Abstract
Background: It has not been fully elucidated which patients with persistent atrial fibrillation (PerAF) should undergo substrate ablation plus pulmonary vein isolation (PVI). This study aimed to identify PerAF patients who required substrate ablation using intraprocedural assessment of the baseline rhythm and the origin of atrial fibrillation (AF) triggers.
Methods and Results: This was a post hoc subanalysis using extended data of the EARNEST-PVI trial, a prospective multicenter randomized trial comparing PVI-alone and PVI-plus (i.e., PVI with added catheter ablation) arms. We divided 492 patients into 4 groups according to baseline rhythm and the location of AF triggers before PVI: Group A (n=22), sinus rhythm with pulmonary vein (PV)-specific AF triggers (defined as reproducible AF initiation from PVs only); Group B (n=211), AF with PV-specific AF triggers; Group C (n=94), sinus rhythm with no PV-specific AF trigger; Group D (n=165), AF with no PV-specific AF trigger. Among the 4 groups, only in Group D (AF at baseline and no PV-specific AF triggers) was arrhythmia-free survival significantly lower in the PVI-alone than PVI-plus arm (P=0.032; hazard ratio 1.68; 95% confidence interval 1.04–2.70).
Conclusions: Patients with sinus rhythm or PV-specific AF triggers did not receive any benefit from substrate ablation, whereas patients with AF and no PV-specific AF trigger benefited from substrate ablation.
Catheter ablation with pulmonary vein isolation (PVI) is an important treatment strategy for the maintenance of sinus rhythm in patients with atrial fibrillation (AF). However, catheter ablation with PVI is less effective for persistent AF (PerAF) than for paroxysmal AF, and the clinical impact of an additional substrate ablation plus PVI remains contentious.1 Although it is true that some patients with PerAF can be treated with PVI alone, it has also been reported that patients who could not be successfully treated with a durable PVI can be made free of arrhythmia recurrence with additional ablation.2 The PerAF population is heterogeneous. For example, pulmonary vein (PV) triggers are known to play an important role in patients with PerAF and an unsuccessful ablation of non-PV triggers is related to arrhythmia recurrence.3,4 The appropriate selection of patients requiring an additional procedure beyond PVI during the initial catheter ablation procedure should be elucidated.
The results of the EARNEST-PVI (Efficacy of Pulmonary Vein Isolation Alone in Patients with Persistent Atrial Fibrillation) trial suggested the benefit of adding substrate ablation (“PVI-plus” strategy) at both the 1- and 3-year follow-up.5,6 The benefit of the PVI-plus strategy persists throughout the 3-year follow-up, especially in patients who have undergone linear ablation.6 Using the 1-year follow-up results, we reported that a DR-FLASH score (based on diabetes, renal dysfunction, PerAF, left atrial [LA] diameter >45 mm, age >65 years, female sex, and hypertension) >3, which is a predictor of a low voltage area, could distinguish patients who would and would not benefit from additional substrate ablation.7 Considering the results of previous studies that could not prove the efficacy of additional substrate ablation,1,8 there is likely to be a certain proportion of the population who will not benefit from additional substrate ablation. Theoretically, additional ablation has an increased risk of complications and it is worth detecting specific patients who would benefit from the PVI-plus strategy.
It has been hypothesized that patients with AF triggers exclusively from the PVs would be a good population for the PVI-alone strategy by us, OCVC-arrhythmia investigators.9 However, using the 1-year follow-up results of the EARNEST-PVI trial, we found that in patients with PerAF there was no difference in the efficacy of the PVI-alone vs. PVI-plus strategy regardless of whether or not there were PV triggers.9 One of the reasons why we could not identify the patient population that could be treated by the PVI-alone strategy was the heterogeneity of the PerAF patient population. The presence of sinus rhythm during the procedure has been reported to be a predictor of arrhythmia-free survival in patients with PerAF using the PVI-alone strategy,10,11 and baseline sinus rhythm would be related to a PV related mechanism and low substrate progression.
We hypothesized that sinus rhythm at baseline prior to the procedure and reproducible initiation of AF from PVs could be predictors that ablation with the PVI-alone strategy could be sufficient and that non-sinus rhythm at baseline and no PV-specific AF triggers could help select a limited population of patients who would require additional substrate ablation to maintain sinus rhythm. The aim of this study was to evaluate patient selection using intraprocedural assessments of baseline rhythm and AF trigger origins, drawing on extended data from the EARNEST-PVI trial.5,6
Methods
Study Population
This study used extended data of the EARNEST-PVI trial, a prospective multicenter randomized open-label trial registered with ClinicalTrials.gov (ID: NCT03514693). The study design and main results have been published previously.5,12
Patients were enrolled in 8 experienced centers to catheter ablation of AF. To be eligible for inclusion, patient had to have undergone an initial catheter ablation with a sustained episode of AF lasting for ≥7 days and <5 years. Exclusion criteria were a LA diameter ≥50 mm, valvular AF, a history of cardiac surgery, hemodialysis, left ventricular ejection fraction <30%, and heart failure with New York Heart Association functional class ≥3. Blood tests were performed within 30 days prior to the AF ablation procedures.
Written informed consent was obtained from all patients before the procedure and the study was approved by the ethics committees of each participating hospital. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Detection of AF Triggers
Anti-arrhythmic drugs were stopped at least 5 half-lives before the procedure, except for amiodarone. At the beginning of the procedure, the origin of the AF triggers was assessed. AF triggers were defined as reproducible ectopy that initiated AF more than once. To assess the origins of AF triggers, a multipolar spiral catheter and an ablation catheter were positioned within the left and right PVs, respectively. Additional electrode catheters were inserted into the coronary sinus (CS) and right atrium (RA). When AF was triggered, if the potential in either PV preceded that in the other PV or in the CS and RA, the origin was suspected to be the respective PV. The origin PV was identified based on the activation sequence when the trigger reappeared by placing a spiral catheter and an ablation catheter in the upper and lower PVs, respectively.
In case of AF at baseline, electrical cardioversion was performed to assess for any spontaneous AF triggers. If AF triggers were identified before isoproterenol loading, isoproterenol was not administered, and ablation of the PVI or non-PV AF triggers was first attempted. Successful ablation of AF triggers was followed by isoproterenol loading and the induction of AF triggers. If no spontaneous AF initiation was observed, AF was induced with isoproterenol, starting with a 0.1 μg/kg bolus injection followed by continuous infusion starting at a rate of 0.05 μg/kg/min and doubling every 2 min to a maximum rate of 0.4 μg/kg/min. The criteria for finishing the isoproterenol test were continuation of a maximum dose of isoproterenol for ≥5 min, systolic blood pressure ≤80 mmHg, heart rate ≥130 beats/min, and detection of AF triggers. After discontinuing the isoproterenol infusion, AF triggers were continually monitored for 5 min to assess the late initiation of AF.
Patients with PV-specific AF triggers were defined as those whose AF triggers consistently and exclusively originated from the PVs only. Patients with no PV-specific AF triggers were defined as those with no apparent AF triggers from the PVs or those with both non-PV triggers and PV triggers.
Catheter Ablation Procedures
In the EARNEST-PVI trial, all patients underwent PVI first, and it was recommended that the large antrum of the ipsilateral PVs was isolated, but individual PVIs were also allowed. The procedure was performed with radiofrequency energy, with a recommended energy setting of 25–35 W. The PVIs were reconfirmed at least 20 min after the first successful PVI.
For complex fractionated atrial electrogram (CFAE) ablation, CFAEs were detected using the automated algorithms of the 3-dimensional electroanatomical mapping systems as described in the protocol paper.12 The endpoint of the CFAE ablation was the elimination of all local CFAE sites in the LA and CS or AF termination.
For linear ablation, at least 2 linear lesion sets were required. One was selected as either an anterior or posterior mitral isthmus line and the other as either a roof or bottom line. The endpoint of the linear ablation was complete bidirectional block across the linear lesions, which was reconfirmed at least 20 min after the first successful block.
Additional ablation procedures recommended by the guidelines13 were accepted, such as ablation of non-PV triggers, common atrial flutter ablation if documented clinically, or induced supraventricular tachycardia ablation. In the PVI-plus group, patients underwent CFAE ablation, linear ablation, or both at the discretion of the attending physician. The induction of non-PV triggers after the PVI was left to the discretion of the operator.
The decision to finish the procedure without completing the protocol, including CFAE elimination, linear conduction block, and non-PV trigger ablation, was left to the operator’s discretion after their best efforts to complete it.
Intraprocedural Assessment According to Baseline Rhythm and AF Trigger Origin
The study population was divided into 4 groups by intraprocedural assessment based on baseline rhythm and AF trigger origin, as shown in Figure 1. The baseline rhythm was evaluated at the beginning of the procedure. Group A consisted of patients who were in sinus rhythm at baseline and had PV-specific AF triggers. Group B consisted of patients who were in AF at baseline and had PV-specific AF triggers. Group C consisted of patients who were in sinus rhythm at baseline and had no PV-specific AF triggers. Group D consisted of patients who were in AF at baseline and had no PV-specific AF triggers.
Patient Follow-up
Patients were scheduled to visit the hospital every 1, 3, 6, and 9 months, and then every 12 months after the ablation. Follow-up included an interview, electrocardiogram (ECG), and blood tests. A 24-h Holter monitoring ECG was scheduled at 6 months, and then 12 months after the ablation. Patients with any symptoms received an additional 24-h Holter ECG or event recorder. In addition to the scheduled follow-up, patients had access to the hospital or a nearby clinic at any time. Patients were strongly encouraged to visit the hospital or a nearby clinic if they had any symptoms that they suspected were arrhythmias or if they noticed irregularities in their peripheral pulse through regular self-measurements.
Arrhythmia recurrence was defined as the documentation of any atrial arrhythmias, including AF, atrial flutter, or atrial tachycardia lasting ≥30 s. The blanking period was defined as 3 months after the procedure. The use of antiarrhythmic drugs was accepted within the blanking period, but was not recommended after the blanking period.
Statistical Analysis
Continuous variables are presented as the median with interquartile range (IQR) and categorical variables are presented as numbers and percentages. Continuous variables were compared using a Wilcoxon signed-rank test. Categorical variables were compared using Fisher’s exact test. Arrhythmia-free survival after the 3-month blanking period was evaluated using Kaplan-Meier analysis with a log-rank test. All analyses were performed with JMP Pro14 software (SAS Institute, Cary, NC, USA). P values two-tailed <0.05 was considered statistically significant.
Results
Study Subjects
In all, 512 patients were enrolled in the EARNEST-PVI trial and were randomly assigned to the PVI-alone or PVI-plus arms. In the present study, using the extended follow-up data of the EARNEST-PVI trial over a median follow-up of 2.8 years, 492 patients were finally assessed after excluding patients because of protocol violations (n=9), errors in the electronic data collection system (n=5), inappropriate outcome measures (n=4), consent withdrawal (n=1), and missing trigger information (n=1; Figure 1). The rhythm at baseline before the ablation procedure was sinus rhythm in 116 (24%) patients and AF in 376 (76.4%). AF was not terminated by electrical cardioversion in 17 (3.5%) patients. PV-specific and non-PV AF triggers were confirmed in 233 (47%) and 24 (5%) patients, respectively. Both PV and non-PV triggers were observed in 13 patients, who were defined as patients with no PV-specific triggers.
There were 22 patients in Group A (12 in the PVI-alone and 10 in the PVI-plus arms), 211 patients in Group B (109 in the PVI-alone and 102 in PVI-plus arms), 94 patients in Group C (48 in the PVI-alone and 46 in PVI-plus arms), and 165 patients in Group D (78 in the PVI-alone and 87 in the PVI-plus arms). Group D had the highest proportion of women. The LA diameter was smallest and the creatine level highest in Group C. The characteristics of Groups A, B, C, and D are summarized in Table 1.
Table 1.
Characteristics of Patients in Groups A–D
| |
Group A
(n=22) |
Group B
(n=211) |
Group C
(n=94) |
Group D
(n=165) |
P value |
| Age (years) |
65 [59–70] |
67 [59–72] |
67 [59–73] |
67 [59–73] |
0.765 |
| Male sex |
16 (73) |
169 (80) |
76 (81) |
112 (68) |
0.028 |
| Longstanding (≥12 months) AF |
4 (18) |
55 (26) |
31 (33) |
34 (21) |
0.146 |
| Duration of persistent AF (days) |
156 [77–330] |
132 [63–392] |
244 [81–462] |
119 [63–271] |
0.074 |
| Hypertension |
12 (55) |
124 (59) |
55 (59) |
105 (64) |
0.697 |
| Diabetes |
4 (18) |
38 (18) |
13 (14) |
28 (17) |
0.840 |
| Heart failure |
2 (9.1) |
39 (18) |
18 (19) |
32 (19) |
0.769 |
| History of MI |
1 (4.6) |
8 (3.8) |
1 (1.1) |
3 (1.8) |
0.363 |
| Chronic kidney disease |
0 (0.0) |
13 (6.2) |
4 (4.3) |
8 (4.9) |
0.788 |
| LV diastolic diameter (mm) |
45 [43–49] |
47 [44–50] |
45 [43–50] |
47 [44–50] |
0.375 |
| LA diameter (mm) |
42 [38–46] |
43 [39–46] |
41 [38–44] |
43 [40–46] |
0.009 |
| Creatinine (mg/dL) |
0.84 [0.74–0.99] |
0.89 [0.81–1.02] |
0.90 [0.78–1.02] |
0.87 [0.74–0.99] |
0.022 |
| BNP (pg/mL) |
140 [68–218] |
145 [99–209] |
148 [96–219] |
148 [99–228] |
0.880 |
| β-blocker |
11 (50) |
90 (43) |
45 (48) |
68 (41) |
0.668 |
| ACEi |
0 (0.0) |
14 (6.7) |
3 (3.2) |
12 (7.3) |
0.432 |
| ARB |
7 (32) |
59 (28) |
27 (29) |
37 (22) |
0.506 |
| DR-FLASH score |
4 [2–4] |
4 [3–5] |
3 [3–4] |
4 [3–5] |
0.341 |
| DR-FLASH score >3 |
12 (55) |
120 (57) |
46 (49) |
98 (59) |
0.423 |
Unless indicated otherwise, data are given as n (%) or the median [interquartile range]. ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; DR-FLASH score, score based on diabetes, renal dysfunction, persistent AF, left atrial diameter >45 mm, age >65 years, female sex, and hypertension; Group A, sinus rhythm with pulmonary vein (PV)-specific AF triggers; Group B, AF with PV-specific AF triggers; Group C, sinus rhythm with no PV-specific AF trigger; Group D, AF with no PV-specific AF trigger; LA, Left atrium; LV, left ventricle; MI, myocardial infarction.
Procedural Data
The procedural characteristics for Groups A, B, C, and D are summarized in Table 2. In total, the a deflectable sheath, irrigation catheter, and contact force-sensing catheter were used in 211 (43%), 482 (98%), and 457 (93%) patients, respectively. Deflectable sheaths were more often used in Groups B and D and contact force-sensing catheters were least used in Group D.
Table 2.
Procedure Characteristics in Groups A–D
| |
Group A
(n=22) |
Group B
(n=211) |
Group C
(n=94) |
Group D
(n=165) |
P value |
| Baseline rhythm |
|
|
|
|
– |
| Sinus rhythm |
22 (100) |
– |
94 (100) |
– |
|
| AF |
– |
211 (100) |
– |
165 (100) |
|
| Deflectable sheath |
3 (14) |
112 (53) |
11 (12) |
85 (52) |
<0.001 |
| Irrigation catheter |
22 (100) |
207 (98) |
93 (99) |
160 (97) |
0.779 |
| Contact force-sensing catheter |
21 (95) |
200 (95) |
92 (98) |
144 (87) |
0.006 |
| CFAE ablation |
0 (0.0) |
13 (6.2) |
3 (3.2) |
21 (13) |
0.015 |
| Linear ablation |
10 (45) |
89 (42) |
43 (46) |
67 (41) |
0.860 |
| Roof |
10 (100) |
89 (100) |
43 (100) |
66 (99) |
0.574 |
| Bottom |
5 (50) |
48 (54) |
32 (74) |
25 (37) |
0.002 |
| LA anterior |
1 (10) |
17 (19) |
0 (0.0) |
21 (31) |
<0.001 |
| Lateral mitral isthmus |
9 (90) |
74 (83) |
43 (100) |
44 (66) |
<0.001 |
| Superior vena cava isolation |
0 (0.0) |
5 (2.4) |
1 (1.1) |
7 (4.2) |
0.475 |
| Non-PV trigger ablation |
0 (0.0) |
1 (0.5) |
5 (5.3) |
22 (13) |
<0.001 |
| Total procedure time (min) |
180 [133–231] |
160 [125–200] |
175 [139–226] |
148 [110–200] |
0.010 |
| Total RF application time (min) |
50 [32–58] |
39 [28–51] |
43 [32–58] |
36 [26–48] |
0.002 |
| Total RF energy 1,000 J |
95 [58–112] |
71 [48–95] |
89 [45–121] |
61 [45–86] |
<0.001 |
Unless indicated otherwise, data are given as the n (%) or median [interquartile range]. CFAE, complex fractionated atrial electrogram; RF, radiofrequency. Other abbreviations as in Table 1.
The origins of the PV triggers are summarized in Table 3. One patient in Group C and 12 patients in Group D had both PV and non-PV triggers. A non-PV trigger ablation was most often performed in Group D. In 4 patients in whom no non-PV triggers were observed during the preoperative electrophysiological study, non-PV triggers were identified during and/or after PVI. Of the 28 patients in total with non-PV triggers, ablations were successful in 13. Of the 233 patients with PV-specific triggers, only 1 (0.4%) patient experienced a non-PV trigger after the PVI.
Table 3.
Characteristics of AF Triggers in Groups A–D
| |
Group A
(n=22) |
Group B
(n=211) |
Group C
(n=94) |
Group D
(n=165) |
| PV triggers |
| Left PV |
19 (86) |
139 (66) |
|
|
| Left superior PV |
13 (59) |
112 (53) |
|
|
| Left inferior PV |
4 (18) |
23 (11) |
|
|
| Unspecified |
2 (9.1) |
13 (6.2) |
|
|
| Right PV |
6 (27) |
115 (55) |
|
|
| Right superior PV |
6 (27) |
99 (47) |
|
|
| Right inferior PV |
0 (0.0) |
11 (5.2) |
|
|
| Unspecified |
0 (0.0) |
7 (3.3) |
|
|
| Non-PV triggers |
| No. non-PV triggers 1/2/3/4 |
0/0/0/0 |
1/0/0/0 |
2/3/0/0 |
13/6/2/1 |
| Superior vena cava |
0 (0.0) |
0 (0.0) |
0 (0.0) |
6 (3.6) |
| High RA |
0 (0.0) |
0 (0.0) |
1 (1.1) |
4 (2.4) |
| Lateral RA |
0 (0.0) |
0 (0.0) |
2 (2.1) |
0 (0.0) |
| RA septum |
0 (0.0) |
0 (0.0) |
1 (1.1) |
5 (3.0) |
| LA septum |
0 (0.0) |
0 (0.0) |
0 (0.0) |
4 (2.4) |
| Posterior LA |
0 (0.0) |
0 (0.0) |
0 (0.0) |
5 (3.0) |
| Anterior LA |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.6) |
| Lateral LA |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.6) |
| Mitral annulus |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (0.6) |
| Coronary sinus |
0 (0.0) |
1 (0.5) |
0 (0.0) |
4 (2.4) |
| Not identified |
0 (0.0) |
0 (0.0) |
4 (4.3) |
4 (2.4) |
Data are given as n (%). RA, right atrium. Other abbreviations as in Table 1.
The sites of the non-PV triggers are also summarized in Table 3. CFAE ablation was most often used in Group D. For the mitral linear ablation strategy, an anterior line was most often selected in Group D. The achievement of a complete linear block is summarized in Table 4. Bidirectional conduction block was similarly achieved among the groups.
Table 4.
Number of Successful Bidirectional Conduction Block/Attempted Linear Ablations in Groups A–D
| Site of ablation |
Group A |
Group B |
Group C |
Group D |
P value |
| Roof |
9/10 (90) |
87/89 (98) |
42/43 (98) |
63/66 (95) |
0.404 |
| Bottom |
5/5 (100) |
45/48 (94) |
31/32 (97) |
23/25 (92) |
0.822 |
| Left atrial anterior |
1/1 (100) |
13/17 (76) |
0/0 |
19/21 (90) |
0.473 |
| Lateral mitral isthmus |
8/9 (89) |
53/74 (72) |
34/43 (79) |
33/44 (75) |
0.703 |
Unless indicated otherwise, data are given as n (%). Abbreviations as in Table 1.
Arrhythmia-Free Survival: Baseline Rhythm and AF Trigger Origins
Arrhythmia-free survival analyses according to baseline rhythm or AF trigger origin are shown in Figure 2. In patients with sinus rhythm at baseline, the arrhythmia-free survival was comparable between the PVI-alone and PVI-plus arms (hazard ratio [HR] 0.98; 95% confidence interval [CI] 0.53–1.81, P=0.950), whereas arrhythmia-free survival was better in the PVI-plus than PVI-alone arm among patients with AF at baseline (HR 1.51; 95% CI 1.08–2.09; P=0.014). In patients with PV-specific AF triggers, arrhythmia-free survival was comparable between the PVI-alone and PVI-plus arms (HR 1.28; 95% CI 0.83–1.98; P=0.264), but tended to be better in the PVI-plus than PVI-alone arm in patients with no PV-specific triggers (HR 1.46; 95% CI 0.99–2.14; P=0.055).
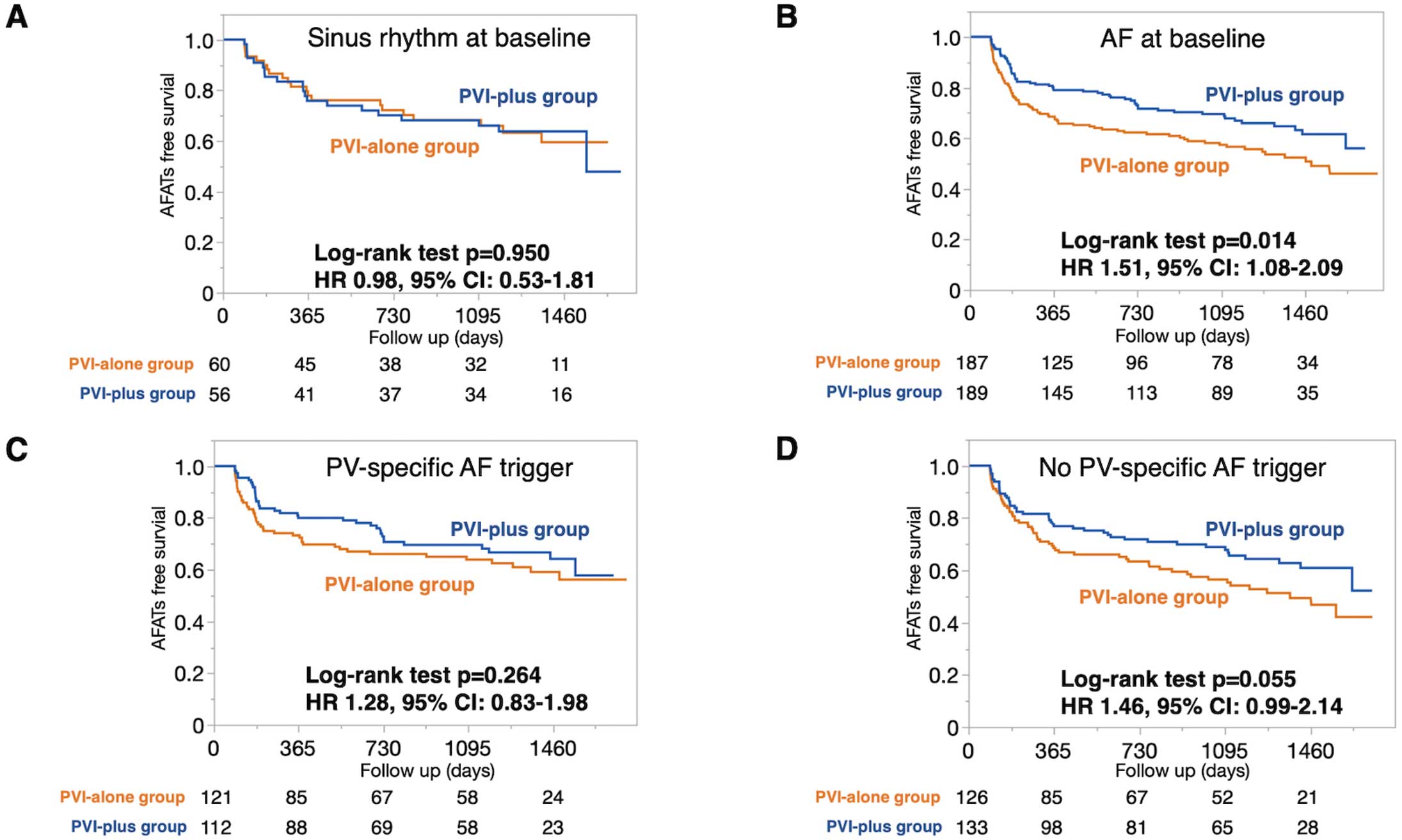
In the no PV-specific AF trigger group, both PV and non-PV triggers were observed in 13 of 24 patients with non-PV triggers. The clinical outcomes were comparable between these 2 groups (HR 0.83; 95% CI 0.31–2.25; log-rank P=0.718; Supplementary Figure 1). After PVI, a non-PV trigger ablation was performed in 28 patients and successful elimination of all of induced non-PV triggers was achieved in 13 (46%) patients. Outcomes were similar in patients in whom non-PV triggers were successfully eliminated and those in whom the non-PV triggers were not eliminated (HR 0.51; 95% CI 0.19–1.37; P=0.172; Supplementary Figure 1).
Arrhythmia-Free Survival in Groups A, B, C, and D
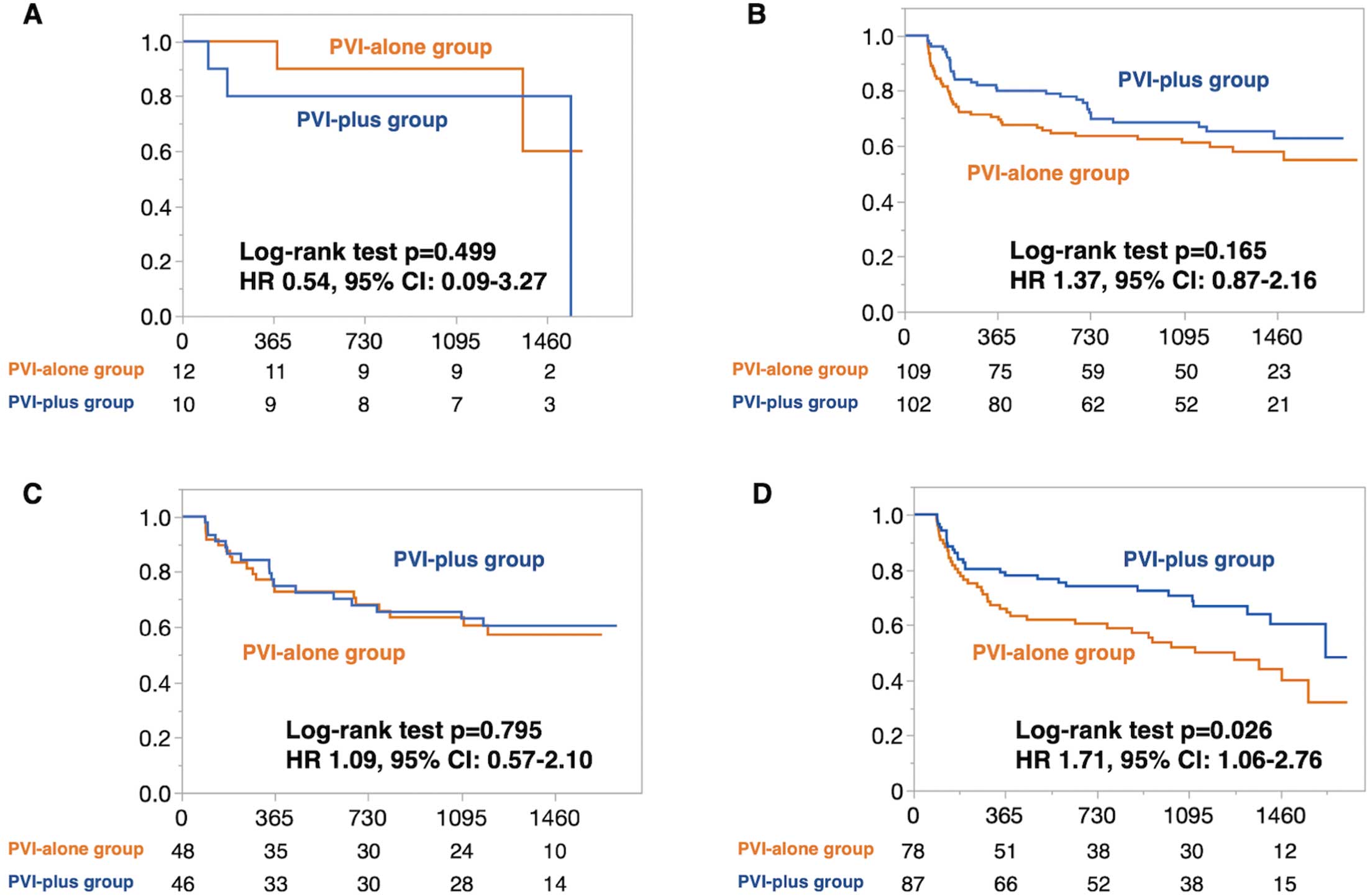
In patients with sinus rhythm at baseline or PV-specific AF triggers (i.e., Groups A, B, and C), the PVI-plus strategy was not superior to the PVI-alone strategy (Group A, HR 0.54 [95% CI 0.09–3.27, P=0.499]; Group B, HR 1.37 [95% CI 0.87–2.16, P=0.165]; Group C, HR 1.09 [95% CI 0.57–2.10, P=0.795]; Figure 3A–C). However, in Group D, the PVI-plus strategy was superior to the PVI-alone strategy (HR 1.71; 95% CI 1.06–2.76; P=0.026; Figure 3D). In patients with a DR-FLASH score >3, the PVI-plus strategy was superior to the PVI-alone strategy (HR=1.85; 95% CI 1.28–2.68; P<0.001; Supplementary Figure 2, left panel), whereas in patients with DR-FLASH score ≤3, no difference was observed between the PVI-plus and PVI-alone strategies (HR 0.87; 95% CI 0.54–1.40; P=0.559; Supplementary Figure 2, right panel). Among patients with a DR-FLASH score >3, those in Groups A and C did not benefit from the PVI-plus strategy, whereas those in Group D benefited most from the PVI-plus strategy (HR 2.08; 95% CI 1.12–3.88). In patients with DR-FLASH score ≤3, even Group D did not benefit from the PVI-plus strategy (HR 0.96; 95% CI 0.55–1.70).
Redo Procedure Assessment
Redo procedures were performed in 126 patients: 69 in the PVI-alone group and 57 in the PVI-plus group. The incidence of any PV reconnections and the incidence of reconnections at the previous linear ablation sites in each group are shown in Supplementary Figure 3.
Procedure-Related Complications
In the EARNEST-PVI trial population, 1 patient in Group D of the PVI-plus arm experienced a massive stroke 3 days after the procedure and died 7 days after the procedure. The details of the safety outcomes within 1 year and the procedure-related complications within 1 week are summarized in Table 5. Regarding the procedure-related complications within 1 week, strokes, cardiac tamponade, and death tended to be observed more often in the PVI-plus arm (3 [1.2%] patients) than PVI-alone arm (0 [0%] patients; P=0.122).
Table 5.
Safety Outcomes Within 1 Year and Procedure-Related Complications Within 1 Week in Groups A–D
| |
PVI-alone arm (n=247) |
PVI-plus arm (n=245) |
Group A
(n=12) |
Group B
(n=109) |
Group C
(n=48) |
Group D
(n=78) |
Group A
(n=10) |
Group B
(n=102) |
Group C
(n=46) |
Group D
(n=87) |
| Safety outcomes within 1 year |
| Death |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.2) |
| Symptomatic stroke |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.3) |
0 (0.0) |
2 (2.0) |
0 (0.0) |
2 (2.3) |
| Procedure-related complications within 1 week |
| Death |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.2) |
| Cardiac tamponade |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.0) |
0 (0.0) |
1 (1.2) |
| Symptomatic stroke |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.2) |
| Systemic thromboembolism |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.0) |
0 (0.0) |
0 (0.0) |
| Atrioesophageal fistula |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Arteriovenous fistula |
0 (0.0) |
1 (0.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Pericarditis |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.0) |
0 (0.0) |
0 (0.0) |
| Hematoma |
0 (0.0) |
1 (0.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.2) |
| Phrenic nerve paralysis |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Atrioventricular block |
0 (0.0) |
1 (0.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Esophageal vagal nerve injury |
0 (0.0) |
1 (0.9) |
0 (0.0) |
1 (1.3) |
1 (10) |
2 (2.0) |
0 (0.0) |
0 (0.0) |
| Heart failure |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (2.2) |
0 (0.0) |
| Infection |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (1.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
Data are given as n (%). PVI-plus, PV isolation (PVI) with additional catheter ablation. Other abbreviations as in Table 1.
Discussion
Main Findings
The main findings of this post hoc analysis of the EARNEST-PVI randomized controlled trial are that arrhythmia-free survival was similar between the PVI-alone and PVI-plus arms in Groups A, B, and C (i.e., patients who were in sinus rhythm at baseline or who had PV-specific AF triggers) and that PVI plus an additional ablation improved the arrhythmia-free survival in Group D (i.e. patients in AF at baseline who had no PV-specific AF triggers). These results indicate that the selection of patients for additional substrate ablation could potentially be suggested by baseline rhythm before the procedure and the origin of the AF triggers. However, further investigations are needed to confirm these findings.
Patient Selection for Additional Ablation
For PerAF, the clinical outcomes with a PVI alone have not been satisfactory,1 but it remains contentious as to whether additional ablation improves clinical outcomes or not,1,5,8,14 and appropriate selection of patients to undergo PVI plus additional ablation is necessary. Inconsistent results following additional substrate ablation could be the result of heterogeneity of the PerAF patient population. A trigger-based mechanism of PerAF has described previously,4 and PVI should play an important role in patients with PV-specific AF triggers. The presence of sinus rhythm during the ablation procedure is reported to be a predictor of improved clinical outcomes with the PVI-alone strategy.10,11 The effectiveness of the PVI-alone strategy in patients with sinus rhythm at baseline indicated that the presence of sinus rhythm at baseline was a surrogate marker of a PV-related AF type. The subgrouping in the present study was based on the reasonable hypothesis that sinus rhythm at baseline was possibly a marker of a less advanced atrial substrate and that the demonstration of PV triggering was a marker of clinical AF arising from the PVs. A combination of the rhythm at baseline and the AF trigger origin to differentiate between patients with triggers and those with a substrate mechanism was used in the present study, and was able to appropriately select patients who should receive an additional ablation strategy and those who should not.
The DR-FLASH score (calculated by the factors diabetes, renal dysfunction, PerAF, LA diameter >45 mm, age >65 years, female sex, and hypertension), could be a surrogate marker of the presence of a low-voltage area.15 In a previous subanalysis of the EARNEST-PVI trial, we reported that a DR-FLASH score >3, based on identifiable preprocedural patient characteristics, indicated the effectiveness of the PVI-plus strategy.7 The present study stratified patients based on electrophysiological findings during ablation procedures, suggesting that combining both approaches could enhance stratification efficacy. Although the analysis was exploratory in nature, we divided patients into groups according to DR-FLASH score (>3 or ≤3) and whether they were part of Group D. The results indicated that patients with DR-FLASH scores >3, particularly those in Group D, may especially benefit from the PVI-plus strategy. These findings require validation in a different patient cohort.
There were more women in Group D than in the other groups. The DR-FLASH scores also include a “female” factor. However, women had worse outcomes independent of the on-treatment strategy group, PVI alone, linear ablation or CFAE, as reported with the 3-year follow-up data.6 To improve the outcomes in women, additional strategies other than linear ablation are needed, or the indications for PerAF ablation should be separated by sex to avoid any negative aspects of additional ablation.
Benefits of the PVI-Alone Strategy
In the present study, the total procedure, radiofrequency application, and total radiofrequency times were shorter in the PVI-alone than PVI-plus arm in both Groups A and B. These results are consistent with those in other randomized trials that compared a PVI-alone to PVI with an additional radiofrequency substrate ablation.1,8 Considerations of additional radiofrequency substrate ablation need to take into account not only the benefits, but also possible disadvantages, such as procedure and radiofrequency times and stiff LA syndrome.16 An extra PV ablation has been reported to increase LA stiffness and is associated with a worse diastolic function after ablation.17 When considering an additional radiofrequency strategy in patients with PerAF, the advantages of the PVI-alone strategy regarding shorter procedure and radiofrequency times and a lower likelihood of the stiff LA syndrome, should be kept in mind.
Benefit and Risk of Substrate Ablation of PerAF
In the present study, additional substrate ablation by linear or CFAE ablation was effective in the selected population. Our on-treatment analysis of the extended follow-up data of the EARNEST-PVI trial revealed that patients with PVI plus linear ablation experienced fewer arrhythmia recurrences than those with PVI alone, whereas patients with PVI plus CFAE ablation had comparable outcomes to those with PVI alone.6 The superiority of PVI plus linear ablation remained after multiple ablations.6 Post-linear ablation reconduction should also be noted as a potential substrate for iatrogenic atrial tachycardia.6 In the present study, we found a tendency for an increased risk of procedure-related complications within 1 week after the procedure. Although the superiority of maintaining sinus rhythm in the PVI-plus arm was demonstrated in the total population of the EARNEST-PVI trial, it becomes crucial to carefully select patients for additional substrate ablation to minimize any potential risk of complications and iatrogenic arrhythmia substrate.
Additional strategies other than linear and CFAE ablation have been reported to improve clinical outcomes after catheter ablation of PerAF. For example, in patients with PerAF, the addition of an infusion of ethanol in the vein of Marshall is associated with an improved arrhythmia-free survival at 6 and 12 months compared with catheter ablation alone.18 Tailored ablation targeting the low-voltage area, using a voltage threshold of 0.5 mV, has been reported to improve clinical outcomes in patients with PerAF.14 Long-term follow-up data for these treatments, including LA tachycardia, are awaited.
Clinical Implications
The practical use of the intraprocedural assessment in the present study was very simple. If the baseline rhythm is sinus rhythm, an additional ablation would not improve the clinical outcomes, and PVI alone is a reasonable strategy. Even if the baseline rhythm is AF, in patients with reproducible PV triggers, an additional ablation would not improve clinical outcomes. In other cases, additional substrate ablation should be considered.
Study Limitations
This study has several limitations. First, this was a post hoc analysis using the extended data of a prospective multicenter randomized trial, and the size of each group was small. Further randomized control studies are needed to confirm the beneficial effects of this patient selection method using rhythm at baseline before the procedure and the trigger origin. Second, arrhythmia recurrences were not monitored with implantable cardiac monitors. Asymptomatic AF probably was underestimated. Third, preprocedural information on electrical cardioversion or pharmacological cardioversion was not available in this study. Fourth, CFAE ablation was more often performed in Group D, because the patients in whom AF could not be terminated by electrical cardioversion were all included in Group D (17 patients). Thus, there was selection bias of the additional ablation strategy for failure of electrical cardioversion in this present study. Fifth, in the present study AF triggers were assessed before PVI with isoproterenol, according to previous reports.19,20 However, there were situations not permitting full investigation of AF triggers, such as immediate recurrence of AF without isoproterenol, persistent AF refractory to electrical cardioversion, and masked AF triggers by easier induced AF triggers. Among these situations, overestimation of PV-specific triggers should be avoided, because our result supports PVI-alone strategy in patients with PV-specific triggers and if the patient had masked AF triggers other than PVs, the patient miss a chance to receive additional ablation. In our study, non-PV triggers were induced after PVI in only 1 (0.4%) patient. Therefore, our results support the trigger assessment for identifying PV-specific AF triggers before PVI with isoproterenol. Sixth, although all centers followed the core protocol of the AF induction test, there may have been slight variations in its execution due to clinical judgment and patient-specific factors.
Conclusions
An intraprocedural assessment according to the baseline rhythm and AF trigger origin could distinguish patients who benefited from PVI with an additional ablation. Patients who were in sinus rhythm at baseline or who had PV-specific AF triggers did not receive any benefit from an additional ablation. Patients who were in AF at baseline and who did not have any PV-specific AF triggers benefitted from PVI with an additional ablation.
Acknowledgment
The authors thank the members of the Osaka Cardiovascular Conference (OCVC) Arrhythmia Investigators (see Appendix), as well as the staff and participants of the EARNEST-PVI trial. The authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection, and Shiro Manabe for his support with the data collection system.
Sources of Funding
This study was funded by Medtronic, Johnson & Johnson, and Abbott.
Disclosures
H.M., T.D., D.N., S.H., and Y. Sakata report receiving grants from Medtronic, Johnson & Johnson, and Abbott during the conduct of the study. Y. Sakata is a member of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Cardiovascular Center, Sakurabashi-Watanabe Hospital (Study no. 17-6); Osaka University Graduate School of Medicine (14377); Kansai Rosai Hospital (15D059 g); Osaka General Medical Center (27-2035); Osaka Police Hospital (548); Osaka Rosai Hospital (28-78); Yao Municipal Hospital (八病H29-5); and Osaka Hospital, Japan Community Healthcare Organization (2016-25).
Data Availability
The study data will not be made available to other researchers for purposes of reproducing the results because of institutional review board restrictions.
Appendix
The Osaka Cardiovascular Conference (OCVC) Arrhythmia Investigators
Yasuhiro Matsuda, Masaharu Masuda, and Toshiaki Mano (Kansai Rosai Hospital, Amagasaki, Japan); Koichi Inoue and Yasushi Matsumura (National Hospital Organization Osaka National Hospital, Osaka, Japan); Masato Kawasaki, Tetsuya Watanabe, and Takahisa Yamada (Osaka General Medical Center, Osaka, Japan); Miwa Miyoshi (Osaka Hospital, Japan Community Healthcare Organization Osaka, Japan); Takashi Kanda, Hitoshi Minamiguchi, Nobuhiko Makino, and Yoshiharu Higuchi (Osaka Police Hospital, Osaka, Japan); Yasuharu Matsunaga, Yasuyuki Egami, Masami Nishino, and Jun Tanouchi (Osaka Rosai Hospital, Sakai, Japan); Taiki Sato, Hirota Kida, Akihiro Sunaga, Tomoaki Nakano, Kentaro Ozu, Yohei Sotomi, Tomoharu Dohi, Katsuki Okada, Takafumi Oka, Toshihiro Takeda, Daisaku Nakatani, Shungo Hikoso, and Yasushi Sakata (Osaka University Graduate School of Medicine, Suita, Japan); Nobuaki Tanaka and Koji Tanaka (Sakurabashi Watanabe Hospital, Osaka, Japan); Tomoko Minamisaka and Shiro Hoshida (Yao Municipal Hospital, Yao, Japan).
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0936
References
- 1.
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015; 372: 1812–1822.
- 2.
Benali K, Barre V, Hermida A, Galand V, Milhem A, Philibert S, et al. Recurrences of atrial fibrillation despite durable pulmonary vein isolation: The PARTY-PVI study. Circ Arrhythm Electrophysiol 2023; 16: e011354, doi:10.1161/CIRCEP.122.011354.
- 3.
Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm 2016; 13: 374–382.
- 4.
Inoue K, Kurotobi T, Kimura R, Toyoshima Y, Itoh N, Masuda M, et al. Trigger-based mechanism of the persistence of atrial fibrillation and its impact on the efficacy of catheter ablation. Circ Arrhythm Electrophysiol 2012; 5: 295–301.
- 5.
Inoue K, Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Y, et al. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: The EARNEST-PVI trial. Europace 2021; 23: 565–574.
- 6.
Masuda M, Inoue K, Tanaka N, Watanabe T, Makino N, Egami Y, et al. Long-term impact of additional ablation after pulmonary vein isolation: Results from EARNEST-PVI Trial. J Am Heart Assoc 2023; 12: e029651, doi:10.1161/JAHA.123.029651.
- 7.
Sato T, Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, et al. DR-FLASH score is useful for identifying patients with persistent atrial fibrillation who require extensive catheter ablation procedures. J Am Heart Assoc 2022; 11: e024916, doi:10.1161/JAHA.121.024916.
- 8.
Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S, et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: The CAPLA randomized clinical trial. JAMA 2023; 329: 127–135.
- 9.
Inoue K, Sotomi Y, Masuda M, Furukawa Y, Hirata A, Egami Y, et al. Efficacy of extensive ablation for persistent atrial fibrillation with trigger-based vs. substrate-based mechanisms: A prespecified subanalysis of the EARNEST-PVI trial. Circ J 2021; 85: 1897–1905.
- 10.
Okawa K, Hara S, Morimoto T, Tsushima R, Sudo Y, Sogo M, et al. Effect of preprocedural pharmacologic cardioversion on pulmonary vein isolation in patients with persistent atrial fibrillation. Heart Rhythm 2021; 18: 1473–1479.
- 11.
Eberly LA, Lin A, Park J, Khoshnab M, Garg L, Chee J, et al. Presence of sinus rhythm at time of ablation in patients with persistent atrial fibrillation undergoing pulmonary vein isolation is associated with improved long-term arrhythmia outcomes. J Interv Card Electrophysiol 2023; 66: 1455–1464.
- 12.
Dohi T, Nakatani D, Inoue K, Hikoso S, Oka T, Hayashi K, et al. Effect of extensive ablation on recurrence in patients with persistent atrial fibrillation treated with pulmonary vein isolation (EARNEST-PVI) trial: Design and rationale. J Cardiol 2019; 74: 164–168.
- 13.
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012; 9: 632–696.e21.
- 14.
Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger FX, et al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evid 2022; 1: EVIDoa2200141, doi:10.1056/EVIDoa2200141.
- 15.
Kosiuk J, Dinov B, Kornej J, Acou WJ, Schonbauer R, Fiedler L, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm 2015; 12: 2207–2212.
- 16.
Reddy YNV, El Sabbagh A, Packer D, Nishimura RA. Evaluation of shortness of breath after atrial fibrillation ablation: Is there a stiff left atrium? Heart Rhythm 2018; 15: 930–935.
- 17.
Park JW, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, et al. Atrial fibrillation catheter ablation increases the left atrial pressure. Circ Arrhythm Electrophysiol 2019; 12: e007073, doi:10.1161/CIRCEP.118.007073.
- 18.
Valderrabano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: The VENUS randomized clinical trial. JAMA 2020; 324: 1620–1628.
- 19.
Haïssaguerre M, Jaïs P, Shah DC, Garrigue S, Takahashi A, Lavergne T, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 2000; 101: 1409–1417.
- 20.
Mountantonakis SE, Elkassabany N, Kondapalli L, Marchlinski FE, Mandel JE, Hutchinson MD. Provocation of atrial fibrillation triggers during ablation: Does the use of general anesthesia affect inducibility? J Cardiovasc Electrophysiol 2015; 26: 16–20.




