Abstract
Background: Potential differences in complications and/or long-term outcomes of perimembranous ventricular septal defect (pmVSD) closures with 3-mm waist vs. 4-mm waist double-disk symmetrical occluders are not known.
Methods and Results: A total of 395 consecutive pediatric patients with pmVSD recruited between January 2017 and March 2021 underwent successful transcatheter closure using symmetrical pmVSD devices. The final analysis involved 208×3-mm and 172×4-mm cases. The median follow-up was 42 months (range: 12–62 months). A total of 175 post-procedure adverse events (AEs) were observed. Most of these AEs were temporary, and there were only 8 major AEs. Compared to the 3-mm waist group, the incidence of residual shunts was significantly higher in the 4-mm waist group (13.4% vs. 6.7%; P=0.030), whereas other AEs showed similar incidences between the 2 groups. Multivariate Cox regression analysis revealed that larger defect, higher ratio between device size and body surface area, and longer procedure time can cause an increased likelihood of AEs, and smaller defect or left disk placement within aneurysmal tissue may reduce it.
Conclusions: Transcatheter closure of pmVSD using a symmetrical double-disk occluder is safe and effective. Compared with a 3-mm waist symmetrical occluder, transcatheter closure with a 4-mm waist symmetrical occluder correlated with higher incidences of residual shunts.
Transcatheter ventricular septal defect (VSD) closure, and particularly perimembranous VSD (pmVSD), represents a great step forward in cardiac device design and procedural techniques.1–5 Multiple studies have shown that transcatheter pmVSD closure has similar safety, efficacy, and clinical outcomes to surgical pmVSD closure.6–8 The early Amplatzer pmVSD device had limited clinical use because it was associated with a high rate of the complete atrioventricular block (cAVB, 3–5%).9,10 In recent years, modified double-disk VSD devices manufactured in China have been widely used in developing countries with satisfactory efficacy.8,11,12 Additionally, the symmetrical device (with a waist length of 3 mm) is associated with a reduced incidence of cAVB.4,13 However, there are still great challenges inherent to transcatheter pmVSD closure, including a small risk of heart block (particularly complete left bundle branch block [CLBBB]) and the possibility of new-onset valve regurgitation following the procedure.14,15 Some studies suggest that possible mechanisms underlying cAVB involve direct compression of, and inflammatory reactions within, conduction tissue.11,16 Thus, some scholars have postulated that increasing the waist length of the modified double-disk symmetrical device from 3 mm to 4 mm might reduce compression and inflammatory edema, which could then translate to decreased occurrences of heart block following the procedure. However, data comparing the clinical outcomes of the 2 different waist-length devices are scarce. It remains unclear whether a symmetrical device with a waist length of 4 mm is less likely to induce heart block than a 3-mm version. Furthermore, little is known about other issues related to the increased waist length of the device.
Thus, this study aimed to compare procedural efficiencies and clinical outcomes for transcatheter device closure between devices with 2 different waist lengths in pediatric patients.
Methods
Study Population
This is a single-center, observational prospective study conducted at Shandong Provincial Hospital Affiliated to Shandong First Medical University. Between January 2017 and March 2021, 395 consecutive pediatric patients with pmVSD who underwent successful transcatheter closure using a modified double-disk symmetrical device were included in the study. All patients were prospectively followed, and at least 1 year of follow-up was required for inclusion in the final dataset. All data are recorded in an online database (http://www.pedhd.cn/).
Indications for transcatheter pmVSD closure were: being aged ≥2 years or weighing ≥10 kg, and having a significant left-to-right shunt, left atrial enlargement and/or left ventricular (LV) volume overload.
The study protocol was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University, and each patient’s guardians provided written informed consent before their participation. This study complied with the ethical standards of the Helsinki Declaration.
Device Implantation
The devices used in this study were modified double-disk symmetrical VSD devices (Shanghai Shape Memory Alloy, Shanghai, China; Lifetech Scientific, Shenzhen, China; Starway Medical, Beijing, China). The diameter of both disks was 4 mm larger than the waist, and the waist length was either 3 or 4 mm (Figure 1). Devices are available in sizes ranging from 4 to 20 mm. Transcatheter VSD closure was performed as described previously.8
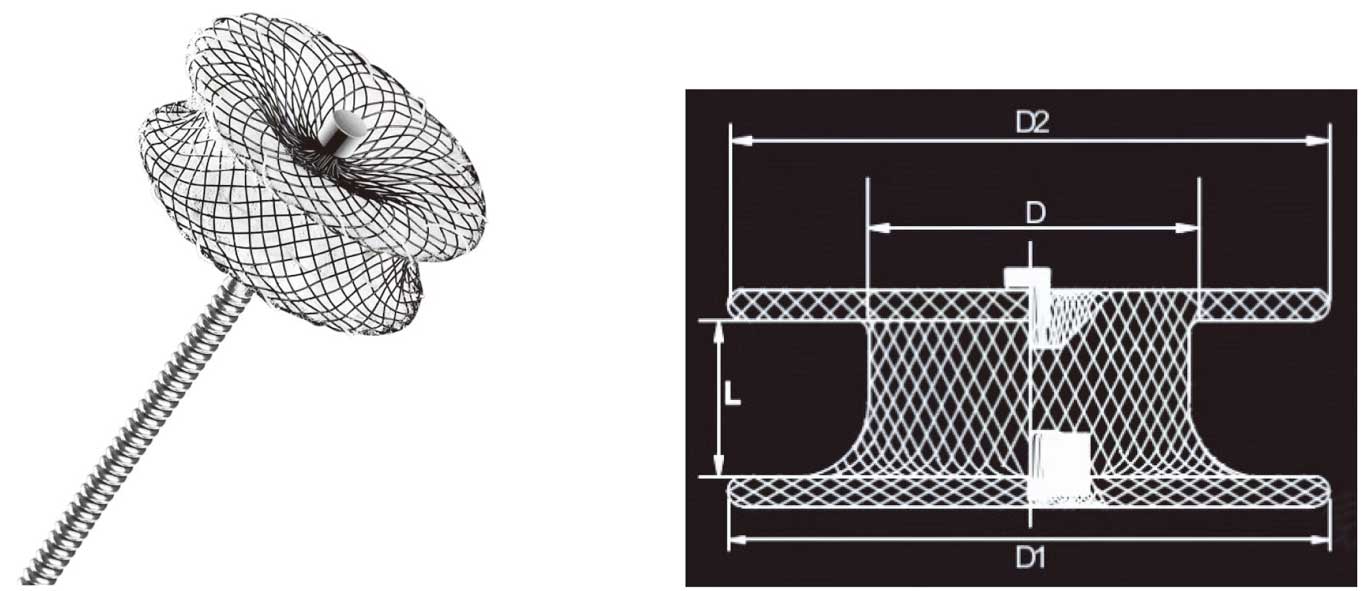
All procedures were performed by 3 surgeons, each of whom had previously completed at least 200 transcatheter VSD closures as the lead operator. A symmetrical device was usually preferred if the subaortic rim was >2 mm, or the subaortic rim was <2 mm, but the left disk could be placed into aneurysmal tissue. Otherwise, an eccentric occluder was usually selected to avoid affecting the aortic valve. For a larger aneurysmal pmVSD with multiple outlets, a thin-waist occluder may be used to reduce or avoid residual shunt. An Amplatzer Duct Occluder II (ADO II) can be used to close a pmVSD with aneurysm or a long tubular pmVSD. The symmetrical device size was usually 2–4 mm larger than the defect diameter. The waist length of the symmetrical device was determined by the operator based on the shape and location of the defect, as well as their experience. If the left disk was placed at the inlet of the defect and the defect shape was longer, a 4-mm waist device was generally selected. Otherwise, a 3-mm waist device was preferred.
Clinical Assessment
During hospitalization, each patient underwent electrocardiography (ECG), chest X-ray, transthoracic echocardiography (TTE), and a 24-h Holter test before and after the procedure. Continuous ECG monitoring was performed following the intervention until discharge. If no adverse events (AEs) occurred, patients were discharged 5–7 days post-procedure. Aspirin (at a dose of 3–5 mg/kg daily) was administered for 6 months after the procedures.
For patients who suffered from the new-onset atrioventricular block, CLBBB, or complete right bundle branch block (CRBBB) after the procedure, intravenous dexamethasone (0.5–1.0 mg/kg daily, maximum 10 mg) was administered for 3–5 days and gradually tapered (to intravenous dexamethasone or oral prednisone) over 2 weeks.
Clinical evaluations were scheduled at 1, 3, 6, and 12 months post-procedure and annually thereafter. These evaluations involved ECGs, chest X-rays, and TTE tests. For patients who presented with postprocedural atrioventricular block, CLBBB, or CRBBB, an additional 24-h Holter test was carried out at each outpatient visit.
AEs
At each evaluation, AEs were identified based on clinical assessment. AEs were recorded and classified as major and minor. Major AEs were defined as, but not limited to, procedure-related deaths, cardiac arrest, cardiac tamponade requiring pericardial drainage, cAVB, CLBBB, device embolization, intracranial hemorrhage, new or increased valvular regurgitation necessitating device removal or surgical repair, ongoing hemolysis requiring device removal, and unplanned cardiac surgery (due to catheterization complications). Minor AEs were defined as, but not limited to, residual shunts (>1 mm), hemolysis, cardiac rhythm/conduction abnormalities (except for cAVB and CLBBB), groin hematomas, blood transfusion due to blood loss, and new or increased valvular regurgitation not requiring surgery.
Statistical Analysis
Continuous variables are summarized with mean±standard deviation (SD), and categorical variables are summarized with counts and/or percentages. χ2
tests or Fisher’s exact tests were used for categorical variables, and Student’s t-tests or Wilcoxon rank-sum tests were used for continuous variables, as appropriate. Freedom from AEs during follow-up was assessed using Kaplan-Meier estimates. Cox proportional hazard regression analysis was performed to explore risk factors associated with the occurrence of AEs. Factors entered into the Cox regression models included sex, age, weight, body surface area (BSA), defect diameter on TTE and left ventriculography (inlet and outlet), subaortic rim (distance between the defect and aortic valve), membranous aneurysms (yes, no), aneurysmal tissue involving the tricuspid valve (yes, no), left disk placement within the aneurysmal tissue (yes, no), device type (3-mm waist and 4-mm waist symmetrical devices), device diameter, device diameter/defective outlet diameter (measured on left ventriculography) ratio, device diameter/BSA ratio, procedure time, fluoroscopic time, and radiation dose. A P value of <0.05 was considered statistically significant. All analyses were performed using SPSS software version 25.0 (SPSS Inc, Chicago, IL, USA).
Results
Baseline Characteristics
Of the 591 children who underwent attempted transcatheter pmVSD closure, 581 (98%) had successful closure, including 395 cases with a symmetrical occluder, 36 cases with an eccentric occluder, 129 cases with an ADO II device, 20 cases with a thin-waist occluder, and 1 case with a vascular plug. Because 15 patients had incomplete follow-up data, 208 cases with 3-mm waist symmetrical devices and 172 cases with 4-mm waist symmetrical devices were included in the final analysis (Figure 2). Baseline patient characteristics are summarized in Table 1. Among the 380 cases, there were 195 females (51.3%) with an average age of 3.66±1.93 years (range 1.6–14 years) and an average weight of 16.14±6.43 kg (range 9–60 kg). TTE showed membranous aneurysms in 267 patients (70.3%), 188 (70.4%) of which had aneurysmal tissue involving the tricuspid valve. At the 1-year follow-up, the average left atrial diameter decreased from 23.79±3.26 mm pre-procedure to 21.45±2.62 mm 1 day post-procedure and then to 20.96±2.66 mm 1 year post-procedure (both P values <0.001).
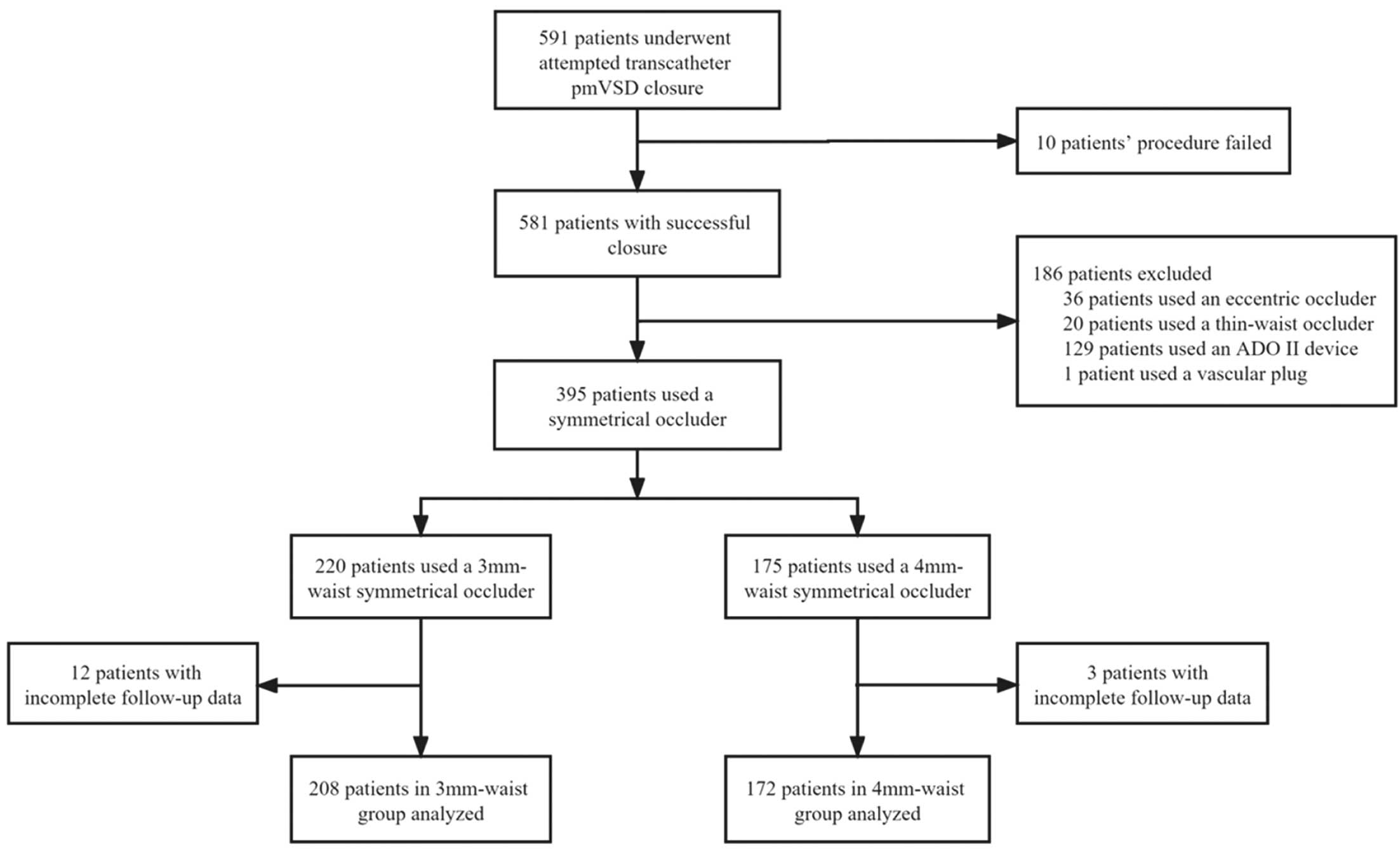
Table 1.
Baseline Patient Characteristics
| |
All patients
(N=380) |
3-mm waist
(n=208) |
4-mm waist
(n=172) |
P value |
| Sex |
|
|
|
0.197 |
| Female |
195 (51.3) |
113 (54.3) |
82 (47.7) |
|
| Male |
185 (48.7) |
95 (45.7) |
90 (52.3) |
|
| Age, years |
3.66±1.93 |
3.61±1.90 |
3.72±1.97 |
0.579 |
| Weight, kg |
16.14±6.43 |
15.88±6.19 |
16.45±6.70 |
0.389 |
| BSA, m2 |
0.78±0.16 |
0.77±0.16 |
0.78±0.17 |
0.600 |
| Hospital stay, days |
9.11±2.47 |
8.79±2.50 |
9.51±2.39 |
0.004 |
| Inlet diameter by TTE, mm |
7.34±2.58 |
7.54±2.60 |
7.10±2.54 |
0.101 |
| Outlet diameter by TTE, mm |
4.44±1.79 |
4.32±1.72 |
4.59±1.86 |
0.147 |
| Subaortic rim, mm |
2.08±1.25 |
1.96±1.26 |
2.22±1.24 |
0.045 |
| Membranous aneurysms |
|
|
|
0.910 |
| No |
113 (29.7) |
61 (29.3) |
52 (30.2) |
|
| Yes |
267 (70.3) |
147 (70.7) |
120 (69.8) |
|
| Aneurysmal tissue involving the tricuspid valve |
|
|
|
0.681 |
| No |
192 (50.5) |
103 (49.5) |
89 (51.7) |
|
| Yes |
188 (49.5) |
105 (50.5) |
83 (48.3) |
|
Data are presented as n (%) or mean±standard deviation. BSA, body surface area; TTE, transthoracic echocardiography.
It showed no difference in terms of sex, age, weight, and BSA (all P>0.05). Meanwhile, there were no significant differences in TTE data, except for subaortic rim size between the 2 groups (Table 1), but the waist length selection for the devices is independent of subaortic rim size.
Procedural Characteristics
Procedural data are shown in Table 2. The mean VSD diameter on angiography was 4.24±1.87 mm, and the device size was 7.31±2.09 mm. Of the 267 patients with membranous aneurysms, the left disk of the device was placed within aneurysmal tissue in 51 cases (19.1%). There were no differences in procedural data such as defect diameter, device size, procedure time, fluoroscopic time and radiation dose.
Table 2.
Procedural Characteristics
| |
All patients
(N=380) |
3-mm waist
(n=208) |
4-mm waist
(n=172) |
P value |
| Inlet diameter on angiography, mm |
7.32±2.78 |
7.45±2.72 |
7.18±2.85 |
0.346 |
| Outlet diameter on angiography, mm |
4.24±1.87 |
4.22±1.89 |
4.27±1.85 |
0.823 |
| Device diameter, mm |
7.31±2.09 |
7.31±2.07 |
7.31±2.12 |
0.983 |
| Device/defect |
1.87±0.54 |
1.89±0.56 |
1.85±0.52 |
0.476 |
| Device/BSA, mm/m2 |
9.74±3.31 |
9.78±3.29 |
9.70±3.34 |
0.827 |
| The left disk placed within the aneurysmal tissue |
|
|
|
0.369 |
| No |
329 (86.6) |
177 (85.1) |
152 (88.4) |
|
| Yes |
51 (13.4) |
31 (14.9) |
20 (11.6) |
|
| Dosage of contrast medium/weight, mL/kg |
4.52±0.99 |
4.48±1.09 |
4.56±0.86 |
0.423 |
| Procedure time, min |
74.77±29.56 |
74.16±29.39 |
75.51±29.84 |
0.657 |
| Fluoroscopic time, min |
11.00±9.96 |
11.24±9.98 |
10.73±9.95 |
0.617 |
| Radiation dose, mGy |
93.25±79.67 |
94.60±78.91 |
91.62±80.77 |
0.717 |
Data are presented as n (%) or mean±standard deviation. BSA, body surface area.
In a low number of cases, devices with different waist lengths were changed because the originally selected size was inappropriate. In the 3-mm waist group, the waist length of the device was changed from 4 mm to 3 mm in 4 patients for the following reasons: 2 cases had accelerated anterior tricuspid blood flow and limited tricuspid valve opening; 1 case had severe tricuspid regurgitation (TR); and 1 case presented with significant residual shunt. These 4 cases were successfully resolved after replacement with a 3-mm waist device with the same diameter. Similarly, in the 4-mm waist group, there were 4 cases that had their devices exchanged. In all of these cases, this was because of the short waist length of the device, which meant that it could not be pulled into the right ventricle and that the right disk failed to deploy.
AEs and Follow-up Evaluation
Follow-up data were collected until April 2022, with a median follow-up period of 42 months (range: 12–62 months). A total of 175 AEs (46.1%) were noted after transcatheter VSD closure, 95.4% of which were minor and the majority of which were temporary and did not require treatment. There were only 29 minor AEs (7.6%) up to the last follow-up. Procedural AEs and follow-up outcomes are reported in Table 3.
Table 3.
Procedural AEs and Follow-up Outcomes
| |
AEs |
Last follow-up |
All
patients
(N=380) |
3-mm
waist
(n=208) |
4-mm
waist
(n=172) |
P value |
All
patients
(N=380) |
3-mm
waist
(n=208) |
4-mm
waist
(n=172) |
P value |
| Major AEs |
8 |
3 |
5 |
0.476 |
0 |
0 |
0 |
– |
| Intracranial hemorrhage |
1 |
1 |
0 |
– |
0 |
0 |
0 |
– |
| New-onset TR needing surgical repair |
1 |
1 |
0 |
– |
0 |
0 |
0 |
– |
| CLBBB |
6 |
1 |
5 |
0.095 |
0 |
0 |
0 |
– |
| Minor AEs |
167 |
87 |
80 |
0.360 |
29 |
14 |
15 |
0.467 |
| Residual shunt (>1 mm) |
37 |
14 |
23 |
0.030 |
5 |
2 |
3 |
0.661 |
Hemolysis not needing surgical device
removal |
12 |
5 |
7 |
0.355 |
0 |
0 |
0 |
– |
New or increased TR not requiring
surgery |
34 |
21 |
13 |
0.388 |
8 |
4 |
4 |
1.000 |
New or increased AR not requiring
surgery |
3 |
2 |
1 |
1.000 |
1 |
0 |
1 |
– |
Cardiac arrhythmias, except for cAVB
and CLBBB |
79 |
43 |
36 |
0.951 |
15 |
8 |
7 |
0.911 |
| IRBBB |
54 |
30 |
24 |
0.896 |
14 |
7 |
7 |
0.717 |
| CRBBB |
9 |
5 |
4 |
1.000 |
0 |
0 |
0 |
– |
| AVB |
2 |
2 |
0 |
– |
0 |
0 |
0 |
– |
| Junctional tachycardia |
10 |
4 |
6 |
0.358 |
1 |
1 |
0 |
– |
| Others |
4 |
2 |
2 |
1.000 |
0 |
0 |
0 |
– |
| Groin hematomas |
2 |
2 |
0 |
– |
0 |
0 |
0 |
– |
| Total |
175 |
90 |
85 |
0.231 |
29 |
14 |
15 |
0.467 |
AEs, adverse events; AR, aortic regurgitation; AVB, atrioventricular block; cAVB, complete atrioventricular block; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; IRBBB, incomplete right bundle branch block; TR, tricuspid regurgitation.
Compared to the 3-mm waist group, the incidence of the residual shunt was significantly higher in the 4-mm waist group (13.4% vs. 6.7%; P=0.030). The incidences of total AEs, major AEs, and other minor AEs were similar between the 2 groups, and follow-up AE outcomes did not differ significantly.
Kaplan-Meier curves showed that there were no differences in the incidences of total AEs in the 4-mm waist group when compared with the 3-mm waist group, and 98.9% of them occurred within 1-week post-procedure. Multivariate Cox regression analysis revealed that larger inlet diameter on angiography (hazard ratio [HR]: 1.16; 95% confidence interval [CI]: 1.063–1.265; P=0.001), higher device/BSA (HR: 1.144; 95% CI: 1.066–1.228; P<0.001), longer procedure time (HR: 1.007; 95% CI: 1.002–1.012; P<0.001), smaller outlet diameter on angiography (HR: 0.779; 95% CI: 0.692–0.877; P<0.001), and left disk placement within the aneurysmal tissue (HR: 0.242; 95% CI: 0.124–0.472; P<0.001) were independent predictors of postprocedural AEs (Table 4).
Table 4.
Cox Regression Analysis for Postprocedural Adverse Events
| Variable |
HR |
95% CI |
P value |
| Inlet diameter on angiography, mm |
1.160 |
1.063–1.265 |
0.001 |
| Outlet diameter on angiography, mm |
0.779 |
0.692–0.877 |
<0.001 |
| Device/defect |
1.017 |
0.966–1.071 |
0.524 |
| Presence of membranous aneurysms |
0.975 |
0.635–1.497 |
0.908 |
| Subaortic rim size, mm |
0.914 |
0.797–1.049 |
0.201 |
| The left disk placed within the aneurysmal tissue |
0.242 |
0.124–0.472 |
<0.001 |
| Device/BSA |
1.144 |
1.066–1.228 |
<0.001 |
| Procedure time, min |
1.007 |
1.002–1.012 |
0.009 |
BSA, body surface area; CI, confidence interval; HR, hazard ratio.
Residual Shunt and Hemolysis
All residual shunts were <3 mm in both groups. Incidences of the residual shunt at the last follow-up did not differ between the 2 groups (1.0% in the 3-mm waist group and 1.7% in the 4-mm waist group; P=0.061), with all of them being <2 mm. The larger inlet diameter (HR: 1.242; 95% CI: 1.015–1.521; P=0.035) was an independent risk factor for residual shunt (Supplementary Table 1). Hemolysis occurred in 5 cases (2.4%) in the 3-mm waist group and 7 cases (4.1%) in the 4-mm waist group, with only 1×4-mm waist case requiring blood transfusion. All hemolysis occurred within 72 h following device implantation and resolved within 7 days with adequate hydration and urine alkalization.
Valve Complications
Aortic regurgitation (AR) occurred in 3 cases (0.8%), all of which were mild and did not require treatment. AR also disappeared in 2 of these cases during follow-up.
There were 22 cases of new TR in the 3-mm waist group, including 1 case of severe regurgitation that underwent surgical repair, 2 cases of moderate regurgitation, and 19 cases of mild regurgitation. The smaller outlet diameter (HR: 0.566; 95% CI: 0.400–0.801; P=0.001) and higher device/BSA (HR: 1.259; 95% CI: 1.065–1.489; P=0.007) were independent predictors of TR (Supplementary Table 2). In the 4-mm waist group, new TR occurred in 13 cases, 1 of which was moderate and 12 of which were mild. Most new TR (26/35, 74.3%) resolved during follow-up. All of the 8 patients with persistent TR had normal right heart function. One of these cases had moderate TR, and the other 7 cases had mild TR.
Arrhythmias
Postprocedural arrhythmias occurred in 85 cases (22.4%), with incomplete right bundle branch blocks of the most common type. Six patients developed CLBBB, but none had cAVB. Most arrhythmias were transient, with persistent arrhythmias in only 15 cases (3.9%). We identified that larger inlet diameter (HR: 1.205; 95% CI: 1.071–1.356; P=0.002) and left disk placement within aneurysmal tissue (HR: 0.183; 95% CI: 0.073–0.462; P<0.001) were independent predictors of postprocedural arrythmia (Supplementary Table 3). All of the 6 patients with CLBBB developed it within 5 days post-procedure, and 3 of them underwent surgical removal of the device and defect repair (at 6, 13, and 40 days post-procedure, respectively). Five cases recovered a normal rhythm, and 1 case reverted to CRBBB. Notably, 1 patient had a recurrent CLBBB 1 month post-procedure that later restored sinus rhythm after the device was surgically removed.
Other AEs
One 31-month-old girl developed ventricular hemorrhage 7 h after successful closure using a 7-mm symmetric device with a 3-mm waist. Fortunately, after lateral ventricular drainage and other treatment, she recovered completely without any sequelae. Two other patients developed groin hematomas. No other AEs, including death, cardiac arrest, cardiac tamponade, device embolization, or cAVB, were recorded in our study.
Discussion
Alongside the widespread application of the modified double-disk VSD devices in developing countries, especially China, several studies have confirmed that using symmetrical devices for pmVSD closure has high success rates, low incidences of serious complications, and favorable results. Compared with a 3-mm waist symmetrical device, transcatheter closure with a 4-mm waist symmetrical device was associated with a higher incidence of the residual shunt, whereas other AEs showed similar incidences between the 2 groups.
Here, we found that larger defect inlet diameter, oversized device size, and longer procedure time could significantly increase AEs incidence after VSD closure, whereas smaller defect outlet diameter and left disk placement within the aneurysmal tissue could significantly reduce it. The larger inlet diameter often means the choice of a larger size occluder, causing an increased likelihood of AEs such as arrhythmia and residual shunt. If the outlet diameter is smaller, the device size generally selected is smaller, and the possibility of resulting in AEs such as arrhythmia and TR is reduced. Similarly, after correcting device size based on BSA, a relatively or absolutely larger device size is more likely to increase AEs incidence. The longer procedure time generally indicates a complicated procedure, and repeated operation will increase the occurrence of AEs. Thus, avoiding the use of oversized devices, as well as placing the left disk within aneurysmal tissue, are beneficial for this procedure. For cases with larger defects and longer procedure times, it is necessary to be vigilant, as postprocedural AEs may occur more easily.
The incidence of the residual shunt in the 4-mm waist group was significantly higher than in the 3-mm waist group. We hypothesize that increasing the waist length by 1 mm resulted in poor fitting between the left and right discs and the ventricular septa, which then contributed to the development of residual shunts across the waist of the device. Although there were no significant differences in the occurrence of hemolysis between the 2 groups, the higher incidence of residual shunts may indicate a higher likelihood of hemolysis. During follow-up, most of the residual shunts were reduced or disappeared with gradual endothelialization around the devices.
There are some studies on the mechanism of arrhythmias after pmVSD closure.11,16–19 Because pmVSD margins are close to the bundle of His and bundle branches, previous studies have speculated that direct mechanical compression, trauma, inflammatory edema, fibrosis or scar formation of the conduction tissue, and friction of the disk on the septal myocardium may underlie the development of postprocedural arrhythmias. A previous study suggested that elongating the waist of the device could reduce septal movement restrictions and disk compression on conduction tissue, thereby decreasing inflammatory responses and lowering the incidence of postprocedural arrhythmias.20 However, we found no significant differences in the occurrence of postprocedural arrhythmias when 4 mm vs. 3 mm devices were used.
Although the incidence of arrhythmias following pmVSD closure is relatively high, right bundle branch blocks dominate these arrhythmias and most of them are temporary. A few recent studies have reported that persistent CLBBB after VSD closure can cause progressively increasing LV end-diastolic diameter and LV dysfunction.13,21,22 Also, CLBBB can cause persistent mechanical ventricular desynchrony, which might induce progressive LV dysfunction and chronic heart failure, even CLBBB-induced cardiomyopathy.23,24
The occurrence of heart block after VSD closure is closely related to the oversized devices and the onset time of heart block.11,17,19,22 Our results demonstrate that larger defects can increase the incidence of arrhythmia, and left disk placement within the aneurysmal tissue may significantly reduce it. Most arrhythmias occur within 7 days after the procedure, but a few may be late-onset or recurrent. It is more difficult to restore normal conduction in recurrent and late-onset cases.11,22 Of our 6 cases of CLBBB, 3 underwent surgical device removal and had restored sinus rhythms, but there was 1 recurrent case. A recent case reported that a 27-month-old patient who developed CRBBB after the procedure progressed to cAVB at 30 months post procedure and reverted to CRBBB after surgical removal of the device.25 In our experience, if cAVB or CLBBB occur following VSD closure, early surgical device removal may be an appropriate approach.
Most studies on valve regurgitation have focused on AR,3,10,26 and TR is often overlooked. The main causes of AR include repeated operations as arteriovenous tracks are established. It is unfortunately easy to cause aortic valve edema or even damage because of the proximity of the defect to the aortic valve — when the left disk is in contact with the aortic valve, AR can result. If a symmetric device contacts the aortic valve, an eccentric device can be used to replace it. In our study, ascending aortic angiography and TTE were performed to assess AR prior to releasing the device.
TR is common after VSD closure.27 However, most of these cases are mild and asymptomatic, and the underlying mechanism remains unclear. In our cohort, we observed that larger occluders may increase the incidence of TR, whereas smaller defects can decrease it, possibly because a bigger right disk may affect TR more easily. Membranous aneurysms were observed in many pmVSD patients, and the membranous tissue was closely tied to the tricuspid valve. Thus, the tricuspid chordae tendineae and/or leaflets may be injured or clamped when the arteriovenous track is established or a device is placed, leading to moderate-to-severe TR and necessitating surgical repair. The delivery sheath may also lead to minor TR prior to the device’s release, which requires careful identification of the cause of TR by an experienced cardiac sonographer using TTE.4 Additionally, with tricuspid chordae tendineae or leaflets clamped by the disk, the patient’s heart rate and blood pressure may also change. In our cases, 1 patient had a tricuspid leaflet tear that resulted in severe TR, but had a successful surgical repair. Mild TR is generally considered to have no effect on cardiac function, with no interventions required.
Compared with TR, tricuspid stenosis is more difficult to detect and identify.8,28 It is usually caused by right disk clamping of the tricuspid chordae tendineae or leaflets, resulting in accelerated anterior tricuspid blood flow. In this study, among the patients with the waist length of the selected device changing from 4 mm to 3 mm, there were 2 cases with limited tricuspid valve opening and 1 case with severe TR due to the use of a 4-mm waist device. Emergency thoracotomy is usually required when tricuspid stenosis develops after device placement. Although there were no between-group differences in TR incidences between the 2 groups, a longer waist (4-mm length) device may be more likely to lead to tricuspid stenosis and severe TR via clamping of the tricuspid valve.
If the defect is suitable for closure with a symmetrical double-disk device, the waist length of the device should be determined based on the shape and size of the defect. In general, a 3-mm waist device is recommended first because using a 4-mm waist device is associated with a higher possibility of postprocedural residual shunt, does not correlate with reduced incidence of arrhythmia, and may even more easily affect the tricuspid valve, leading to tricuspid stenosis or regurgitation. If the left disk is placed at the inlet of the defect but the defect shape is longer, a 4-mm waist device may be a more appropriate choice. Otherwise, the right disk may not be properly pulled into the right ventricle.
Study Limitations
Our study has several limitations. First, although these patients were recruited consecutively and prospectively, they were not randomized. Second, these patients were enrolled from a large pediatric heart center in eastern China, but the experience of a single center may not be universally representative. Moreover, there was a relatively low number of patients included in our analysis. Future studies should incorporate a larger sample size and a longer follow-up period.
Conclusions
For pediatric patients with pmVSD, transcatheter device closure of defects using a symmetrical double-disk device is safe and effective, with excellent long-term follow-up outcomes. Compared with a 3-mm waist symmetrical device, transcatheter closure with a 4-mm waist symmetrical device was associated with a higher incidence of the residual shunt, with no advantage in reducing the incidence of postprocedural arrhythmias.
Disclosures
The authors have no conflicts of interest to declare.
Sources of Funding
This work was supported by the Project of Medicine and Healthcare Sci-tech Development Plan of Shandong Province [202106010792].
IRB Information
The present study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (Reference number: SWYX:NO.2020-258).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0583
References
- 1.
Butera G, Carminati M, Chessa M, Piazza L, Abella R, Negura DG, et al. Percutaneous closure of ventricular septal defects in children aged <12: Early and mid-term results. Eur Heart J 2006; 27: 2889–2895.
- 2.
Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, et al. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur Heart J 2007; 28: 2361–2368.
- 3.
Qin Y, Chen J, Zhao X, Liao D, Mu R, Wang S, et al. Transcatheter closure of perimembranous ventricular septal defect using a modified double-disk occluder. Am J Cardiol 2008; 101: 1781–1786.
- 4.
Wang L, Cao S, Li J, Yang L, Liu Y, Ren J, et al. Transcatheter closure of congenital perimembranous ventricular septal defect in children using symmetric occluders: An 8-year multiinstitutional experience. Ann Thorac Surg 2012; 94: 592–598.
- 5.
Jiang D, Zhang J, Fan Y, Han B, Zhao L, Yi Y, et al. The efficacy and medium to long-term follow-up of transcatheter retrograde closure of perimembranous ventricular septal defects via the femoral artery with amplatzer duct occluder II in children. Front Pediatr 2021; 9: 571407.
- 6.
Yang J, Yang L, Yu S, Liu J, Zuo J, Chen W, et al. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: A randomized controlled trial. J Am Coll Cardiol 2014; 63: 1159–1168.
- 7.
Yang J, Yang L, Wan Y, Zuo J, Zhang J, Chen W, et al. Transcatheter device closure of perimembranous ventricular septal defects: Mid-term outcomes. Eur Heart J 2010; 31: 2238–2245.
- 8.
Jiang D, Han B, Zhao L, Yi Y, Zhang J, Fan Y, et al. Transcatheter device closure of perimembranous and intracristal ventricular septal defects in children: Medium- and long-term results. J Am Heart Assoc 2021; 10: e020417.
- 9.
Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: Early and long-term results. J Am Coll Cardiol 2007; 50: 1189–1195.
- 10.
Zuo J, Xie J, Yi W, Yang J, Zhang J, Li J, et al. Results of transcatheter closure of perimembranous ventricular septal defect. Am J Cardiol 2010; 106: 1034–1037.
- 11.
Bai Y, Xu XD, Li CY, Zhu JQ, Wu H, Chen SP, et al. Complete atrioventricular block after percutaneous device closure of perimembranous ventricular septal defect: A single-center experience on 1046 cases. Heart Rhythm 2015; 12: 2132–2140.
- 12.
Li H, Shi Y, Zhang S, Ren Y, Rong X, Wang Z, et al. Short- and medium-term follow-up of transcatheter closure of perimembranous ventricular septal defects. BMC Cardiovasc Disord 2019; 19: 222.
- 13.
Wang C, Zhou K, Luo C, Shao S, Shi X, Li Y, et al. Complete left bundle branch block after transcatheter closure of perimembranous ventricular septal defect. JACC Cardiovasc Interv 2019; 12: 1631–1633.
- 14.
Shahanavaz S, Winlaw DS, Opotowsky AR. What is blocking transcatheter ventricular septal defect closure? J Am Heart Assoc 2022; 11: e024963.
- 15.
Li D, Zhou X, Li M, An Q. Comparisons of perventricular device closure, conventional surgical repair, and transcatheter device closure in patients with perimembranous ventricular septal defects: A network meta-analysis. BMC Surg 2020; 20: 115.
- 16.
Li Y, Hua Y, Fang J, Wan C, Wang C, Zhou K. Identification of risk factors for arrhythmia post transcatheter closure of perimembranous ventricular septal defect. J Invasive Cardiol 2015; 27: E158–E166.
- 17.
Yang R, Kong XQ, Sheng YH, Zhou L, Xu D, Yong YH, et al. Risk factors and outcomes of post-procedure heart blocks after transcatheter device closure of perimembranous ventricular septal defect. JACC Cardiovasc Interv 2012; 5: 422–427.
- 18.
Wu Z, Yang P, Xiang P, Ji X, Tian J, Li M. Left anterior fascicular block after transcatheter closure of ventricular septal defect in children. Front Cardiovasc Med 2021; 8: 609531.
- 19.
Lin L, Liu J, Guo X, Chen H, Huang Y, Zheng H, et al. Risk factors for atrioventricular block after occlusion for perimembranous ventricular septal defect. Heart Rhythm 2022; 19: 389–396.
- 20.
Zhou Y, Qin Y, Zhao X, Lang X, Zhu N, Bai Y, et al. The impact of short or long transcatheter occluder waist lengths on postprocedure complete atrioventricular block: A retrospective study. J Invasive Cardiol 2015; 27: E231–E235.
- 21.
Tang C, Shao S, Zhou K, Hua Y, Luo C, Wang C. Complete left bundle-branch block after transcatheter closure of perimembranous ventricular septal defect using amplatzer duct occluder II. J Am Heart Assoc 2022; 11: e022651.
- 22.
Jiang D, Fan Y, Han B, Zhao L, Yi Y, Zhang J, et al. Risk factors and outcomes of postprocedure complete left bundle branch block after transcatheter device closure of perimembranous ventricular septal defect. Circ Cardiovasc Interv 2021; 14: e009823.
- 23.
Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, Behar N, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61: 1089–1095.
- 24.
Pouleur AC, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, et al. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: The MADIT-CRT trial. Eur Heart J 2011; 32: 1720–1729.
- 25.
Xie L, Zhang H, Zhang R, Xiao T. Management of late-onset complete atrioventricular block post transcatheter closure of perimembranous ventricular septal defects. Front Pediatr 2020; 7: 545.
- 26.
Zhang W, Wang C, Liu S, Zhou L, Li J, Shi J, et al. Safety and efficacy of transcatheter occlusion of perimembranous ventricular septal defect with aortic valve prolapse: A six-year follow-up study. J Interv Cardiol 2021; 2021: 6634667.
- 27.
Santhanam H, Yang L, Chen Z, Tai BC, Rajgor DD, Quek SC. A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. Int J Cardiol 2018; 254: 75–83.
- 28.
Munirathinam GK, Kumar B, Mishra AK. Tricuspid stenosis: A rare and potential complication of ventricular septal occluder device. Ann Card Anaesth 2018; 21: 195–199.


