Abstract
Background: The long-term effects of cumulative resting heart rate (cumRHR) on the incidence of cardiovascular events and all-cause mortality in older (age ≥60 years) hypertensive populations remain unclear. Therefore, the aim of this study was to investigate the association between cumRHR and cardiovascular events and all-cause mortality.
Methods and Results: This post hoc analysis used data from the Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial of 7,517 patients in whom resting heart rate (RHR) was measured at 0, 3, 6, 9, and 12 months. “cumRHR” refers to the weighted mean of the RHR for each time interval. Participants were divided into quartiles (Q1–Q4) based on cumRHR. After adjustment for potential confounders and using Q3 (72.19–75.88 [beats/min] × year) as the reference, patients in Q4 (75.94–109.44 [beats/min] × year) had higher risks of the primary outcome (a composite of stroke, acute coronary syndrome, acute decompensated heart failure, coronary revascularization, atrial fibrillation, and death from any cardiovascular cause) (hazard ratio [HR] 2.21; 95% confidence interval [CI] 1.42–3.43; P<0.001), major adverse cardiovascular events (HR 1.93; 95% CI 1.18–3.16; P=0.009), and stroke (HR 3.55; 95% CI 1.42–8.86; P=0.007) and those in Q1 (44.50–68.44 [beats/min] × year) had an increased risk of the primary outcome (HR 1.71; 95% CI 1.08–2.71; P=0.02). No such trends were observed for all-cause mortality. A U-shaped relationship was observed with the primary outcome, with higher risk for both very low or very high cumRHR levels compared with midrange values.
Conclusions: Both low and high cumRHR levels were associated with higher risk of cardiovascular events in older patients with hypertension.
Cardiovascular diseases (CVDs), and principally ischemic heart disease and stroke, remain the leading cause of mortality globally and contribute to the rising costs of healthcare.1,2 Hypertension is the leading modifiable risk factor involved in the global burden of CVD.3–6 Between 1990 and 2019, the number of people worldwide with hypertension doubled, leading to a heavy disease burden, and hypertension poses a serious threat to life.7 The Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial showed that intensive treatment, with a systolic blood pressure (SBP) target of 110–129 mmHg, is associated with a lower incidence of cardiovascular events than standard treatment, with a target of 130–149 mmHg.8
High heart rate is an independent risk factor for cardiovascular events and mortality, and is closely associated with hypertension. High heart rate is a common clinical phenotype of hypertension. Most studies define a resting heart rate (RHR) >80 beats/min as an increased heart rate. In a large French population, untreated hypertensive patients had an approximately 6-beats/min faster heart rate than normotensive individuals, and the proportion of patients with a heart rate ≥85 beats/min was significantly higher in the hypertensive group than among those with normal blood pressure.9 According to the Tensiopulse study, which evaluated 38,145 patients cared for by 2,000 general practitioners across Italy, more than 30% of hypertensive patients had an RHR ≥80 beats/min.10 Previous studies have shown that a high RHR is significantly associated with CVD in hypertensive populations.11,12 Furthermore, a prospective cohort study showed that high RHR appears to be an independent determinant of all-cause mortality and cardiovascular events in older (age ≥60 years) but not younger (age <60 years) individuals.13 A post hoc analysis of data from the Systolic Blood Pressure Intervention Trial (SPRINT) also showed that high RHR is a key cardiovascular risk factor in older patients with hypertension, independent of any reduction in SBP.14 However, the heart rates of participants were measured at single time points in most of these studies, and may have been influenced by various factors, such as the external environment, emotion, body temperature, and disease status. The concept of cumulative exposure has been proposed as a more valid index.15–18 However, little is known about the influence of cumulative RHR (cumRHR) exposure on the incidence of cardiovascular events and all-cause mortality in older (age ≥60 years) patients with hypertension.
To assess the influence of cumRHR exposure on the risks of cardiovascular events and all-cause mortality, we performed a post hoc analysis of the STEP trial, using RHR measurements at 5 time points.
Methods
Study Design and Participants
We performed a post hoc analysis of data from the STEP clinical trial (NCT03015311). Detailed information on the design, rationale, and primary outcomes of the STEP trial has been published previously.8,19 Briefly, the STEP trial was an open-label multicenter randomized controlled trial that compared the effects of intensive (SBP target 110–129 mmHg) vs. standard (SBP target 130–149 mmHg) SBP treatment on the cardiovascular outcomes of 8,511 patients with hypertension who attended 42 clinical centers in China. The present study was approved by the Ethics Committee of Fuwai Hospital, as well as the ethics committees of all collaborating centers. All participants provided written informed consent. The procedures followed were in accordance with the Declaration of Helsinki and the ethical standards of the responsible committee on human experimentation (institutional or regional).
Eligible patients were required to be aged 60–80 years and to have essential hypertension, defined as an SBP of 140–190 mmHg or the administration of antihypertensive medication. Patients with a history of ischemic or hemorrhagic stroke were excluded. Furthermore, for the present post hoc analysis, patients with missing RHR data at any of the 0-, 3-, 6-, 9-, or 12-month follow-up examinations (n=593), those who used a β-blocker (n=322), and those who experienced a primary outcome event or died from any cause before their 12-month visit (n=79) were excluded. After the application of these criteria, data from 7,517 participants were included in the trial (Figure 1).
Data Collection and Definitions
The sociodemographic characteristics of each participant were collected at baseline by trained STEP physicians and included age, sex, body mass index (BMI), smoking status (never, ever, or current), alcohol consumption (never, ever, or current), and use of antihypertensive medication. The 10-year risk of CVD was estimated using the Framingham risk score.20 Current smokers were defined as those who had smoked at least 1 cigarette per day for more than the preceding 6 months. Current alcohol drinkers were defined as those who had consumed alcohol at least once per month for more than the preceding 6 months. BMI was calculated by dividing body mass (kg) by the height squares (m2).
Clinical information, including office blood pressure and heart rate, was collected at baseline and every 3 months during the follow-up period. At each visit, office blood pressure and heart rate were measured using the same validated automatic BP monitor device (Omron HBP-1100U; Omron Healthcare, Kyoto, Japan). Sitting brachial blood pressure and heart rate were measured in the upper right arm using an appropriately sized cuff after at least 5 min rest and calculated as the average of 3 readings obtained at 1-min intervals by a trained trial staff member. Laboratory examinations, including measurements of circulating uric acid, creatinine, fasting glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (LDL-C) concentrations, were performed at baseline and annually thereafter.
Calculation of cumRHR
To calculate cumRHR, we used data from 5 follow-up visits, namely those at 0, 3, 6, 9, and 12 months. cumRHR was calculated as the weighted sum of the mean RHR value for each visit as follows:
cumRHR = ([RHR1 + RHR2] / 2 × time1–2) + ([RHR2 + RHR3] / 2 × time2–3) + ([RHR3 + RHR4] / 2 × time3–4) + ([RHR4 + RHR5] / 2 × time4–5)
where RHR1, RHR2, RHR3, RHR4, and RHR5 are the RHRs at the first, second, third, fourth, and fifth examinations, respectively, and time1–2, time2–3, time3–4, and time4–5 represent the time intervals between consecutive RHR measurements (0.25 years).21 As in previous studies,22 participants were stratified into cumRHR quartiles: Q1, Q2, Q3, or Q4. The cumRHR for Q1–Q4 was 44.50–68.44, 68.50–72.12, 72.19–75.88, and 75.94–109.44 (beats/min) × year, respectively. Q3 was used as the reference group.
Outcomes
The primary outcome was a composite of stroke (ischemic or hemorrhagic), acute coronary syndrome (acute myocardial infarction or hospitalization for unstable angina), acute decompensated heart failure, coronary revascularization, atrial fibrillation, and death from any cardiovascular cause (cardiovascular death includes fatal coronary artery disease (CAD), fatal stroke, death from heart failure, and sudden cardiac death). This was the same as that used in the STEP trial.8 The secondary outcomes were major adverse cardiovascular events (MACE), stroke, and all-cause mortality. MACE was defined as a composite of all the components of the primary outcome except stroke. For the present analysis, we included all the outcome events known to the authors up to December 31, 2020.
Statistical Analysis
Continuous data are presented as the mean±SD and categorical data are presented as numbers and percentages. The baseline characteristics of participants were compared across baseline cumRHR groups using one-way analysis of variance for continuous data and the Chi-squared test for categorical data.
A univariate Cox regression model was used to analyze the relationship between baseline heart rate and outcome events. We used the cause-specific Cox model to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for the primary and secondary outcomes. The proportional hazards assumption was tested using Schoenfeld residuals, and no violations were identified. Model 1 was unadjusted. Model 2 was adjusted for the clinical center, age, sex, baseline SBP, baseline diastolic blood pressure, baseline BMI, baseline fasting blood glucose concentration, baseline triglyceride concentration, baseline LDL-C, baseline estimated glomerular filtration rate, history of arteriosclerotic CVD at baseline, smoking status, and alcohol consumption status. Model 3 was adjusted for all variables in Model 2 and further adjusted for baseline heart rate. Cumulative incidence curves were constructed to compare the incidence of outcomes among the 4 groups. The data were also analyzed after stratification with respect to age (60–69 vs. 70–80 years), sex (male vs. female), and SBP target (intensive treatment vs. standard treatment), and their interactions were tested. To test the robustness of our findings, the following sensitivity analyses were performed: (1) imputing missing RHR data at follow-up; (2) including those patients using β-blocker in the analysis; and (3) using the Fine–Gray subdistribution model. The restricted cubic spline method with 3 knots was used to analyze non-linear relationships between cumRHR and outcome events.
Data were analyzed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P<0.05 was considered statistically significant. Multiple imputation methods were used to replace missing data.
Results
Baseline Characteristics of Participants
Of the 7,517 participants (mean age 66.19 years, 53.48% female) included in the study, 1,875 were assigned to Q1, 1,854 were assigned to Q2, 1,905 were assigned to Q3, and 1,883 were assigned to Q4. The baseline characteristics of the participants stratified according to cumRHR are presented in Table 1. Compared with other groups, the participants in cumRHR Q4 were more likely to be younger and male and had higher baseline BMI, SBP, diastolic blood pressure, fasting blood glucose, triglyceride, total cholesterol, LDL-C, and uric acid. Moreover, Q4 had a higher proportion of participants with a history of smoking, drinking, and diabetes, and those in Q4 had a high 10-year risk of CVD (estimated using a Framingham risk score ≥15%). There were no significant differences in the number of antihypertensive agents used or in the number of individuals not taking antihypertensive medication among the 4 groups.
Table 1.
Baseline Patient Demographics and Clinical Characteristics According to Cumulative RHR Quartile
| |
Q1 |
Q2 |
Q3 |
Q4 |
P value |
| No. patients |
1,875 |
1,854 |
1,905 |
1,883 |
|
| Age (years) |
66.61±4.94 |
66.36±4.85 |
66.02±4.80 |
65.77±4.61 |
<0.001 |
| Age 70–80 years |
510 (27.20) |
451 (24.33) |
424 (22.26) |
395 (20.98) |
<0.001 |
| Male sex |
862 (45.97) |
835 (45.04) |
850 (44.62) |
950 (50.45) |
<0.001 |
| Body mass index (kg/m2) |
25.41±3.12 |
25.56±3.10 |
25.50±3.19 |
25.81±3.18 |
<0.001 |
| Heart rate (beats/min) |
67.59±9.20 |
72.26±8.86 |
74.62±8.83 |
79.76±10.49 |
<0.001 |
| Blood pressure |
| Systolic (mmHg) |
145.57±16.57 |
146.50±16.91 |
146.49±16.70 |
146.73±16.59 |
0.04 |
| Diastolic (mmHg) |
80.77±10.80 |
82.29±10.59 |
83.20±10.28 |
84.36±10.50 |
<0.001 |
| Smoking status |
|
|
|
|
<0.001 |
| Never |
1,394 (74.35) |
1,366 (73.68) |
1,375 (72.18) |
1,282 (68.08) |
|
| Former |
255 (13.60) |
209 (11.27) |
201 (10.55) |
232 (12.32) |
|
| Current |
226 (12.05) |
279 (15.05) |
329 (17.27) |
369 (19.60) |
|
| Drinking status |
|
|
|
|
0.02 |
| Never |
1,294 (69.20) |
1,261 (68.13) |
1,338 (70.53) |
1,230 (65.46) |
|
| Former |
107 (5.72) |
88 (4.75) |
84 (4.43) |
103 (5.48) |
|
| Current |
469 (25.08) |
502 (27.12) |
475 (25.04) |
546 (29.06) |
|
| Fasting blood glucose (mmol/L) |
5.94±1.51 |
6.00±1.69 |
6.13±1.77 |
6.21±1.65 |
<0.001 |
| Lipid profile |
| TC (mmol/L) |
4.80±1.09 |
4.91±1.06 |
4.89±1.07 |
4.97±1.10 |
<0.001 |
| TG (mmol/L) |
1.46±0.94 |
1.55±0.96 |
1.64±1.16 |
1.71±1.18 |
<0.001 |
| HDL-C (mmol/L) |
1.27±0.32 |
1.26±0.31 |
1.26±0.31 |
1.25±0.30 |
0.09 |
| LDL-C (mmol/L) |
2.64±0.88 |
2.72±0.86 |
2.66±0.86 |
2.72±0.90 |
0.047 |
| UA (mmol/L) |
343.93±87.34 |
347.99±91.44 |
345.87±89.29 |
353.81±90.58 |
0.006 |
| Serum Cr (μmol/L) |
73.85±18.35 |
73.01±17.95 |
72.09±17.55 |
74.03±18.25 |
0.80 |
| eGFR (mL/min/1.73 m2) |
82.96±13.84 |
83.74±13.45 |
84.60±13.42 |
83.96±13.99 |
0.01 |
| eGFR <60 mL/min/1.73 m2 |
31 (1.85) |
29 (1.82) |
16 (0.99) |
34 (2.14) |
0.07 |
| Medical history |
| Diabetes |
326 (17.39) |
339 (18.28) |
340 (17.85) |
391 (20.76) |
0.04 |
| ASCVD |
383 (20.61) |
327 (17.78) |
286 (15.11) |
297 (15.85) |
<0.001 |
| Antihypertensive agents |
| No. antihypertensive agents |
1.39±0.64 |
1.40±0.67 |
1.41±0.64 |
1.40±0.63 |
0.56 |
| Not using antihypertensive agents |
87 (4.64) |
105 (5.66) |
87 (4.57) |
88 (4.67) |
0.35 |
| Framingham risk score ≥15 |
1,262 (75.57) |
1,242 (77.87) |
1,241 (77.03) |
1,272 (80.20) |
0.01 |
Unless indicated otherwise, data are give as n (%) or mean±SD. ASCVD, arteriosclerotic cardiovascular disease (acute coronary syndrome, stable coronary artery disease, revascularization, ischemic cardiomyopathy, ischemic stroke, transient ischemic attack, and peripheral atherosclerotic diseases); Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RHR, resting heart rate; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Baseline characteristics of STEP participants were also compared between those included in and excluded from this analysis (Supplementary Table 1). Most characteristics were similar between the 2 groups, with the exception of age, blood pressure, antihypertensive medications, and diabetes, which may be related to the exclusion of patients using β-blockers from our analysis.
Characteristics of RHR Between Baseline and Follow-up
The mean baseline RHR of participants was 73.58 beats/min. The mean RHR during the first 3 months (0-, 1- and 2-month follow-up) was 72.79 beats/min, that during the first year (3-, 6-, 9-, and 12-month follow-up) was 72.20 beats/min, that during the second year (15-, 18-, 21-, and 24-month follow-up) was 72.24 beats/min, and that during the third year (27-, 30-, 33-, and 36-month follow-up) was 71.72 beats/min. Univariate Cox proportional hazard regression showed that baseline heart rate was significantly associated with stroke (P<0.05; Supplementary Table 2), but not with the primary outcome, MACE, and all-cause mortality.
Association of cumRHR With Outcomes
During a median follow-up of 3.33 years, there were 206 primary outcome events, 152 MACE, 63 strokes, and 87 deaths from any cause, and the absolute risks for these outcomes were 27.40%, 20.22%, 0.84% and 1.16%, respectively. Associations between cumRHR and outcomes are summarized in Table 2. In unadjusted models, and compared with Q3, the risk of the primary outcome was significantly higher for Q1 (HR 1.76; 95% CI 1.15–2.69; P=0.009) and Q4 (HR 2.20; 95% CI 1.46–3.30; P<0.001). Similar trends were observed for MACE (HR 1.69 [95% CI 1.06–2.70; P=0.03] and HR 1.89 [95% CI 1.19–2.99; P=0.007] for Q1 and Q4, respectively), but only Q4 had a higher risk of stroke relative to Q3 (HR 3.64; 95% CI 1.58–8.42; P=0.003). After adjustment for multiple potential confounders, participants in Q4 were found to be at significantly higher risk of the primary outcome (HR 2.24; 95% CI 1.45–3.45; P<0.001), MACE (HR 1.89; 95% CI 1.17–3.08; P=0.01), and stroke (HR 3.79, 95% CI 1.54–9.33, P=0.004) than those in Q3. Participants in Q1 were also at a higher risk of the primary outcome relative to those in Q3 (HR 1.68, 95% CI 1.07–2.63, P=0.02). These associations persisted after an additional adjustment for baseline heart rate based on Model 2 (P<0.05). Similar trends were not observed for all-cause mortality (Table 2).
Table 2.
Association Between Cumulative RHR and Outcomes
| |
Q1 (n=1,875) |
Q2 (n=1,854) |
Q3 (n=1,905) |
Q4 (n=1,883) |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
HR (95% CI) |
HR (95% CI) |
P value |
| Primary outcome |
| Model 1 |
1.76 (1.15–2.69) |
0.009 |
1.25 (0.79–1.97) |
0.34 |
1 (Ref.) |
2.20 (1.46–3.30) |
<0.001 |
| Model 2 |
1.68 (1.07–2.63) |
0.02 |
1.24 (0.77–2.00) |
0.38 |
1 (Ref.) |
2.24 (1.45–3.45) |
<0.001 |
| Model 3 |
1.71 (1.08–2.71) |
0.02 |
1.25 (0.77–2.02) |
0.37 |
1 (Ref.) |
2.21 (1.42–3.43) |
<0.001 |
| MACE |
| Model 1 |
1.69 (1.06–2.70) |
0.03 |
0.96 (0.56–1.63) |
0.87 |
1 (Ref.) |
1.89 (1.19–2.99) |
0.007 |
| Model 2 |
1.53 (0.93–2.51) |
0.10 |
0.98 (0.57–1.70) |
0.95 |
1 (Ref.) |
1.89 (1.17–3.08) |
0.01 |
| Model 3 |
1.49 (0.89–2.48) |
0.13 |
0.98 (0.56–1.69) |
0.92 |
1 (Ref.) |
1.93 (1.18–3.16) |
0.009 |
| Stroke |
| Model 1 |
1.90 (0.76–4.76) |
0.17 |
2.67 (1.11–6.39) |
0.03 |
1 (Ref.) |
3.64 (1.58–8.42) |
0.003 |
| Model 2 |
2.04 (0.76–5.46) |
0.16 |
2.59 (1.00–6.69) |
0.05 |
1 (Ref.) |
3.79 (1.54–9.33) |
0.004 |
| Model 3 |
2.21 (0.81–5.99) |
0.12 |
2.65 (1.03–6.86) |
0.04 |
1 (Ref.) |
3.55 (1.42–8.86) |
0.007 |
| All-cause mortality |
| Model 1 |
0.98 (0.55–1.76) |
0.94 |
0.90 (0.49–1.64) |
0.73 |
1 (Ref.) |
0.92 (0.51–1.66) |
0.78 |
| Model 2 |
0.95 (0.49–1.84) |
0.87 |
1.03 (0.53–2.00) |
0.93 |
1 (Ref.) |
0.93 (0.47–1.85) |
0.84 |
| Model 3 |
0.93 (0.46–1.85) |
0.83 |
1.02 (0.52–1.99) |
0.96 |
1 (Ref.) |
0.94 (0.47–1.89) |
0.87 |
Participants were divided into quartiles (Q1–Q4) based on cumulative RHR. Model 1 was unadjusted. Model 2 was adjusted for clinical center, age, sex, baseline systolic blood pressure (SBP), baseline diastolic blood pressure, baseline body mass index, baseline fasting blood glucose concentration, baseline TG concentration, baseline LDL-C concentration, baseline eGFR, history of arteriosclerotic cardiovascular disease at baseline, smoking status, and alcohol consumption status. Model 3 was adjusted for variables in Model 2 and further adjusted for baseline heart rate. The primary outcome was a composite of stroke, acute coronary syndrome, acute decompensated heart failure, coronary revascularization, atrial fibrillation, or death from cardiovascular causes. Major adverse cardiovascular events (MACE) were a composite of the individual components of the primary outcome except for stroke. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
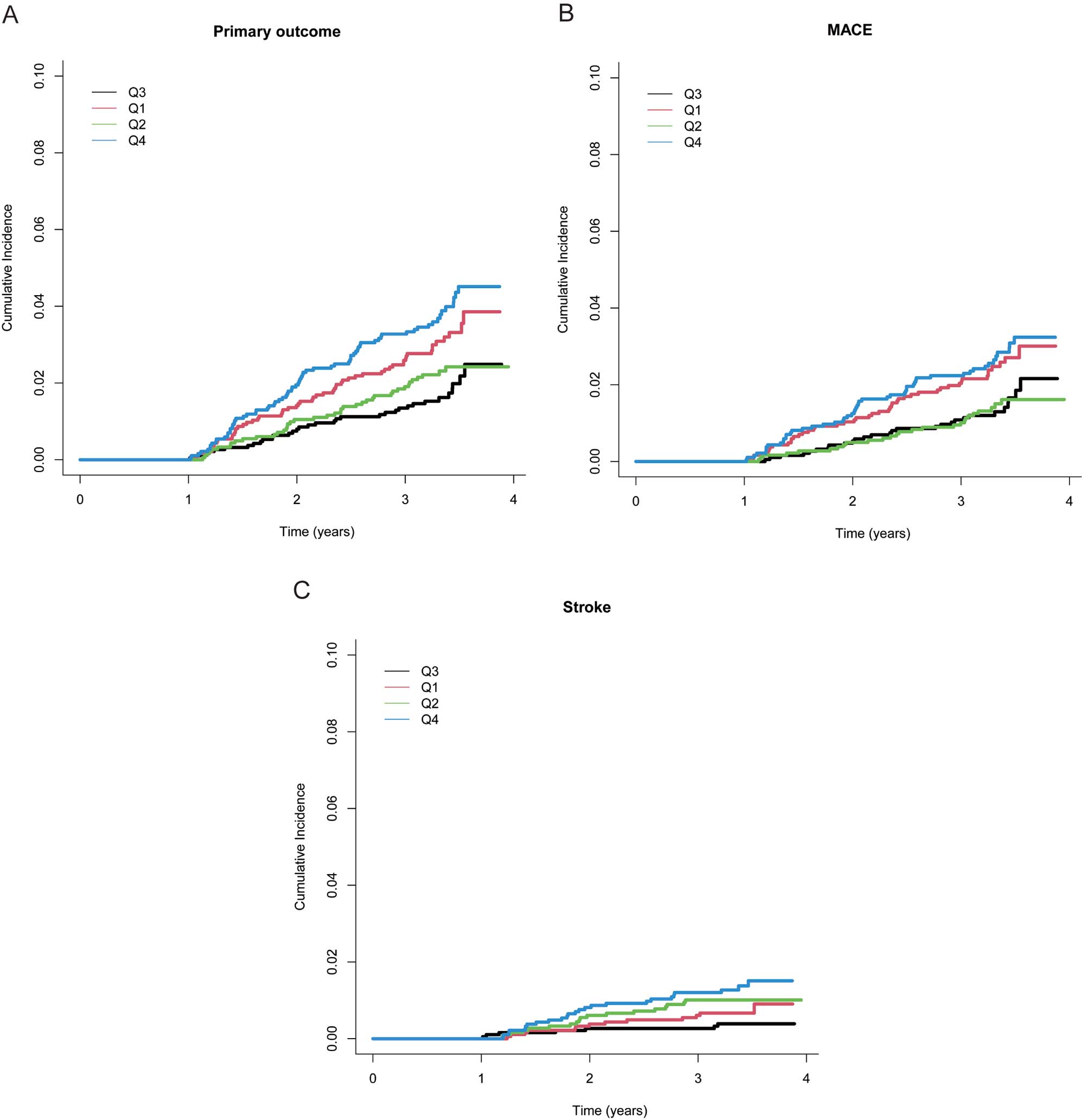
Cumulative incidence curves for cumRHR are shown in Figure 2. The restricted cubic spline curve showed that cumRHR had a non-linear relationship with the incidence of the primary outcome (Figure 3). The lowest risk of the primary outcome was present when the cumRHR was 72 (beats/min) × year.
Subgroup Analyses and Sensitivity Analyses
To further explore the relationship between cumRHR and the outcome events, we conducted subgroup analyses according to SBP target, age, and sex (Table 3). The results of these analyses were similar to those of the primary analysis, suggesting that intensive treatment did not adversely affect these patients. After adjustment for confounding factors, the risk of the primary outcome was significantly higher in the standard-treatment group, among those aged 60–69 years, and females in the Q4 group (Table 3). Compared with Q3, participants in the Q4 group who were aged 60–69 years or female had a higher risk of MACE. Participants who received standard treatment or were female in Q4 had a higher risk of stroke compared with those in Q3. Similar results were obtained in sensitivity analyses in which missing RHR data at follow-up were imputed, patients using β-blockers were included, or the Fine–Gray subdistribution model was used (Table 4).
Table 3.
Subgroup Analyses of the Relationships Between Cumulative RHR and Outcomes
| |
Q1 |
Q2 |
Q3 |
Q4 |
P for
interaction |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
HR (95% CI) |
HR (95% CI) |
P value |
| Primary outcome |
| TreatmentA |
| Intensive (n=78) |
2.53 (1.14–5.61) |
0.02 |
2.24 (1.01–4.97) |
0.05 |
1 (Ref.) |
3.19 (1.48–6.85) |
0.003 |
0.33 |
| Standard (n=121) |
1.43 (0.81–2.54) |
0.21 |
0.85 (0.46–1.59) |
0.61 |
1 (Ref.) |
1.87 (1.09–3.20) |
0.02 |
| Age |
60–69 years
(n=132) |
1.60 (0.90–2.85) |
0.11 |
1.18 (0.65–2.14) |
0.59 |
1 (Ref.) |
2.30 (1.34–3.94) |
0.002 |
0.99 |
70–80 years
(n=74) |
2.02 (0.93–4.40) |
0.08 |
1.44 (0.63–3.28) |
0.39 |
1 (Ref.) |
2.09 (0.98–4.49) |
0.06 |
| Sex |
| Male (n=110) |
1.25 (0.68–2.31) |
0.47 |
0.95 (0.50–1.82) |
0.87 |
1 (Ref.) |
1.77 (1.01–3.13) |
0.048 |
0.52 |
| Female (n=96) |
2.48 (1.21–5.09) |
0.01 |
1.80 (0.86–3.78) |
0.12 |
1 (Ref.) |
2.99 (1.48–6.03) |
0.002 |
| MACE |
| TreatmentA |
| Intensive (n=63) |
2.64 (1.08–6.44) |
0.03 |
1.59 (0.61–4.12) |
0.34 |
1 (Ref.) |
3.08 (1.29–7.34) |
0.01 |
0.47 |
| Standard (n=89) |
1.11 (0.59–2.11) |
0.74 |
0.74 (0.37–1.48) |
0.40 |
1 (Ref.) |
1.53 (0.83–2.82) |
0.17 |
| Age |
60–69 years
(n=98) |
1.13 (0.60–2.13) |
0.70 |
0.75 (0.37–1.49) |
0.41 |
1 (Ref.) |
2.02 (1.13–3.63) |
0.02 |
0.82 |
70–80 years
(n=54) |
2.65 (1.06–6.63) |
0.04 |
1.74 (0.66–4.58) |
0.26 |
1 (Ref.) |
1.89 (0.74–4.78) |
0.18 |
| Sex |
| Male (n=81) |
1.03 (0.52–2.06) |
0.92 |
0.77 (0.37–1.60) |
0.48 |
1 (Ref.) |
1.49 (0.79–2.83) |
0.22 |
0.45 |
| Female (n=71) |
2.29 (1.03–5.09) |
0.04 |
1.34 (0.57–3.17) |
0.50 |
1 (Ref.) |
2.74 (1.25–6.02) |
0.01 |
| Stroke |
| TreatmentA |
| Intensive (n=23) |
1.68 (0.27–10.45) |
0.58 |
4.58 (0.96–21.73) |
0.06 |
1 (Ref.) |
3.98 (0.80–19.73) |
0.09 |
0.42 |
| Standard (n=40) |
2.52 (0.76–8.44) |
0.13 |
1.81 (0.53–6.23) |
0.35 |
1 (Ref.) |
3.53 (1.15–10.82) |
0.03 |
| Age |
60–69 years
(n=40) |
3.72 (0.97–14.31) |
0.06 |
3.99 (1.11–14.36) |
0.03 |
1 (Ref.) |
4.04 (1.13–14.53) |
0.03 |
0.43 |
70–80 years
(n=23) |
0.93 (0.20–4.36) |
0.93 |
1.25 (0.28–5.66) |
0.77 |
1 (Ref.) |
3.35 (0.88–12.66) |
0.07 |
| Sex |
| Male (n=34) |
1.86 (0.53–6.58) |
0.33 |
1.62 (0.46–5.80) |
0.45 |
1 (Ref.) |
2.86 (0.90–9.02) |
0.07 |
0.72 |
| Female (n=29) |
2.96 (0.56–15.72) |
0.20 |
4.80 (1.03–22.78) |
0.046 |
1 (Ref.) |
4.89 (1.05–22.78) |
0.04 |
| All-cause mortality |
| TreatmentA |
| Intensive (n=46) |
1.19 (0.47–3.00) |
0.72 |
1.24 (0.51–3.03) |
0.63 |
1 (Ref.) |
0.82 (0.31–2.18) |
0.69 |
0.90 |
| Standard (n=41) |
0.63 (0.22–1.81) |
0.39 |
0.76 (0.27–2.13) |
0.6 |
1 (Ref.) |
1.13 (0.41–3.11) |
0.82 |
| Age |
60–69 years
(n=46) |
0.39 (0.13–1.19) |
0.10 |
0.98 (0.42–2.28) |
0.96 |
1 (Ref.) |
0.97 (0.40–2.32) |
0.94 |
0.16 |
70–80 years
(n=41) |
1.83 (0.65–5.17) |
0.26 |
1.16 (0.38–3.56) |
0.79 |
1 (Ref.) |
0.85 (0.26–2.76) |
0.79 |
| Sex |
| Male (n=49) |
0.86 (0.36–2.09) |
0.75 |
1.13 (0.49–2.59) |
0.78 |
1 (Ref.) |
0.62 (0.24–1.57) |
0.31 |
0.56 |
| Female (n=38) |
0.94 (0.31–2.75) |
0.92 |
0.88 (0.28–2.75) |
0.82 |
1 (Ref.) |
1.60 (0.54–4.68) |
0.39 |
Participants were divided into quartiles (Q1–Q4) based on cumulative RHR. AIntensive treatment had a SBP target of 110–129 mmHg; standard treatment had an blood pressure target of 130–149 mmHg. Abbreviations as in Tables 1,2.
Table 4.
Sensitivity Analyses of the Relationship Between Cumulative RHR and the Primary Outcome
| |
Q1 |
Q2 |
Q3 |
Q4 |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
HR (95% CI) |
HR (95% CI) |
P value |
| Analysis 1 |
| Model 1 |
1.76 (1.15–2.69) |
0.009 |
1.25 (0.79–1.97) |
0.34 |
1 (Ref.) |
2.20 (1.46–3.30) |
<0.001 |
| Model 2 |
1.68 (1.07–2.63) |
0.02 |
1.24 (0.77–2.00) |
0.38 |
1 (Ref.) |
2.24 (1.45–3.45) |
<0.001 |
| Model 3 |
1.71 (1.08–2.71) |
0.02 |
1.25 (0.77–2.01) |
0.37 |
1 (Ref.) |
2.21 (1.42–3.43) |
<0.001 |
| Analysis 2 |
| Model 1 |
1.66 (1.09–2.51) |
0.02 |
1.24 (0.80–1.92) |
0.34 |
1 (Ref.) |
2.16 (1.46–3.21) |
<0.001 |
| Model 2 |
1.63 (1.05–2.55) |
0.03 |
1.26 (0.79–2.02) |
0.33 |
1 (Ref.) |
2.22 (1.45–3.40) |
<0.001 |
| Model 3 |
1.66 (1.05–2.62) |
0.03 |
1.27 (0.79–2.03) |
0.32 |
1 (Ref.) |
2.19 (1.42–3.38) |
<0.001 |
| Analysis 3 |
| Model 1 |
1.75 (1.15–2.66) |
0.009 |
1.23 (0.78–1.94) |
0.37 |
1 (Ref.) |
2.16 (1.44–3.23) |
<0.001 |
| Model 2 |
1.62 (1.03–2.53) |
0.03 |
1.21 (0.74–1.97) |
0.44 |
1 (Ref.) |
2.22 (1.44–3.42) |
<0.001 |
| Model 3 |
1.65 (1.05–2.61) |
0.03 |
1.22 (0.75–1.99) |
0.43 |
1 (Ref.) |
2.19 (1.41–3.42) |
<0.001 |
Participants were divided into quartiles (Q1–Q4) based on cumulative RHR. Analysis 1, imputing missing RHR data at follow-up; Analysis 2, including those using β-blockers; Analysis 3, using the Fine-Gray subdistribution model. Model 1 was unadjusted. Model 2 was adjusted for the clinical center, age, sex, baseline SBP, baseline diastolic blood pressure, baseline body mass index, baseline fasting blood glucose concentration, baseline TG concentration, baseline LDL-C concentration, baseline eGFR, history of arteriosclerotic cardiovascular disease at baseline, smoking status, and alcohol consumption status. Model 3 was adjusted for variables in Model 2 and further adjusted for baseline heart rate. The primary outcome was a composite of stroke, acute coronary syndrome, acute decompensated heart failure, coronary revascularization, atrial fibrillation, or death from cardiovascular causes. Abbreviations as in Tables 1,2.
Discussion
We have shown that in older (age ≥60 years) individuals with hypertension, compared with those with a cumRHR of 72.19–75.88 (beats/min) × year (Q3), those with very high cumRHR levels (75.94–109.44 [beats/min] × year; Q4) had a higher risk of the primary outcome, MACE, and stroke, and those with very low cumRHR levels (44.50–68.44 [beats/min] × year; Q1) also had a higher risk of the primary outcome. Similar trends were not observed for all-cause mortality. In addition, we identified a U-shaped non-linear relationship between cumRHR and the incidence of cardiovascular events, with the lowest risk being present at a cumRHR of 72 (beats/min) × year. High cumRHR was found to be a long-term predictor of cardiovascular events in older patients with hypertension.
High heart rate is a common clinical manifestation of hypertension. In recent years, the management of heart rate in patients with hypertension and high heart rate have been of significant clinical interest. The European Society of Cardiology/European Society of Hypertension guidelines state that an RHR >80 beats/min should be used as a predictor of cardiovascular risk,23 and many studies have suggested that keeping the RHR <60 beats/min may be beneficial.10 Recent evidence suggests that a high heart rate is an important risk factor for adverse cardiovascular outcomes. In the International Verapamil-SR/Trandolapril Study (INVEST) trial of patients with CAD and hypertension, high baseline and follow-up RHRs were associated with a high risk of adverse outcomes, with a linear relationship with respect to baseline RHR and a J-shaped relationship with respect to follow-up RHR; specifically, the risk of MACE increased by 6% for every 5-beat/min increase in RHR.24 In patients with hypertension and left ventricular hypertrophy, the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE) study showed that every 10-beat/min increase in HR was associated with a 16% higher adjusted risk of cardiovascular mortality, and the persistence or development of a heart rate ≥84 beats/min was associated with a 55% greater risk of cardiovascular-related death.25 In the Glasgow Clinic study, the development or progression of tachycardia during follow-up was associated with a higher incidence of adverse events in hypertensive individuals.26 However, the optimal target range for cumRHR in older patients with hypertension is not yet clear. High cumRHR was also found to increase CVD risk in the present study, consistent with the results of previous studies.22 This finding may be the result of sympathetic nervous system activation, peripheral vascular resistance, and endothelial cell dysfunction.27–29
Our findings indicate that participants in the intensive treatment group still had a higher risk of the primary outcome with elevated cumRHR compared with Q3. In the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial of patients with hypertension, the highest heart rate quintile experienced cardiac events 73% sooner than the lowest heart rate quintile, and this was not affected by intensive blood pressure control.12 Similar conclusions were drawn in SPRINT trial.14 These results also emphasize that elevated RHR helps identify individuals at higher risk, even when blood pressure is well controlled. The well-known Systolic Heart Failure Treatment with the If inhibitor ivabradine Trial (SHIFT) showed that the use of ivabradine to lower heart rate can significantly improve the prognosis of patients with chronic heart failure and in sinus rhythm with a heart rate of ≥70 beats/min.30 A study found that heart rate ≥75 beats/min was independently associated with major adverse cardiac and cerebral events in patients with CAD.31 The morBidity-mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left-ventricULar dysfunction (BEAUTIFUL) trial enrolled patients with CAD and left ventricular dysfunction and showed that lowering heart rate with ivabradine reduced the incidence of CAD outcomes in a subgroup of patients with heart rate ≥70 beats/min.32 On the basis of the results of these studies, it is reasonable to believe that lowering heart rate can improve prognosis. Therefore, long-term RHR levels should be taken more seriously in the evaluation of CVD risk in each hypertensive patient.
Importantly, we found that very low cumRHR was also associated with higher CVD risk, indicating a U-shaped relationship between them. This finding is supported by some previous studies. In a study of Chinese patients with hypertension, a heart rate of <65 beats/min was found to be associated with a higher incidence of MACE.33 The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trials found that low mean heart rate (<60 beats/min) was independently associated with incident atrial fibrillation, and this was particularly evident in subjects who were not receiving treatment with β-blockers.34 These findings differ from those of previous studies,35 which may be explained by differences in the patient populations studied. A lower heart rate may reflect underlying pathophysiological changes, such as conduction system disorders. A slow heart rate leads to atrial repolarization dispersion, which can trigger cardiovascular events, such as atrial fibrillation.36 Moreover, bradycardia reflects lower cardiac function and sympathetic nerve tone, which may lead to lower cardiac output, inadequate perfusion of target organs, and less ability to respond to stress, thereby predisposing towards cardiovascular events. This suggests that we should keep the cumRHR in an appropriate range (72.19–75.88 [beats/min]×year) when treating hypertension in older adults. The targeting of this range of cumRHR may help reduce the risk of cardiovascular events.
Many previous studies have shown that RHR is associated with all-cause mortality,10 but we did not make a similar finding in the present study. This may be because the “older adults” included in our study were younger on average, had lower blood pressure at baseline and during follow-up, and had less underlying disease than those included in the Systolic Hypertension in Europe (Syst-Eur) study.37 In subgroup analyses, we found that both very high and very low cumRHR were closely related to cardiovascular outcomes in women. Continuous monitoring and management of heart rate may potentially improve the outcomes of hypertensive patients, especially in women and those aged 60–69 years.
We excluded patients who were using β-blockers from this study. Beta-blockers are widely used by patients with hypertension to control heart rate, but were not routinely used by participants in the STEP trial. Older (age ≥60 years) patients show large variations in heart rate, and previous studies have not provided sufficient evidence that the use of β-blockers and the resulting reduction in RHR significantly improve prognosis.38 In The Losartan Intervention For Endpoint reduction (LIFE) study, the use of atenolol reduced RHR more effectively than losartan in patients with similar blood pressures, but was associated with a higher mortality rate.39 In addition, in the INternational VErapamil-SR/trandolapril STudy (INVEST) and the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), the use of atenolol was not associated with a superior outcome.24,40 A meta-analysis of the efficacy of β-blockers on mortality and cardiovascular outcomes in hypertensive patients found that metoprolol, propranolol, and oxprenolol significantly reduce the risk of cardiovascular mortality compared with atenolol.41 However, there is a lack of evidence regarding the most appropriate doses of these drugs for use in older patients. Furthermore, older people often have comorbid chronic diseases, such as chronic obstructive pulmonary disease or atrioventricular blockade, and the use of β-blockers may have other adverse effects. Therefore, excluding patients using heart rate control drugs from this study helped us identify the effects of RHR on the physiological state of older people. Certainly, a more thorough understanding of heart rate control, achieved through further high-quality studies, would help in designing appropriate treatment strategies for older patients with hypertension.
To the best of our knowledge, this is the first study to characterize the relationships of cumRHR with cardiovascular events and all-cause mortality in older patients with hypertension. The cumulative exposure index used in the present study reflects the RHR of participants over a period of time and the cumulative effect of RHR. This reduces the limitation of measurement error and we predict that it will become a new way of evaluating heart rate.
This study also has several limitations. First, the median follow-up period of this post hoc study was 3.33 years, which may not be sufficient to capture all endpoint events. Second, although we adjusted for potential risk factors for cardiovascular events and all-cause death, other unmeasured or residual confounders, such as medication and diseases, are likely to have affected the study results. Finally, our findings may not be directly generalizable to individuals from other race or ethnicity cohorts, because the STEP trial participants were all Han Chinese.
In conclusion, both low and high cumRHR levels were associated with a higher risk of cardiovascular events in older patients with hypertension. Thus, the monitoring and maintenance of an appropriate RHR may help with the treatment and prognosis of hypertension. In the future, more rigorous prospective randomized clinical trials and foundational research should be conducted to further explore the relationships of cumRHR with cardiovascular events and all-cause mortality, which may provide more evidence regarding the optimal management of heart rate in patients with hypertension.
Acknowledgments
The authors thank all staff members of the STEP team for their contribution.
Sources of Funding
This work was supported by the Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province [2019]113–55 (No. 190826155555147) and the Shantou Medical Health Science and Technology Plan [2020]66 (No. ST2020027).
Disclosures
The authors declare that they have no conflicts of interest.
IRB Information
This study was approved by the Ethics Committee of Fuwai Hospital (Reference no. 2016-838).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-24-0690
References
- 1.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol 2020; 76: 2982–3021.
- 2.
Wei D, Xiao W, Zhou L, Guo J, Lu W, Wang Y. Age-period-cohort analysis of ischemic heart disease morbidity and mortality in China, 1990–2019. Circ J 2022; 86: 1437–1443.
- 3.
Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1223–1249.
- 4.
Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks. J Am Coll Cardiol 2020; 76: 2980–2981.
- 5.
Kubo K, Hirata A, Kadota A, Harada A, Nakamura Y, Hayakawa T, et al. Risk factors for heart failure and coronary artery disease mortality based on the National Vital Statistics during a 25-year follow-up in Japan: NIPPON DATA90. Circ J 2024; 88: 1478–1487.
- 6.
Suzuki Y, Kaneko H, Okada A, Fujiu K, Takeda N, Morita H, et al. BP classification using the 2017 ACC/AHA BP guidelines with risk of cardiovascular events in older individuals. J Cardiol 2024; 84: 394–403.
- 7.
Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398: 957–980.
- 8.
Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med 2021; 385: 1268–1279.
- 9.
Morcet JF, Safar M, Thomas F, Guize L, Benetos A. Associations between heart rate and other risk factors in a large French population. J Hypertens 1999; 17: 1671–1676.
- 10.
Palatini P. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension 2011; 58: 745–750.
- 11.
Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am Heart J 1993; 125: 1148–1154.
- 12.
Julius S, Palatini P, Kjeldsen SE, Zanchetti A, Weber MA, McInnes GT, et al. Usefulness of heart rate to predict cardiac events in treated patients with high-risk systemic hypertension. Am J Cardiol 2012; 109: 685–692.
- 13.
Li K, Yao C, Yang X, Dong L. Effect of resting heart rate on all-cause mortality and cardiovascular events according to age. J Am Geriatr Soc 2017; 65: 989–994.
- 14.
Sobieraj P, Siński M, Lewandowski J. Resting heart rate and cardiovascular outcomes during intensive and standard blood pressure reduction: An analysis from SPRINT trial. J Clin Med 2021; 10: 3264.
- 15.
Jenkins LM, Garner CR, Kurian S, Higgins JP, Parrish TB, Sedaghat S, et al. Cumulative blood pressure exposure, basal ganglia, and thalamic morphology in midlife. Hypertension 2020; 75: 1289–1295.
- 16.
Huang Y, Zhou H, Zhang S, Zhong X, Lin Y, Xiong Z, et al. Mid- to late-life time-averaged cumulative blood pressure and late-life retinal microvasculature: The ARIC study. J Am Heart Assoc 2022; 11: e25226.
- 17.
Tian X, Wang A, Wu S, Zuo Y, Chen S, Zhang L, et al. Cumulative serum uric acid and its time course are associated with risk of myocardial infarction and all-cause mortality. J Am Heart Assoc 2021; 10: e020180.
- 18.
Wang X, Feng B, Huang Z, Cai Z, Yu X, Chen Z, et al. Relationship of cumulative exposure to the triglyceride-glucose index with ischemic stroke: A 9-year prospective study in the Kailuan cohort. Cardiovasc Diabetol 2022; 21: 66.
- 19.
Zhang S, Wu S, Ren J, Chen X, Zhang X, Feng Y, et al. Strategy of blood pressure intervention in the elderly hypertensive patients (STEP): Rational, design, and baseline characteristics for the main trial. Contemp Clin Trials 2020; 89: 105913.
- 20.
D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008; 117: 743–753.
- 21.
Zang EA, Wynder EL. Cumulative tar exposure. A new index for estimating lung cancer risk among cigarette smokers. Cancer 1992; 70: 69–76.
- 22.
Yu J, Dai L, Zhao Q, Liu X, Chen S, Wang A, et al. Association of cumulative exposure to resting heart rate with risk of stroke in general population: The Kailuan cohort study. J Stroke Cerebrovasc Dis 2017; 26: 2501–2509.
- 23.
Mancia G, Rosei EA, Azizi M, Burnier M, Clement DL, Coca A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104.
- 24.
Kolloch R, Legler UF, Champion A, Cooper-DeHoff RM, Handberg E, Zhou Q, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: Findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur Heart J 2007; 29: 1327–1334.
- 25.
Okin PM, Kjeldsen SE, Julius S, Hille DA, Dahlof B, Edelman JM, et al. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertensive patients with electrocardiographic left ventricular hypertrophy. Eur Heart J 2010; 31: 2271–2279.
- 26.
Paul L, Hastie CE, Li WS, Harrow C, Muir S, Connell JMC, et al. Resting heart rate pattern during follow-up and mortality in hypertensive patients. Hypertension 2010; 55: 567–574.
- 27.
Grassi G, Quarti-Trevano F, Seravalle G, Dell’Oro R, Facchetti R, Mancia G. Association between the European Society of Cardiology/European Society of Hypertension heart rate thresholds for cardiovascular risk and neuroadrenergic markers. Hypertension 2020; 76: 577–582.
- 28.
Whelton SP, Blankstein R, Al-Mallah MH, Lima JAC, Bluemke DA, Hundley WG, et al. Association of resting heart rate with carotid and aortic arterial stiffness: Multi-Ethnic Study of Atherosclerosis. Hypertension 2013; 62: 477–484.
- 29.
Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2014; 113: 644–649.
- 30.
Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376: 886–894.
- 31.
Oba Y, Kabutoya T, Kohro T, Imai Y, Kario K, Sato H, et al. Relationships among heart rate, β-blocker dosage, and prognosis in patients with coronary artery disease in a real-world database using a multimodal data acquisition system. Circ J 2023; 87: 336–344.
- 32.
Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet 2008; 372: 807–816.
- 33.
Sun N, Chen Y, Xi Y, Wang H, Wang L. Association between heart rate and major adverse cardiovascular events among 9,991 hypertentive patients: A multicenter retrospective follow-up study. Front Cardiovasc Med 2021; 8: 741784.
- 34.
Böhm M, Schumacher H, Linz D, Reil JC, Ukena C, Lonn E, et al. Low resting heart rates are associated with new-onset atrial fibrillation in patients with vascular disease: Results of the ONTARGET/TRANSCEND studies. J Intern Med 2015; 278: 303–312.
- 35.
Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: An analysis of ONTARGET/TRANSCEND. Clin Res Cardiol 2014; 103: 149–159.
- 36.
Ferrari R, Fox K. Heart rate reduction in coronary artery disease and heart failure. Nat Rev Cardiol 2016; 13: 493–501.
- 37.
Palatini P. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med 2002; 162: 2313.
- 38.
Bangalore S, Sawhney S, Messerli FH. Relation of Beta-blocker–induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol 2008; 52: 1482–1489.
- 39.
Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002; 359: 995–1003.
- 40.
Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet 2005; 366: 895–906.
- 41.
Zhang Y, Sun N, Jiang X, Xi Y. Comparative efficacy of β-blockers on mortality and cardiovascular outcomes in patients with hypertension: A systematic review and network meta-analysis. J Am Soc Hypertens 2017; 11: 394–401.