Abstract
Background: Despite an increased incidence of chronic heart failure (HF) and sudden cardiac death (SCD), the use of implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) is much lower in Japan than in Western countries. The HF Indication and SCD Prevention Trial Japan (HINODE) prospectively assessed the mortality rate, appropriately treated ventricular arrhythmias (VA), and HF in Japanese patients with a higher risk of HF.
Methods and Results: HINODE consisted of ICD, CRT-defibrillator (CRT-D), pacing, and non-device treatment cohorts. This subanalysis evaluated the impact of the implantation of high-voltage devices (HVD; ICD and CRT-D) in 171 Japanese patients. We compared all-cause mortality, VA, and HF events between elderly (age >70 years at study enrollment) and non-elderly HVD recipients. The estimated survival rate through 24 months in the HVD cohort was 85.8% (97.5% lower control limit 77.6%). The risk of all-cause mortality was increased for the elderly vs. non-elderly (hazard ratio [HR] 2.82; 95% confidence interval [CI] 1.01–7.91; P=0.039), but did not differ after excluding ICD patients with CRT-D indication (HR 2.32; 95% CI 0.79–6.78; P=0.11). There were no differences in VA and HF event-free rates between elderly and non-elderly HVD recipients (P=0.73 and P=0.55, respectively).
Conclusions: Although elderly patients may have a higher risk of mortality in general, the benefit of HVD therapy in this group is comparable to that in non-elderly patients.
Implantable cardioverter-defibrillators (ICDs) have been shown to reduce total mortality when used for primary and secondary prevention of sudden cardiac death (SCD),1,2 and previous landmark trials have shown the efficacy of prophylactic ICD implantation (primary prevention) in patients with heart failure (HF) with reduced ejection fraction regardless of underlying heart disease (i.e., ischemic or non-ischemic cardiomyopathy).3,4 However, the results of these studies, primarily from the US and Europe, may not be fully applicable to Japanese clinical practice because of differences in the proportions of underlying heart disease, genetics, and comorbidities. Furthermore, despite an increase in the incidence of chronic HF, the current use of ICD and cardiac resynchronization therapy (CRT) is much lower in Japan than in Western countries.5,6 Thus, there is a need for a large-scale prospective study with Japanese HF patients to clarify the effectiveness of ICD therapy for primary prevention.
The Heart Failure Indication and Sudden Cardiac Death Prevention Trial Japan (HINODE) was designed to prospectively determine the rates of mortality, appropriately treated ventricular arrhythmias (VA), and HF and to compare them with references from historical landmark trials, such as Multicenter Automatic Defibrillator Implantation Trial‒Reduce Inappropriate Therapy (MADIT-RIT),7 in Japanese patients who meet the European Society of Cardiology (ESC) guidelines for SCD primary prevention or CRT for HF across 4 clinical therapy cohorts:8 ICD, CRT-D, pacing (PA), and a non-device (ND) cohort.9 HINODE demonstrated that mortality and VA event rates from landmark trials are applicable for primary prevention in patients in Japan.10
Conversely, previous studies have shown that the benefit of a high-voltage device (HVD), especially in CRT-D therapy, is questionable in elderly patients because a defibrillator backup had subtle or no survival benefit in symptomatic HF patients requiring a CRT device,11 and that increased age is seen as an argument against the use of cardiac implantable electric devices (CIEDs), especially defibrillator device therapy. Therefore, this subanalysis of HINODE evaluated the impact of ICD or CRT-D implantation as primary prevention in elderly Japanese patients who were at higher risk of HF.
Methods
Patients and Study Protocol
The present study was a subanalysis of HINODE that evaluated the impact of HVD (ICD or CRT-D) implantation in elderly Japanese patients who were at higher risk of HF. A detailed description of HINODE can be found elsewhere.9 Briefly, HINODE is a prospective multicenter registry designed to collect data on VA, HF events, and mortality in Japanese patients with HF. Patients who underwent de novo implantation of an ICD, CRT-D, pacemaker (PM), or CRT pacing (CRT-P) were screened for eligibility for one of these four particular forms of therapy with physician discretion, with the decision regarding device implantation left solely to the discretion of individual physicians. After the treatment decision had been made, patients were prospectively enrolled into 1 of 4 cohorts:
• an ICD cohort (transvenous or subcutaneous, reduced left ventricular ejection fraction [LVEF] ≤35%), New York Heart Association (NYHA) Class II or III, prescribed guideline-directed medical therapy for 3 months
• a CRT-D cohort (reduced LVEF ≤35%), left bundle branch block (LBBB) with QRS >130 ms or non-LBBB with QRS ≥150 ms despite optimal medical therapy (OMT), or, alternatively, in atrial fibrillation (AF) with NYHA Class III, and QRS duration ≥130 ms despite OMT
• a pacing cohort: PM or CRT-P (expectation of >40% right ventricular pacing, reduced LVEF ≤50%, QRS >90 ms, and any previous hospitalization for HF)
• a non-device cohort, who met ICD or CRT-D implantation criteria but were not implanted with a cardiac device.
Patients with a history of previous PM, ICD, CRT-P, or CRT-D implantation were excluded from the ICD/CRT-D cohort, and all criteria for device implantation were based on ESC guidelines for the diagnosis and treatment of acute and chronic HF.11 Enrollment in the ICD, CRT-D, and non-device cohorts was limited to patients meeting 2–5 of the following risk factors: LVEF ≤35%; NYHA Class III or IV; LBBB with QRS ≥130 ms or any QRS morphology ≥150 ms; renal dysfunction (defined as chronic blood urea nitrogen [BUN] >26 mg/dL or ≥9.28 mmol/L); type 1 or 2 diabetes; chronic AF; prior myocardial infarction; age >70 years; and/or current smoker or smoking during the past 5 years. ICD device programming required a high cut-off rate or delayed therapy and followed the principles of MADIT-RIT trial.7
All patients were followed up for a minimum of 12 months and device recipients were required to be connected to a home monitoring system. In the present study we compared clinical events (i.e., all-cause mortality, VA, and HF events) between elderly (defined as age >70 years at the time of study enrolment) and non-elderly HVD recipients using the HINODE dataset.
The HINODE protocol was approved by the institutional review boards of each participating center, including Toho University Ohashi Medical Center (No. H17012) and written informed consent was obtained from each enrolled subject. The investigation conformed to the principles outlined in the Declaration of Helsinki.
Definitions and Study Endpoints
As the primary endpoint, the present study assessed all-cause mortality, VA, and HF events in elderly and non-elderly patients with an HVD implant (ICD and CRT-D recipients) among the overall HINODE study cohort. As described previously,9,10 VA events were adjudicated by an independent events committee and defined as causing hemodynamic instability and impacting patient management, including all shock or antitachycardia pacing (ATP) treatment events, all non-sustained events in the therapeutic range (≥200 beats/min), and any event that was sustained for ≥30 s in the monitoring range (>170 beats/min). To support VA event data collection in ICD or CRT-D recipients, the home monitoring system submitted reports on device programing and recorded electrograms of detected sustained or non-sustained VA.
HF events were also adjudicated by an independent events committee and needed to meet the following conditions: patients had to be admitted and discharged with a calendar date change (hospitalization) and receive a new or increased decongestive HF regimen or inotropes with oral or parenteral medications during an in-hospital stay (at least 1 night); or patients were not hospitalized but received intravenous decongestive therapy with ≥1 drugs, including diuretics, inotropes, or vasodilators, or other parenteral therapies.10
Because age >70 years at the time of enrollment is defined as one of the risk factors for SCD, we defined elderly patients in the present study as those aged >70 years.
Statistical Analysis
Descriptive statistics are presented as the mean±SD, counts (%), or the median, when specified. Categorical data were compared with the Chi-squared test and continuous data were compared using Student’s t-test. Two-tailed P<0.05 was considered statistically significant. Kaplan-Meier analyses estimated the freedom from all-cause mortality, first VA event, and first HF event through 24 months of follow-up for ICD and CRT-D recipients. Event-free rates were compared for elderly vs. non-elderly patients with P values derived from log-rank tests and hazard ratios (HRs) with 95% confidence intervals (CIs) derived from Cox proportional hazards models. HRs were further adjusted for patient cardiomyopathy by including cardiomyopathy as a covariate in each Cox proportional hazards model. Events in the elderly cohort were subdivided by elderly age group (70–74, 75–79, and ≥80 years) and binary event rates were compared using Fisher’s exact test. The analyses used all patients enrolled in the CRT-D cohort and either all patients enrolled in the ICD cohort or patients enrolled in the ICD cohort with an ESC guideline-based ICD implant.
Elderly patients were defined as those aged >70 years based on the predefined study risk factors in the enrollment criteria. The age cut-off was further evaluated using receiver operating characteristic (ROC) curves to evaluate the sensitivity and specificity of various age cut-off values in predicting all-cause mortality. ROC curves were built using logistic regression with event as a binary outcome and age as a continuous predictor. Youden’s index was calculated from the sensitivity and specificity measures, and the age resulting in the maximum Youden’s index was considered the optimal cut-off point.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
HINODE enrolled 354 patients. There were 102, 69, 68, and 115 patients in the ICD, CRT-D, pacing, and non-device cohorts, respectively, with a corresponding mean age for each cohort of 68±10, 66±13, 75±11, and 69±12 years. The ICD and CRT-D cohorts had a median of 4 predefined study risk factors, whereas the pacing and non-device cohorts had 3 (Table 1). Elderly patients (n=191) comprised 49.0% of the ICD cohort, 46.4% of the CRT-D cohort, 67.6% of the pacing cohort, and 54.8% of the non-device cohort. Patients were followed for a mean of 19.6±6.5 months.
Table 1.
Characteristics of Each of the 4 Cohorts (Total n=354)
| |
ICD cohort
(n=102) |
CRT-D cohort
(n=69) |
Pacing cohort
(n=68) |
Non-device cohort
(n=115) |
| Age (years) |
68.2±10.4 |
66.4±13.4 |
75.0±10.9 |
68.8±11.8 |
| Age >70 years (n=191) |
50 (49.0) |
32 (46.4) |
46 (67.6) |
63 (54.8) |
| Median no. predefined risk factors |
4 |
4 |
3 |
3 |
Unless indicated otherwise, data are given as the mean±SD or n (%). CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator.
Patient Characteristics
Detailed demographic and clinical characteristics of ICD and CRT-D recipients (elderly vs. non-elderly) are presented in Table 2. In the elderly group, the mean age of patients at the time of implant was 76.4±4.3 years (range 71–88 years), most were male (61 patients; 74.4%), the mean LVEF was 26.4±6.2%, and the underlying heart disease included ischemic cardiomyopathy in 46 patients (56.1%). Although the N-terminal pro B-type natriuretic peptide (NT-proBNP) concentrations did not differ significantly between the elderly and non-elderly patients (P=0.53), the elderly group had a higher proportion of patients with hypertension (53.7% vs. 38.2%; P=0.043), renal dysfunction (29.3% vs. 13.5%; P=0.011), ischemic cardiomyopathy (56.1% vs. 29.2%; P<0.001), and number of predefined risk factors (4.2±0.8 vs. 3.2±1.0; P<.001) and a lower proportion of patients with NYHA Class III or IV and a smoking history.
Table 2.
Baseline Characteristics of Elderly (Age >70 Years) and Non-Elderly Patients in the Cohort Implanted With a High-Voltage DeviceA
| |
Total
(n=171) |
Elderly
(n=82) |
Non-elderly
(n=89) |
P value |
| Age (years) |
67.4±11.6 |
76.4±4.33 |
59.1±10.1 |
<0.001 |
| Male sex |
134 (78.4) |
61 (74.4) |
73 (82) |
0.226 |
| NYHA Class III or IV |
58 (33.9) |
21 (25.6) |
37 (41.6) |
0.028 |
| LVEF (%) |
25.9±6.2 |
26.4±6.21 |
25.4±6.18 |
0.31 |
| NT-proBNP (pg/mL) |
2,981±2,839 |
3,322±2,966 |
2,662±2,772 |
0.527 |
| No. risk factors |
3.64±1.05 |
4.17±0.83 |
3.16±1.0 |
<0.001 |
| Ischemic cardiomyopathy |
72 (42.1) |
46 (56.1) |
26 (29.2) |
<0.001 |
| HT |
78 (45.6) |
44 (53.7) |
34 (38.2) |
0.043 |
| Renal dysfunction |
36 (21.1) |
24 (29.3) |
12 (13.5) |
0.011 |
| Diabetes |
69 (40.4) |
34 (41.5) |
35 (39.3) |
0.776 |
| Chronic AF |
28 (16.4) |
14 (17.1) |
14 (15.7) |
0.813 |
| QRS duration (ms) |
132.8±28.53 |
134.8±28.88 |
131.0±28.2 |
0.391 |
| Smoking history |
46 (26.9) |
13 (15.9) |
33 (37.1) |
0.002 |
| Prior MI |
59 (34.5) |
36 (43.9) |
23 (25.8) |
0.013 |
| Diuretics |
130 (76) |
62 (75.6) |
68 (76.4) |
0.903 |
| β-blocker |
151 (88.3) |
66 (80.5) |
85 (95.5) |
0.002 |
| ACEi |
80 (46.8) |
38 (46.2) |
42 (47.2) |
0.911 |
| ARB |
46 (26.9) |
21 (25.6) |
25 (28.1) |
0.715 |
| Antiarrhythmic agent |
60 (35.1) |
31 (37.8) |
29 (32.6) |
0.475 |
Unless indicated otherwise, data are given as the mean±SD or n (%). AHigh-voltage devices include both implantable cardioverter-defibrillators and cardiac resynchronization therapy. ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; HT, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
Elderly patients in the ICD and CRT-D group were implanted with single-chamber ICD implants (n=8 patients; 9.8%), dual-chamber ICD implants (n=35; 42.7%), subcutaneous ICD (S-ICD) implants (n=7; 8.5%), and CRT-D implants (n=32; 39.0%). The QRS duration on electrocardiograms (ECG) at baseline did not differ significantly between the elderly and non-elderly groups (134.8±28.9 vs. 131.0± 28.2 ms, respectively; P=0.39). All devices used were manufactured by Boston Scientific (Marlborough, MA, USA).
Most patients in the elderly and non-elderly groups achieved OMT, including β-blockers (66 [80.5%] vs. 85 [95.5%] patients; P=0.002), diuretics (62 [75.6%] vs. 68 [76.4%] patients; P=0.903), angiotensin-converting enzyme inhibitors (38 [46.3%] vs. 42 [47.2%] patients; P=0.91), and angiotensin receptor blockers (21 [25.6%] vs. 25 [28.1%] patients; P=0.72). Approximately one-third of patients in the elderly and non-elderly groups received antiarrhythmics (31 [37.8%] vs. 29 [32.6%] patients, respectively; P=0.48).
All-Cause Mortality
During the follow-up period, 23 patients died from any cause for an absolute death rate of 13.7% (14/102) in the ICD cohort and 13.0% (9/69) in the CRT-D cohort. There were 8 (7.8%) cardiac deaths in the ICD group and 7 (10.1%) in the CRT-D group. The estimated survival rate for ICD and CRT-D device recipients through 24 months was 85.8% (97.5% lower control limit [LCL] 77.6%). In the HVD (ICD and CRT-D device) cohort, the risk of all-cause mortality was greater for elderly than non-elderly patients (HR 2.82; 95% CI 1.01–7.91). However, some elderly patients received ICD despite an ESC guideline indication for CRT-D; excluding those ICD patients with a CRT-D indication resulted in no significant difference in mortality between the elderly and non-elderly groups (HR 2.32; 95% CI 0.79–6.78). Furthermore, after adjusting for ischemic cardiomyopathy, the mortality risk remained similar between the 2 groups (elderly vs. non-elderly) but was no longer significant (adjusted [a] HR 2.87; 95% CI 0.99–8.35).
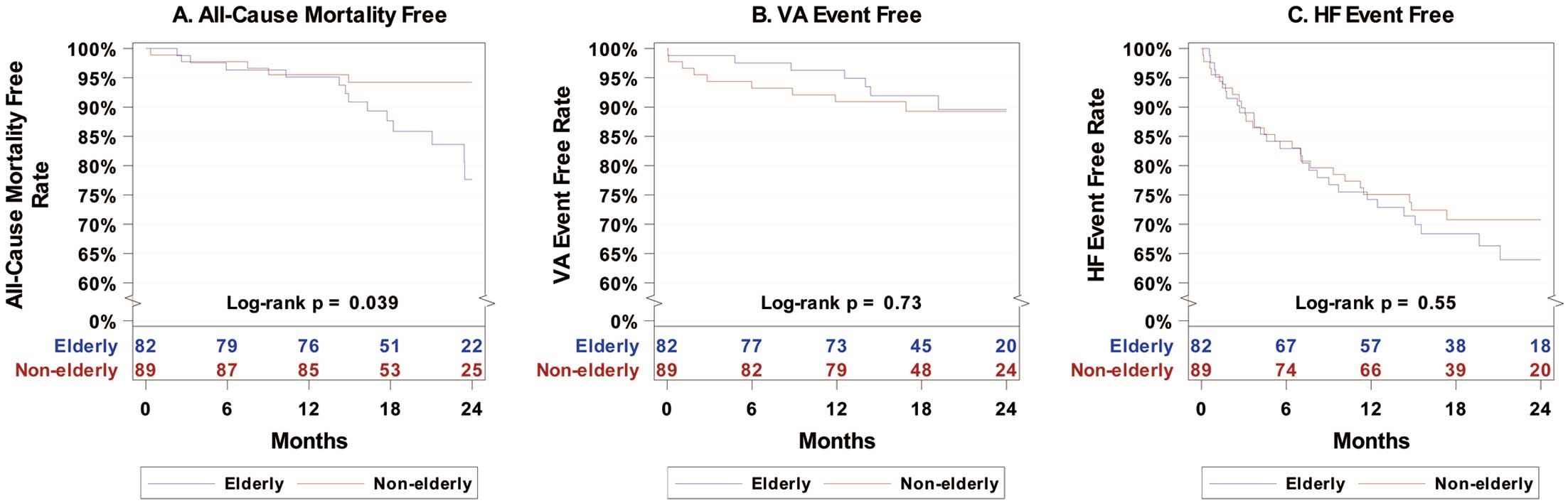
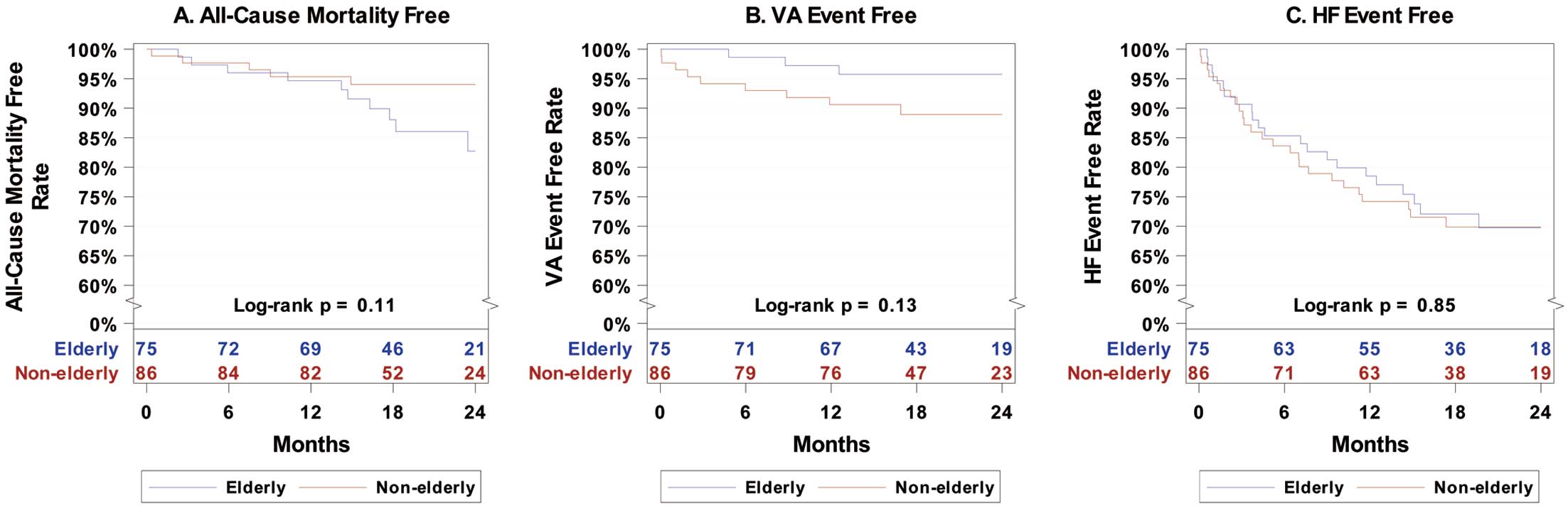
Kaplan-Meier analysis for all-cause mortality showed a statistically significant difference between the elderly and non-elderly groups during the follow-up period (log-rank P=0.039; Figure 1A), whereas there was no significant difference between the 2 groups after excluding those ICD patients with a CRT-D indication (log-rank P=0.11; Figure 2A).
VA Events
The VA event-free rate in elderly ICD and CRT-D patients was not significantly different from that in non-elderly patients in either the enrolled cohort (HR 0.84; 95% CI 0.31–2.25) or the ESC guideline indication cohort (HR 0.37; 95% CI 0.10–1.39). Subanalysis according to ICD or CRT-D implantation in entire enrolled cohort also showed no significant difference (CRT-D: HR 0.37 [95% CI 0.04–3.59; P=0.39]; ICD: HR 1.07 [95% CI 0.35–3.32; P=0.91]). The difference remained non-significant after adjusting for ischemic cardiomyopathy (aHR 0.75; 95% CI 0.27–2.11).
Kaplan-Meier analysis for VA events showed no significant difference between the elderly and non-elderly cohorts during the follow-up period for either the entire enrolled cohort (log-rank P=0.73; Figure 1B) or the ESC guideline indication cohort (log-rank P=0.13; Figure 2B).
HF Events
There was no significant difference in the HF event-free rate between elderly and non-elderly ICD and CRT-D patients in either entire enrolled cohort (HR 1.18; 95% CI 0.68–2.03) or the ESC guideline indication cohort (HR 0.94; 95% CI 0.53–1.69). The difference remained non-significant after adjusting for ischemic cardiomyopathy (aHR 1.24; 95% CI 0.70–2.21). Subanalysis according to ICD or CRT-D implantation in entire cohort also showed no significant difference (CRT-D: HR 0.98 [95% CI 0.40–2.42; P=0.97]; ICD: HR 1.31 [95% CI 0.66–2.60; P=0.44]). Kaplan-Meier analysis for HF events showed no significant difference between the elderly and non-elderly cohort during the follow-up period for either the entire enrolled cohort (log-rank P=0.55; Figure 1C) or the ESC guideline indication cohort (log-rank P=0.85; Figure 2C).
Best Age Cut-Off Value
As described above, because age >70 years is one of the risk factors defined as part of the HINODE enrollment criteria, we defined elderly patients in the present study as those aged >70 years. The optimal age cut-off value to predict all-cause mortality, VA, and HF events was further evaluated using ROC curves and Youden’s index. In the ICD and CRT-D cohorts, the optimal age cut-off value for predicting all-cause mortality was 70 years, with a sensitivity of 59.5%, a specificity of 72.2%, a negative predictive value of 17.3%, and a positive predictive value of 94.8% (Figure 3). The area under the ROC curve for predicting all-cause mortality was 0.65 (95% CI 0.54–0.76). Age was not a significant predictor of VA or HF events, so no optimal cut-off point was identified for these outcomes. We also investigated each endpoint among the elderly patients categorized by age (70–74 vs. 75–79 vs. ≥80 years). There was no significant difference in the event rates by elderly age group (Table 3).
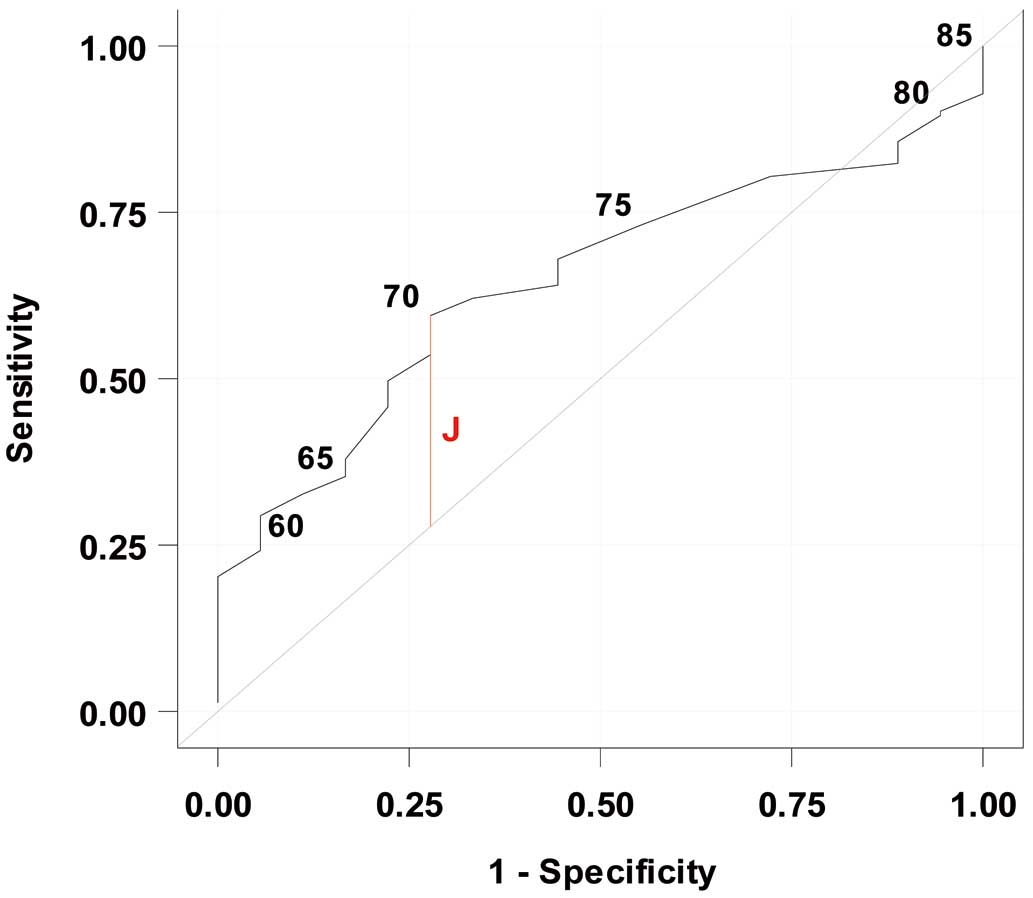
Table 3.
Number of Patients Experiencing Events Among Elderly Patients by Age Group
| Endpoint |
Age group (years) |
P value |
| 70–74 (n=28) |
75–79 (n=37) |
≥80 (n=17) |
| Ventricular arrhythmia |
1 (3.6) |
5 (13.5) |
1 (5.9) |
0.4074 |
| All-cause mortality |
4 (14.3) |
8 (21.6) |
1 (5.9) |
0.3555 |
| HF events |
13 (46.4) |
10 (27.0) |
4 (23.5) |
0.1913 |
Data show the number of patients experiencing events from 0 to 24 months with percentages in parentheses. HF, heart failure.
Discussion
To the best of our knowledge, this is the first study to evaluate the impact of ICD or CRT-D implantation for primary prevention in elderly Japanese patients at higher risk of HF. The findings of the present study can be summarized as follows:
• The estimated survival rate for HVD recipients at higher risk of HF (n=171) through 24 months was 85.8% (97.5% LCL 77.6%).
• The risk of all-cause mortality was greater for the elderly than non-elderly cohort (HR 2.82; 95% CI 1.01–7.91; P=0.039). However, after excluding ICD patients with an indication for CRT-D from the analysis, there was no longer any significant difference in the risk of all-cause mortality for elderly patients (HR 2.32; 95% CI 0.79–6.78; P=0.11).
• Among ICD and CRT-D patients, there was no significant difference in VA and HF event-free rates or between elderly and non-elderly patients.
In general, a greater number of comorbidities are more common in elderly patients. Indeed, the number of defined risk factors was significantly higher among elderly than non-elderly patients (4.2±0.8 vs. 3.2±1.0; P<0.001) in the present study, and this may contribute to the question as to whether elderly patients will receive any survival benefit from ICD therapy, especially in terms of primary prevention. Because the current guidelines do not provide any age cut-off for ICD therapy as primary prevention, it is controversial as to whether elderly patients should receive the ICD or CRT-D therapy. Zakine et al reported the risk and benefit balance in elderly patients using multicenter real-world data.12 In that paper, Zakine et al compared clinical outcomes, including mortality, between ICD recipients aged ≥80 and <80 years implanted for primary prevention. Although the overall rates of device-related complications and appropriate therapy did not differ between older and younger subjects (complications: 24% vs. 26.7%, respectively [P=0.60]; appropriate therapy: 19.4% vs. 21.6%, respectively [P=0.34]), the overall death rate was higher in ICD recipients aged ≥80 years than in younger subjects (36.3% vs. 12.9%, respectively; P<0.0001).12
Consistent with that prior study,12 we showed in the present study that the risk of all-cause mortality was greater for elderly than non-elderly patients in the entire HVD (ICD and CRT-D) cohort (HR 2.82; 95% CI 1.01–7.91; P=0.039). However, interestingly, after excluding patients with an indication for CRT-D based on ESC guidelines from the ICD cohort resulted in no significant difference in the risk of all-cause mortality between elderly and non-elderly patients (HR 2.32; 95% CI 0.75–18.35; P=0.11). Because the decision as to which device was implanted was solely at the discretion of each individual physician in HINODE, even if it were indicated, CRT-D implantation may be avoided in Japanese real-world clinical practice. In fact, recent subanalysis of the CHART-2 study reported that less than half the patients with an indication for CRT according to the Japanese Circulation Society (JCS) guidelines received CRT and the risk factor for not receiving CRT was age.13 Furthermore, Satake et al reported that the use of an ICD for the primary prevention of SCD in patients hronic HF is validated, but still underused in Japan.14 Taken together, the findings indicate that the outcomes of HVD therapy in elderly patients are comparable to those in non-elderly patients if age is taken into account as one of several risk factors, and that ICD and CRT therapy may be considered, despite age being a standalone risk factor in contemporary Japanese clinical practice.
Comparison With Other Japanese High-Voltage Registries
HINODE was designed to prospectively assess the rates of mortality, appropriately treated VA, and HF in Japanese patients, including a non-device cohort. In the present subanalyses, we focused on the HVD cohort. The Nippon Storm Study is the largest prospective registry study for assessing VA events and electrical storm in HVD recipients.15 Compared with HINODE, patient background and the occurrence of VA events were comparable in the Nippon Storm Study excluding baseline cardiomyopathy in the primary prevention cohort.16 However, the study enrollment periods differed between HINODE (2017–2018) and the Nippon Storm Study (2010–2012), which may have affected the observed rates of underlying heart disease.
Event Risk According to Etiology
More recently, Yafasova et al reported the result of long-term follow-up (median 9.5 years) of The Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality (DANISH), which assessed the efficacy of ICD implantation for primary prevention in patients with non-ischemic systolic HF against mortality.17 The authors reported that ICD implantation did not provide an overall survival benefit in patients with non-ischemic systolic HF, whereas ICD implantation was associated with a lower incidence of all-cause mortality, cardiovascular death, and SCD in patients aged ≤70 years.17 That study suggests that baseline heart disease and age are important factors in primary HVD recipients. In contrast, although unable to perform a propensity score-matched analysis, we found no significant differences in all-cause mortality, VA events, and HF events after adjusting for underlying cardiomyopathy between elderly and non-elderly HVD recipients. These results may be attributable to differences in baseline characteristics in elderly and non-elderly HVD recipients between HINODE and DANISH.
To identify patients who are more likely to benefit from ICD therapy for primary prevention, Younis et al reported the MADIT-ICD benefit score, which asses the individualized predicted risk for life-threating ventricular tachycardia/ventricular fibrillation and competing risk of non-arrhythmic mortality based on the MADIT trial series.18 Because we found age to be one of several risk factors, the risk stratification scoring system may help more informed decisions to be made based on patient background. Future randomized controlled trials are needed to identify elderly Japanese patients (e.g., age >80 years) who gain true clinical benefit (i.e., ischemic or non-ischemic cardiomyopathy) from HVD therapy as primary prevention.
Study Limitations
The present study has several limitations. First, although we tried to compare the landmark historical data in elderly patients, the number of patients in the HINODE database was too small for advanced (i.e., propensity score matched or adjusted) comparisons that compare outcomes while taking into consideration differences in patient characteristics. Second, it may be argued that the age cut-off used to define elderly patients (>70 years) in the present study may be relatively young compared with previous studies.10,11 However, because age >70 years at the time of enrollment was defined as one of the risk factors in HINODE, we defined elderly patients in the present study as those aged >70 years. Although the area under the ROC curve (0.65) suggests only moderate ability to predict all-cause mortality from age, the maximum Youden’s index identified that optimal sensitivity and specificity were achieved using an age cut-off of 70 years. Third, because the device implantation criteria were based on ESC guidelines for the diagnosis and treatment of acute and chronic HF to enable a comparison with Western landmark trials, the study results may have differed if HINODE had used JCS guidelines. Finally, analyses were performed post hoc with no prespecified performance goals or power calculations, and data were collected observationally, which may introduce confounding variables that influence model estimates.
Conclusions
Although elderly patients may have a higher risk of mortality in general, the benefit of device therapy in the elderly is comparable to that in non-elderly patients if age is taken into account as one of several risk factors. Thus, our data support reconsideration of ICD and CRT therapy in the elderly, despite age as a standalone risk in contemporary Japanese clinical practice.
Acknowledgments
The authors sincerely thank the HINODE enrolling sites, physicians, and study coordinators. The authors also thank all members of the adjudication committees who reviewed endpoint events and electrocardiograms. Special thanks to the Boston Scientific technical support team and the ICON study team, who supported all trial-related preparations and monitoring. In addition, the authors thank Hiroshi Kuradomi, and Shuichi Matsumoto from the Boston Scientific clinical team, who were essential for project progress and conduct.
Sources of Funding
This work was supported and funded by Boston Scientific.
Disclosures
T.I. has received scholarship funds or donations towards scholarship funds from Medtronic Japan Co., Ltd., Japan Lifeline Co., Ltd., Daiichi Sankyo Co., Ltd.; honoraria for lectures from Bayer Co., Ltd., Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb Co., Ltd.; and consulting honoraria from Boston Scientific. M. Nakamura has received grants and personal fees from Bayer Yakuhin, Daiichi Sankyo, and Sanofi, and personal fees from Bristol-Myers Squibb and Nippon Boehringer Ingelheim. K.A. has received speaker honoraria from Abbott Japan, Boehringer-Ingelheim, and Daiichi-Sankyo Co., Ltd.; consulting honoraria from Boston Scientific; and belongs to an endowment department of Abbott Japan. T.S. and T.K. are employees of Boston Scientific. T.I. and K.A. are members of Circulation Journal’s Editorial Team. The remaining authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
IRB Information
This study was approved by the Toho University Ohashi Medical Center (No. H17012).
Data Availability
The deidentified analysis data will be shared on a request basis. Please contact the corresponding author directly to request data sharing. Data will be shared as soon as approved by the HINODE steering committee, and will be available until end of December, 2024. The data will be shared with anyone wishing to access the data. Any analyses on the data will be approved and data will be shared as Excel file via email.
References
- 1.
Lee DS, Green LD, Liu PP, Dorian P, Newman DM, Grant FC, et al. Effectiveness of implantable defibrillators for preventing arrhythmic events and death: A meta-analysis. J Am Coll Cardiol 2003; 41: 1573–1582.
- 2.
Nanthakumar K, Epstein AE, Kay GN, Plumb VJ, Lee DS. Prophylactic implantable cardioverter-defibrillator therapy in patients with left ventricular systolic dysfunction: A pooled analysis of 10 primary prevention trials. J Am Coll Cardiol 2004; 44: 2166–2172.
- 3.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883.
- 4.
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237.
- 5.
Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur Heart J 2022; 43: 716–799.
- 6.
Japan Arrhythmia Device Industry Association. Annual trends in the number of ICDs and CRT-Ds installed by prefecture [in Japanese]. http://www.jadia.or.jp/medical/crt-d.html (accessed March 11, 2024).
- 7.
Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012; 367: 2275–2283.
- 8.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200.
- 9.
Yamasaki H, Ando K, Ikeda T, Mitsuhashi T, Murohara T, Nishii N, et al. Rationale and design of the HINODE study: Heart Failure Indication and Sudden Cardiac Death Prevention Trial Japan. J Arrhythm 2021; 37: 1031–1037.
- 10.
Aonuma K, Ando K, Kusano K, Asai T, Inoue K, Inamura Y, et al. Primary results from the Japanese Heart Failure and Sudden Cardiac Death Prevention Trial (HINODE). ESC Heart Fail 2022; 9: 1584–1596.
- 11.
Laish-Farkash A, Bruoha S, Katz A, Goldenberg I, Suleiman M, Michowitz Y, et al. Morbidity and mortality with cardiac resynchronization therapy with pacing vs. with defibrillation in octogenarian patients in a real-world setting. Europace 2017; 19: 1357–1363.
- 12.
Zakine C, Garcia R, Narayanan K, Gandjbakhch E, Algalarrondo V, Lellouche N, et al. Prophylactic implantable cardioverter-defibrillator in the very elderly. Europace 2019; 21: 1063–1069.
- 13.
Hayashi H, Yasuda S, Nakano M, Sakata Y, Nochioka K, Shiroto T, et al. Utilization and efficacy of cardiac resynchronization therapy in patients with chronic heart failure: A report from the CHART-2 study. Circ Rep 2022; 4: 264–273.
- 14.
Satake H, Fukuda K, Sakata Y, Miyata S, Nakano M, Kondo M, et al. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure: A report from the CHART-2 study. Circ J 2015; 79: 381–390.
- 15.
Noda T, Kurita T, Nitta T, Chiba Y, Furushima H, Matsumoto N, et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol 2018; 255: 85–91.
- 16.
Kondo Y, Noda T, Takanashi Y, Sasaki S, Sato Y, Nitta T, et al. Two-year outcomes of primary prophylactic use of defibrillators for ischemic and non-ischemic cardiomyopathy: Propensity score-matched analysis from the Nippon Storm Study. Circ J 2023, doi:10.1253/circj.CJ-23-0613.
- 17.
Yafasova A, Butt JH, Elming MB, Nielsen JC, Haarbo J, Videbak L, et al. Long-term follow-up of DANISH (The Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality). Circulation 2022; 145: 427–436.
- 18.
Younis A, Goldberger JJ, Kutyifa V, Zareba W, Polonsky B, Klein H, et al. Predicted benefit of an implantable cardioverter-defibrillator: The MADIT-ICD benefit score. Eur Heart J 2021; 42: 1676–1684.