Abstract
Background: Despite the widespread use of PROPATEN®, a bioactive heparin-bonded expanded polytetrafluoroethylene graft, in bypass surgery, there are only a few reports of long-term results. We evaluated the long-term results of PROPATEN®
use for above-knee femoropopliteal bypass (AKFPB).
Methods and Results: After PROPATEN®-based AKFPB, patients were prospectively registered at 20 Japanese institutions between July 2014 and October 2017 to evaluate long-term results. During the median follow-up of 76 months (interquartile range 36–88 months) for 120 limbs (in 113 patients; mean [±SD] age 72.7±8.1 years; 66.7% male; ankle-brachial index [ABI] 0.45±0.27; lesion length 26.2±5.7 cm; chronic limb-threatening ischemia in 45 limbs), there were 8 major amputations; however, clinical improvement was sustained (mean [±SD] ABI 0.87±0.23) and the Rutherford classification grade improved in 105 (87.5%) limbs at the latest follow-up. At 8 years, the primary patency, freedom from target-lesion revascularization, secondary patency, survival, and amputation-free survival, as estimated by the Kaplan-Meier method, were 66.3±4.8%, 71.5±4.4%, 86.5±3.4%, 53.1±5.0%, and 47.4±5.3%, respectively.
Conclusions: This multicenter prospective registry-based analysis showed sustained excellent clinical improvement and secondary patency for up to 8 years following PROPATEN®-based AKFPB. PROPATEN®
constitutes a durable and good revascularization option for complex superficial femoral artery lesions, especially when endovascular treatment is inappropriate or an adequate venous conduit is unavailable.
With recent advances in endovascular technologies and techniques, endovascular treatment (EVT) for the revascularization of lower extremity arterial disease (LEAD) has been increasingly used.1–5 Even in complex superficial femoral artery (SFA) lesions, EVT, especially with the Viabahn stent graft (W.L. Gore & Associates, Flagstaff, AZ, USA) for long lesions, has conferred good clinical results.6,7 These results, together with the high morbidity and longer hospital stay associated with bypass surgery (BS),8 supported the recommendations in guidelines for EVT of the SFA lesion;4,5 the 2017 European Society of Cardiology guidelines recommended EVT for SFA lesions with a length of up to 25 cm.3
For BS, all guidelines recommend the use of a good venous conduit as the graft due to its superior patency compared with prosthetic grafts.3–5 However, in Japan, the expanded polytetrafluoroethylene (ePTFE) graft (70%) is preferred over venous conduits (20%) for above-knee femoropopliteal bypass (AKFPB).2 Currently, PROPATEN®
(W.L. Gore & Associates), a bioactive heparin-bonded ePTFE graft, has replaced conventional ePTFE grafts and, owing to its sustained thromboresistant features9 and superior patency,10 is widely used for BS. However, there are few reports of the long-term results of PROPATEN®
for BS,11–13 and, in Japan, only one prospective study has evaluated PROPATEN®
thus far and only reported medium-term results.14 This paper updates that study and reports on the long-term results of PROPATEN®
for AKFPB in the Japanese population.
Methods
Study Design and Participants
The original study design has been described previously.14 In summary, this was a prospective multicenter registry performed in 20 Japanese institutions from July 2014 to October 2017. In total, 120 limbs from 113 patients who were undergoing AKFPB using PROPATEN®
for symptomatic LEAD (Rutherford classification 2–6) were registered in a unified database, and data were prospectively collected until October 2017. The exclusion criteria were as follows: age <20 years, expected life expectancy <2 years, past history of coagulopathy or contraindication to anticoagulation and antiplatelet therapy, history of deep vein thrombosis of the index limb, and history of Type II heparin-induced thrombocytopenia.
To evaluate the long-term results, a retrospective review of patient data, obtained from chart review and registry information, was performed. All procedures were performed in accordance with the Declaration of Helsinki and the study protocol was reviewed and approved by the institutional review board of each participating hospital. In the initial study, all participants provided written informed consent. However, because the new study was a retrospective observational study that reviewed charts only, informed consent was not obtained from patients, with an opt-out method used instead.
Surgical Procedure
All procedures, according to each institution’s standard of care, were performed by board-certified vascular surgeons or by vascular residents under the guidance of board-certified vascular surgeons. The characteristics of the surgical procedure varied at the discretion of each surgeon, including: graft size; the use of intraoperative angiography and concomitant additional treatment, including EVT, endarterectomy, and femoro-femoral crossover bypass; the choice of suture; the amount of heparin and protamine; and use of topical hemostatic agents. The type of anastomosis created was also left to the surgeon’s discretion and some cases had distal anastomosis with a protruding area created around the anastomotic toe (cuffed anastomosis).15 No vein patch or cuff was used for anastomosis. Furthermore, postoperative antiplatelet or anticoagulation therapy was administered at the physician’s discretion; however, at least one antiplatelet or anticoagulation drug was administered continuously lifelong unless there was a clinical event (e.g., gastrointestinal bleeding) that precluded treatment. In the case of patients receiving double antiplatelet therapy initially, they were usually weaned to single antiplatelet therapy within 1 year and continued lifelong.
Follow-up and Data Collection
The mandated assessment points of the study included: baseline; 1, 3, 6, and 12 months after surgery; and at least every 6 months thereafter for up to 3 years. Follow-up was continued according to the standard of care at each institution. At each assessment point, major adverse events (i.e., death, major amputation, and target lesion revascularization [TLR]) and the LEAD stages according to the Rutherford classification were ascertained. The ankle-brachial index (ABI) and duplex ultrasound were usually performed at each clinic visit, and computed tomography (CT) was performed at the discretion of the attending physician.
Endpoints and Definition
The primary endpoint was primary patency at the most recent follow-up visit. Secondary endpoints included secondary patency, freedom from TLR, mortality, and major amputations. Primary patency was defined as the absence of occlusion, restenosis of the treated segment (including the 1 cm proximal and distal to the anastomosis), or TLR. Restenosis was defined as a peak systolic velocity ratio ≥2.4 on duplex ultrasound or >50% stenosis on CT angiography. Secondary patency was defined as the revascularization of a completely occluded graft by reintervention and failure of revascularization was defined as loss of secondary patency. Perioperative complications were defined as complications that occurred within 30 days of the procedure. Major amputation was defined as an above-ankle amputation of the index limb. Wound complication was defined as any wound-related event that led to prolonged hospitalization, including both surgical site infection and lymphocele.
Statistical Analysis
All data were analyzed on a limb basis, and additional data cleaning resulted in minor changes from that of the previous study, which used patient-based analysis. Continuous variables are presented as the mean±SD and categorical variables are presented as frequencies and proportions. Statistical significance was set at P<0.05, and 95% confidence intervals (CIs) are reported as appropriate.
Primary patency, secondary patency, freedom from TLR, and mortality were estimated using the Kaplan-Meier method. The association between baseline characteristics and restenosis risk was investigated using the Cox proportional hazards regression model. Variables with P<0.10 in the univariate model were entered into the multivariate model. Because there were only 36 events, 4 variables were selected on the basis of previously reported results.14 All analyses were performed using IBM®SPSS®
version 27 (IBM Corp., Armonk, NY, USA).
Results
Patient Demographics and Lesion Characteristics
Participant demographics and lesion characteristics, on a per-limb basis, are summarized in Table 1. The mean age of patients was 72.7±8.1 years, 66.7% were men, and chronic limb-threatening ischemia (CLTI) was observed in 45 (37.5%) limbs. The most frequent comorbidity was hypertension (75.8%), followed by chronic kidney disease (57.5%) and diabetes (52.5%). Seventeen (14.2%) limbs were from patients who were dependent on hemodialysis, and 98 (81.7%) limbs were from either current smokers or those who had a smoking history. The preoperative mean ABI was 0.45±0.27, and only 35 (29.2%) limbs had all 3 tibial arteries patent. The mean lesion length was 26.2±5.7 cm; Trans-Atlantic Inter-Society Consensus (TASC) II Grade C and D lesions were observed in 20 (16.7%) and 94 (78.3%) limbs, respectively. The mean outflow popliteal artery diameter was 4.9±1.1 mm, and EVT to the target lesion was performed prior to BS in 26.7% of limbs.
Table 1.
Patient Demographics and Lesion Characteristics (n=120 Limbs)
| Age (years) |
72.7±8.1 |
| Male sex |
80 (66.7) |
| Body mass index (kg/m2) |
22.5±3.8 |
| Rutherford classification |
| 2 |
30 (25.0) |
| 3 |
45 (37.5) |
| 4 |
13 (10.8) |
| 5 |
31 (25.8) |
| 6 |
1 (0.8) |
| Ankle-brachial index |
0.45±0.27 |
| No. run-off vessels |
| 0 |
1 (0.8) |
| 1 |
43 (35.8) |
| 2 |
41 (34.2) |
| 3 |
35 (29.2) |
| TASC II classification |
| Grade A |
0 (0.0) |
| Grade B |
6 (5.0) |
| Grade C |
20 (16.7) |
| Grade D |
94 (78.3) |
| Lesion length (cm) |
26.2±5.7 |
| Outflow popliteal artery diameter (mm) |
4.9±1.1 |
| Key comorbidities and medical history |
| Smoking, currentA |
31 (25.8) |
| Smoking, formerB |
67 (55.8) |
| Prior superficial femoral artery treatment |
32 (26.7) |
| Arterial hypertension |
91 (75.8) |
| Diabetes |
63 (52.5) |
| Dyslipidemia |
57 (47.5) |
| Chronic kidney diseaseC |
69 (57.5) |
| Hemodialysis |
17 (14.2) |
| Prior coronary disease |
38 (31.7) |
| Prior cerebrovascular disease |
24 (20.8) |
| Chronic obstructive pulmonary disease |
10 (8.3) |
| Serum albumin <3.0 (g/dL) |
24 (20.8) |
Categorical variables are presented as n (%) and continuous variables are presented as the mean±SD. AA current smoker was defined as a patient who did not stop smoking before the operation. BA former smoker was defined as a patient who was not smoking at the time of the operation but who had a history of smoking in the past. CChronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. TASC, Trans-Atlantic Inter-Society Consensus.
Operative Results
The operative results are summarized in Table 2. The mean operative time was 213.8±81.7 min, and 59.2% of procedures were performed by a senior surgeon. The most frequently used graft size was 6 mm (60.0%) and a cuffed distal anastomosis was used in 93 (77.5%) limbs. Additional procedures were performed in 40.8% of cases, with the most frequently performed additional procedure being EVT to the inflow artery in 29 limbs (24.2%), followed by common femoral artery endarterectomy in 19 limbs (15.8%). There was one intraoperative complication, which was distal embolization. Postoperatively, at least one antiplatelet medication was administered in the case of 116 (96.7%) limbs and oral anticoagulants for 23 (19.2%) limbs.
Table 2.
Operative Characteristics and Postoperative Results (n=120 Limbs)
| Operator |
| Senior surgeon |
71 (59.2) |
| Resident |
49 (40.8) |
| Procedure time (min) |
213.8±81.7 |
| Blood loss (mL) |
274.4±287.7 |
| Intraoperative angiography |
79 (65.8) |
| Additional procedureA |
49 (40.8) |
| Graft diameter (mm) |
| 6 |
72 (60.0) |
| 7 |
28 (23.3) |
| 8 |
20 (16.7) |
| Cuffed distal anastomosis |
93 (77.5) |
| Intraoperative complicationB |
1 (0.8) |
| Postoperative medical treatment |
| Single antiplatelet agent |
42 (35.0) |
| Cilostazol |
50 (41.7) |
| Two or more antiplatelet agents |
74 (61.7) |
| Oral anticoagulants |
23 (19.2) |
| Statin |
54 (45.0) |
| Perioperative complicationC |
8 (6.7) |
| Ankle-brachial index |
| Within 30 days after surgery (missing in 23 limbs) |
0.96±0.14 |
| At latest follow-up (missing in 28 limbs) |
0.87±0.23 |
| Improvement in Rutherford classification |
| Within 30 days after surgery |
98 (81.7) |
| At latest follow-up |
105 (87.5) |
| Major amputation |
8 (6.7) |
Categorical variables are presented as n (%) and continuous variables are presented as the mean±SD. AAdditional procedures included endovascular therapy for iliac artery or tibial artery, femoral crossover bypass, or endarterectomy of the ipsilateral common femoral artery. BThe intraoperative complication was distal embolization. CPostoperative complications included 6 wound complications, 1 case of takotsubo cardiomyopathy, and 1 case of renal failure requiring hemodialysis.
Postoperative Results
There were 8 perioperative complications, which included 6 wound complications, 1 case of takotsubo cardiomyopathy, and 1 case of renal failure that necessitated hemodialysis; the case of takotsubo cardiomyopathy ultimately led to the only mortality (0.8%) within 30 days after the operation. The mean ABI improved from 0.45±0.27 to 0.96±0.14 postoperatively, and improvement in the Rutherford classification grade was achieved in 98 limbs (81.7%). These results were sustained as a mean ABI of 0.87±0.23, and the improvement in the Rutherford classification grade was 87.5% at the latest follow-up. During the median follow-up of 76 months (interquartile range 36–88 months), 8 major amputations and 51 deaths occurred; 12 patients were lost to follow-up (Table 2).
Endpoints and Risk Analysis for Primary Patency
Primary patency, freedom from TLR, secondary patency, survival, and amputation-free survival at 8 years estimated by the Kaplan-Meier method were 66.3±4.8%, 71.5±4.4%, 86.5±3.4%, 53.1±5.0%, and 47.4±5.3%, respectively (Figures 1–4). Graft occlusion was observed in 14 limbs, leading to 4 major amputations. Univariate analysis for the risk factors of primary patency revealed that the number of run-off vessels (3 vs. ≤2; P=0.06), diabetes (P=0.08), hemodialysis (P<0.01), prior coronary disease (P=0.01), and cuffed distal anastomosis (P=0.08) were significant factors (P<0.10; Table 3). In the multivariate analysis using the number of run-off vessels, hemodialysis, prior coronary disease, and cuffed distal anastomosis, only prior coronary disease was significant (adjusted hazard ratio [HR] 2.04; 95% CI 1.02–4.06; P=0.04; Table 3).

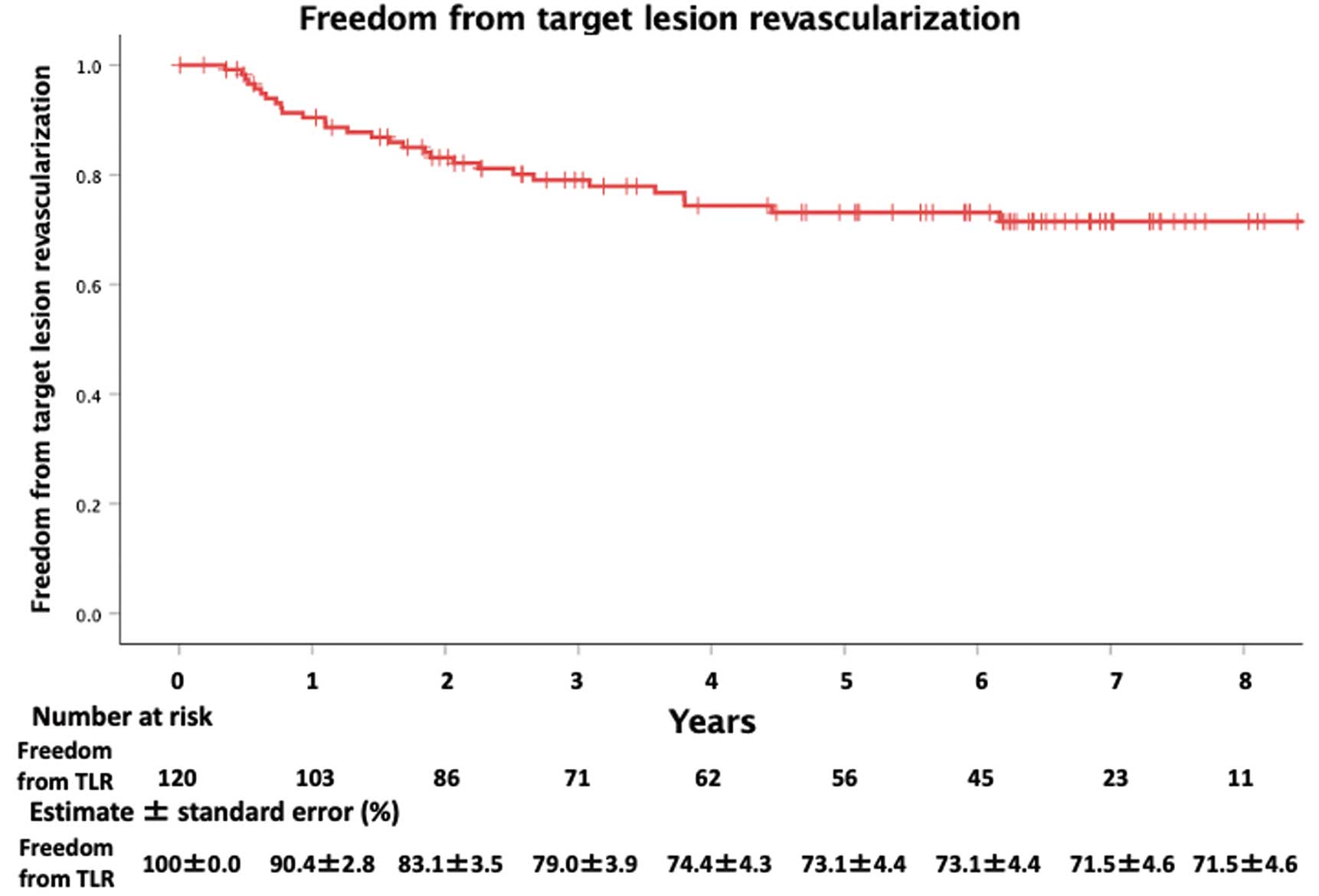


Table 3.
Association Between Clinical Features and Primary Patency
| |
Crude |
Adjusted |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Age (≥75 vs. <75 years) |
0.72 (0.36–1.42) |
0.34 |
N/I |
|
| Sex (male vs. female) |
1.17 (0.57–2.38) |
0.67 |
N/I |
|
| BMI (≥25 vs. <25 kg/m2) |
0.75 (0.35–1.60) |
0.46 |
N/I |
|
| CLTI |
0.99 (0.49–2.02) |
0.99 |
N/I |
|
| No. run-off vessels (3 vs. ≤2) |
0.47 (0.21–1.04) |
0.06 |
0.55 (0.25–1.24) |
0.15 |
| Lesion length >25 cm |
0.96 (0.49–1.88) |
0.91 |
|
|
| Outflow diameter <5 mm |
1.21 (0.62–2.36) |
0.57 |
N/I |
|
| Arterial hypertension |
1.06 (0.50–2.27) |
0.87 |
N/I |
|
| Diabetes |
1.84 (0.93–3.64) |
0.08 |
N/I |
|
| Dyslipidemia |
1.38 (0.72–2.67) |
0.33 |
N/I |
|
| Chronic kidney diseaseA |
1.02 (0.53–1.97) |
0.96 |
N/I |
|
| Hemodialysis |
2.89 (1.30–6.40) |
0.009 |
1.94 (0.79–4.72) |
0.15 |
| Prior coronary disease |
2.40 (1.23–4.68) |
0.01 |
2.04 (1.02–4.06) |
0.04 |
| Prior cerebrovascular disease |
0.90 (0.38–2.18) |
0.82 |
N/I |
|
| COPD |
1.40 (0.50–3.98) |
0.52 |
N/I |
|
| Prior SFA treatment |
1.08 (0.52–2.25) |
0.83 |
N/I |
|
| Serum albumin <3.0 g/dL |
1.53 (0.67–3.50) |
0.32 |
N/I |
|
| Smoking historyB |
0.77 (0.35–1.70) |
0.52 |
N/I |
|
| Operator (senior surgeon vs. resident) |
0.59 (0.29–1.20) |
0.14 |
N/I |
|
| Intraoperative angiography |
0.78 (0.40–1.52) |
0.46 |
N/I |
|
| Additional procedure |
0.93 (0.48–1.82) |
0.84 |
N/I |
|
| Graft diameter ≥7 mm |
0.55 (0.27–1.11) |
0.10 |
N/I |
|
| Cuffed distal anastomosis |
0.56 (0.27–1.16) |
0.08 |
0.77 (0.35–1.71) |
0.52 |
| Two or more antiplatelet agents |
1.42 (0.70–2.89) |
0.34 |
N/I |
|
| Cilostazol |
1.14 (0.59–2.20) |
0.69 |
N/I |
|
| Oral anticoagulant |
1.34 (0.63–2.86) |
0.45 |
N/I |
|
| Statin |
1.72 (0.89–3.34) |
0.11 |
N/I |
|
| Perioperative complication |
2.17 (0.76–6.20) |
0.15 |
N/I |
|
Adjusted hazard ratios (HRs) were obtained from the multivariate model in which variables with statistical significance in the univariate model and variables that were significant in previous reports were entered. AChronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2. BSmoking history includes both current and former smokers. BMI, body mass index; CI, confidence interval; CLTI, chronic limb-threatening ischemia; COPD, chronic obstructive pulmonary disease; N/I, not included; SFA, superficial femoral artery.
Discussion
Owing to its superior long-term patency compared with a prosthetic graft, a good venous conduit is the gold standard for BS.3–5 However, a good venous conduit is not always available16 and prosthetic grafts, especially ePTFE grafts, are often used for AKFPB in real-world clinical practice.2 In the present study, analysis of data from a multicenter prospectively registered registry showed that PROPATEN®
for AKFPB demonstrated acceptable long-term primary patency and excellent secondary patency, which resulted in sustained clinical improvement, even in a complex SFA lesion.
Revascularization of the SFA lesion has recently been dominated by EVT, even in the case of complex lesions, due to advances in both endovascular technologies and techniques.1–5 One example is the wide application of drug-eluting technology, especially the use of paclitaxel. A global multicenter registry for high-dose paclitaxel-coated balloons has shown good clinical results in complex SFA lesions,17,18 and a randomized controlled trial that compared ZILVER PTX paclitaxel-eluting stents (Cook Medical, Bloomington, IN, USA) and AKFPB using prosthetic grafts in TASC Grade C and D lesions showed no significant differences in long-term results for up to 5 years.19 Another prominent device that has driven EVT for complex SFA lesions, the Viabahn stent graft, has the same bioactive heparin-bonded ePTFE as PROPATEN®, which promises a thromboresistant feature, and because restenosis only occurs at the edge of the device, this graft has shown good clinical outcomes in long SFA lesions.6,7,20 In a randomized controlled trial that compared the Viabahn stent graft with BS, no significant difference was noted at 12 months.21
Nonetheless, EVT is ineffective and, in some cases, inappropriate for some SFA lesions. For example, worse clinical outcomes following EVT have been reported for lesions with severe calcification,22–24 those with a small diameter,22,25 and long (>25 cm) lesions.26 In these cases, BS should still be provided, especially when multiple risk factors for EVT are present22 or the patient is young and needs long-term results.
Our study demonstrated that PROPATEN®
for AKFPB had acceptable long-term results, even in a population in which a significant proportion of patients had unfavorable characteristics for EVT, including a long mean lesion length of 26.2±5.7 cm, a small mean outflow diameter of the popliteal artery of 4.9±1.1 mm, a history of EVT failure for the target lesion (26.7% of patients), CLTI (37.5% of patients), and poor (0 or 1) run-off vessels (36.6% of patients). If a good venous conduit is available, AKFPB using a venous conduit is superior to that using a prosthetic graft; however, a good venous conduit is not always available.16 Our acceptable long-term results supports the use of AKFPB with PROPATEN®
in cases with lesion characteristics unfavorable for EVT and when a good venous conduit is unavailable. Furthermore, the preservation of a good venous conduit for future coronary artery bypass grafting or distal bypass for AKFPB failure or in the progression of tibial artery disease is facilitated by using PROPATEN®. In fact, in the present study, 4 limbs (three AKFPB failure and 1 progression of the tibial artery) required distal bypass using a venous conduit during follow-up.
Multivariate analysis for risk factors affecting primary patency showed that a long (>25 cm) lesion (HR 0.96; 95% CI 0.49–1.88; P=0.91) and outflow diameter <5 mm (HR 1.21; 95% CI 0.62–12.36; P=0.57) are not risk factors for AKFPB using PROPATEN®, further supporting BS over EVT for these lesions. In contrast, a previous study of mid-term results showed that having 3 run-off vessels, cuffed distal anastomosis, no coronary artery disease, and no hemodialysis were associated with favorable primary patency at 2 years on univariate analysis.14 However, our study showed that only prior coronary artery disease was a significant risk factor for restenosis using multivariate analysis. This difference may be attributable to the simple fact that the previous study did not perform a multivariate analysis. Furthermore, factors affecting mid- and long-term results may differ, because technical factors probably affect the early stage and the progression of atherosclerosis will probably affect the later stage after BS. Prior coronary artery disease may be an indicator of an advanced atherosclerotic state; however, because other suggestive factors such as CLTI, diabetes, and the number of run-off vessels are not significant, further investigation is warranted.
This study has several limitations. First, this multicenter registry was prospectively registered until October 2017. However, the present study of the evaluation of long-term results was a retrospective cohort study. Second, because most clinical decisions, including the revascularization method, were left to the discretion of the attending physician, selection bias for the inclusion of only good candidates for AKFPB cannot be denied. Third, we only collected information on the history of coronary interventions, not chronic heart failure, which has been associated with worse clinical outcomes. Furthermore, owing to the limited number of patients, the multivariate analysis may have been underpowered to detect factors that would have been significant in a larger population. However, despite these limitations, we believe our study’s results reflect the real-world treatment of LEAD, because 690 EVTs were performed for SFA lesions during the study period. This indicates that the selection bias was minimized.
Conclusions
Our multicenter prospective registry-based analysis showed that excellent clinical improvement, acceptable primary patency, and excellent secondary patency were sustained for up to 8 years following AKFPB using PROPATEN®. Thus, AKFPB using PROPATEN®
is a durable, good revascularization option for complex SFA lesions, especially when EVT is inappropriate or when an adequate venous conduit is unavailable.
Acknowledgments
The authors thank Hideyuki Hayashi, MD (Department of Surgery, Keio University School of Medicine), Shinsuke Mii, MD, PhD (Department of Vascular Surgery, Saiseikai Yahata General Hospital), Makiko Ohmori, MD (Division of Vascular Surgery, Department of Surgery, The Jikei University School of Medicine), Shigeshi Ono, MD, PhD (Department of Surgery, Tokyo Dental College Ichikawa General Hospital), Daijirou Akamatsu, MD, PhD (Division of Vascular Surgery, Department of Surgery, Tohoku University Hospital), Yasuhito Sekimoto, MD, PhD (Department of Surgery, Tokyo Medical Center), Keita Hayashi, MD (Department of Vascular Surgery, Hiratsuka City Hospital), Norihide Sugano, MD, PhD (Department of Surgery, Okubo Hospital), Kenjiro Kaneko, MD (Department of Vascular Surgery, Shin-Yurigaoka General Hospital), Atsunori Asami, MD, PhD (Department of Surgery, Saitama Municipal Hospital), and Ryota Sugisawa, MD, PhD (Department of Vascular Surgery, Hamamatsu Red Cross Hospital) for their contributions to this study.
Sources of Funding
This investigator-sponsored study was partly supported by W.L. Gore and Associates. The sponsors were not involved in the study design, data collection, data analysis and interpretation, or the decision to publish the manuscript.
Disclosures
T.O. has received consulting fees from W.L. Gore and Associates and Endovascular Japan, Inc. The remaining authors declare that there are no conflicts of interest.
IRB Information
The study’s protocol was reviewed and approved by the institutional review board of each participating hospital; the Institutional Review Board of Saiseikai Central Hospital first reviewed and approved (2022-28-1) the study protocol.
Data Availability
Deidentified participant data will be shared upon reasonable request to the corresponding author. All datasets used, including the study protocol, will be available with the permission of the Institutional Review Board of Saiseikai Central Hospital until the end of December 2029. Data will be shared with anyone wishing to access the data. Any analyses of the data will be approved and the data will be shared as an Excel file via email.
References
- 1.
Iida O, Takahara M, Kohsaka S, Soga Y, Fujihara M, Mano T, et al. Impact of institutional volume on critical in-hospital complications adjusted for patient- and limb-related characteristics: An analysis of a nationwide Japanese registry of endovascular interventions for PAD. J Endovasc Ther 2020; 27: 739–748.
- 2.
The Japanese Society for Vascular Surgery Database Management Committee Member; NCD Vascular Surgery Data Analysis Team. Vascular surgery in Japan: 2016 annual report by the Japanese Society for Vascular Surgery. Ann Vasc Dis 2021; 14: 419–438.
- 3.
Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816.
- 4.
Nordanstig J, Behrendt CA, Baumgartner I, Belch J, Bäck M, Fitridge R, et al. Editor’s choice: European Society for Vascular Surgery (ESVS) 2024 clinical practice guidelines on the management of asymptomatic lower limb peripheral arterial disease and intermittent claudication. Eur J Vasc Endovasc Surg 2024; 67: 9–96.
- 5.
Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J Vasc Surg 2015; 61(Suppl): 2S–41S.
- 6.
Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, et al. Sustained benefit at 2 years for covered stents versus bare-metal stents in long SFA lesions: The VIASTAR trial. Cardiovasc Intervent Radiol 2015; 38: 25–32.
- 7.
Ohki T, Kichikawa K, Yokoi H, Iida O, Yamaoka T, Maeda K, et al. Long-term results of the Japanese multicenter Viabahn trial of heparin bonded endovascular stent grafts for long and complex lesions in the superficial femoral artery. J Vasc Surg 2021; 74: 1958–1967.e2.
- 8.
van de Weijer MA, Kruse RR, Schamp K, Zeebregts CJ, Reijnen MM. Morbidity of femoropopliteal bypass surgery. Semin Vasc Surg 2015; 28: 112–121.
- 9.
Freeman J, Chen A, Weinberg RJ, Okada T, Chen C, Lin PH. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded polytetrafluoroethylene PROPATEN graft in a chronic canine femoral artery bypass model. Ann Vasc Surg 2018; 49: 295–303.
- 10.
Samson RH, Morales R, Showalter DP, Lepore MR, Nair DG. Heparin-bonded expanded polytetrafluoroethylene femoropopliteal bypass grafts outperform expanded polytetrafluoroethylene grafts without heparin in a long-term comparison. J Vasc Surg 2016; 64: 638–647.
- 11.
Piffaretti G, Dorigo W, Castelli P, Pratesi C, Pulli R, Pratesi C, et al. Results from a multicenter registry of heparin-bonded expanded polytetrafluoroethylene graft for above-the-knee femoropopliteal bypass. J Vasc Surg 2018; 67: 1463–1471.e1.
- 12.
Piffaretti G, Dorigo W, Ottavi P, Pulli R, Castelli P, Pratesi C, et al. Results of infrainguinal revascularization with bypass surgery using a heparin-bonded graft for disabling intermittent claudication due to femoropopliteal occlusive disease. J Vasc Surg 2019; 70: 166–174.e1.
- 13.
Uhl C, Grosch C, Hock C, Töpel I, Steinbauer M. Comparison of long-term outcomes of heparin bonded polytetrafluoroethylene and autologous vein below knee femoropopliteal bypasses in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg 2017; 54: 203–211.
- 14.
Shibutani S, Obara H, Matsubara K, Toya N, Isogai N, Ogino H, et al. Midterm results of a Japanese prospective multicenter registry of heparin-bonded expanded polytetrafluoroethylene grafts for above-the-knee femoropopliteal bypass. Circ J 2020; 84: 501–508.
- 15.
Inoue Y, Sugano N, Jibiki M, Kitamura S, Iwai T. Cuffed anastomosis for above-knee femoropopliteal bypass with a stretch expanded polytetrafluoroethylene graft. Surg Today 2008; 38: 679–684.
- 16.
Hata Y, Iida O, Takahara M, Asai M, Masuda M, Okamoto S, et al. Saphenous vein size as a surrogate marker for mortality of patients with chronic limb-threatening ischemia undergoing endovascular therapy. J Cardiol 2021; 78: 341–346.
- 17.
Brodmann M, Lansink W, Guetl K, Micari A, Menk J, Zeller T. Long-term outcomes of the 150 mm drug-coated balloon cohort from the IN.PACT Global Study. Cardiovasc Intervent Radiol 2022; 45: 1276–1287.
- 18.
Tepe G, Brodmann M, Micari A, Scheinert D, Choi D, Menk J, et al. 5-year outcomes of drug-coated balloons for peripheral artery in-stent restenosis, long lesions, and CTOs. JACC Cardiovasc Interv 2023; 16: 1065–1078.
- 19.
Bosiers MJ, De Donato G, Torsello G, Silveira PG, Scheinert D, Veroux P, et al. ZILVERPASS Study: ZILVER PTX stent versus prosthetic above-the-knee bypass surgery in femoropopliteal lesions, 5-year results. Cardiovasc Intervent Radiol 2023; 46: 1348–1358.
- 20.
Bosiers M, Deloose K, Callaert J, Verbist J, Hendriks J, Lauwers P, et al. Superiority of stent-grafts for in-stent restenosis in the superficial femoral artery: Twelve-month results from a multicenter randomized trial. J Endovasc Ther 2015; 22: 1–10.
- 21.
Reijnen MMPJ, van Walraven LA, Fritschy WM, Lensvelt MMA, Zeebregts CJ, Lemson MS, et al. 1-Year results of a multicenter randomized controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. JACC Cardiovasc Interv 2017; 10: 2320–2331.
- 22.
Soga Y, Takahara M, Iida O, Tomoi Y, Kawasaki D, Tanaka A, et al. Vessel patency and associated factors of drug-coated balloon for femoropopliteal lesion. J Am Heart Assoc 2023; 12: e025677.
- 23.
Fanelli F, Cannavale A, Gazzetti M, Lucatelli P, Wlderk A, Cirelli C, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol 2014; 37: 898–907.
- 24.
Ichihashi S, Sato T, Iwakoshi S, Itoh H, Kichikawa K. Technique of percutaneous direct needle puncture of calcified plaque in the superficial femoral artery or tibial artery to facilitate balloon catheter passage and balloon dilation of calcified lesions. J Vasc Interv Radiol 2014; 25: 784–788.
- 25.
Krishnan P, Farhan S, Schneider P, Kamran H, Iida O, Brodmann M, et al. Determinants of drug-coated balloon failure in patients undergoing femoropopliteal arterial intervention. J Am Coll Cardiol 2022; 80: 1241–1250.
- 26.
Böhme T, Noory E, Brechtel K, Scheinert D, Bosiers M, Beschorner U, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: 36-month results of the Viabahn 25 cm Trial. J Endovasc Ther 2021; 28: 222–228.





