induced VF and successfully defibrillate using a <65-J shock.10,12–14 Conversion testing was successful in most cases. However, a significant proportion of patients (10%) with S-ICDs did not respond to shock therapy. Although the present study does not provide clear cut-off values for the amount of adipose tissue, fat mass, or basal heart anteroposterior diameter, it may be helpful in identifying such high-risk patients preoperatively. Moreover, of the patients requiring ICDs, approximately 4% are clinically unstable, hesitant to undergo shock testing, or unable to have VF induced by transthoracic pacing.15 In such cases, preoperative CT may allow for the prediction of the potential risk of defibrillation failure, and the validity of performing the conversion test in high-risk patients may be warranted.
Furthermore, this study showed that patients with a greater amount of adipose tissue were more likely to have a suboptimal system position. It would be expected that the likelihood of delivering the system to the subcutaneous adipose tissue rather than the fascial plane would be greater in patients with greater amounts of adipose tissue to avoid the risk of increased intraoperative bleeding. Preoperative CT measurements may identify patients at risk for suboptimal postoperative system positioning and alter the preoperative preparation and procedural strategies, such as fluoroscopic guidance during the implant procedure.11
Study LimitationsThis was a retrospective study with a relatively small cohort of patients referred for S-ICD implantation. Because all induced VFs of eligible patients were successfully terminated with a 65-J shock, this study did not include patients with high DFT or conversion failure. No patients presented a shock impedance >100 Ω; therefore, clinically high-risk patients were not included. A recent study has shown that most Japanese patients have a low risk of defibrillation failure with S-ICD,16 and the results of the present study are consistent with the previous report. In addition, no patients had system repositioning. Moreover, we did not perform multiple tests at the same shock output to validate reproducibility or step-down the defibrillation testing protocol. The implant technique changed to a 2-incisional technique from a 3-incisional technique during the study period. Francia et al reported that the 2-incisional technique provides lower shock impedance and is associated with a low-risk PRAETORIAN score.17,18 However, in our study, there were no significant differences between each incisional technique. Finally, a lower LVEF has been associated with a higher defibrillation energy requirement.19 However, most patients in the present study had a preserved LVEF, and thus we could not assess the effect of the myocardium. Further studies are warranted, including in patients with much higher shock impedance or lower LVEF. In addition, the lack of evaluation regarding the impact of radiation exposure and cost on CT is a concern. However, despite advances in implantation technology, the present study showed that patient-specific factors, such as adipose tissue, have a significant impact on shock impedance. Although CT imaging of all patients may not be recommended due to cost and radiation exposure, we believe that it is worth investigating to stratify risk and unnecessary shock testing when preoperative CT imaging is performed for other purposes. Finally, shock impedance is not the only relevant variable in predicting the DFT of the S-ICD. The combination of anatomic system positioning and system impedance is the strongest predictor of successful conversion.5,11 The implantation technique can alter the energy requirements for conversion efficacy, and appropriate system positioning should be important.
Currently, there is no tool to preoperatively predict the incidence of high shock impedance in patients awaiting S-ICD implantation. Preoperative CT-measured amount of adipose tissue and basal heart anteroposterior diameter are independent predictors of shock impedance. These may provide better accuracy in identifying higher shock impedance in patients with S-ICDs.
We are grateful to Dr. Makoto Takano and Mr. Yasuyoshi Ogawa for data accumulation and helpful discussions about image proccessing.
This study did not receive any specific funding.
Y.J.A. is a number of Circulation Journal’s Editorial Team. All other authors have no relationships to disclose relevant to this paper’s contents.
This study was approved by the Ethics Committee of St. Marianna University Hospital (Approval no. 4673).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0229