Abstract
Background: This prospective multicenter study assessed the prevalence of myocardial injury in patients with COVID-19 using cardiac magnetic resonance imaging (CMR).
Methods and Results: We prospectively screened 505 patients with moderate to severe COVID-19 disease from 7 hospitals in Japan. Of these patients, 31 (mean [±SD] age 63.5±10.4 years, 23 [74%] male) suspected of myocardial injury, based on elevated serum troponin or B-type natriuretic peptide concentrations either upon admission or 3 months after discharge, underwent CMR 3 months after discharge. The primary endpoint was the presence of myocardial injury, defined by any of the following: (1) contrast enhancement in the left or right ventricle myocardium on late gadolinium enhancement CMR; (2) left or right ventricular dysfunction (defined as <50% and <45%, respectively); and (3) pericardial thickening on contrast enhancement. The mean (±SD) duration between diagnosis and CMR was 117±16 days. The primary endpoint was observed in 13 of 31 individuals (42%), with 8 (26%) satisfying the modified Lake Louise Criteria for the diagnosis of acute myocarditis.
Conclusions: This study revealed a high incidence of myocardial injury identified by CMR in patients with moderate to severe COVID-19 and abnormal findings for cardiac biomarkers.
The COVID-19 pandemic was a considerable public health crisis.1 Initially identified as a cluster of cases of pneumonia, it swiftly became apparent that COVID-19 was more than a mere respiratory ailment. The clinical trajectory of COVID-19 is frequently complicated by cardiovascular conditions, encompassing both venous and arterial thrombosis, electrophysiological anomalies, and myocardial injury.2,3 Acute myocardial injury associated with COVID-19 is considered a rare but serious cardiovascular complication,4,5 but information on its prevalence and pathogenesis is limited. Although, blood troponin6,7 and echocardiography8–10 have been used to evaluate acute cardiovascular complications, neither test is ideal, in terms of sensitivity and specificity, for detecting myocardial injury.
Cardiovascular magnetic resonance (CMR) is an imaging modality that can evaluate myocardial histology and can accurately detect pathological changes such as myocardial necrosis, edema, and fibrosis. CMR is particularly useful in detecting the myocarditis-like myocardial injury seen in COVID-19 infection.11 Previous studies have used CMR to investigate the pattern of myocardial injury in COVID-19, mainly in the recovery phase.12,13 However, most of these previous studies have been retrospective studies, with only a limited number of prospective studies using CMR and focusing on COVID-19 patients suspected of myocardial injury.
Therefore, we conducted a national multicenter study to elucidate the prevalence of myocardial injury in COVID-19 patients, as well as the pattern and severity of myocardial injury on CMR.
Methods
Among the hospitals that participated in the multicenter prospective cohort study conducted by the Japanese Respiratory Society, 7 hospitals in Japan capable of performing CMR took part in the present study. This research is distinct from the COVID-19 study led by the Japanese Respiratory Society and involved prospective patient enrollment. To be eligible for inclusion in the present study, patients had to have COVID-19 of moderate or higher severity according to the Ministry of Health, Labour and Welfare severity criteria14 and to meet any one of the following: (1) high-sensitivity troponin positivity confirmed at least once during hospitalization or at the 3-month follow-up; (2) high-sensitivity troponin value recorded during hospitalization or up to 3 months after discharge, with a confirmed increase of ≥50% from the time of admission (or ≥150% of the measured sensitivity concentration if the time of admission was below the measured sensitivity); and (3) B-type natriuretic peptide (BNP) ≥100 pg/mL or N-terminal pro BNP (NT-proBNP) ≥300 pg/mL during hospitalization or up to 3 months after discharge. Patients with known coronary artery disease (myocardial infarction or angina pectoris) or heart failure before admission and patients for whom contrast-enhanced magnetic resonance imaging (MRI) is contraindicated (i.e., patients with claustrophobia, metal implants, severe renal dysfunction [estimated glomerular filtration rate <30 mL/min/1.73 m2], a history of serious side effects to gadolinium contrast media [anaphylactic reaction], and pregnant women) were excluded from the study. Patient enrollment is shown in Figure 1.
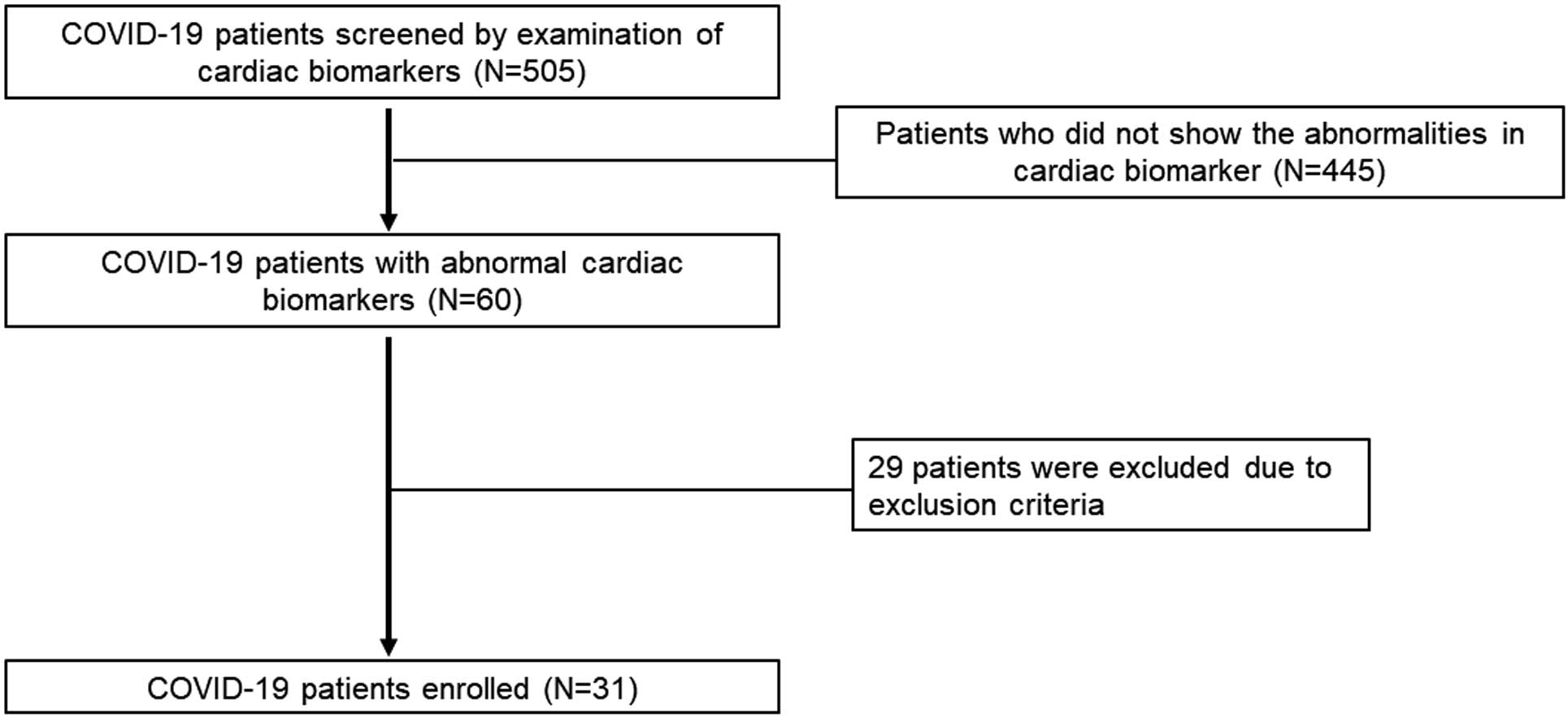
In all, 505 patients were screened for participation in the study. Of these, 60 showed abnormalities in cardiac biomarkers (troponin and/or BNP/NT-proBNP). Twenty-nine patients were excluded based on the exclusion criteria, leaving 31 patients who were enrolled in the study and underwent CMR (Figure 1).
This study was approved by the Institutional Review Board of Kochi University (Reference no. 2020-140) and the local ethics committees of all participating institutions. Written informed consent was obtained from all patients prior to study inclusion.
MRI Examination
In this study, CMR was performed 3 months after discharge. CMR was performed at each facility using 1.5- or 3.0-T MRI systems. With regard to the MRI sequences, cine MRI, late gadolinium enhancement (LGE)-MRI, and T2-weighted images (T2WI) were mandatory. The data for T1
mapping were obtained from 6 facilities capable of imaging, whereas T2
mapping was performed at only 1 facility. Cine MRI was performed using the steady-state free precession method, and left ventricular (LV) short-axis, 2-chamber, and 4-chamber images were acquired. For cine MRI, the entire right ventricle (RV) was included in the imaging range. LGE-MRI was based on the inversion recovery method, and LV short-axis, 2-chamber, and 4-chamber images were acquired. For black-blood T2WI, fat suppression by short tau inversion recovery was performed and LV short-axis, 2-chamber, and 4-chamber images were acquired. T1
mapping was performed using LV short-axis images. The Modified Look–Locker inversion recovery method was used for the T1
mapping sequence.15
MRI Image Analysis
CMR images were collected and subsequently analyzed at Yokohama City University, which serves as the core laboratory for image analysis. Images were analyzed by an analyst who was unaware of the relevant clinical information. CMR images were analyzed using dedicated software (Vitrea; Canon Medical Systems, Otawara, Japan). This software facilitates the semi-automatic tracing of endocardial and epicardial boundaries. The Simpson method was used to compute various parameters, including LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), stroke volume, and LV ejection fraction (LVEF). For the RV, short-axis images were used for volume analysis, enabling the calculation of RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV), RV stroke volume (RVSV), and RV ejection fraction (RVEF).
Regarding LGE-MRI, 2 types of assessment were conducted: visual and quantitative analysis. The visual evaluation involved a consensus reading by 2 observers to determine the presence of LGE in the myocardium of the LV or RV. In addition, visual assessment included evaluation of contrast enhancement in the pericardium. Quantitative evaluation of LGE-MRI was performed using the planimetry method in the aforementioned software (Vitrea; Canon Medical Systems). All LGE images used in the analysis were acquired using the inversion recovery method.
The percentage of LGE (%LGE) was calculated by dividing the volume of enhanced myocardium by the volume of non-enhanced myocardium and multiplying the result by 100.16 Myocardial edema in black-blood T2WI was defined as a signal intensity exceeding 2 standard deviations (SD) of the skeletal muscle signal intensity.17 Native T1
time was measured at the mid-level of the LV septum. The extracellular volume (ECV) fraction at the mid-level of the LV septum was also calculated using the following formulas:18
|
λ=
ΔR1
myo
ΔR1
blood
=
R1myo post−R1myo pre
R1blood post−R1blood pre
|
where λ is the partition coefficient, R1 is the inverse of T1, T1 is the T1
relaxation time, myo is the myocardium, pre and post refer to pre-contrast and post-contrast, respectively, and Hct is the hematocrit.
The cut-off value for T2
mapping was not set at 2SD above the facility-specific reference value, but rather was based on prior research, which indicated an indicative threshold for active myocarditis of >60 ms.19
Outcomes
The primary outcome in this study was myocardial injury, which was defined by either: (1) atypical enhancement on LGE-MRI in the LV, RV, or both; or (2) impaired LVEF (<50%) or RVEF (<45%),20 identified via cine MRI; or (3) discernible pericardial thickening or enhancement.12 In this study, we focused on evaluating CMR findings in the chronic phase at 3 months after diagnosis of COVID-19. Anticipating variability in these findings, we chose a composite endpoint as the primary outcome, rather than relying on a single CMR abnormality.
Secondary endpoints included an examination of the frequency of native T1
abnormalities by T1
mapping. Native T1
abnormalities were defined as an elevation >2SD of the facility-specific reference value (Supplementary Table). This approach is in accordance with the clinical recommendations for myocardial mapping of the Society for Cardiovascular Magnetic Resonance and the European Association for Cardiovascular Imaging.21 In addition, the number of patients fulfilling the modified Lake Louise criteria11 for the diagnosis of acute myocarditis via CMR was assessed.
Statistical Analysis
Data were analyzed using SPSS version 29.0 (SPSS Inc., Chicago, IL, USA). Continuous data are presented as the mean±SD or median with interquartile range (IQR), whereas categorical data are presented as numbers with percentages. The significance of differences in continuous values was evaluated using an unpaired t-test or the Mann-Whitney U test, depending on the normality of data distribution, assessed using the Shapiro-Wilk test. The significance of differences in categorical values was evaluated using Chi-squared tests. In consideration of the potential variability in the evaluation of chronic myocardial edema 3 months later, T2WI assessment was conducted independently by 2 observers. The reproducibility of the assessment was evaluated by calculating the intraclass correlation coefficient (ICC). P<0.05 was considered statistically significant.
Results
Patient Characteristics
The characteristics of the COVID-19 patients included in this study are presented in Table 1. The mean patient age was 63.5±10.4 years, 23 (74%) patients were male, and the mean body mass index was 24.6±6.0 kg/m2. Hypertension was present in 16 (52%) patients, diabetes in 11 (35%), and dyslipidemia in 7 (23%). Nine (29%) patients had renal impairment (eGFR <60 mL/min/1.73 m2) and 7 (23%) were current smokers. Upon admission, the mean SpO2
was 92.4±4.5%, indicating a slight decline. Blood analyses revealed mildly elevated d-dimer concentrations (mean 2.0±3.3 μg/mL), whereas Krebs von den Lungen 6 (KL-6) concentrations (mean 332±301 U/mL) were within the normal range. Median concentrations of the myocardial biomarkers were as follows: troponin T, 0.013 ng/mL (IQR 0.010–0.016 ng/mL); BNP, 127.5 pg/mL (IQR 80.6–189.9 pg/mL); and NT-proBNP, 124.5 pg/mL (IQR 48.2–343.7 pg/mL). Abnormalities in troponin T were observed in 48% (15/31) of patients, and 68% (21/31) had abnormalities in BNP or NT-proBNP. The mean interval between the diagnosis of COVID-19 and CMR was 117±16 days (range 94–149 days).
Table 1.
Characteristics of the 31 Patients With COVID-19 Included in the Study
| Age (years) |
63.5±10.4 |
| Male sex |
23 (74) |
| Height (cm) |
164.9±7.4 |
| Weight (kg) |
67.5±16.0 |
| BMI (kg/m2) |
24.6±6.0 |
| Hypertension |
16 (52) |
| Diabetes |
11 (35) |
| Dyslipidemia |
7 (23) |
| Renal impairmentA |
9 (29) |
| Smoking history |
| Current smoker |
7 (23) |
| Ex-smoker |
14 (45) |
| Never smoker |
10 (32) |
| SpO2 on admission (%) |
92.4±4.5 |
| Blood testing |
| WBC count (/μL) |
7,417±4,607 |
| Hemoglobin (g/dL) |
14.3±1.6 |
| Hematocrit (%) |
41.0±6.4 |
| D-dimer (μg/mL) |
2.0±3.3 |
| KL-6 (U/mL) |
332±301 |
| Troponin T (ng/mL) |
0.013 [0.010–0.016] |
| BNP (pg/mL) |
127.5 [80.6–189.9] |
| NT-proBNP (pg/mL) |
124.5 [48.2–343.7] |
| % Patients with abnormal troponin T |
48 (15/31) |
| % Patients with abnormal BNP or NT-proBNP |
68 (21/31) |
Values are presented as mean±SD, median [interquartile range], or n (%). ARenal impairment was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. BMI, body mass index; BNP, B-type natriuretic peptide; KL-6, sialylated carbohydrate antigen; NT-proBNP, N-terminal pro B-type natriuretic peptide; WBC, white blood cell.
Prevalence of Myocardial Injury in COVID-19 Assessed by CMR
Table 2 provides a comprehensive overview of the CMR findings in COVID-19 patients. Among the patient cohort, 26% (8/31) exhibited LV LGE. Conversely, none of the patients exhibited RV LGE. Cine MRI analysis revealed LV systolic dysfunction (LVEF <50%) in 4 (13%) patients and RV systolic dysfunction (RVEF <45%) in 6 (19%) patients. No contrast effects on the pericardium were observed. Native T1
abnormalities in the LV myocardium were found in 48% (14/29) of patients, whereas myocardial edema, as indicated by T2WI or T2
mapping, was present in 10 (32%) patients. The reproducibility of T2WI assessment showed high interobserver reproducibility, with an ICC of 0.93 (95% confidence interval [CI] 0.85–0.96). Based on these anomalous CMR findings, 8 patients (26%) met the modified Lake Louise criteria, and 12 patients (42%) reached the primary endpoint (Table 2). ECV of the septum of the LV myocardium was measured in 29 patients, in whom the mean ECV fraction was 0.30±0.06.
Table 2.
Results of Cardiac MRI in Patients With COVID-19
| Abnormal findings on CMR sequences |
| LGE on the LV |
8/31 (26) |
| LGE on the RV |
0/31 (0) |
| %LGE |
15.1±13.1 |
| LV dysfunction (LVEF <50%) |
4/31 (13) |
| RV dysfunction (RVEF <45%) |
6/31 (19) |
| Pericardial enhancement |
0/31 (0) |
| Native T1 abnormalityA |
14/29 (48) |
| ECV fraction |
0.30±0.06 |
| Myocardial edema on T2WI/T2 mapping |
10/31 (32) |
| Positive for criteria based on abnormal CMR findings |
| Modified Lake Louise criteria |
8/31 (26) |
| Primary endpointB |
12/31 (42) |
Values are presented as mean±SD or n (%). AAn abnormality in native T1
was defined as a high value exceeding 2SD of each facility’s reference value. BThe primary endpoint was myocardial injury defined by any one of the following: (1) abnormal enhancement in the left (LV) or right ventricle (RV) or both on late gadolinium enhancement (LGE) magnetic resonance imaging (MRI); (2) LV dysfunction (LV ejection fraction [LVEF] <50%) or RV dysfunction (RV ejection fraction [RVEF] <45%) on cine MRI; or (3) pericardial thickening or abnormal enhancement. CMR, cardiac magnetic resonance; ECV, extracellular volume; T2WI, T2-weighted image.
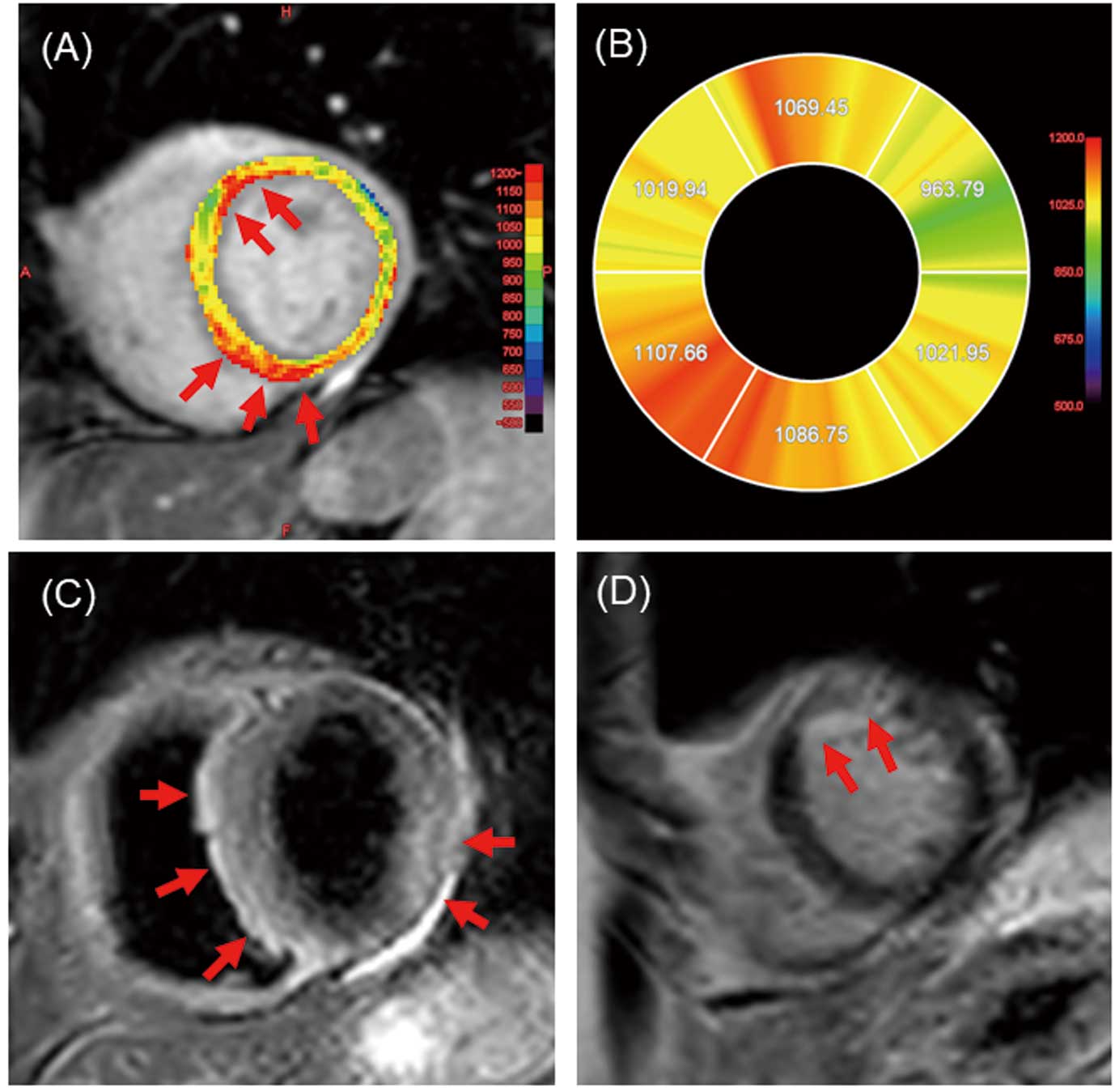
Anomalies in T1
mapping, T2WI, and LGE in a patient are shown in Figure 2. Native T1
mapping revealed elevated values in the anterior wall and septum (Figure 2A,B), whereas T2WI showed myocardial edema in the septum and posterior wall (Figure 2C). LGE showed irregular contrast enhancement in the anterior wall (Figure 2D). These observations culminated in positive fulfillment of the Lake Louise criteria.
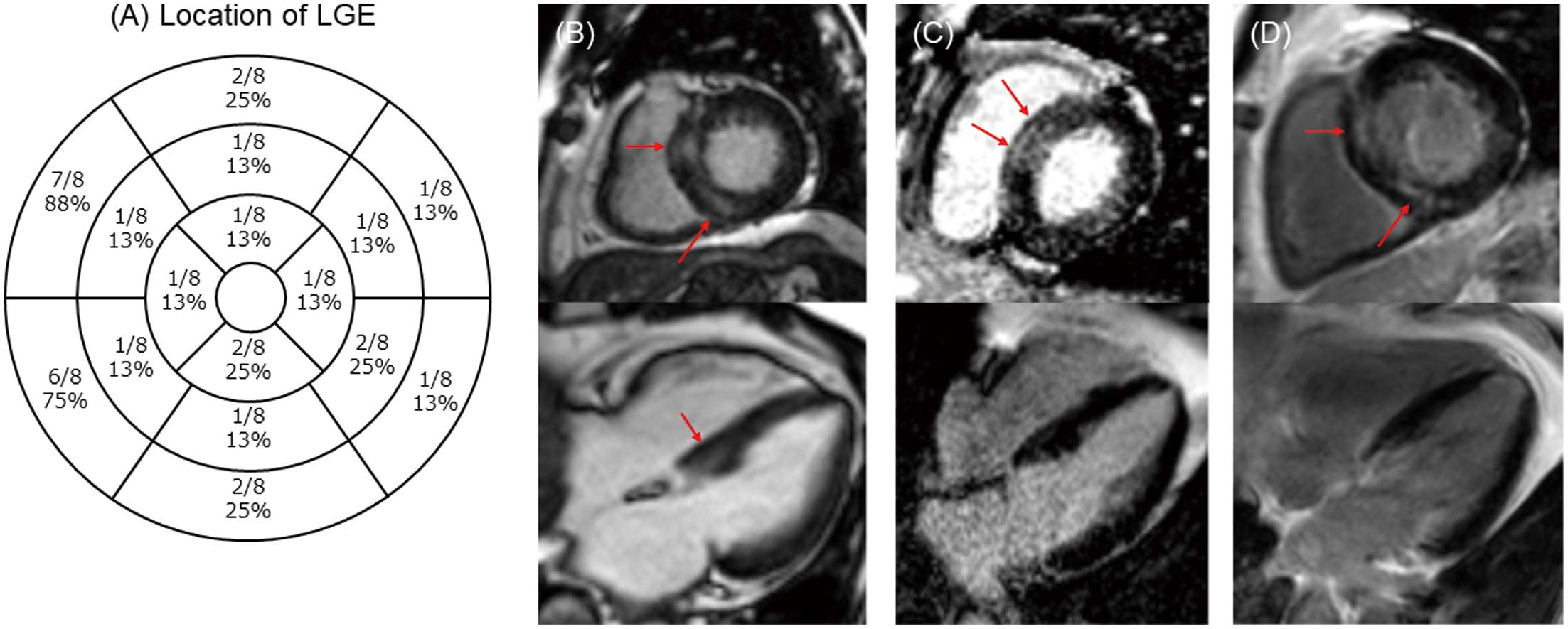
Location and Pattern of Myocardial Injury on CMR
Figure 3A shows the precise positioning of LGE within the LV according to the American Heart Association (AHA) 16-segment model. All 8 patients in this study who presented with LV LGE had LGE along the mid-myocardial or epicardial side, reflecting a non-ischemic myocardial injury pattern. In addition, 1 (13%) patient showed subendocardial LGE, implying a potential coexisting myocardial infarction. Images in Figure 3B–D are from 3 representative COVID-19 patients, each of whom had LGE in the middle layer of the ventricular septal wall. These findings suggest that the basal septum may be a preferred site of myocardial injury in COVID-19. The mean %LGE obtained from quantitative analysis of LGE was 15.1±13.1% (Table 2).
Presence or Absence of Abnormal CMR Findings and Clinical Observations
Patients were divided into 2 groups based on the presence or absence of the primary endpoint; Table 3 compares clinical information, troponin T, and BNP/NT-pro-BNP concentrations between these 2 groups. The most significant difference was observed for troponin T during the acute phase, suggesting its value in predicting the primary endpoint. In addition, we compared patients in terms of the presence or absence of myocardial edema (Table 4). Troponin T concentrations at both admission and the 3-month follow-up were significantly higher in the group with than without myocardial edema (at admission, 0.017 [IQR 0.017–0.018] vs. 0.011 [IQR 0.01–0.015] ng/mL [P=0.001]; at 3 months, 0.016 [IQR 0.008–0.020] vs. 0.010 [IQR 0.007–0.011] ng/mL [P=0.043]; Table 4). However, there were no significant differences in BNP or NT-proBNP concentrations between the 2 groups. These findings suggest the potential utility of measuring troponin T to evaluate the presence of myocardial edema, particularly during the acute phase.
Table 3.
Clinical Information in Patients With and Without the Primary Endpoint (the Presence of Myocardial Injury)
| |
Primary endpoint |
P value |
| Yes (n=12) |
No (n=19) |
| Age (years) |
65.6±8.2 |
61.9±11.7 |
0.33 |
| Male sex |
83% (10/12) |
71% (15/21) |
0.72 |
| BMI (kg/m2) |
24.6±5.8 |
24.6±6.3 |
0.99 |
| SpO2 on admission (%) |
91.9±3.6 |
92.8±5.1 |
0.61 |
| WBC count (/μL) |
7,701±4,398 |
7,211±4,867 |
0.78 |
| Hemoglobin (g/dL) |
15.0±1.0 |
13.8±1.8 |
0.046 |
| Hematocrit (%) |
42.2±8.3 |
40.2±4.6 |
0.38 |
| D-dimer (μg/mL) |
1.2±10 |
2.6±4.2 |
0.27 |
| KL-6 (U/mL) |
356±350 |
315±270 |
0.72 |
| Troponin T (ng/mL) |
0.017 [0.017–0.018] |
0.011 [0.010–0.015] |
0.001 |
| BNP (pg/mL) |
123.6 [20–202] |
123.3 [79.6–176.2] |
0.19 |
| NT-proBNP (pg/mL) |
127.95 [91.3–164.2] |
107.9 [33.8–300.2] |
0.085 |
| At 3 months |
| Troponin T (ng/mL) |
0.016 [0.008–0.020] |
0.010 [0.007–0.011] |
0.043 |
| BNP (pg/mL) |
40.8 [16.8–76.5] |
11.6 [10.1–14.3] |
0.62 |
| NT-proBNP (pg/mL) |
113.5 [84.0–141.2] |
49.2 [27.4–125] |
0.083 |
Unless indicated otherwise, values are presented as the mean±SD, median [interquartile range], or n (%). Abbreviations as in Table 1.
Table 4.
Clinical Information in Patients With and Without Myocardial Edema Detected by CMR on T2WI/T2 Mapping
| |
Myocardial edema |
P value |
| Yes (n=10) |
No (n=21) |
| Age (years) |
66.7±8.9 |
61.9±10.8 |
0.42 |
| Male sex |
90% (9/10) |
67% (7/21) |
0.34 |
| BMI (kg/m2) |
26.0±6.1 |
23.9±5.9 |
0.82 |
| SpO2 on admission (%) |
92.3±4.3 |
92.5±4.7 |
0.92 |
| WBC count (/μL) |
8,522±4,755 |
6,890±4,556 |
0.37 |
| Hemoglobin (g/dL) |
15.1±1.0 |
13.9±1.7 |
0.043 |
| Hematocrit (%) |
44.5±2.9 |
39.4±7.0 |
0.033 |
| D-dimer (μg/mL) |
1.1±0.9 |
2.5±3.9 |
0.26 |
| KL-6 (U/mL) |
357±397 |
320±254 |
0.75 |
| Troponin T (ng/mL) |
0.017 [0.017–0.018] |
0.011 [0.01–0.015] |
0.001 |
| BNP (pg/mL) |
123.6 [20–202] |
131.4 [108.05–180.3] |
0.19 |
| NT-proBNP (pg/mL) |
127.95 [91.3–164.2] |
124.5 [48.2–570] |
0.085 |
| At 3 months |
| Troponin T (ng/mL) |
0.016 [0.008–0.020] |
0.010 [0.007–0.011] |
0.043 |
| BNP (pg/mL) |
40.8 [16.8–76.5] |
11.6 [10.125–14.35] |
0.62 |
| NT-proBNP (pg/mL) |
113.5 [84.0–141.2] |
49.2 [26.1–181] |
0.083 |
Unless indicated otherwise, values are presented as the mean±SD, median [interquartile range], or n (%). Abbreviations as in Tables 1,2.
Discussion
In this prospective multicenter study, the prevalence of myocardial injury in convalescent COVID-19 patients was determined using CMR. LGE-MRI revealed a substantial prevalence of anomalous contrast enhancement within the basal septum of the LV. Specifically, 42% of patients exhibited cardiac injury as per the primary endpoint, whereas 26% of patients fulfilled the Lake Louise criteria for acute myocarditis on CMR. The outcomes of this study have the potential to yield valuable insights into cardiac injury during the post-COVID-19 convalescence phase.
Myocardial injury resulting from COVID-19 represents an infrequent yet consequential complication. Several mechanisms for the myocardial injury have been postulated, including direct viral invasion of the myocardium, injury linked to inflammation, an imbalance in oxygen supply and demand, and microvascular dysfunction.10 The renin-angiotensin system (RAS), particularly angiotensin-converting enzyme 2 (ACE2), plays a role in COVID-19 pneumonia, where infection occurs via ACE2. However, ACE2 is also expressed in myocardial tissue and is suspected to be involved in COVID-19-induced myocardial injury.22 Pathological investigations have also indicated the possibility of viral infection of endothelial cells and endotheliitis,23 and it is recognized that Type 2 myocardial infarctions can manifest in COVID-19 patients.5,24 Initial myocardial injury in COVID-19 primarily presents with abnormalities in myocardial troponin concentrations. A report from New York State indicated that 36% (985/2,736) of COVID-19 patients exhibited elevated troponin I concentrations (>0.03 ng/dL), with even minor elevations being associated with an unfavorable prognosis.6 A study from Wuhan also noted that abnormalities in cardiac markers such as troponin and BNP were associated with a poorer prognosis, particularly among elderly patients.7 Subsequently, numerous non-invasive echocardiographic reports have highlighted the association between right heart system dilatation, RV systolic dysfunction, and an adverse prognosis.9,25
CMR is an exceptional modality for evaluating myocardial histology, promptly reflecting pathological changes such as myocardial fibrosis, edema, and necrosis. Consequently, CMR has emerged as a promising and accurate diagnostic tool for assessing myocardial injury. An early study by Puntmann et al demonstrated abnormal CMR findings in 78 of 100 COVID-19 patients, with native T1
abnormalities being the most prevalent.12 Since then, several studies have reported abnormal CMR findings in COVID-19 patients, particularly from the US, European countries, and China. A recent meta-analysis revealed that the frequency of CMR abnormalities was 27.5% for recent LV LGE, 11.9% for pericardial contrast enhancement, 39.5% for abnormal T1
values, and 38.0% for abnormal T2
values.22 These findings align closely with the outcomes of the present study, demonstrating similarity in LV contrast effects, abnormal T1
values, and abnormal T2
values. However, the finding of pericardial contrast enhancement in the present study was less frequent compared with the findings of the meta-analysis. The precise reasons for this disparity remain unclear, but previous meta-analyses have revealed substantial variability in the frequency of abnormal CMR findings in COVID-19, likely influenced by different eligibility criteria and imaging evaluation methods across the different studies.22 To minimize the variability encountered in different reports, a meticulously conducted prospective study adhering to rigorous criteria would be immensely valuable.
Some previous investigations have yielded outcomes consistent with the findings of the present study. For example, Wang et al documented comparable results, with LGE detected in 30% of 44 convalescent COVID-19 patients, all of whom exhibited mid-myocardial or subepicardial distribution.26 Similarly, Knight et al reported myocarditis-like LGE in 45% of 29 COVID-19 patients.27 Recently, 2 large multicenter retrospective cohort studies of COVID-19-related myocardial injury were conducted.28,29 The large retrospective analysis of 1,047 COVID-19 patients from 18 international centers reported that 20.9% of patients had a non-ischemic injury pattern and that elevated troponin was a significant risk factor for cardiac injury (odds ratio 25.27).28 In the other study, the prevalence of myocardial injury was reported to be 2.4–4 per 1,000 patients and correlated with poor prognosis.29 In the present study, 26% of patients also had myocarditis-like myocardial injury, consistent with the findings of these previous studies.
The observation of myocardial edema 3 months after diagnosis in the present study is also intriguing. Myocardial edema is typically observed only in the acute phase, yet we found that it persisted in 32% of patients in the chronic phase. Previous studies have shown that despite a median of 71 days from diagnosis to CMR, myocardial edema was found in approximately 60% of patients,12 which is roughly twice as many as in in the present study. Furthermore, basic research suggests that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can sustain a persistent infection in the myocardium, and high-titer infections can lead to abnormal cardiac contractile function, potentially acting as a trigger for future heart failure.30 Our findings may also be indicative of persistent myocardial infection due to SARS-CoV-2.
Another unique aspect of our study is that we were able to assess the frequency of cardiac impairment using the entire screened patient population as the denominator. Overall, we screened 505 patients with moderate or higher severity COVID-19, of whom 60 (11%) had abnormal cardiac biomarkers and 12 (2.3%) ultimately met the primary endpoint. Compared with a previous study,29 we observed a slightly higher frequency of cardiac impairment in the present study. The high frequency may be due to the inclusion criteria used for this study, which included patients with moderate disease or higher.
Another discovery of note in this study pertains to the analysis of the sites and patterns of myocardial injury. Among the CMR sequences used, LGE holds particular significance due to its specificity in detecting myocardial fibrosis, particularly focal fibrosis. This study showed a prevalent occurrence of LGE with a non-ischemic pattern in the septum of the cardiac base among COVID-19 patients suspected of having myocardial injury (Figure 2). A recent study by Fronza et al also demonstrated a higher frequency of LGE in the basal septum and inferior wall of COVID-19 patients, distinguishing it from patterns observed in viral myocarditis and post-vaccine myocarditis.31 Our observation of the location of LGE in the basal septum aligns with the findings of that study.
In terms of %LGE, the mean value in the present study was 15.1%. A multicenter study conducted in Poland reported a %LGE of 7.2±17.1% in 150 patients with COVID-19 myocarditis, which was significantly lower than the %LGE observed in patients with viral myocarditis (9.6±8.1%; P<0.01).32 At 15.1%, the %LGE in our patient group is slightly higher than the findings reported by the study from Poland. This may be due to the slightly higher severity of the patients included in our study compared with the previous study, because we included COVID-19 patients with moderate or higher disease severity.
Study Limitations
Our study has several important limitations that should be acknowledged. First, it is important to acknowledge that the frequency and severity of myocardial injury may vary across different strains of COVID-19, and the present study primarily focused on the Delta variant outbreak. Second, due to the absence of post-CMR imaging follow-up, the significance of abnormal CMR findings to patient prognosis remains undetermined. Third, because of the strain on the healthcare system caused by worsening of COVID-19 outbreak, we were only able to enroll a smaller number of patients than initially calculated in our sample size estimation. (In past reports, CMR examinations conducted on COVID-19 patients revealed abnormal findings in 78% of cases.12 For the present study, we aimed to investigate a population with a higher pretest probability, expecting to find abnormal CMR findings in approximately 85% of cases. When estimating a relative precision of 7.5% (with a 95% CI of 6.4%), the actual analyzable sample size required would be 126 cases. Considering dropouts and variations in the quality of CMR images obtained, we set the anticipated sample size at 150 cases.) Fourth, we do not have detailed patient information for the COVID-19 patients who underwent screening at each facility but did not undergo CMR. Fifth, it is important to acknowledge that the validity of using LVEF <50% and RVEF <45% as cut-off criteria for COVID-19 myocardial injury is not firmly established. However, we based these criteria for abnormal contractility on the fact that the cut-off value for heart failure with mildly reduced ejection fraction is an LVEF of 50%,33 and an RVEF cut-off of 45% is used for prognostic prediction in cardiomyopathy.20 Therefore, although we used these values in the present study, it should be noted that their validity needs to be explored in future research. Sixth, in this multicenter study, the type and dose of contrast media varied from center to center (Supplementary Table). The possibility that these differences may have influenced the quantification of LGE cannot be disregarded.
Conclusions
In this prospective multicenter study, myocardial injury observed by CMR in patients with COVID-19 was identified at a high frequency of approximately 40%. Although further investigations are needed to assess the prognostic impact, the findings of this study provide valuable insights into myocardial injury in COVID-19 patients.
Sources of Funding
This work was supported by the Ministry of Health, Labour and Welfare (20CA2052).
Disclosures
Y.M. has received honoraria from Otsuka Pharmaceutical Co., Novartis Pharma K.K., Bayer Inc., and AstraZeneca; and research grants from Pfizer Japan Inc., Otsuka Pharmaceutical Co., EN Otsuka Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. K.H. and K.N. are members of Circulation Journal’s Editorial Board. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Institutional Review Board of Kochi University (Reference no. 2020-140), and the local ethics committees of all participating institutions.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0729
References
- 1.
Narayan KMV, Curran JW, Foege WH. The COVID-19 pandemic as an opportunity to ensure a more successful future for science and public health. JAMA 2021; 325: 525–526, doi:10.1001/jama.2020.23479.
- 2.
Tomidokoro D, Hiroi Y. Cardiovascular implications of the COVID-19 pandemic. J Cardiol 2022; 79: 460–467, doi:10.1016/j.jjcc.2021.09.010.
- 3.
Nishimoto Y, Yachi S, Takeyama M, Tsujino I, Nakamura J, Yamamoto N, et al. The current status of thrombosis and anticoagulation therapy in patients with COVID-19 in Japan: From the CLOT-COVID study. J Cardiol 2022; 80: 285–291, doi:10.1016/j.jjcc.2022.03.015.
- 4.
Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020; 141: 1903–1914, doi:10.1161/circulationaha.120.047349.
- 5.
Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-segment elevation in patients with COVID-19: A case series. N Engl J Med 2020; 382: 2478–2480, doi:10.1056/NEJMc2009020.
- 6.
Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020; 76: 533–546, doi:10.1016/j.jacc.2020.06.007.
- 7.
Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020; 41: 2070–2079, doi:10.1093/eurheartj/ehaa408.
- 8.
Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation 2020; 142: 342–353, doi:10.1161/circulationaha.120.047971.
- 9.
Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging 2020; 13: 2459–2461, doi:10.1016/j.jcmg.2020.05.010.
- 10.
Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020; 76: 2043–2055, doi:10.1016/j.jacc.2020.08.069.
- 11.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176, doi:10.1016/j.jacc.2018.09.072.
- 12.
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1265–1273, doi:10.1001/jamacardio.2020.3557.
- 13.
Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021; 42: 1866–1878, doi:10.1093/eurheartj/ehab075.
- 14.
Yamakawa K, Yamamoto R, Terayama T, Hashimoto H, Ishihara T, Ishimaru G, et al. Japanese rapid/living recommendations on drug management for COVID-19: Updated guidelines (July 2022). Acute Med Surg 2022; 9: e789, doi:10.1002/ams2.789.
- 15.
Kellman P, Hansen MS. T1-mapping in the heart: Accuracy and precision. J Cardiovasc Magn Reson 2014; 16: 2, doi:10.1186/1532-429x-16-2.
- 16.
Axelsson Raja A, Farhad H, Valente AM, Couce JP, Jefferies JL, Bundgaard H, et al. Prevalence and progression of late gadolinium enhancement in children and adolescents with hypertrophic cardiomyopathy. Circulation 2018; 138: 782–792, doi:10.1161/circulationaha.117.032966.
- 17.
Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson 2011; 13: 13, doi:10.1186/1532-429x-13-13.
- 18.
Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012; 126: 1206–1216, doi:10.1161/circulationaha.111.089409.
- 19.
Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, et al. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 2015; 8: e003073, doi:10.1161/circimaging.114.003073.
- 20.
Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013; 128: 1623–1633, doi:10.1161/circulationaha.113.002518.
- 21.
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017; 19: 75, doi:10.1186/s12968-017-0389-8.
- 22.
Kato S, Azuma M, Fukui K, Kodama S, Nakayama N, Kitamura H, et al. Cardiac involvement in coronavirus disease 2019 assessed by cardiac magnetic resonance imaging: A meta-analysis. Heart Vessels 2022; 37: 1570–1582, doi:10.1007/s00380-022-02055-6.
- 23.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418, doi:10.1016/s0140-6736(20)30937-5.
- 24.
Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, et al. ST-elevation myocardial infarction in patients with COVID-19: Clinical and angiographic outcomes. Circulation 2020; 141: 2113–2116, doi:10.1161/circulationaha.120.047525.
- 25.
Giustino G, Pinney SP, Lala A, Reddy VY, Johnston-Cox HA, Mechanick JI, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol 2020; 76: 2011–2023, doi:10.1016/j.jacc.2020.08.059.
- 26.
Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, et al. Cardiac involvement in COVID-19 patients: Mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021; 23: 14, doi:10.1186/s12968-021-00710-x.
- 27.
Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, et al. COVID-19: Myocardial injury in survivors. Circulation 2020; 142: 1120–1122, doi:10.1161/circulationaha.120.049252.
- 28.
Vidula MK, Rajewska-Tabor J, Cao JJ, Kang Y, Craft J, Mei W, et al. Myocardial injury on CMR in patients with COVID-19 and suspected cardiac involvement. JACC Cardiovasc Imaging 2023; 16: 609–624, doi:10.1016/j.jcmg.2022.10.021.
- 29.
Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, et al. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation 2022; 145: 1123–1139, doi:10.1161/circulationaha.121.056817.
- 30.
Murata K, Makino, A, Tomonaga K, Masumoto H. Predicted risk of heart failure pandemic due to persistent SARS-CoV-2 infection using a three-dimensional cardiac model. iScience 2023; 27: 108641, doi:10.1016/j.isci.2023.108641.
- 31.
Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, et al. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology 2022; 304: 553–562, doi:10.1148/radiol.212559.
- 32.
Haberka M, Rajewska-Tabor J, Wojtowicz D, Jankowska A, Miszalski-Jamka K, Janus M, et al. Perimyocardial injury specific for SARS-cov-2-induced myocarditis in comparison with non-COVID-19 myocarditis: A multicenter CMR study. JACC Cardiovasc Imaging 2022; 15: 705–707, doi:10.1016/j.jcmg.2021.11.002
- 33.
Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ J 2021; 85: 2252–2291, doi:10.1253/circj.CJ-21-0431.