Abstract
Background: Whether comprehensive risk assessment predicts post-referral outcome in patients with pulmonary arterial hypertension (PAH) referred for lung transplantation (LT) in Japan is unknown.
Methods and Results: We retrospectively analyzed 52 PAH patients referred for LT. Risk status at referral was assessed using 3- and 4-strata models from the 2022 European Society of Cardiology and European Respiratory Society guidelines. The 3-strata model intermediate-risk group was further divided into 2 groups based on the median proportion of low-risk variables (modified risk assessment [MRA]). The primary outcome was post-referral mortality. During follow-up, 9 patients died and 13 patients underwent LT. There was no survival difference among 3-strata model groups. The 4-strata model classified 33, 16, and 3 patients as low intermediate, high intermediate, and high risk, respectively. The 4-strata model identified high-risk patients with a 1-year survival rate of 33%, but did not discriminate survival between the intermediate-risk groups. The MRA classified 15, 28, 8, and 1 patients as low, low intermediate, high intermediate, and high risk, respectively. High intermediate- or high-risk patients had worse survival (P<0.001), with 1- and 3-year survival rates of 64% and 34%, respectively. MRA high intermediate- or high-risk classification was associated with mortality (hazard ratio 12.780; 95% confidence interval 2.583–63.221; P=0.002).
Conclusions: Patients classified as high intermediate or high risk by the MRA after treatment should be referred for LT.
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by increased pulmonary arterial pressure and vascular resistance due to remodeling of the pulmonary arterioles, eventually leading to progressive right heart failure and death.1–3 Advances in PAH-targeted therapy have improved the symptoms and prognosis of PAH patients over the past 20 years.4 However, PAH remains an incurable disease, with an estimated 3-year mortality rate of 30%.5 Hence, lung transplantation (LT) remains an important curative treatment for patients who are refractory to optimal combination therapy and are at high risk of death.6
Current guidelines recommend risk stratification based on an estimated 1-year mortality rate in order to optimize management, such as escalation of combination therapy or timely referral for LT.6 These guidelines recommend that patients at intermediate or high risk based on the 3-strata model, or patients at high intermediate or high risk based on the 4-strata model, are referred for LT.6–8 Recently, Vicaire et al showed that risk status at the time of LT listing could predict post-listing survival in a cohort of 102 PAH patients.5
However, given that organ allocation system differs by country, the optimal timing for referring PAH patients for LT may also vary and remains to be solved for each country. In Japan, where the waiting period is approximately 3 years due to the small number of brain-dead donors, clinicians have started to refer PAH patients for LT at an earlier stage of the disease, while facing the challenge of predicting medium- to long-term outcomes after referral.9,10 Therefore, a risk assessment tool for predicting post-referral outcome is warranted.
The aims of this retrospective study were to investigate the predictive value of current PAH risk assessment at the time of LT referral and to develop a decision-making tool for timely referral of patients for LT.
Methods
Study Patients and Data Collection
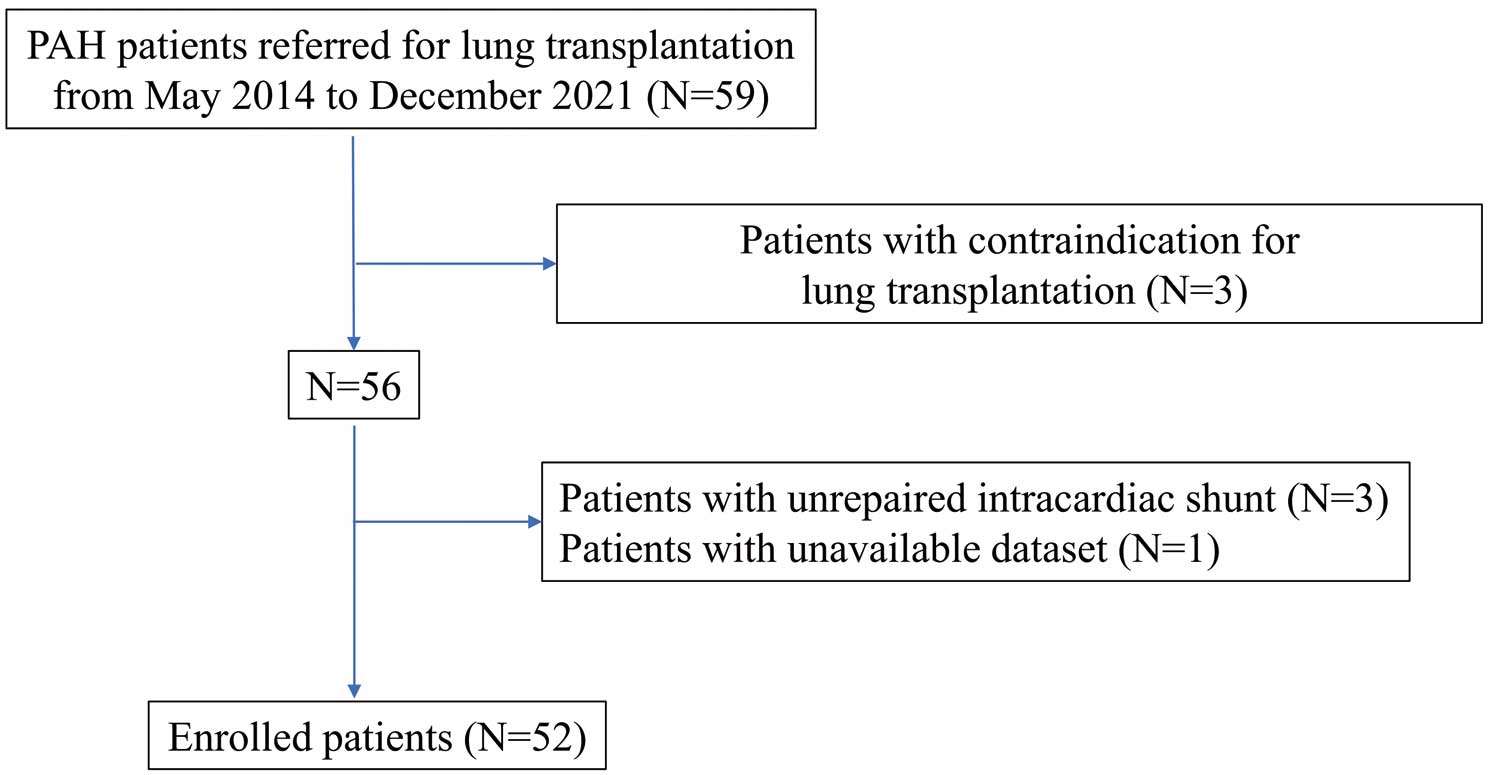
We performed a retrospective cohort study by analyzing the records of 59 consecutive patients with Group 1 pulmonary hypertension referred for LT evaluation at The University of Tokyo Hospital between May 2014 and December 2021 (Figure 1). Among these 59 patients, 3 with contraindications for LT (hypertrophic cardiomyopathy, malignancy, or lack of family support) were excluded. Another 3 patients with unrepaired intracardiac shunt were also excluded from the study and 1 patient was excluded because the last hemodynamic study had been conducted more than 1 year before referral. Thus, the final cohort in this study consisted of 52 patients. The data used for analysis was that obtained at the time of referral.
This study was performed according to the ethical guidelines of The University of Tokyo and in accordance with the Declaration of Helsinki. Approval for the study was granted by the Ethics Committee of The University of Tokyo (No. 2650), which waived the requirement for written informed consent because of the retrospective nature of the study. As an alternative, patients were provided the opportunity to withdraw from the study (https://cardiovasc.m.u-tokyo.ac.jp/consultation/clinicalresearch/retrospective_data).
Clinical Measurements
Echocardiographic measurements were performed as described previously.11 Briefly, patients underwent 2-dimensional echocardiography performed by experienced operators using commercially available devices in accordance with the guidelines of the American Society of Echocardiography.12 Tricuspid annular plane systolic excursion (TAPSE) was measured on the M-mode tracing obtained from the right ventricle (RV)-focused apical 4-chamber view using the distance of systolic excursion of the RV annular segment along its longitudinal plane. The tricuspid regurgitation (TR) pressure gradient was calculated from the continuous-wave Doppler TR velocity using the simplified Bernoulli equation. Pulmonary artery systolic pressure (PASP) was calculated using the following formula:
PASP = 4 × (peak velocity of TR)2 + estimated RAP
where RAP is right atrial pressure. RAP was estimated based on inferior vena cava diameter and collapsibility, following the guidelines.13 The TAPSE/PASP ratio, a known parameter for assessing RV-pulmonary circulation coupling, was also calculated.6
Hemodynamic parameters were measured in the cardiac catheterization laboratory during right heart catheterization. Cardiac output was measured by the thermodilution method, the use of which is recommended when the direct Fick method is not available.6,14
PAH Risk Assessment
As shown in Figure 2, patients were classified as having low-, intermediate- or high-risk status using the 3-strata model from the 2022 European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines, as well as being at low, low intermediate, high intermediate or high risk according to the 4-strata model.6 As variables for risk assessment, World Health Organization (WHO) functional class, 6-minute walk distance, B-type natriuretic peptide (BNP), TAPSE/PASP, mean RAP, and cardiac index were included, with cut-off values defined in the risk table of the 2022 ESC/ERS guidelines used.6
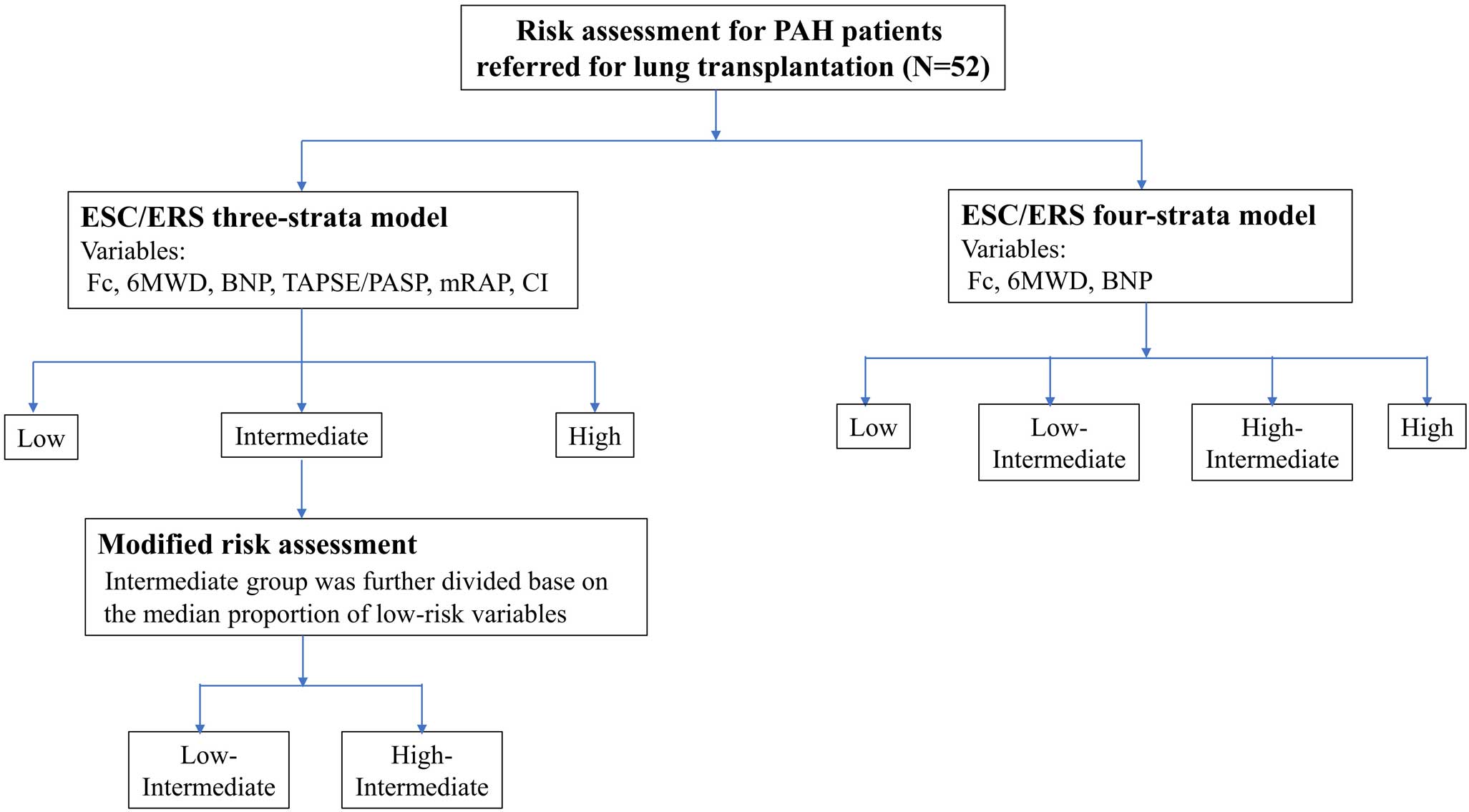
In the 3-strata model, patients were categorized as “low risk”, “intermediate risk”, or “high risk” according to cut-off values for each variable. Each variable was graded from 1 to 3, where 1=“low risk”, 2=“intermediate risk”, and 3=“high risk”. The mean was calculated by dividing the sum of all grades by the number of variables available for each patient. The mean grade was rounded off to the nearest integer, which was used to define the patient’s risk group.15–17
In the 4-strata model, each variable was graded from 1 to 4, where 1=“low risk”, 2=“low intermediate risk”, 3=“high intermediate risk” and 4=“high risk”. The mean was calculated by dividing the sum of all grades by the number of variables and rounding to the next integer, which was used to define the patient’s risk group.18
In the exploratory analysis, patients in the intermediate-risk group according to the 3-strata model were further divided into 2 groups based on the median proportion of low-risk variables achieved among 6 variables (termed “modified risk assessment”): a low intermediate-risk group and a high intermediate-risk group.
Follow-up Data Analysis
For follow-up data analysis, the BNP concentration at the final evaluation was collected. N-Terminal pro BNP (NT-proBNP) N was converted into BNP using the following conversion formula based on previous reports:19
log10[NT-proBNP] = 1.1 × log10[BNP] + 0.570
Outcome
Patients were followed up from the date of referral for LT evaluation to January 31, 2023. After LT evaluation, patients were registered with the Japan Organ Transplantation Network, where the waitlist order system is adopted for allocating cadaveric lungs. The primary outcome was all-cause mortality after referral. Patients who survived without LT were censored on January 31, 2023. Patients who underwent LT were censored on the date of LT.
Statistical Analyses
All statistical analyses were performed using SPSS statistics 19 (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean±SD or median with interquartile range (IQR) unless specified otherwise. Continuous variables were compared between groups using the Mann-Whitney test for non-normally distributed data. Kaplan-Meier analysis was performed to assess the survival of the overall cohort and subgroups stratified according to the etiology or risk stratification, and the log-rank test was used to compare the distribution of survival. Univariate and multivariate Cox proportional hazards analyses were used to investigate the association of risk stratification with all-cause mortality after referral. In multivariate analysis, age, sex, etiologies of PAH, and risk stratification were used as covariates. For all analyses, two-tailed P<0.05 was considered statistically significant.
Results
Study Population
In all, 52 PAH patients were included in the present study. The demographic, functional, laboratory, echocardiographic, and hemodynamic characteristics of the study population at the time of referral are summarized in Table 1. The median age was 30.5 years (IQR 22.0–40.0 years), and 34 (65%) patients were female. PAH etiology included idiopathic or heritable PAH (I/HPAH) in 43 (83%) patients, PAH related to connective tissue disease (CTD-PAH) in 5 (9%) patients, PAH with feature of venous involvement (pulmonary veno-occlusive disease [PVOD]) in 3 (6%) patients, and PAH related to repaired congenital heart disease (CHD-PAH) in 1 (2%) patient. All patients were on combination therapy, and 47 (91%) patients were receiving high-dose parental prostacyclin therapy (epoprostenol, 81.0 ng/kg/min [IQR 52.1–112.8 ng/kg/min]; treprostinil, 103.2±42.2 ng/kg/min). Despite optimal combination therapy, all patients were in WHO functional class III or IV at the time of referral.
Table 1.
Baseline Characteristics of Pulmonary Arterial Hypertension Patients Referred for Lung Transplantation (n=52)
| Observation period (years) |
2.5 [1.8–3.4] |
| Outcome |
| Lung transplantation |
13 (25) |
| Death |
9 (17) |
| Age (years) |
30.5 [22.0–40.0] |
| Male sex |
18 (35) |
| BMI (kg/m2) |
19.8 [18.1–21.3] |
| Diagnosis |
| I/HPAH |
43 (83) |
| CTD-PAH |
5 (9) |
| PVOD |
3 (6) |
| CHD-PAH |
1 (2) |
| Medication management |
| Epoprostenol |
32 (62) |
| Treprostinil |
15 (29) |
| Selexipag |
7 (13) |
| PDE5 inhibitor |
30 (58) |
| sGC stimulator |
21 (40) |
| ERA |
51 (98) |
| Dose of parental prostacyclin |
| Epoprostenol (ng/kg/min) |
81.0 [52.1–112.8] |
| Treprostinil (ng/kg/min) |
103.2±42.2 |
| Combination therapy |
| 2 drugs |
6 (12) |
| 3 or more drugs |
46 (88) |
| WHO functional class |
| III |
49 (94) |
| IV |
3 (6) |
| 6-minute walk distance (m) |
409 [360–484] |
| Laboratory data |
| BNP (pg/mL) |
38.5 [14.1–169.5] |
| Echocardiography |
| TAPSE/PASP (mm/mmHg; n=50) |
0.30±0.12 |
| Right heart catheterization |
| mRAP (mmHg) |
7.0 [5.0–9.0] |
| mPAP (mmHg) |
53.2±14.3 |
| Cardiac index (L/min/m2) |
4.03 [3.01–4.92] |
| PVR (Wood units) |
7.88±3.85 |
| Risk stratification |
| 3-strata model |
| Low risk |
15 (29) |
| Intermediate risk |
36 (69) |
| High risk |
1 (2) |
| 4-strata model |
| Low risk |
0 |
| Low intermediate risk |
33 (63) |
| High intermediate risk |
16 (31) |
| High risk |
3 (6) |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). BMI, body mass index; BNP, B-type natriuretic peptide; CHD-PAH, pulmonary arterial hypertension (PAH) related to repaired congenital heart disease; CTD-PAH, PAH related to connective tissue disease; ERA, endothelin receptor antagonist; I/HPAH, idiopathic/hereditary PAH; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PASP, pulmonary arterial systolic pressure; PDE5, phosphodiesterase 5; PVOD, pulmonary veno-occlusive disease; PVR, pulmonary vascular resistance; sGC, soluble guanylate cyclase; TAPSE, tricuspid annular plane systolic excursion; WHO, World Health Organization.
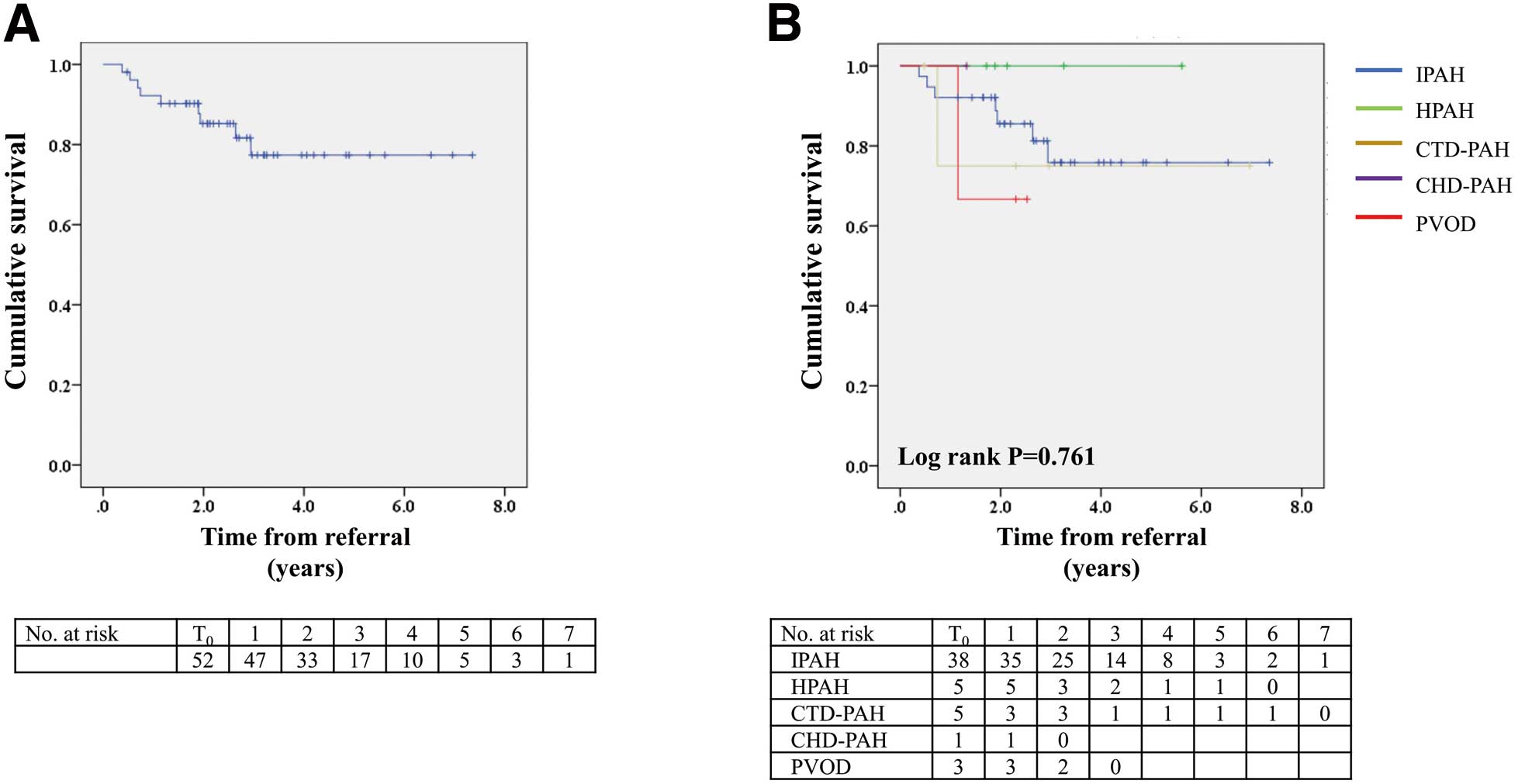
Over a median follow-up period of 2.5 years (IQR 1.8–3.4 years), 9 patients (7 with IPAH, 1 with CTD-PAH, and 1 with PVOD) died of severe respiratory and circulatory failure, and 13 patients underwent LT. Overall survival rates after referral were 92%, 85%, and 77% at 1, 2, and 3 years, respectively (Figure 3A). Kaplan-Meier analysis showed no significant difference in post-referral survival among subgroups stratified by PAH etiologies (Figure 3B).
3-Strata Risk Assessment at the Time of Referral
According to the 2022 ESC/ERS 3-strata model, 15 (29%), 36 (69%), and 1 (2%) patients in this study were classified as having low, intermediate and high mortality risk status. The median values of the calculated scores were 1.33 (range 1.17–1.40) and 1.67 (range 1.50–2.33) in the low- and intermediate-risk groups, and the 1 patient in the high-risk group had a score of 2.50. Kaplan-Meier analysis showed no significant difference in post-referral survival between the low- and intermediate-risk groups (log-rank test, P=0.334); survival rates at 1, 2, and 3 years were 100%, 92%, and 92%, respectively, in the low-risk group and 89%, 82%, and 70%, respectively, in the intermediate-risk group (Figure 4A). Cox proportional analysis (with the low-risk group as the reference) showed that intermediate risk was not associated with increased mortality (hazard ratio [HR] 4.085; 95% confidence interval [CI] 0.510–32.719; P=0.185; Table 2).

Table 2.
Cox Proportional Analysis for Overall Survival in Subgroups Stratified by the (A) 3-Strata and (B) 4-Strata Models and (C) Modified Risk Assessment
| Risk assessment |
HR (95% CI) |
P value |
| (A) 3-strata model |
| Low |
Reference |
– |
| Intermediate |
4.085 (0.510–32.719) |
0.185 |
| High |
0.000 (0.000–infinity) |
0.990 |
| (B) 4-strata model |
| Low intermediate |
Reference |
– |
| High intermediate |
1.806 (0.403–8.079) |
0.440 |
| High |
20.597 (2.979–142.428) |
0.002 |
| (C) Modified risk assessment |
| Low |
Reference |
– |
| Low intermediate |
1.823 (0.189–17.547) |
0.603 |
| High intermediate |
17.187 (1.984–148.917) |
0.010 |
| High |
0.000 (0.000–infinity) |
0.993 |
CI, confidence interval; HR, hazard ratio.
4-Strata Risk Assessment at the Time of Referral
According to the 2022 ESC/ERS 4-strata model, 33 (63%), 16 (31%), and 3 (6%) patients were classified as having low intermediate, high intermediate, and high mortality risk status. The median values of calculated scores were 2.00 (range 1.67–2.00) and 2.33 (range 2.33–3.00) in the groups classified as low intermediate and high intermediate risk, respectively, and the 3 patients in the high-risk group had a score of 3.67. Kaplan-Meier analysis showed that the high-risk group had a poor prognosis (log-rank test, P<0.001); estimated mean survival was 0.8 years (95% CI 0.4–1.3 years), and the survival rate at 1 year was 33% (Figure 4B). Conversely, there was no significant difference in post-referral survival between the low intermediate- and high intermediate-risk groups (log-rank test, P=0.432). Survival rates at 1, 2, and 3 years were 97%, 90%, and 83%, respectively, in the low intermediate-risk group and 93%, 87%, and 74%, respectively, in the high intermediate-risk group. Estimated mean survival time in the low intermediate- and high intermediate-risk groups was 6.5 years (95% CI 5.6–7.3 years) and 3.7 years (95% CI 3.0–4.4 years), respectively. Cox proportional analysis (with the low intermediate-risk group as the reference) revealed that high intermediate risk was not associated with increased mortality (HR 1.806; 95% CI 0.403–8.079; P=0.440), whereas patients at high risk showed impaired survival (HR 20.597; 95% CI 2.979–142.428; P=0.002; Table 2).
When combined into a single group, patients in the high intermediate- or high-risk group did not show a significant difference in survival compared with the low intermediate-risk group (log-rank test, P=0.117).
Modified Risk Assessment at the Time of Referral
As an exploratory analysis, the 3-strata model intermediate-risk group was further divided into 2 subgroups based on the median proportion of low-risk variables (33%) achieved at the time of referral: a group with low intermediate risk, in which the proportion of low-risk variables was ≥33%; and a group with high intermediate risk, in which the proportion of low-risk variables was ≤32%. Using this modified risk assessment classified 15 (29%), 28 (54%), 8 (15%), and 1 (2%) patients as having low, low intermediate, high intermediate, and high mortality risk. Kaplan-Meier analysis revealed that, among these groups, patients at high intermediate risk had significantly worse survival (log-rank test, P<0.001; Figure 4C). Cox proportional analysis (with the low-risk group as the reference) showed that a low intermediate risk was not associated with increased mortality (HR 1.823; 95% CI 0.189–17.547; P=0.603), whereas patients at high intermediate risk showed impaired survival (HR 17.187; 95% CI 1.984–148.917; P=0.010; Table 2). When combined into one group, patients with high intermediate or high risk had significantly worse survival than patients at low and low intermediate risk (log-rank test, P<0.001); respective survival rates at 1, 2, and 3 years were 92%, 92%, and 92% in the low-risk group; 96%, 80%, and 80% in the group with low intermediate risk; and 64%, 34%, and 34% in the combined high intermediate- and high-risk group. Estimated mean survival time in the groups with low, low intermediate, and high intermediate/high risk was 6.9 years (95% CI 6.2–7.7 years), 5.0 years (95% CI 4.3–5.6 years), and 2.2 years (95% CI 1.0–3.4 years), respectively.
The result of univariate Cox regression analysis of factors associated with post-referral mortality are summarized in Table 3. Modified Risk Assessment categorization as being at high intermediate or high risk at the time of referral was significantly associated with the primary outcome in univariate Cox analysis (HR 10.363; 95% CI 2.726–39.397; P=0.001). Furthermore, the risk status remained significantly associated with the primary outcome in multivariate Cox analysis adjusted for age, sex, and PAH etiologies (HR 12.780; 95% CI 2.583–63.221; P=0.002).
Table 3.
Univariate and Multivariate Cox Proportional Analysis of Factors Associated With Mortality After Referral for Lung Transplantation
| Parameter |
Univariate |
Multivariate |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Sex (male) |
0.578 (0.120–2.788) |
0.495 |
1.309 (0.220–7.785) |
0.767 |
| Age (years) |
1.056 (0.993–1.124) |
0.081 |
1.066 (0.992–1.145) |
0.082 |
| I/HPAH (vs. non-I/HPAH) |
0.579 (0.119–2.808) |
0.498 |
2.143 (0.307–14.984) |
0.442 |
High intermediate or high risk by MRA (vs. low or low
intermediate risk) |
10.363 (2.726–39.397) |
0.001 |
12.780 (2.583–63.221) |
0.002 |
CI, confidence interval; HR, hazard ratio; I/HPAH, idiopathic/heritable pulmonary arterial hypertension; LT, lung transplantation; MRA, modified risk assessment.
Data for BNP at the final follow-up evaluation were available for 45 patients: 38 patients at low or low intermediate risk and 7 patients at high intermediate or high risk based on modified risk assessment at LT referral. The interval between LT referral and final follow-up evaluation was 2.2 years (IQR 1.7–2.9 years). The median BNP concentration was significantly higher in the group at high intermediate/high risk than in the group at low/low intermediate risk (508.1 [IQR 274.0–1,002.2] vs. 35.6 [IQR 6.2–128.0] pg/mL, respectively; P=0.002), indicative of more advanced heart failure.
Discussion
Risk stratification is recommended in order to optimize treatment and prognosis in PAH patients.6 Recent guidelines suggest that PAH patients at intermediate or high risk according to the 3-strata model, or at high intermediate or high risk according to the 4-strata model, under optimal medical therapy are referred for LT.6–8 However, given that these risk assessment tools have been developed based on an estimated 1-year mortality rate, it remains unclear whether the same risk stratification could be applicable for LT referral in Japan, where the waiting period is approximately 3 years. Therefore, we investigated the predictive value of current PAH risk stratification at the time of LT referral in this retrospective study. Our main findings were as follows: (1) according to the 3-strata model, most patients were classified as being at intermediate risk, and there was no survival difference among the groups; (2) the 4-strata model did not discriminate survival between low intermediate and high intermediate risk, but identified patients at high mortality risk; and (3) the modified risk assessment, which divided the intermediate-risk group into 2 based on the proportion of low-risk variables achieved, discriminated patients at low or low intermediate risk who could continue medical therapy, and patients at high intermediate or high risk who should be referred for LT.
Because the International Society for Heart and Lung Transplantation (ISHLT) and the 2015 ESC/ERS guidelines recommend that patients who are classified as intermediate or high risk by the 3-strata model should be referred for LT,7,8 we first stratified the overall cohort based on the 3-strata model. In this analysis, we found that most patients, who were in WHO Class III or IV under combination therapy including a parental prostacyclin analog, were actually classified as low or intermediate risk at the time of referral. This supports previous reports that the long waiting time in Japan makes clinicians refer PAH patients for LT earlier than suggested in the current guidelines.9 In the survival analysis, there was no significant difference between the low- and intermediate-risk groups. However, the low-risk group notably had a favorable prognosis, with a survival rate of 92% at 3 years, suggesting that LT referral was premature and medical treatment could be continued for those in the low-risk group. Conversely, the 3-strata model could not detect patients who had a poor prognosis and should be referred for LT. This result is consistent with the main limitation of the 3-strata model, namely that it could not fully discriminate the severity of those in the intermediate-risk group, in which 60–70% of PAH patients are classified, indicating the necessity to substratify this group.6
Next, we classified the overall cohort by using the 4-strata model, which is recommended for use at follow-up in the most recent guideline.6 The 4-strata model identified patients at high risk, who had poor survival rates (33%) at 1 year after referral. This result is consistent with the findings of Vicaire et al that patients at high risk at the time of LT listing showed poor survival after listing.5 A previous study that reported high mortality rates of 50–70% within a year in high-risk patients at follow-up also supports our finding.20 In addition to patients in the high-risk group, the 2022 ESC/ERS guidelines suggest that patients in the high intermediate-risk group are referred for LT. Olsson et al reported that patients who maintained high intermediate-risk status according to the COMPERA 2.0 method on combination therapy had poor outcomes and were likely to benefit from LT.5,21 However, in our analysis, the high intermediate-risk group had better survival than the high-risk group, and there was no survival difference between the high intermediate- and low intermediate-risk groups. This discrepancy may be due to differences in study design, sample size, cohort background, healthcare system, and graft allocation rules. Our findings are similar to those of previous studies in that the prognosis of patients classified as being in the high-risk group using the 4-strata model was too poor for them to survive the waiting period, and early referral before patients achieve a high-risk status is recommended.5,21 However, the 4-strata model could not fully discriminate patients in the intermediate-risk group who should be referred for LT, leading us to perform an exploratory analysis with the aim of classifying patients in the intermediate-risk group in another way.
Achieving a low-risk status is considered a key goal in managing PAH patients.6 Recently, Kylhammar et al reported that survival rates were better for patients with a larger proportion of low-risk variables at follow-up.15 Thus, we modified the 3-strata model by dividing the intermediate-risk group based on the median proportion of low-risk variables achieved among 6 functional and RV-related parameters. As a result, this modified risk assessment newly identified 8 patients at high intermediate risk under optimal combination therapy. In the survival analysis, patients at high intermediate or high risk had poorer survival than patients at low and low intermediate risk, with survival rates at 1, 2, and 3 years in the former group (high intermediate/high risk) being 64%, 34%, and 34%, respectively. The elevated BNP value at follow-up in the high intermediate- or high-risk group was also supportive of more advanced heart failure in these patients. Given that LT should be generally considered for patients with an estimated mortality ≥50% at 2 years, it seems reasonable that those in the high intermediate- or high-risk group according to the modified risk assessment even after combination therapy should be referred for LT.8,22 Conversely, patients at low and low intermediate risk according to the modified risk assessment showed favorable 3-year survival (≥80%) that was equal to or better than the 3-year survival after cadaveric LT in Japan.23 This means that referring patients at low and low intermediate risk according to the modified risk assessment for LT was premature, and these patients were rather likely to benefit from receiving combination therapy. Thus, risk stratification could help clinicians make decisions as to whether to refer patients for LT or continue medical therapy. However, further work that considers the circumstances of each country is needed to validate and refine the assessment tool in the context of LT referral.
Our study has several limitations. First, it was a single-center retrospective study with a small sample size in Japan, where the lung allocation score and high-priority list have not yet been introduced. In order to validate our findings, future research with a larger sample size is needed. Second, there was small number of patients classified as high risk, possibly due to selection bias. In addition, our cohort included some patients who survived for more than 5 years without LT, similar to a previous report from Japan.24 Patients with WHO functional class III and IV under optimal medical therapy have been considered suitable for LT. However, long-term survivors in our study may have been referred for LT too early.24 Third, we could not incorporate variables such as cardiopulmonary exercise testing, echocardiographic index other than TAPSE/PASP, cardiac magnetic resonance imaging, or mixed venous oxygen saturation due to a lack of available data. We did not incorporate qualitative variables such as signs of right heart failure, progression of symptoms and clinical manifestations, and syncope because qualitative measurements may vary substantially among clinicians.25 In order to investigate which variables should be incorporated when deciding LT referral, further research is needed. Fourth, while using the 4-strata model at the follow-up is recommended in the most recent guidelines,6 we modified the 3-strata model, which is recommended for use at the time of diagnosis. Current guidelines recommend that additional variables, such as imaging and hemodynamics, could be taken into account as necessary in the 4-strata model.6 In addition, evaluation of potential LT candidates is a comprehensive process. For these reasons, we incorporated variables obtained from right heart imaging and hemodynamics, which are not included in the original 4-strata model but are repeatedly performed as part of the follow-up assessment in Japan. However, because cut-off values for echocardiographic or hemodynamic variables in the 4-strata model have been not established, we developed a new model by modifying the established 3-strata model. Further research is needed in order to use cut-off values for each variable in the 4-strata model.
Conclusions
The prognosis of the high-risk group based on the 4-strata model was too poor considering the waiting period in Japan. However, the modified risk assessment could discriminate patients who could continue medical therapy or should be referred for LT. Risk stratification could help clinicians make decisions to refer patients for LT. Further work is needed to refine the assessment tool in the context of LT referral.
Sources of Funding
This study did not receive any specific funding.
Disclosures
I.K. is a member of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Ethics Committee of The University of Tokyo (No. 2650).
References
- 1.
Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43(Suppl S): 13S–24S.
- 2.
Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: Pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8: 443–455.
- 3.
Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: The clinical syndrome. Circ Res 2014; 115: 115–130.
- 4.
Sitbon O, Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur Respir Rev 2017; 26: 160055, doi:10.1183/16000617.0055-2016.
- 5.
Vicaire H, Pavec JL, Mercier O, Montani D, Boucly A, Roche A, et al. Risk stratification in patients with pulmonary arterial hypertension at the time of listing for lung transplantation. J Heart Lung Transplant 2022; 41: 1285–1293.
- 6.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731.
- 7.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.
- 8.
Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021; 40: 1349–1379.
- 9.
Maki H. Should Patients choose lung transplantation or optimal drug therapy?: The clinical situation in the treatment of pulmonary arterial hypertension in Japan. Circ J 2020; 84: 158–160.
- 10.
Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, et al. Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J 2019; 83: 842–945.
- 11.
Ishii S, Minatsuki S, Hatano M, Saito A, Yagi H, Shimbo M, et al. The ratio of TAPSE to PASP predicts prognosis in lung transplant candidates with pulmonary arterial hypertension. Sci Rep 2023; 13: 3758, doi:10.1038/s41598-023-30924-1.
- 12.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14.
- 13.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–788.
- 14.
Manek G, Gupta M, Chhabria M, Bajaj D, Agrawal A, Tonelli AR. Hemodynamic indices in pulmonary hypertension: A narrative review. Cardiovasc Diagn Ther 2022; 12: 693–707.
- 15.
Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181.
- 16.
Hjalmarsson C, Kjellström B, Jansson K, Nisell M, Kylhammar D, Kavianipour M, et al. Early risk prediction in idiopathic versus connective tissue disease-associated pulmonary arterial hypertension: Call for a refined assessment. ERJ Open Res 2021; 7: 00854-2020, doi:10.1183/23120541.00854-2020.
- 17.
Sugiyama Y, Matsubara H, Shimokawahara H, Ogawa A. Outcome of mean pulmonary arterial pressure-based intensive treatment for patients with pulmonary arterial hypertension. J Cardiol 2022; 80: 432–440.
- 18.
Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, et al. COMPERA 2.0: A refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102311, doi:10.1183/13993003.02311-2021.
- 19.
Hamatani Y, Iguchi M, Ueno K, Aono Y, Esato M, Tsuji H, et al. Prognostic significance of natriuretic peptide levels in atrial fibrillation without heart failure. Heart 2021; 107: 705–712.
- 20.
Kylhammar D, Hjalmarsson C, Hesselstrand R, Jansson K, Kavianipour M, Kjellström B, et al. Predicting mortality during long-term follow-up in pulmonary arterial hypertension. ERJ Open Res 2021; 7: 00837-2020, doi:10.1183/23120541.00837-2020.
- 21.
Olsson KM, Richter MJ, Kamp JC, Gall H, Ghofrani HA, Fuge J, et al. Refined risk stratification in pulmonary arterial hypertension and timing of lung transplantation. Eur Respir J 2022; 60: 2103087, doi:10.1183/13993003.03087-2021.
- 22.
Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G, et al. External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J 2022; 59: 2102419, doi:10.1183/13993003.02419-2021.
- 23.
Date H. Current status and problems of lung transplantation in Japan. J Thorac Dis 2016; 8(Suppl 8): S631–S636.
- 24.
Akagi S, Matsubara H, Nakamura K, Oto T, Ejiri K, Ito H. Marked reduction of pulmonary artery pressure after registration for lung transplantation is associated with long-term survival in patients with pulmonary arterial hypertension: Cohort study. Circ J 2020; 84: 245–251.
- 25.
Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 390–398.