Article ID: CJ-23-0877
Article ID: CJ-23-0877
Background: The 1-year clinical outcomes of the Absorb GT1 Japan post-market surveillance (PMS) suggested that an appropriate intracoronary imaging-guided bioresorbable vascular scaffold (BVS) implantation technique may reduce the risk of target lesion failure (TLF) and scaffold thrombosis (ST) associated with the Absorb GT1 BVS. The long-term outcomes through 5 years are now available.
Methods and Results: This study enrolled 135 consecutive patients (n=139 lesions) with ischemic heart disease in whom percutaneous coronary intervention (PCI) with the Absorb GT1 BVS was attempted. Adequate lesion preparation, imaging-guided appropriate sizing, and high-pressure post-dilatation using a non-compliant balloon were strongly encouraged. All patients had at least 1 Absorb GT1 successfully implanted at the index procedure. Intracoronary imaging was performed in all patients (optical coherence tomography: 127/139 [91.4%] lesions) and adherence to the implantation technique recommendations was excellent: predilatation, 100% (139/139) lesions; post-dilatation, 98.6% (137/139) lesions; mean (±SD) post-dilatation pressure, 18.8±3.5 atm. At 5 years, the follow-up rate was 87.4% (118/135). No definite/probable ST was reported through 5 years. The cumulative TLF rate was 5.1% (6/118), including 2 cardiac deaths, 1 target vessel-attributable myocardial infarction, and 3 ischemia-driven target lesion revascularizations.
Conclusions: Appropriate intracoronary imaging-guided BVS implantation, including the proactive use of pre- and post-balloon dilatation during implantation may be beneficial, reducing the risk of TLF and ST through 5 years.
Metallic drug-eluting stents (DES) have led to significant improvements in event-free survival in patients undergoing percutaneous coronary intervention (PCI) for the treatment of ischemic coronary heart disease (IHD) by providing mechanical support to the artery to prevent recoil and delivering antiprolific agents to prevent restenosis.1 However, DES are still associated with an approximate 2% per year accrued rate of stent-related ischemic events out to 5 years after revascularization;2,3 some studies suggest very late adverse events may occur beyond 10 years.4–6 The pathophysiological mechanisms underlying these very late adverse events may be due to the permanent metallic implant that mechanically cages the coronary artery, preventing coronary vasomotion, autoregulation, and adaptive coronary remodeling.7,8 Furthermore, the permanent presence of foreign material in the coronary artery may promote chronic inflammation, neoatherosclerosis, and strut fracture, serving as a nidus for very late adverse events.9
Bioresorbable vascular scaffolds (BVS) were designed to provide mechanical support and drug delivery similar to DES early after PCI and to then completely resorb over a 3-year period, resulting in the recovery of cyclic pulsatility, vasoregulation, and adaptive remodeling, effectively eliminating the nidus for very late adverse events attributed to DES.7,10 The Absorb GT1 everolimus-eluting poly(L-lactide) BVS (Abbott Vascular, Santa Clara, CA, USA) is the most widely studied Bioabsorbable Scaffold.11 Although several randomized controlled trials demonstrated non-inferiority of the Absorb BVS compared with the XIENCE cobalt chromium everolimus-eluting stent up to 1 year,12–14 mid-term15,16 and long-term results11,17,18 showed increased rates of composite device-oriented adverse events and device thrombosis. Post hoc analyses suggested that inadequate scaffold expansion/apposition due, at least in part, to suboptimal implantation technique was a potential cause of the increased adverse event rates.19–21 To mitigate the risk of adverse events (specifically, scaffold thrombosis [ST]), Abbott Vascular recommended an appropriate BVS implantation technique that included good lesion preparation, quantitative vessel sizing and selection of a matched-size BVS, and post-dilatation with high pressure using a non-compliant balloon.22
The Absorb GT1 Japan post-marketing surveillance (PMS) was the conditional study for the approval of the Absorb GT1 BVS device mandated by the Pharmaceuticals and Medical Devices Agency in Japan. All patients who were treated with Absorb GT1 were required to be registered in the PMS, and participating physicians were required to follow the appropriate implantation technique provided by the sponsor. Although Absorb GT1 was withdrawn from the global market due to low sales volume, the clinical significance of an appropriate implantation technique guided by intravascular imaging modalities, especially optical coherence tomography (OCT),23 on short- and long-term clinical outcomes is valuable. In all, 135 patients were enrolled prior to product withdrawal. This 135-patient cohort demonstrated 100% lesion success rate and no cases of ST (primary endpoint), death, or myocardial infarction (MI) through 1 year.24 Herein, we report the final 5-year outcomes from the Absorb GT1 Japan PMS. One of the study objectives was to confirm the sponsor-proposed “PSP” (defined as adequate lesion preparation [P], appropriate sizing [S], and post-dilatation [P]) for BVS implantation could improve clinical outcomes. Therefore, a descriptive comparison to the ABSORB Japan randomized controlled trial (RCT; NCT01844284), for which the PSP concept had not yet been introduced during enrollment, is also provided.25 Of the 266 patients in the ABSORB Japan RCT, 83 (31.2%) were assigned to the group for postoperative OCT observation, and 86 lesions in 82 subjects were available for analysis in the core laboratory. However, imaging guidance was not provided by the protocol.25
The Absorb GT1 Japan PMS was a prospective multicenter PMS of the Absorb GT1 that enrolled patients with ischemic coronary artery disease in Japan. The trial design and methods have been described previously.24,26 The protocol was designed by the principal investigators and sponsor (Abbott Vascular), in collaboration with the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT).
The study protocol was approved by Toho University Ohashi Medical Center Institutional Review Board (MI 16-2) and the institutional review boards or ethics committees at each participating center. The sponsor funded the trial, was involved in protocol design, site selection and management, and data analysis. The principal investigators had unrestricted data access, prepared the manuscript and vouch for the accuracy and completeness of the reported data. The Absorb GT1 Japan PMS is registered at ClinicalTrials.gov (ID: NCT03409731). This single-arm multicenter observational study was performed in compliance with the articles of the revised Declaration of Helsinki October 2013 and the Good Post marketing Surveillance Practice (GPSP) ordinance from the Ministry of Health, Labor and Welfare in Japan.
Patients, Procedure, and Follow-upPatients provided written informed consent prior to the index procedure. Patients were registered in the PMS when Absorb GT1 BVS implantation was attempted. Patients were not eligible to receive the investigational device and were excluded from the PMS if: the reference vessel diameter (RVD) was <2.5 or >3.75 mm; there was a left main coronary artery lesion, aorta ostial lesion, severe calcification, hinge motion, or ST-elevation MI; and/or the patient was undergoing kidney dialysis, was at high bleeding risk, or could not tolerate at least 12 months of dual antiplatelet therapy (DAPT) with aspirin and ADP receptor antagonists per the site standard practice.
As described previously,24 the Absorb GT1 implanted procedure was performed per optimal technique recommended by the sponsor, and included “PSP” (adequate lesion preparation [P], appropriate sizing [S], and post-dilatation [P]), to minimize final residual diameter stenosis and incomplete scaffold apposition (ISA). DAPT was recommended for at least 12 months following the index procedure. Clinical follow-up was performed at 1 and 3 months, and 1, 2, 3, 4, and 5 years after the procedure (Supplementary Figure).
EndpointsThe primary endpoint of the PMS was the ST rate at 3 months, per the Academic Research Consortium definition.27 Other clinical endpoints included the rate of target lesion failure (TLF; a composite of cardiac death, MI attributable to the target vessel [TV-MI], or ischemia-driven target lesion revascularization [ID-TLR]) at each follow-up.13,24
To evaluate whether successful implantation was achieved, angiograms and intravascular (intravascular ultrasound/OCT) images from the first 150 patients taken before and after the procedure were sent to an independent core laboratory (Cardiocore, Tokyo, Japan) for analysis. The image analysis has been described previously in detail. ISA was adjudicated when the distance between the lumen and endoluminal contour was >157 µm (scaffold thickness).24 An independent clinical events committee (CEC; Baim Clinical Research, Boston, MA, USA) adjudicated all deaths and suspected cases of MI. Periprocedural MI, which was defined as a post-procedural creatine kinase-MB >5-fold the upper limit of normal, was also included. ST events were adjudicated by a ST image review committee.
Statistical AnalysisThe success rate was calculated among the intent-to-treat population. Other analyses were performed in the full analysis set, defined as patients who received at least 1 Absorb GT1 device. Data were summarized using descriptive statistics. Continuous variables are presented as the mean±SD and categorical variables are presented as percentages by categories. Time-to-first event rates were estimated using Kaplan-Meier methodology and compared by log-rank tests. All analyses were performed using SAS for Windows, version 9.3 or higher (SAS Institute, Cary, NC, USA).
In all, 135 patients were enrolled from December 13, 2016 to December 11, 2017 at 15 sites in Japan (Supplementary Figure). All 135 patients registered in the PMS successfully received at least 1 Absorb GT1 BVS during the index procedure. Therefore, all analyses were based on 135 patients. Five-year follow-up was completed in 87.4% (118/135) of patients. Seventeen patients were lost to follow-up without any death, MI, or revascularization (DMR) and excluded from the 5-year analysis (Supplementary Figure).
Patient Demographics and Baseline Risk FactorsPatients were predominately male (84.4% [114/135]). On average, patients were older (64.0±10.9 years) and of normal weight (BMI: 24.4±3.18 kg/m2). Approximately 26% of patients had a prior coronary intervention, whereas 87% had stable coronary artery disease (Table 1).
Baseline Characteristics of Patients in the Present Study and ABSORB Japan Randomized Controlled Trial
| GT1 PMS (n=135) |
AVJ-301 (n=266) |
|
|---|---|---|
| Demographics and risk factors | ||
| Age (years) | 64.0±10.9 (134) | 67.1±9.4 (266) |
| Male sex (%) | 84.4 (114/135) | 78.9 (210/266) |
| BMI (kg/m2) | 24.40±3.18 (135) | 24.01±3.03 (260) |
| Any diabetes | 39.3 (53/135) | 36.1 (96/266) |
| Diabetes treated with insulin | 4.4 (6/135) | 9.0 (24/266) |
| Lipid disorder requiring medication | 62.2 (84/135) | 74.1 (197/266) |
| Hypertension requiring medication | 68.9 (93/135) | 72.2 (192/266) |
| Renal failure | 3.7 (5/135) | NA |
| On dialysis | 0.0 (0/5) | Excluded |
| Current smoker | 23.7 (32/135) | 19.9 (53/266) |
| Family history of CAD | 10.4 (14/135) | 6.5 (16/246) |
| Prior coronary intervention | 25.9 (35/135) | 36.1 (96/266) |
| Prior MI | 9.6 (13/135) | 16.0 (42/262) |
| Ischemic status | ||
| NSTEMI | 0.7 (1/135) | Excluded |
| Unknown MI | 1.5 (2/135) | Excluded |
| Unstable angina | 6.7 (9/135) | 9.8 (26/266) |
| Stable angina | 49.6 (67/135) | 63.9 (170/266) |
| Silent ischemia | 37.0 (50/135) | 26.3 (70/266) |
| Old MI | 3.0 (4/135) | NA |
Data are presented as the mean±SD or as percentages, with n or n/N values in parentheses. AVJ-301, ABSORB Japan randomized controlled trial; BMI, body mass index; CAD, coronary artery disease; GT1 PMS, Absorb GT1 Japan post-market surveillance (present study); MI, myocardial infarction; NA, not applicable; NSTEMI, non-ST-elevation myocardial infarction.
Procedural Results
Procedural results, including quantitative coronary angiography (QCA) measurements and OCT analysis data, of the present study and of the ABSORB Japan RCT are presented in Tables 2 and 3. Preprocedure QCA measurements were similar between the 2 studies. Predilatation was performed in 100% of patients in both studies, whereas post-dilation was performed in 99% and 82% of patients in the PMS and ABSORB Japan RCT, respectively. More frequent use of non-compliant balloons, larger balloons and higher-pressure inflations were observed in the PMS, per the PSP recommendations.
Lesion and Procedure Characteristics
| GT1 PMS (135 patients, 139 lesions) |
AVJ-301 (266 patients, 275 lesions) |
|
|---|---|---|
| Preprocedure QCA | ||
| Lesion length (mm) | 13.8±4.7 | 13.4±5.3 |
| RVD (mm) | 2.73±0.41 | 2.71±0.45 |
| MLD (mm) | 1.00±0.30 | 0.96±0.33 |
| %DS | 63.1±9.5 | 64.5±11.1 |
| Procedure | ||
| Predilatation performed | 100 | 100 |
| Maximum balloon diameter (mm) | 2.91±0.36 | 2.80±0.37 |
| Balloon/QCA-RVD ratio | 1.08±0.16 | 1.05±0.13 |
| BR ratio ≥1.1 | 71 | 64 |
| Total scaffold length (mm) | 20.0±5.1 | 20.2±5.8 |
| Nominal scaffold diameter (mm) | 3.12±0.34 | 3.07±0.38 |
| Scaffold/RVD ratio | 1.16±0.14 | 1.15±0.13 |
| Post-dilation performed | 99 | 82 |
| With a non-compliant balloon | 88 | 64 |
| Nominal balloon diameter (mm) | 3.33±0.40 | 3.18±0.44 |
| Larger than scaffold | 62 | 36 |
| >0.5 mm larger than scaffold | 2.2 | 0.4 |
| BR ratio | 1.20±0.15 | 1.17±0.11 |
| BR ratio ≥1.1 | 80 | 57 |
| ≥16 atm | 87 | 42 |
| ≥18 atm | 69 | 31 |
| Post-procedure QCA | ||
| In-scaffold | ||
| Acute gain (mm) | 1.48±0.44 | 1.47±0.40 |
| MLD (mm) | 2.49±0.36 | 2.43±0.37 |
| %DS | 12.9±5.6 | 11.6±7.5 |
| In-segment | ||
| Acute gain (mm) | 1.29±0.44 | 1.25±0.41 |
| MLD (mm) | 2.30±0.38 | 2.20±0.39 |
| %DS | 18.5±6.5 | 20.0±6.7 |
Data are presented as the mean±SD or the percentage of patients. AVJ-301, ABSORB Japan randomized controlled trial; BR ratio, ratio of balloon diameter to reference vessel diameter; %DS, percentage diameter stenosis; GT1 PMS, Absorb GT1 Japan post-market surveillance (present study); MLD, minimum lumen diameter; QCA, quantitative coronary angiography; RVD, reference vessel diameter.
Optical Coherence Tomography Observations
| GT1 PMS (135 patients, 139 lesions) |
AVJ-301 (82 patients, 86 lesions) |
|
|---|---|---|
| Preprocedure | ||
| Mean lumen area (mm2) | ||
| Proximal | 8.04±2.51 (110) | NA |
| Target | 5.09±1.49 (122) | NA |
| Distal | 6.29±2.30 (115) | NA |
| Minimum lumen area, segment (mm2) | 1.94±0.94 (122) | NA |
| Post-procedure | ||
| Mean lumen area (mm2) | ||
| Proximal | 8.21±2.47 (121) | 7.67±2.86 (58) |
| Scaffold | 8.18±1.85 (127) | 7.38±2.02 (80) |
| Distal | 6.54±2.21 (123) | 6.60±2.77 (63) |
| Minimum lumen area, scaffold (mm2) | 6.86±1.77 (127) | 6.09±1.82 (80) |
| Acute disruption | 1.59 (2/126) | 0.0 (0/80) |
| % Lesions with ISA | 56.7 (72/127) | 82.5 (66/80) |
| % Struts with ISA | 1.89±3.63 (127) | 4.70±6.68 (80) |
| Maximum distance of malapposed strut (mm) | 0.33±0.12 (72) | 0.37±0.16 (66) |
Unless indicated otherwise, data are presented as the mean±SD, with n or n/N in parentheses. AVJ-301, ABSORB Japan randomized controlled trial; GT1 PMS, Absorb GT1 Japan post-market surveillance; ISA, incomplete scaffold apposition.
Although post-procedural QCA in-scaffold and in-segment measurements were similar in both studies (Table 2), numerical improvements were observed in the PMS compared with the ABSORB Japan RCT in post-procedural OCT, including the proximal area (8.21±2.47 vs. 7.67±2.86 mm2), scaffold mean lumen area (8.18±1.85 vs. 7.38±2.02 mm2), and the minimal lumen area within the scaffold (6.86±1.77 vs. 6.09±1.82 mm2; Table 3). In the present study, the percentage of lesions with ISA was also improved compared with the ABSORB Japan RCT (56.7% vs. 82.5%, respectively).
Antiplatelet Medication Use Through 5 YearsPost-procedural antiplatelet medication use through 5 years is presented in Table 4. DAPT was initiated in most patients (131/135) after the procedure. DAPT use was maintained in 90.4%, 83.7%, 72.6%, and 45.2% of patients at 1, 2, 3, and 5 years after the index procedure, respectively. In comparison, DAPT use was continued in only 29.8% of patients through 5 years in the Absorb arm of the ABSORB Japan RCT, with only 47.9% and 40.0% of patients on DAPT at 2 and 3 years, respectively.18 Importantly, in the present study, 11 bleeding events were reported (5 of these were considered serious and occurred while on DAPT).
Antiplatelet Use Over the 5-Years Period After the Procedure
| Drugs | Time after procedure | ||||||
|---|---|---|---|---|---|---|---|
| 7 days | 90 days | 0–1 year | 0–2 years | 0–3 years | 0–4 years | 0–5 years | |
| Aspirin | 97.0 | 96.3 | 93.3 | 89.6 | 86.9 | 78.5 | 70.4 |
| ADP receptor inhibitor | 100 | 97.8 | 94.8 | 89.6 | 79.3 | 67.4 | 57 |
| Prasugrel | 80.0 | 77.8 | 72.6 | 66.7 | 59.3 | 48.1 | 41.5 |
| Clopidogrel | 18.5 | 18.5 | 20.7 | 22.0 | 20.0 | 19.3 | 15.6 |
| Ticagrelor | 0.0 | 0.0 | 0.0 | 0 | 0 | 0 | 0 |
| Ticlopidine | 2.2 | 1.5 | 1.5 | 0 | 0 | 0 | 0 |
| DAPT | 97.0 | 94.8 | 90.4 | 83.7 | 72.6 | 58.5 | 45.2 |
Data show the percentage of patients using each medication at each time point. DAPT, dual antiplatelet therapy.
Clinical Outcomes Through 5 Years
Clinical outcomes through 5 years are presented in Tables 5 and 6 and in Figure 1. No ST was reported throughout the 5-year follow-up (Table 5). In the ABSORB Japan RCT, the cumulative number of ST events at 5 years was 3.8% (in the Absorb BVS arm). The time-to-first event curve comparing the rates of ST in the present study and in the ABSORB Japan RCT is shown in Figure 1A.
ST per Academic Research Consortium Definition
| Items | GT1-PMS (n=135) |
AJ-301 (n=266) |
|---|---|---|
| Acute/Subacute ST (0–30 days) | ||
| Definite/Probable | 0.0 (0/134) | 1.1 (3/265) |
| Definite | 0.0 (0/134) | 1.1 (3/265) |
| Probable | 0.0 (0/134) | 0.0 (0/265) |
| Overall ST (0–90 days) | ||
| Definite/Probable | 0.0 (0/134) | 1.1 (3/262) |
| Definite | 0.0 (0/134) | 1.1 (3/262) |
| Probable | 0.0 (0/134) | 0.0 (0/262) |
| Late ST (31–365 days) | ||
| Definite/Probable | 0.0 (0/134) | 0.4 (1/262) |
| Definite | 0.0 (0/134) | 0.4 (1/262) |
| Probable | 0.0 (0/134) | 0.0 (0/262) |
| Cumulative ST (0–1,825 days) | ||
| Definite/Probable | 0.0 (0/116) | 3.8 (9/237) |
| Definite | 0.0 (0/116) | 3.8 (9/237) |
| Probable | 0.0 (0/116) | 0.0 (0/237) |
Data are presented as percentages, with n/N in parentheses. ST, scaffold thrombosis.
Clinical Outcomes Through 5 Years in the Present Study and ABSORB Japan Randomized Controlled Trial
| Time point / Clinical event | GT1 PMS (n=135) |
AVJ-301 (n=266) |
|---|---|---|
| 1 year (365 days)A | ||
| DMR | 2.2 (3/134) | 9.8 (26/265) |
| TVF | 0.0 (0/134) | 6.0 (16/265) |
| MACE | 0.0 (0/134) | 4.2 (11/265) |
| TLF | 0.0 (0/134) | 4.2 (11/265) |
| Cardiac death/all MI | 0.0 (0/134) | 3.4 (9/265) |
| All death | 0.0 (0/134) | 0.8 (2/265) |
| Cardiac death | 0.0 (0/134) | 0.0 (0/265) |
| All MI | 0.0 (0/134) | 3.4 (9/265) |
| Target vessel MI | 0.0 (0/134) | 3.4 (9/265) |
| TLR | 0.7 (1/134) | 2.6 (7/265) |
| ID-TLR | 0.0 (0/134) | 2.6 (7/265) |
| TVR | 0.7 (1/134) | 4.9 (13/265) |
| ID-TVR | 0.0 (0/134) | 4.9 (13/265) |
| All revascularization | 2.2 (3/134) | 7.9 (21/265) |
| 3 years (1,095 days)A | ||
| DMR | 12.4 (16/129) | 24.0 (62/258) |
| TVF | 6.2 (8/129) | 13.2 (34/258) |
| MACE | 4.7 (6/129) | 9.7 (25/258) |
| TLF | 3.9 (5/129) | 8.9 (23/258) |
| Cardiac death/all MI | 2.3 (3/129) | 6.6 (17/258) |
| All death | 2.3 (3/129) | 2.7 (7/258) |
| Cardiac death | 1.6 (2/129) | 0.4 (1/258) |
| All MI | 1.6 (2/129) | 6.2 (16/258) |
| Target vessel MI | 0.8 (1/129) | 5.4 (14/258) |
| TLR | 3.1 (4/129) | 8.1 (21/258) |
| ID-TLR | 2.3 (3/129) | 7.0 (18/258) |
| TVR | 5.4 (7/129) | 12.0 (31/258) |
| ID-TVR | 3.9 (5/129) | 10.9 (28/258) |
| All revascularization | 10.1 (13/129) | 20.5 (53/258) |
| 5 years (1,825 days)A | ||
| DMR | 19.5 (23/118) | 29.1 (74/254) |
| TVF | 9.3 (11/118) | 16.1 (41/254) |
| MACE | 5.9 (7/118) | 11.8 (30/254) |
| TLF | 5.1 (6/118) | 11.0 (28/254) |
| Cardiac death/all MI | 3.4 (4/118) | 7.9 (20/254) |
| All death | 3.4 (4/118) | 5.9 (15/254) |
| Cardiac death | 1.7 (2/118) | 1.2 (3/254) |
| All MI | 2.5 (3/118) | 7.5 (19/254) |
| Target vessel MI | 1.7 (2/118) | 6.7 (17/254) |
| TLR | 5.1 (6/118) | 10.2 (26/254) |
| ID-TLR | 3.4 (4/118) | 8.3 (21/254) |
| TVR | 9.3 (11/118) | 15.0 (38/254) |
| ID-TVR | 6.8 (8/118) | 13.4 (34/254) |
| All revascularization | 16.1 (19/118) | 24.4 (62/254) |
Data are presented as percentages, with n/N in parentheses. AClinical event results for the AVJ-301 include follow-up windows. DMR, all death, all MI, all revascularization; GT1 PMS, Absorb GT1 Japan post-market surveillance (present study); ID, ischemia driven; MACE, major adverse cardiac event; MI, myocardial infarction; TLF, target lesion failure; TVF, target vessel failure; TVR, target vessel revascularization.
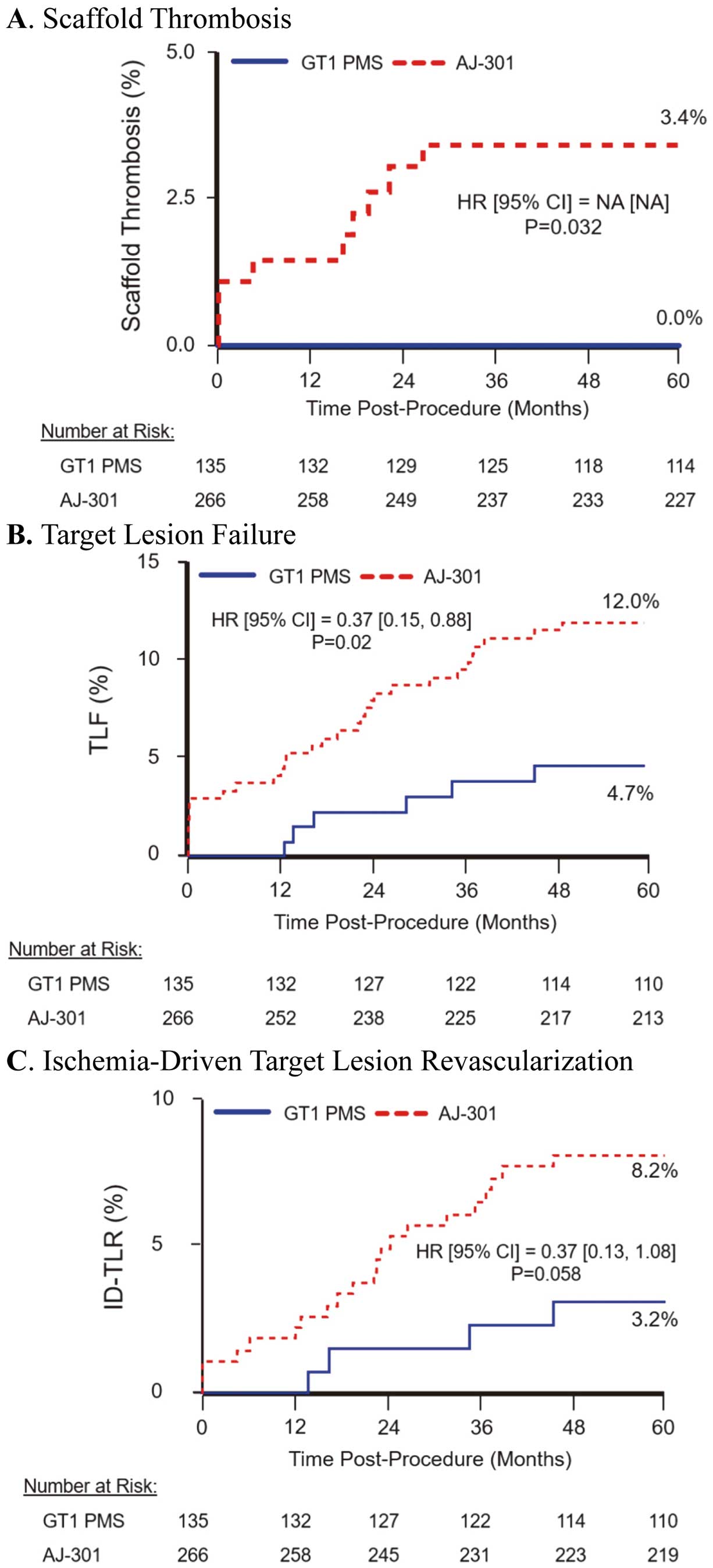
Time-to-first event curves through 5-year follow-up for clinical outcomes in the present study (Absorb GT1 Japan Post-Market Surveillance; GT1 PMS) and the ABSORB Japan randomized controlled trial (AJ-301). (A) Scaffold thrombosis; (B) target lesion failure (TLF); (C) ischemia-driven target lesion revascularization (ID-TLR). CI, confidence interval; HR, hazard ratio; NA, not applicable.
All composite and component endpoints through 5 years are presented in Table 6. The cumulative 5-year rates of DMR, target vessel failure, major adverse cardiac events, TLF, and cardiac death/all MI were 19.5%, 9.3%, 5.9%, 5.1%, and 3.4%, respectively. These rates are all lower than those reported in the Absorb BVS (29.1%, 16.1%, 11.8%, 11.0%, and 7.9%, respectively) arm of the ABSORB Japan RCT.18 In both studies, the rate of TLF (4.7% vs. 12.0% PMS vs. ABSORB Japan RCT, respectively; hazard ratio [HR] 0.37; Figure 1B) was primarily driven by ID-TLR (3.2% vs. 8.2% PMS vs. ABSORB Japan RCT, respectively; HR 0.37; Figure 1C).
In the present study, at 5 years, the rates for all-cause death (3.4%), cardiac death (1.7%), all MI (2.5%), TV-MI (1.7%), all target lesion revascularization (TLR; 5.1%), ID-TLR (3.4%), all target vessel revascularization (TVR; 9.3%), ischemia-driven (ID-) TVR (6.8%), and all revascularization (16.1%) were low (Table 6). Of note, in the majority of outcomes, events were limited to the first 3 years, with very few events occurring between 3 and 5 years. The rates of all component endpoints in the present study were lower than in the ABSORB Japan RCT for all MI (7.5% in the ABSORB Japan RCT), TV-MI (6.7%), all TLR (10.2%), ID-TLR (8.3%), all TVR (15.0%), ID-TVR (13.4%), and all revascularization (24.4%), but not cardiac death (1.2%), although the numbers of events were numerically similar.18 Similarly, in the ABSORB Japan RCT, events were limited to the first 3 years after implantation.
5-Year OCT ResultsAlthough OCT imaging at follow-up was not mandated, OCT imaging at the 5-year follow-up was obtained in 2 patients (Figure 2). Patient background information and OCT movie images are provided in the online appendix.
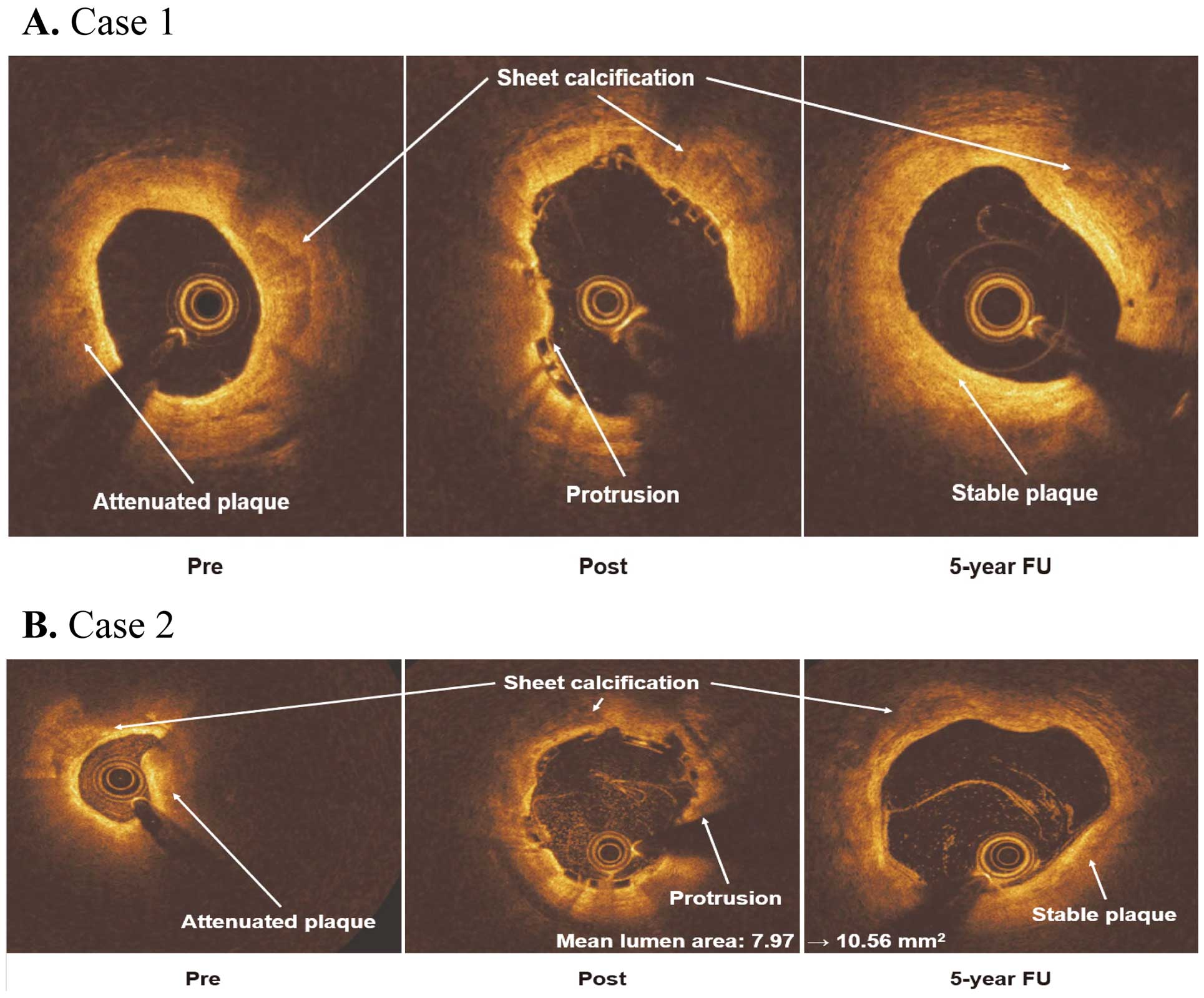
Optical coherence tomography (OCT) imaging at the 5-year follow-up. (A) Case 1. OCT images before (Pre) and after (Post) percutaneous coronary intervention (PCI) and at the 5-year follow-up (FU). The pre-PCI image shows the attenuated plaque that resulted in the plaque protrusion (Post) after deployment of the scaffold. At the 5-year follow-up, the region of plaque protrusion has stabilized. (B) Case 2. OCT images before (Pre) and after (Post) PCI and at the 5-year follow-up (FU). The pre-PCI image shows the coexistence of attenuated plaque and sheet calcification. Plaque protrusion (Post) appeared after the deployment of the scaffold. However, at 5-year follow-up, plaque stabilization was achieved. Mean lumen area increased (from 7.97 to 10.56 mm2), despite the sheet calcification present at all time points. This case demonstrates that positive remodeling can be obtained regardless of calcification.
Case 1 OCT images of the mid-left anterior descending coronary artery (RVD 2.28 mm; minimum lumen diameter [MLD] 1.15 mm) of a 68-year-old man before PCI, after PCI, and 5 years after Absorb BVS implantation are shown in Figure 2A. The MLD after the procedure was 2.67 mm. At the 5-year follow-up, the MLD was 2.48 mm, corresponding to a late lumen loss of 0.19 mm. The scaffold was mostly resorbed, but remnants could be seen in some frames. Neither neoatherosclerosis (defined as the presence of thin-cap fibroatheroma, macrophage, calcification, or visible vasa vasorum) nor thrombus was observed. Interestingly, there was evidence of stabilized plaque attenuation where the lesion had been covered by the scaffold (Supplementary Movie 1).
Case 2 OCT images of the distal right coronary artery (RVD 2.68 mm; MLD 0.47 mm) of a 65-year-old man before PCI, after PCI, and 5 years after Absorb BVS implantation are shown in Figure 2B. Positive arterial remodeling was observed, even at the site of calcified plaque. Immediately after the procedure, the mean lumen area (MLA) was 7.97 mm2. At the 5-year follow-up, the MLA was 10.56 mm2, corresponding to an MLA gain of 2.59 mm2. The dissection observed at the distal edge of the scaffold after the procedure was shown to be healed on 5-year follow-up imaging. The scaffold had been completely fully resorbed, even at the portion of the lesion with the calcified plaque. Neither neoatherosclerosis nor thrombus was observed (Supplementary Movie 2).
With follow-up now complete through 5 years, the novel findings from this PMS were no reported cases of ST and low rates of both composite and individual device-oriented outcome measures through 5 years. These rates were consistently lower than those reported in the ABSORB Japan RCT Absorb BVS. In addition, most events occurred within the first 3 years, with very few events occurring between 3 and 5 years, consistent with previous Absorb trials.11,18,28
In this study, there were no early or very late instances of ST through 5 years. These promising results may be attributed to the high compliance to the recommended implantation technique and the routine use of intravascular imaging.19,20,29,30 For example, Puricel et al demonstrated that the adoption and strict adherence of a BVS-specific implantation strategy was independently associated with an approximate 70% reduction in the incidence of ST compared with patients treated without such a strategy.19 However, most Absorb trials reported poor adoption of a BVS-appropriate implantation technique, resulting in higher TLF and ST rates compared with contemporary DES. Stone et al20 reported that in an analysis of the ABSORB II, ABSORB Japan, ABSORB China, and ABSORB III trials, all 3 optimal PSP techniques were performed in only 4.9% of patients. Interestingly, this small subgroup had the lowest HR for TLF (HR 0.57), demonstrating the importance of technique with BVS implantation. In the earlier Absorb trials, the use of intravascular imaging was not encouraged.20 Most recently, the ABSORB IV RCT11 strongly recommended an improved patient and lesion selection process and implantation technique to address the effects of suboptimal BVS implantation techniques on patient outcomes;19,20 however, the use of intravascular imaging guidance was not strongly promoted and only used in 15.6% of BVS implantation procedures. Despite high rates of pre- and post-dilatation (99.9% and 84.3%, respectively) and the very low number of lesions with RVD <2.25 mm, the mean in-device acute gain was lower and residual diameter stenosis was higher with BVS than DES. Correspondingly, device and procedural success rates were lower in the Absorb arm. The 5-year Kaplan-Meier rates of TLF and ST were 17.5% and 1.7%, respectively, in the BVS arm compared with 14.5% and 1.1%, respectively, in the DES arm.
In the present study, the 5-year rates of TLF (Table 6) and ST (Table 5) were 5.1% and 0%, respectively. Although the differences in study design and sample size make any comparisons between the present and previous studies, more specifically the ABSORB Japan RCT, hypothesis generating only, many potential reasons explaining the discrepancies in results between studies can be suggested. Protocol-based exclusion of small vessels and recommendation for prolonged prescription of DAPT (especially prasugrel) may have contributed to the improved outcome. Routine imaging guidance, including before and after the BVS deployment, allows for the selection of appropriate BVS size, strut attachment to the vessel wall, and avoidance of underexpansion of the BVS, which is an important determinant of BVS-related events. This observation supports that ensuring appropriate vessel sizing and optimal device expansion and apposition to the vessel wall, which are best addressed with intravascular imaging, promotes complete re-endothelialization and, subsequently, the necessary coverage of the resorbable struts to prevent intraluminal scaffold dismantling during bulk erosion, thought to be the leading cause of the excess risk of thrombosis with BVS.11,30,31
As with previous Absorb trials,11,18,28 most events in the present study occurred within the first 3 years, the approximate time needed for complete resorption of the Absorb GT1 BVS, with very few events occurring between 3 and 5 years (Tables 5,6; Figure 1). For example, in the ABSORB Japan RCT, all ST events were restricted to the first 3 years following BVS implantation, with no instances of ST occurring after 3 years. These data provide additional support that the risk period associated with the Absorb BVS was limited to the initial 3 years and may be further reduced with strict compliance to a BVS-specific implantation technique and the routine use of intravascular imaging.
In the present study, DAPT continuation for at least 12 months was strongly encouraged. At the 5-year follow-up, 45.2% (61/135) of patients were on DAPT (Figure 1). In the ABSORB Japan RCT, the number of participants receiving the Absorb BVS that were on DAPT at 2 and 3 years was substantially lower than in the present study (47.9% [127/265] vs. 83.7% [113/135] at 2 years; 40.0% [106/265] vs. 72.6% [98/135] at 3 years). However, optimal DAPT duration after BVS implantation and its relationship to the rate of adverse events require further investigation.
In addition, we present 5-year follow-up OCT images for 2 representative cases. In both cases, plaque protrusion turned to stabilized plaque. Notably, positive remodeling was seen in 1 case despite sheet calcification. Using serial OCT and pathological findings in a porcine model, Nakatani et al showed late lumen enlargement after BVS implantation.32 Human OCT data from the EVERBIO II study subanalysis also presented the possibility of positive remodeling after BVS implantation.33 Our findings from OCT images are in line with these previous reports and reproduced the “golden tube”,34 although the number of cases is quite limited.
Study LimitationsThere are several limitations that should be considered. First, we must acknowledge the possibility of Type 2 error due to the limited sample size. The present study had initially planned to enroll 2,000 consecutive patients. However, in September 2017, Abbott Vascular decided to stop selling the Absorb GT1 system globally, including Japan. Surveillance sites could continue to use the device and register patients until December 15, 2017. As a result, only 135 patients were enrolled prior to the retirement of the Absorb BVS device. Next, complex lesions were largely excluded from the PMS. Highly selected lesions were treated based on the CVIT guidelines. In addition, intravascular imaging was used for lesions and monitored by a core laboratory. The low frequency of intracoronary imaging use may be perceived as a barrier to generalizing this result outside Japan. As is the nature of a PMS, this trial did not include a control group to compare the 5-year clinical outcomes. In conjunction with the small sample size, interstudy comparisons of clinical outcome rates should be considered hypothesis generating only. Finally, subjects were predominantly male, which may restrict the generalizability of the results to women.
Adherence to a BVS-specific implantation technique, in addition to the consistent use of intravascular imaging, may reduce the incidence of very late adverse events through 5 years.
The authors thank Sunao Nakamura (Shin-Tokyo Hospital), Kotaro Hasegawa (Saitama Saiseikai Hospital), Shigeru Saito (Shonan Kamakura General Hospital), Yoshisato Shibata (Miyazaki Medical Association Hospital), Mamoru Nanasato (Nagoya Daini Red Cross Hospital), Toshiro Shinke (Kobe University Hospital), and Kazushige Kadota (Kurashiki Central Hospital) for enrolling patients to this PMS. The authors also thank Scaffold Thrombosis committee members (Takashi Akasaka [Wakayama Medical University Hospital] and Shinpei Nakatani [Osaka Police Hospital]). The authors acknowledge Christine VandeLinde of PharmTrials for data analysis.
This PMS was sponsored by Abbott Vascular.
M.N., T. Kawasaki, T.S., K.T., and K.K. received ad hoc honoraria from Abbott Vascular (the sponsor of this PMS) during the 5-year study period. K.K. is a member of Circulation Journal’s Editorial Team. H.K. and K.S. are full-time employees of Abbott Vascular. M.N. has received endowed department from Boston Scientific Japan, Kaneka, Terumo, Nipro, Otsuka Medical, Japan Lifeline, Asahi Intec, and Biotronik Japan. N.S., K.F., J.F. and T. Kimura have nothing to disclose.
This study was approved by Toho University Ohashi Medical Center Institutional Review Board (MI16-2) and review boards or ethics committee at each of the participating centers.
The deidentified participant data, study protocol, and statistical analysis plan are available at https://clinicaltrials.gov/ct2/show/NCT03409731. The 4-year data are currently available. The 5-year data will become available by April 2024.
Supplementary Movie 1. Serial OCT finding pre, post BVS implantation and 5 year follow-up.
Supplementary Movie 2. Serial OCT finding pre, post BVS implantation and 5 year follow-up.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0877