Abstract
Background: Although the efficacy of an implantable cardioverter defibrillator (ICD) in preventing sudden cardiac death is well established, the incidence and predictors of appropriate ICD therapy in Japanese ischemic heart disease (IHD) patients remain unclear.
Methods and Results: We retrospectively studied Japanese 141 IHD patients undergoing transvenous ICD or cardiac resynchronization therapy with a defibrillator (CRT-D) implantation for primary or secondary prevention at Hirosaki University Hospital. Over a mean (±SD) follow-up period of 5.5±2.8 years, the incidence of appropriate ICD therapy was similar in the primary and secondary prevention groups, although it was relatively more frequent in the first 2 years in the secondary prevention group. Four patients died due to sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), mainly due to post-shock pulseless electrical activity. Once patients had received their first appropriate ICD therapy, 49.2% received second appropriate ICD therapy within 6 months. Cox proportional hazard analysis revealed that sustained VT as an index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation was an independent predictor of appropriate ICD therapy, but VF was not.
Conclusions: The incidence of appropriate ICD therapy was comparable in primary and secondary prevention among Japanese IHD patients. We need to recognize the high-risk period for second appropriate ICD therapy after the first therapy and sustained VT as index life-threatening ventricular tachyarrhythmia as a risk factor for appropriate ICD therapy.
The effectiveness of an implantable cardioverter defibrillator (ICD) for the prevention of sudden cardiac death (SCD) has been well established in past clinical trials,1 and the number of ICD implantations has increased in developed countries, including Japan.2 However, most previous clinical trials have been performed in the US and Europe, and evidence in Japanese patients is limited, especially in the case of ischemic heart disease (IHD). Therefore, it is unclear whether the recommendations regarding ICD implantation based on past clinical trials are suitable for Japanese IHD patients because of differences in patient characteristics and outcomes, such as the prognosis of IHD patients with reduced left ventricular ejection fraction (LVEF) and the proportion of ischemic etiology in ICD recipients.3–7 In the present study, we retrospectively studied Japanese IHD patients who underwent ICD or cardiac resynchronization therapy with a defibrillator (CRT-D) implantation for primary or secondary prevention to determine patient characteristics, outcomes, and predictors of appropriate ICD therapy.
Methods
Study Patients and Data Collection
The study population included 141 patients with IHD who underwent ICD or CRT-D implantation for primary (n=56) or secondary prevention (n=85) at Hirosaki University Hospital between January 2005 and October 2022. These patients were followed up for a mean (±SD) of 5.5±2.8 years after ICD/CRT-D implantation. All patients met the Class I indication for ICD/CRT-D implantation according to the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.8 According to Japanese Heart Rhythm Society guidelines,9 CRT-D was indicated if the QRS duration was ≥120 ms with left bundle branch block (LBBB), LVEF ≤35%, and New York Heart Association (NYHA) Class II or III symptoms of heart failure. All data including baseline characteristics, laboratory data, and medications at hospital discharge, were collected from medical records and the Hirosaki University Hospital database. Subsequent clinical events were collected from medical records or from patients’ primary care physicians up to 8 years after ICD/CRT-D implantation.
This study was conducted based on the ethical guidelines for medical research on humans in the Declaration of Helsinki and was approved by the Ethics Committee of the Hirosaki University Graduate School of Medicine (Approval no. 2022-153).
ICD Therapy Analysis
The ICDs were interrogated every 6 months, and unscheduled device interrogations were performed in a case of symptomatic episodes of arrhythmia and/or shock therapy. ICD therapies were defined as either antitachycardia pacing (ATP) or shock therapy, including cardioversion and defibrillation. Therapies were classified as appropriate when they were delivered in response to sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). The appropriateness of ICD therapy was determined by independent review of device-stored electrograms by expert reviewers. The device settings basically followed the manufacturers’ protocols, with programming customized to specific circumstances at the discretion of the electrophysiologist. The time to the appropriate ICD therapy was determined as the time from the date of ICD implantation to the date of the first appropriate ICD therapy. The time to the second ICD therapy was determined as the time from the date of the first appropriate ICD therapy to the date of the second appropriate ICD therapy. Regarding analysis of second ICD therapy, any subsequent appropriate ICD therapy occurring >24 h after the first appropriate ICD therapy was determined to be the second ICD therapy.
Results
Baseline and Angiographic Characteristics
Table 1 presents patients’ baseline characteristics, laboratory data, and medications at hospital discharge after ICD/CRT-D implantation. Of the 56 patients implanted with ICD/CRT-D for primary prevention, 47 (83.9%) met the Class I indication for ICD according to ACC/AHA guidelines, namely LVEF ≤30%. The remaining 9 (16.1%) patients had LVEF ≤35% with NYHA Class II or III (Figure 1A). Of the 85 patients implanted with ICD/CRT-D for secondary prevention, 52 (61.1%) had sustained VT as an index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation. The remaining 33 (38.9%) patients had VF (Figure 1B).
Table 1.
Baseline Characteristics, Laboratory Data, and Medications at Hospital Discharge
| |
Primary prevention
(n=56) |
Secondary prevention
(n=85) |
P value |
| Age (years) |
68.4±7.9 |
65.4±9.5 |
0.053 |
| Male sex |
52 (92.9) |
75 (88.2) |
0.36 |
| BMI (kg/m2) |
24.4±4.4 |
24.3±4.1 |
0.68 |
| Prior PCI |
40 (71.4) |
57 (67.1) |
0.58 |
| Prior CABG |
25 (44.6) |
19 (22.6) |
0.005 |
| Diabetes |
32 (57.1) |
40 (47.1) |
0.24 |
| Maintenance hemodialysis |
7 (12.5) |
7 (8.2) |
0.41 |
| Atrial fibrillation |
17 (30.4) |
16 (18.8) |
0.11 |
| CRT-D |
35 (62.5) |
8 (9.4) |
<0.0001 |
| LVEF (%) |
25.6±5.6 |
35.5±11.0 |
<0.0001 |
| Laboratory data |
| Hemoglobin (g/dL) |
12.2±2.1 |
12.2±2.0 |
0.90 |
| Serum creatinine (mg/dL) |
1.7±1.4 |
1.5±1.7 |
0.50 |
| Creatinine clearance (mL/min) |
53±29 |
62±33 |
0.13 |
| Total cholesterol (mg/dL) |
159±35 |
159±43 |
0.95 |
| Triglyceride (mg/dL) |
128±72 |
138±108 |
0.56 |
| HDL-C (mg/dL) |
43±13 |
40±15 |
0.96 |
| LDL-C (mg/dL) |
90±23 |
90±32 |
0.96 |
| BNP (pg/mL) |
510±852 |
316±571 |
0.11 |
| Medications at hospital discharge |
| ACEi/ARB |
40 (71.4) |
69 (81.2) |
0.18 |
| β-blocker |
50 (89.3) |
77 (90.6) |
0.80 |
| Statin |
41 (73.2) |
61 (71.8) |
0.85 |
| Loop diuretics |
46 (82.1) |
47 (55.3) |
0.0007 |
| MRA |
31 (55.4) |
36 (42.4) |
0.13 |
| Thiazide |
5 (8.9) |
0 (0.0) |
0.002 |
| Warfarin |
33 (58.9) |
28 (32.9) |
0.002 |
| DOACs |
4 (7.1) |
4 (4.7) |
0.54 |
| Amiodarone |
16 (28.6) |
59 (69.4) |
<0.0001 |
| Sotalol |
0 (0.0) |
6 (7.1) |
0.01 |
Unless indicated otherwise, data are given as the mean±SD or n (%). ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CRT-D, cardiac resynchronization therapy with a defibrillator; DOACs, direct oral anticoagulants; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention.

Survival Curves and Causes of Death
Figure 2A–C shows survival curves for all-cause death (Figure 2A), cardiac death (Figure 2B), and death due to sustained VT/VF (Figure 2C) in patients implanted with an ICD/CRT-D for primary and secondary prevention. The incidence of all-cause death, cardiac death, and death due to sustained VT/VF was similar between the primary and secondary prevention groups. For patients implanted with an ICD/CRT-D for primary prevention, incidence of all-cause death was 5.5%, 21.5%, and 30.0% at 1, 3, and 5 years, respectively; in comparison, in patients implanted with an ICD/CRT-D for secondary prevention, the incidence was 8.3%, 13.3%, and 26.1% at 1, 3, and 5 years, respectively.

Table 2 presents causes of death during the follow-up period. Among the 52 patients who died during follow-up period, cardiac death accounted for almost half the deaths. The most common cause of cardiac death was congestive heart failure followed by SCD of undetermined origin and death due to sustained VT/VF. In the 5 cases of SCD of undetermined origin, postmortem analysis of device-stored electrograms was not performed, so that the etiology could not be determined. Among the 4 cases of death due to sustained VT/VF, pulseless electrical activity (PEA) occurred after appropriate shock therapy in 3 patients, and sustained VT/VF was not terminated by ATP or shock therapy in one patient.
Table 2.
Causes of Death During Follow-up in Patients With ICD/CRT-D for Primary or Secondary Prevention
| Cause of death |
Primary prevention
(n=25) |
Secondary prevention
(n=27) |
| Cardiac |
| CHF |
11 (44.0) |
8 (29.6) |
| Sustained VT/VF |
2 (8.0) |
2 (7.4) |
| SCD of undetermined origin |
2 (8.0) |
3 (11.1) |
| AMI |
1 (4.0) |
0 (0.0) |
| Non-cardiac |
| Cancer |
2 (8.0) |
3 (11.1) |
| Renal failure |
3 (12.0) |
1 (3.7) |
| Pneumonia |
0 (0.0) |
1 (3.7) |
| Stroke |
0 (0.0) |
1 (3.7) |
| Others |
5 (20.0) |
5 (18.5) |
| Unknown |
0 (0.0) |
3 (11.1) |
Data are given as n (%). AMI, acute myocardial infarction; CHF, congestive heart failure; CRT-D, cardiac resynchronization therapy with a defibrillator; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
Incidence of the First Appropriate ICD Therapy
Figure 2D shows survival curves for the first appropriate ICD therapy, including ATP and shock therapy, in patients implanted with an ICD/CRT-D for primary or secondary prevention. Although there was no statistically significant difference in the incidence of the first appropriate ICD therapy between the 2 groups, first appropriate ICD therapy was relatively more frequent in the first 2 years after implantation in the secondary prevention group. The incidence of the first appropriate ICD therapy at 1, 3, and 5 years was 19.1%, 27.0%, and 29.8%, respectively, in the secondary prevention group, compared with 7.3%, 14.2%, and 19.9%, respectively, in the primary prevention group. Most of the appropriate ICD therapy was delivered for sustained VT (Figure 3A), and approximately 40% of appropriate ICD therapy was ATP followed by ATP plus shock and shock (Figure 3B).
Incidence of Second ICD Therapy
Figure 2E shows the survival curve for the second appropriate ICD therapy after the first appropriate ICD therapy over a mean (±SD) of 1.3±1.6 years of follow-up. The second appropriate ICD therapy commonly occurred in the early period after the first appropriate ICD therapy. Of note, once patients received their first appropriate ICD therapy, 49.2% received second appropriate ICD therapy within 6 months. Half of the second appropriate ICD therapy was shock therapy. Analyzing 37 patients who received first appropriate ICD therapy (21 who received shock therapy, 16 who received only ATP), we found no difference in the incidence of the second appropriate ICD therapy between these 2 groups (log rank P=0.59).
Predictors of the First Appropriate ICD Therapy
Table 3 presents Cox proportional hazard models for the first appropriate ICD therapy in the primary and secondary prevention groups. Among patients in the primary prevention group, univariate analysis revealed maintenance hemodialysis and medication without statin as predictors for the first appropriate ICD therapy, although neither was a significant predictor in multivariate analysis. A previous study demonstrated that CRT potentially leads to reverse remodeling of the left ventricle, such as a reduction in left ventricle volume and an increase in LVEF.10 We obtained LVEF data at least 3 months after CRT-D implantation for 25 of 35 CRT-D recipients in the primary prevention group, and found no difference in LVEF between baseline and follow-up for these 25 patients (26.4±5.8% vs 29.7±10.3%, respectively; P=0.16). Because of the favorable effect of the presence of LBBB on LVEF improvements after CRT,11 we further examined 10 CRT-D recipients with left LBBB in whom LVEF data were obtained at follow-up. In these 10 patients, there was no significant difference in LVEF between baseline and follow-up (27.2±5.3% vs 29.3±8.7%, respectively; P=0.52). NYHA functional class at the time of ICD/CRT-D implantation was obtained for 43 of 56 patients implanted with an ICD for primary prevention. Of these patients, 2 were classified as NYHA Class I, 24 were classified as NYHA Class II, and 17 were classified as NYHA Class III. In Cox proportional hazards analysis, NYHA Class III was not predictor of appropriate ICD therapy in the primary prevention group (Table 3A).
Table 3.
Cox Proportional Hazards Models for the First Appropriate ICD Therapy
| |
Univariate analysis |
Multivariate analysis |
| HR |
95% CI |
P value |
HR |
95% CI |
P value |
| (A) Primary prevention |
| Age ≥70 years |
0.42 |
0.12–1.50 |
0.18 |
|
|
|
| Male sex |
1.26 |
0.16–9.62 |
0.82 |
|
|
|
| LVEF ≤30% |
1.57 |
0.35–6.99 |
0.55 |
|
|
|
| CRT-D |
1.45 |
0.49–4.24 |
0.50 |
|
|
|
| NYHA Class III |
0.38 |
0.08–1.76 |
0.21 |
|
|
|
| Diabetes |
1.31 |
0.45–3.85 |
0.62 |
|
|
|
| Maintenance hemodialysis |
3.72 |
1.15–12.00 |
0.03 |
2.61 |
0.72–9.46 |
0.14 |
| Atrial fibrillation |
1.06 |
0.34–3.34 |
0.92 |
|
|
|
| ACEi ARB |
1.09 |
0.35–3.45 |
0.88 |
|
|
|
| β-blocker |
0.55 |
0.12–2.50 |
0.44 |
|
|
|
| Statin |
0.38 |
0.14–1.04 |
0.06 |
0.49 |
0.16–1.54 |
0.23 |
| Amiodarone |
0.50 |
0.11–2.23 |
0.36 |
|
|
|
| (B) Secondary prevention |
| Age ≥70 years |
1.30 |
0.57–2.93 |
0.53 |
|
|
|
| Male sex |
1.00 |
0.30–3.33 |
1.00 |
|
|
|
| LVEF ≤35% |
2.53 |
1.10–5.86 |
0.03 |
1.69 |
0.68–4.20 |
0.26 |
| CRT-D |
2.38 |
0.81–6.94 |
0.11 |
|
|
|
| Sustained VT as index arrhythmia |
3.42 |
1.28–9.09 |
0.01 |
2.79 |
1.03–7.58 |
0.04 |
| Diabetes |
0.76 |
0.35–1.66 |
0.49 |
|
|
|
| Maintenance hemodialysis |
1.07 |
0.25–4.54 |
0.93 |
|
|
|
| Atrial fibrillation |
1.04 |
0.39–2.77 |
0.93 |
|
|
|
| ACEi/ARB |
0.32 |
0.14–0.74 |
0.008 |
0.48 |
0.19–1.19 |
0.11 |
| β-blocker |
0.62 |
0.19–2.07 |
0.44 |
|
|
|
| Statin |
1.64 |
0.62–4.35 |
0.32 |
|
|
|
| Amiodarone |
0.68 |
0.31–1.51 |
0.34 |
|
|
|
| Sotalol |
2.12 |
0.63–7.11 |
0.22 |
|
|
|
CI, confidence interval; HR, hazard ratio; NYHA, New York Heart Association. Other abbreviations as in Tables 1,2.
In the secondary prevention group, univariate analysis revealed that LVEF ≤35%, sustained VT as the index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation, and medication without an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker were predictors of appropriate ICD therapy. In multivariate analysis, sustained VT as the index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation was an independent predictor of the first appropriate ICD therapy (Table 3B).
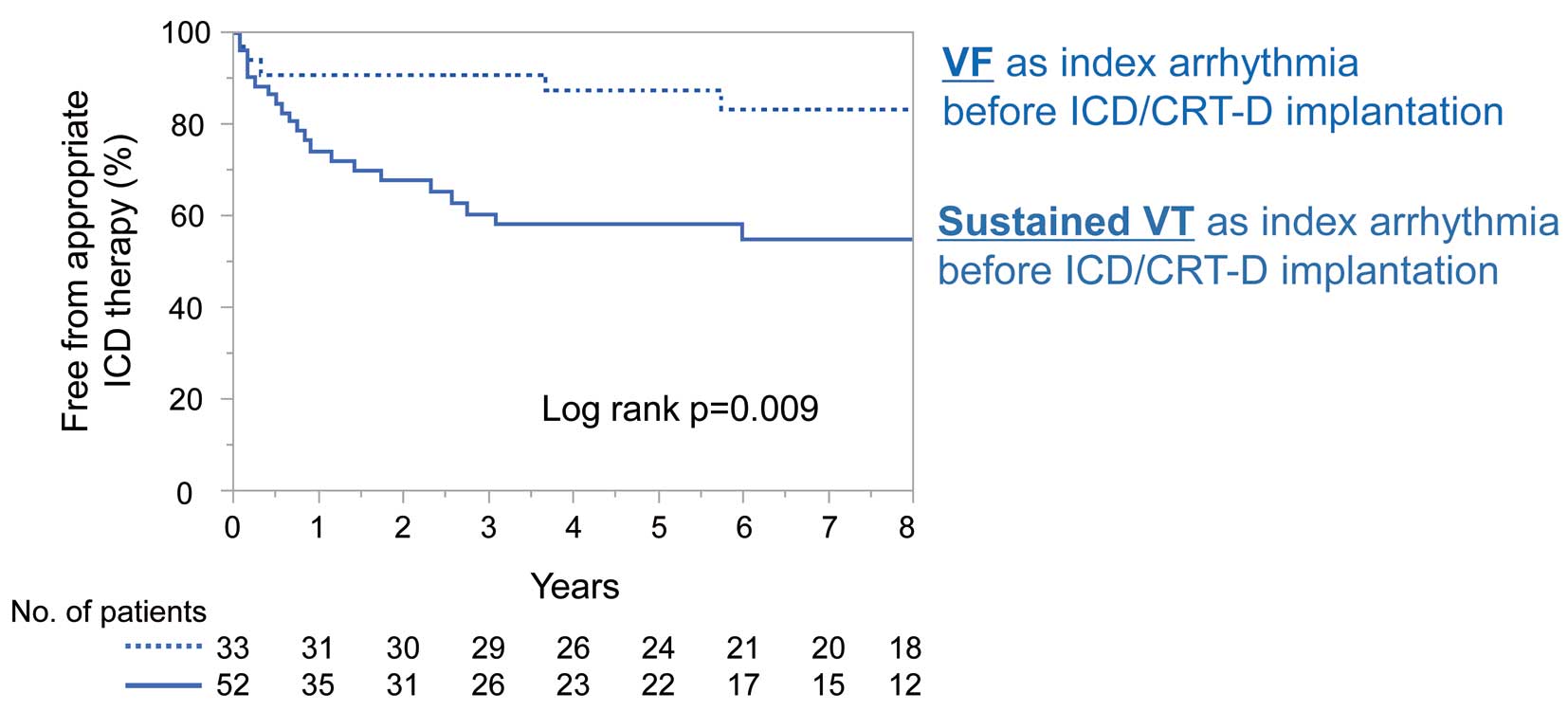
Figure 4 shows Kaplan-Meier survival curves for the first appropriate ICD therapy in the secondary prevention group. The incidence of appropriate ICD therapy was significantly higher among patients with sustained VT as the index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation than among those with VF (log rank P=0.009). Of the 52 patients with VT as the index ventricular tachyarrhythmia before ICD/CRT-D implantation, the first appropriate ICD therapy was delivered for VT in 19 patients and for VF in 2 patients. Among these patients, the second appropriate ICD therapy was delivered to 13, and all the therapy was delivered for VT. In contrast, among the 33 patients with VF as the index ventricular tachyarrhythmia before ICD/CRT implantation, the first appropriate ICD therapy was delivered for VT in 2 patients and for VF in 3. Of the 56 patients in the primary prevention group, 15 underwent a first appropriate ICD therapy. All the therapy was delivered for VT, and 6 patients underwent a second appropriate ICD therapy, with 5 receiving second appropriate therapy for VT. Mean LVEF was significantly lower in patients with VT as the index arrhythmia than in patients with VF (33.3±10.6% vs. 38.9±10.9%; P=0.02).
Discussion
Incidence of Appropriate ICD Therapy in Japanese Patients With IHD
Table 4 summarizes previous studies specifically addressing appropriate ICD therapy in ICD recipients.12–21 The incidence of appropriate ICD therapy could be affected by baseline characteristics such as LVEF, NYHA classification, the presence or absence of non-sustained VT, device setting, medications, and revascularization. Although the inclusion and exclusion criteria of these studies varied, and indirect comparisons should be made with caution, these previous findings indicate that the incidence of appropriate ICD therapy for primary and secondary prevention in Japanese IHD patients seems to be comparable to that in the US and Europe, and the recommendations of ICD implantation based on past clinical trials performed in the US and Europe seems to be suitable for Japanese IHD patients. In addition, our results also demonstrated that the efficacy of ICD for primary prevention is comparable to that for secondary prevention, indicating the usefulness of ICD for primary prevention for Japanese IHD patients. It is well established that the incidence of appropriate ICD therapy is higher for secondary prevention than primary prevention according to studies performed in the US and Europe.14,15,22,23 In contrast, the incidence of appropriate ICD therapy was comparable between primary and secondary prevention in Japanese IHD patients.18,19 Previously, the incidence of SCD in IHD patients was reported to be much less frequent in Japan than in the US and Europe;3–6,24 therefore, the prophylactic use of ICD in Japanese IHD patients has been recognized as being less effective than in the US and Europe. However, data from the World Health Organization regarding the leading causes of death showed that the incidence of death due to IHD has been increasing in Japan.25 In addition, in the Hisayama Study, the prevalence of sudden unexpected death increased significantly from 2.5% (in 1962) to 7.6% (in 2009) concomitant with an increase in the prevalence of IHD from 2.1% to 6.6%.26 Furthermore, in the Chronic Heart Failure Analysis and Registry in the Tohoku District-2 study, the prevalence of IHD in patients with Stage C/D heart failure increased from 25.4% to 47.1%,27 indicating the rapid trend of Westernization of heart failure etiology. However, the proportion of ischemic etiology of ICD recipients was 36%, and the proportion of primary prevention was less than half that of secondary prevention in 2016,2 both of which were much lower in the US and Europe,14,22,23 indicating that there may be underuse of ICD in Japanese IHD patients.
Table 4.
Summary of Previous Studies Evaluating Appropriate ICD Therapy in ICD Recipients
| Study |
Enrollment
period |
Sample
size |
IHD
(%) |
Mean
follow-up
period
(years) |
Mean (±SD)
age (years) |
CRT-D
(%) |
LVEF
(%) |
Incidence of
appropriate ICD
therapy |
| Primary prevention |
| Singh et al.12 |
1997–2001 |
719 |
100 |
1.4 |
64±10 |
N/A |
23±5 |
24% at 1.4 years |
| Alsheikh-Ali et al.13 |
1995–2005 |
525 |
100 |
2.0 |
67±11 |
N/A |
23±7 |
22% at 2.0 years |
| van Welsenes et al.14 |
1996–2008 |
1,302 |
68 |
3.4 |
63±11 |
55 |
29±12 |
37% at 5.0 years |
| Sabbag et al.15 |
2010– |
1,766 |
65 |
2.5 |
64±12 |
43 |
N/A |
9% at 2.5 years |
| An et al.17 |
2000–2012 |
118 |
100 |
3.9 |
69±10 |
52 |
25±5 |
37% at 3.9 years |
| Kabutoya et al.18 |
2014–2016 |
165 |
100 |
2.1 |
70 |
50 |
28 |
20% at 2.0 years |
| Kondo et al.19 |
2010–2012 |
178 |
100 |
2.0 |
70±9 |
53 |
31±11 |
15% at 2.0 years |
| Kotake et al.20 |
2010–2012 |
531 |
32 |
≥2 |
66 |
67 |
27% |
27% at 3 years |
| Secondary prevention |
| Borleffs et al.16 |
1996– |
456 |
100 |
4.5 |
65±10 |
N/A |
35±14 |
30% at 4.5 years |
| van Welsenes et al.14 |
1996–2008 |
832 |
82 |
3.4 |
63±13 |
15 |
37±15 |
51% at 5.0 years |
| Sabbag et al.15 |
2010– |
583 |
61 |
2.5 |
64±15 |
17 |
N/A |
21% at 2.5 years |
| Kabutoya et al.18 |
2014–2016 |
227 |
100 |
2.1 |
68 |
15 |
37 |
20% at 2.0 years |
| Kondo et al.19 |
2010–2012 |
315 |
100 |
2.0 |
67±11 |
17 |
39±14 |
24% at 2.0 years |
| Kotake et al.20 |
2010–2012 |
454 |
54 |
≥2 |
66 |
30 |
33 |
36% at 3 years |
| Nagahara et al.21 |
1999–2015 |
147 |
34 |
3.2 |
59 |
7 |
45 |
54% at 5 years |
IHD, ischemic heart disease. Other abbreviations as in Tables 1,2.
The patient characteristics and results of our study are similar to the recently published Japan Implantable Devices in Coronary Artery Disease (JID-CAD) study,18 but there are some important differences, including follow-up period and the incidence of appropriate ICD therapy. According to previous studies, appropriate ICD therapy occurs over years in primary prevention patients;14,15,23 therefore, longer follow-up is required to evaluate the effect of ICD/CRT-D. In addition, the survival curve of the incidence of appropriate ICD therapy in the primary prevention group caught up to the curve for the secondary prevention group at 2 years in the JID-CAD study18 and after 5 years in our study. Our results demonstrate the important clinical usefulness of ICD for primary prevention because of the occurrence of appropriate therapy over the years, concomitant with a relatively higher incidence of ICD therapy in early period after ICD implantation in the secondary prevention group.
Death Due to Sustained VT/VF in Patients Implanted With ICD/CRT-D
In the present study, 4 patients died due to post-shock PEA or refractory sustained VT/VF under a functioning ICD. Previously, Mitchell et al. performed a postmortem analysis of 68 patients with ICD and identified the mechanism of SCD as post-shock PEA in 29%, uncorrected sustained VT/VF in 25%, and incessant sustained VT/VF in 13%; they also found that lower LVEF and NYHA Class III/IV were associated with post-shock PEA.28 In another study analyzing ICD recordings of 176 patients including 376 defibrillation attempts in the out-of-hospital setting for sustained VT/VF, a return of an organized rhythm was obtained after the first defibrillation in 112 episodes, whereas there were 206 episodes of post-shock asystole, 24 of which resulted in sustained asystole.29 In a study of 22 patients for whom postmortem ICD interrogations and/or electrocardiogram monitoring were available, 3 patients died due to post-shock PEA and 7 patients died due to refractory VT/VF.30 In another study, of 22 patients with out-of-hospital sudden death (mean LVEF of 25.5±7.6%) and postmortem ICD interrogation, 11 patients received ICD shocks for sustained VT/VF, but the first ICD therapy was unsuccessful in 7.31 Of these patients, 4 required all 6 shocks (the maximum number delivered by the ICD for any given tachyarrhythmia), and 1 patient died due to refractory VF.31 In the era of strategic programming, contemporary ICD systems deliver appropriate ICD therapy for life-threatening VT/VF accurately;32 however, failure to treat sustained VT/VF still occurs due to post-shock PEA28,29 or refractory VT/VF.28,31 At the time of ICD implantation, post-shock PEA or refractory VT/VF during defibrillation threshold (DFT) testing are not common, but prolonged hemodynamic deterioration or failure to terminate VT/VF does occur.33,34 In fact, at the end of DFT testing, it was reported that patients with LVEF ≤30% exhibited significant impairment of the cardiac index compared with before defibrillation, and 1 of 13 patients required inotropic support.35 Thus, although the majority of sustained VT/VF episodes are successfully terminated by ICD therapy,32 it needs to be kept in mind that death due to sustained VT/VF can potentially occur even in patients with a functioning ICD, especially in patients with impaired left ventricular systolic function.28,32
Second ICD Therapy After the First ICD Therapy
In the present study, it was surprising that 49.2% of patients received second appropriate ICD therapy within 6 months after the first appropriate ICD therapy. Freedberg et al. reported that 79% of patients received a second appropriate ICD therapy within 1 year after the first appropriate ICD therapy, and that the mean time from the first to the second therapy was 22 days.36 In the Leiden Out-of-Hospital Cardiac Arrest (LOHCAT), appropriate ICD therapy within 6 months after ICD implantation was a risk factor for subsequent ICD therapy.16 Another study by van Welsenes et al. reported that 49 of 141 (35%) ICD recipients for primary prevention received a second appropriate ICD shock at a mean (±SD) of 275±455 days after the first shock.14 Thus, previous studies have demonstrated a high-risk period for second appropriate ICD therapy after the first; however, the study by Freedberg et al. was reported in 2001,36 and van Welsenes et al.14 only analyzed appropriate shock. In the LOHCAT, the risk after 6 months after ICD implantation was analyzed.16 In our study, we analyzed the risk of the second appropriate ICD therapy occurring >24 h after the first therapy in Japanese IHD patients. Our results strongly suggest an urgent need to reduce the incidence of the second appropriate ICD therapy after the first therapy regardless of the type of the therapy, as well as the importance of recognizing the high-risk period for appropriate patient management, because there is consistent evidence of a positive association between the number of ICD therapies, especially ICD shock, and an increased risk of mortality, heart failure, shock anxiety, and depression.37,38 Recently, additional treatment to reduce repeated appropriate ICD therapy has been highlighted, and some randomized clinical trials have been conducted to test whether catheter ablation could reduce the incidence of second therapy.39 Considering the fact that approximately half of all patients receiving a first appropriate ICD therapy will experience second appropriate ICD therapy within 6 months, those with sustained VT should be treated more aggressively with catheter ablation in addition to antiarrhythmics.
Predictor of Appropriate ICD Therapy
Our results clearly indicated that sustained VT as the index life-threatening ventricular tachyarrhythmia before ICD/CRT-D implantation was an independent predictor of appropriate ICD therapy. Previous studies support our finding.21,36,40,41 In the Antiarrhythmics versus Implantable Defibrillators Trial, the incidence of appropriate ICD therapy was higher in patients with sustained VT as the index life-threatening arrhythmia than in those with VF.40 The cumulative incidence of appropriate ICD therapy was 68% at 1 year in patients with sustained VT, whereas it was 39% in patients with cardiac arrest.40 Similarly, Boulé et al. showed that 115 of 187 (61%) patients with sustained VT as the index arrhythmia and 24 of 52 (46%) patients with VF as the index arrhythmia received appropriate ICD therapy over the course of 7.8 years of follow-up.41 Nagahara et al. reported that the incidence of appropriate ICD therapy was 70% in patients with sustained VT as the index arrhythmia and 34% in patients with cardiopulmonary arrest over a 5-year follow-up.21 In our study, the incidence of appropriate ICD therapy was 25.7% at 1 year and 41.9% at 5 year in patients with VT as the index ventricular tachyarrhythmia, compared with 9.1% and 12.6%, respectively, in patients with VF; these rates are much lower than in previous studies,21,36,41 presumably because in previous studies the enrollment period was in the 1990 s or the early 2000s,36,40,41 a single-chamber ICD was implanted in 24.3% of patients,41 and there was a lower proportion of ischemic etiology.21 In addition, the incidence of appropriate ICD therapy in patients with a prior history of VT was compared with that in patients with a prior history of cardiac arrest.21,36,40 Our findings clearly indicate the important differences in the electrophysiologic characteristics of these 2 life-threatening ventricular tachyarrhythmias for risk stratification of future recurrence. VT often has multiple coexisting VT circuits surrounded by large scars at the endocardium, epicardium, or deep within the myocardium, which may become substrate for VT recurrence in the long term.42 In fact, VT commonly occurs in patients with a prior history of VT and is much less likely to be detected in patients with a history of only VF, and higher reproducibility of VT has been demonstrated in previous studies.43 In current guidelines regarding ICD recommendations for secondary prevention, VT and VF are not distinguished, but the findings of previous studies and the present study clearly indicate that patients with prior VT should be considered as being at high risk of recurrence.
Previous studies have demonstrated that LVEF improvement after CRT-D implantation was associated with reduced risk of appropriate ICD therapy.44 Conversely, the improvement in LVEF and the incidence of appropriate ICD therapy after CRT are possibly affected by the etiology of the cardiac dysfunction, and the effect of CRT seems to be limited in the case of ischemic etiology.10,17 Current guidelines regarding ICD recommendations are mainly based on the LVEF and NYHA classification.8,9,45 Although all-cause mortality was correlated with NYHA classification driven by an increase in cardiovascular death, recent evidence suggests that it is controversial whether the NYHA classification is associated with the incidence of appropriate ICD therapy.46 It is clinically important to identify patients at high risk of appropriate ICD therapy for appropriate patient management, but it remains unclear whether the predictors of appropriate ICD therapy in the studies performed in the US and Europe are suitable for Japanese patients because of differences in cardiovascular mortality, the proportion of ischemic etiology among ICD recipients, genetic background, lifestyle, and healthcare insurance. Our study provides important clinical evidence of the need to identify patients at high risk of appropriate ICD therapy in Japanese ICD recipients with IHD.
Study Limitations
This study has several limitations. First, this was a retrospective single-center study that covered a period of 15 years. During this time, there have been changes in the medications used to manage IHD,47 and the ICD technologies and programming strategies for the detection of and therapy for sustained VT/VF have advanced, which may influence the outcomes of patients and the incidence of appropriate ICD therapy. Second, the presence or absence of non-sustained VT was not included. Third, we analyzed both ATP and shock therapy; therefore, sustained VT, which is not life threatening, was included. However, analysis of slower sustained VT could be important because syncope, hemodynamic compromise, or secondary tachyarrhythmias are potentially induced even by slower stable sustained VT. Fourth, we analyzed the data at hospital discharge, therefore subsequent changes, including medication and laboratory data, were not considered. Fifth, follow-up data were missing for some patients because of the retrospective nature of the study. Thirteen patients withdrew from the study during the follow-up period. Finally, the number of patients included in the study was not large enough and a longer follow-up period could be required to assess long-term outcomes.
Conclusions
The incidence of appropriate ICD therapy in patients with ICD/CRT-D implanted for primary and secondary prevention is significant in Japanese IHD patients, especially in patients with sustained VT as the index life-threatening ventricular tachyarrhythmia. Once patients received appropriate ICD therapy, careful management is required because of a high risk of second appropriate ICD therapy relatively soon thereafter. Further studies are clearly needed to evaluate the efficacy of ICD for Japanese IHD patients to prevent SCD.
Acknowledgments
The authors thank Machiko Kogawa for technical support.
Sources of Funding
This study did not receive any specific funding.
Disclosures
H.T. has received research grant support from Medtronic Japan Co., Ltd., Fukuda Denshi Kita-Tohoku Hanbai Co., Ltd., Boston Scientific Co., and BIOTRONIK Japan Co., Ltd, and scholarship grants from Japan Lifeline Co. K.O. has received speakers’ bureau/honoraria from Johnson and Johnson, Medtronic, Daiichi-Sankyo, and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Ethics Committee of Hirosaki University Graduate School of Medicine (Approval no. 2022-153).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-24-0436
References
- 1.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883.
- 2.
Yokoshiki H, Shimizu A, Mitsuhashi T, Ishibashi K, Kabutoya T, Yoshiga Y, et al. Trends in the use of implantable cardioverter-defibrillator and cardiac resynchronization therapy device in advancing age: Analysis of the Japan cardiac device treatment registry database. J Arrhythm 2020; 36: 737–745.
- 3.
Tanno K, Miyoshi F, Watanabe N, Minoura Y, Kawamura M, Ryu S, at al. Are the MADIT II criteria for ICD implantation appropriate for Japanese patients? Circ J 2005; 69: 19–22.
- 4.
Kuga K, Yamasaki H, Hattori A, Xu DZ, Watanabe S, Arimoto T, et al. Prognosis of myocardial infarction with left ventricular dysfunction in the coronary revascularization era: Subanalysis of the Japanese Coronary Artery Disease (JCAD) study. Circ J 2014; 78: 2483–2491.
- 5.
Shiga T, Hagiwara N, Ogawa H, Takagi A, Nagashima M, Yamauchi T, et al. Sudden cardiac death and left ventricular ejection fraction during long-term follow-up after acute myocardial infarction in the primary percutaneous coronary intervention era: Results from the HIJAMI-II Registry. Heart 2009; 95: 216–220.
- 6.
Hanada K, Sasaki S, Seno M, Kimura Y, Ichikawa H, Nishizaki F, et al. Reduced left ventricular ejection fraction is a risk for sudden cardiac death in the early period after hospital discharge in patients with acute myocardial infarction. Circ J 2022; 86: 1490–1498.
- 7.
Ueda N, Noda T, Kusano K, Yasuda S, Kurita T, Shimizu W. Use of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in Asia. JACC Asia 2023; 3: 335–345.
- 8.
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation 2018; 138: e210–e271.
- 9.
Nogami A, Kurita T, Kusano K, Goya M, Shoda M, Tada H, et al. JCS/JHRS 2021 guideline focused update on non-pharmacotherapy of cardiac arrhythmias. Circ J 2022; 86: 337–363.
- 10.
Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: Multicenter automatic defibrillator implantation trial: Cardiac resynchronization therapy. Circulation 2010; 122: 985–992.
- 11.
Rickard J, Kumbhani DJ, Popovic Z, Verhaert D, Manne M, Sraow D, et al. Characterization of super-response to cardiac resynchronization therapy. Heart Rhythm 2010; 7: 885–889.
- 12.
Singh JP, Hall WJ, McNitt S, Wang H, Daubert JP, Zareba W, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: Findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J Am Coll Cardiol 2005; 46: 1712–1720.
- 13.
Alsheikh-Ali AA, Homer M, Maddukuri PV, Kalsmith B, Estes NA 3rd, Link MS. Time-dependence of appropriate implantable defibrillator therapy in patients with ischemic cardiomyopathy. J Cardiovasc Electrophysiol 2008; 19: 784–789.
- 14.
van Welsenes GH, van Rees JB, Borleffs CJ, Cannegieter SC, Bax JJ, van Erven L, et al. Long-term follow-up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace 2011; 13: 389–394.
- 15.
Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm 2015; 12: 2426–2433.
- 16.
Borleffs CJ, van Erven L, Schotman M, Boersma E, Kiès P, van der Burg AE, et al. Recurrence of ventricular arrhythmias in ischaemic secondary prevention implantable cardioverter defibrillator recipients: Long-term follow-up of the Leiden Out-of-Hospital Cardiac Arrest study (LOHCAT). Eur Heart J 2009; 30: 1621–1626.
- 17.
An Y, Ando K, Soga Y, Nomura A, Nagashima M, Hayashi K, et al. Mortality and predictors of appropriate implantable cardioverter defibrillator therapy in Japanese patients with Multicenter Automatic Defibrillator Implantation Trial II criteria. J Arrhythm 2017; 33: 17–22.
- 18.
Kabutoya T, Mitsuhashi T, Shimizu A, Nitta T, Mitamura H, Kurita T, et al. Prognosis of Japanese patients with coronary artery disease who underwent implantable cardioverter defibrillator implantation: The JID-CAD study. Circ Rep 2021; 3: 69–76.
- 19.
Kondo Y, Noda T, Sato Y, Ueda M, Nitta T, Aizawa Y, et al. Comparison of 2-year outcomes between primary and secondary prophylactic use of defibrillators in patients with coronary artery disease: A prospective propensity score-matched analysis from the Nippon Storm Study. Heart Rhythm O2 2021; 2: 5–11.
- 20.
Kotake Y, Yasuoka R, Tanaka M, Noda T, Nitta T, Aizawa Y, et al. Comparison of second appropriate defibrillator therapy occurrence in patients implanted for primary prevention and secondary prevention: Sub-analysis of the Nippon Storm Study. Int J Cardiol Heart Vasc 2021; 32: 100704.
- 21.
Nagahara D, Fujito T, Mochizuki A, Shimoshige S, Hashimoto A, Miura T. Predictors of appropriate ICD therapy in Japanese patients with structural heart diseases: A major role of prior sustained ventricular tachycardia in secondary prevention. J Arrhythm 2018; 34: 527–535.
- 22.
Kim MH, Zhang Y, Sakaguchi S, Goldberger JJ. Time course of appropriate implantable cardioverter-defibrillator therapy and implications for guideline-based driving restrictions. Heart Rhythm 2015; 12: 1728–1736.
- 23.
Darma A, Nedios S, Kosiuk J, Richter S, Doering M, Arya A, et al. Differences in predictors of implantable cardioverter-defibrillator therapies in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2016; 18: 405–412.
- 24.
Hanada K, Kinjo T, Yokoyama H, Tsushima M, Senoo M, Ichikawa H, et al. Incidence, predictors, and outcome associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Circ J 2024; 88: 1254–1264.
- 25.
World Health Organization. Global health estimates: Leading causes of death. Accessed October 2, 2022. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
- 26.
Nagata M, Ninomiya T, Doi Y, Hata J, Ikeda F, Mukai N, et al. Temporal trends in sudden unexpected death in a general population: The Hisayama study. Am Heart J 2013; 165: 932–938.e1.
- 27.
Satake H, Fukuda K, Sakata Y, Miyata S, Nakano M, Kondo M, et al. Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure: A report from the CHART-2 Study. Circ J 2015; 79: 381–390.
- 28.
Mitchell LB, Pineda EA, Titus JL, Bartosch PM, Benditt DG. Sudden death in patients with implantable cardioverter defibrillators: The importance of post-shock electromechanical dissociation. J Am Coll Cardiol 2002; 39: 1323–1328.
- 29.
Pierce AE, Roppolo LP, Owens PC, Pepe PE, Idris AH. The need to resume chest compressions immediately after defibrillation attempts: An analysis of post-shock rhythms and duration of pulselessness following out-of-hospital cardiac arrest. Resuscitation 2015; 89: 162–168.
- 30.
Duray GZ, Schmitt J, Richter S, Israel CW, Hohnloser SH. Arrhythmic death in implantable cardioverter defibrillator patients: A long-term study over a 10 year implantation period. Europace 2009; 11: 1462–1468.
- 31.
Pires LA, Lehmann MH, Steinman RT, Baga JJ, Schuger CD. Sudden death in implantable cardioverter-defibrillator recipients: Clinical context, arrhythmic events and device responses. J Am Coll Cardiol 1999; 33: 24–32.
- 32.
Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm 2016; 13: e50–e86.
- 33.
Birnie D, Tung S, Simpson C, Crystal E, Exner D, Ayala Paredes FA, et al. Complications associated with defibrillation threshold testing: The Canadian experience. Heart Rhythm 2008; 5: 387–390.
- 34.
Mollerus M, Naslund L. Myocardial stunning following defibrillation threshold testing. J Interv Card Electrophysiol 2007; 19: 213–216.
- 35.
Steinbeck G, Dorwarth U, Mattke S, Hoffmann E, Markewitz A, Kaulbach H, et al. Hemodynamic deterioration during ICD implant: Predictors of high-risk patients. Am Heart J 1994; 127: 1064–1067.
- 36.
Freedberg NA, Hill JN, Fogel RI, Prystowsky EN. Recurrence of symptomatic ventricular arrhythmias in patients with implantable cardioverter defibrillator after the first device therapy: Implications for antiarrhythmic therapy and driving restrictions. CARE Group. J Am Coll Cardiol 2001; 37: 1910–1915.
- 37.
Elsokkari I, Parkash R, Tang A, Wells G, Doucette S, Yetisir E, et al. Mortality risk increases with clustered ventricular arrhythmias in patients with implantable cardioverter-defibrillators. JACC Clin Electrophysiol 2020; 6: 327–337.
- 38.
Suzuki T, Shiga T, Kuwahara K, Kobayashi S, Suzuki S, Nishimura K, et al. Prevalence and persistence of depression in patients with implantable cardioverter defibrillator: A 2-year longitudinal study. Pacing Clin Electrophysiol 2010; 33: 1455–1461.
- 39.
Samuel M, Healey JS, Nault I, Sterns LD, Essebag V, Gray C, et al. Ventricular tachycardia and ICD therapy burden with catheter ablation versus escalated antiarrhythmic drug therapy. JACC Clin Electrophysiol 2023; 9: 808–821.
- 40.
The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997; 337: 1576–1583.
- 41.
Boulé S, Sémichon M, Guédon-Moreau L, Drumez É, Kouakam C, Marquié C, et al. Long-term outcome of implantable cardioverter-defibrillator implantation in secondary prevention of sudden cardiac death. Arch Cardiovasc Dis 2016; 109: 517–526.
- 42.
Komatsu Y, Hocini M, Nogami A, Maury P, Peichl P, Iwasaki YK, et al. Catheter ablation of refractory ventricular fibrillation storm after myocardial infarction. Circulation 2019; 139: 2315–2325.
- 43.
Raitt MH, Dolack GL, Kudenchuk PJ, Poole JE, Bardy GH. Ventricular arrhythmias detected after transvenous defibrillator implantation in patients with a clinical history of only ventricular fibrillation: Implications for use of implantable defibrillator. Circulation 1995; 91: 1996–2001.
- 44.
Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald AC, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: Results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation 2014; 130: 2278–2286.
- 45.
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867.
- 46.
Sabbag A, Glikson M, Suleiman M, Boulos M, Goldenberg I, Beinart R, et al. Arrhythmic burden among asymptomatic patients with ischemic cardiomyopathy and an implantable cardioverter-defibrillator. Heart Rhythm 2019; 16: 813–819.
- 47.
Higuma T, Akashi YJ, Fukumoto Y, Obara H, Kakuma T, Asaumi Y, et al. Residual coronary risk factors associated with long-term clinical outcomes in patients with coronary artery disease treated with high- vs. low-dose statin therapy: REAL-CAD substudy. Circ J 2024; 88: 995–1003.





