Article ID: CJ-24-0801
Article ID: CJ-24-0801
Background: The prognostic utility of high-sensitivity cardiac troponin T (hs-cTnT) on clinical outcomes in cardiac sarcoidosis (CS) remains unknown, so we evaluated hs-cTnT in the chronic phase of CS.
Methods and Results: We enrolled 92 consecutive patients with CS in the chronic phase after medical therapies. Patients were divided into 2 groups according to hs-cTnT level: 0.014 ng/mL: high hs-cTnT (n=37); normal hs-cTnT (n=55). The primary endpoint was cardiac death and the secondary endpoint was cardiac death, ventricular tachyarrhythmias, or hospitalization for heart failure. The mean age of patients was 63±11 years, and 75 received steroid treatment. During a median follow-up of 63 months, there were 9 cardiac deaths: 7 (19%) patients with high hs-cTnT and 2 (4%) patients with normal hs-cTnT. The rate of cardiac death was higher in patients with high hs-cTnT than in those with normal hs-cTnT (log-rank, P<0.01). Cox proportional hazard analysis showed that hs-cTnT was an independent predictor of cardiac death. The events rate was higher in patients with high hs-cTnT than in those with normal hs-cTnT (log-rank, P<0.01): cardiac death, ventricular tachyarrhythmias or hospitalization for heart failure occurred in 24 (65%) patients with high hs-cTnT and 11 (20%) patients with normal hs-cTnT.
Conclusions: Elevated hs-cTnT was linked with adverse outcomes in CS patients, suggesting it is an effective prognostic biomarker.
Sarcoidosis is a systemic granulomatous disease of unknown etiology.1 Cardiac sarcoidosis (CS) induces ventricular tachyarrhythmias and congestive heart failure, which are linked to an increased risk of death.2–4 CS is a progressive disease, and long-term continuous medical therapies, including steroid treatment, are required to improve the prognosis.5,6 In the clinical setting, risk stratification of CS in the chronic phase is important to manage therapeutic strategies. Although several studies have shown that electrocardiography, transthoracic echocardiography, and cardiac magnetic resonance (CMR) imaging are useful for assessing clinical outcomes,7–9 the utility of biomarkers has not been established.
Cardiac troponin is a sensitive and specific biomarker of myocardial injury, and high-sensitivity cardiac troponin T (hs-cTnT) is reportedly associated with both disease activity and treatment response in patients with CS.10,11 However, because the efficacy of hs-cTnT for prognostication in the chronic phase of CS after medical therapies remains unknown, we peformed the present study to evaluate the impact of hs-cTnT on clinical outcomes for patients with CS.
Between August 2013 and July 2022, we prospectively enrolled 92 consecutive patients with CS in the chronic phase after initiation of medical therapies. CS was diagnosed according to guidelines after clinical examination that included endomyocardial biopsy, transthoracic echocardiography, gallium-67 (67Ga) citrate scintigraphy, fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET), and CMR.12–14 This study was performed according to the principles of the Declaration of Helsinki and was approved by the institutional ethics committee. Informed consent was obtained from participants before entering the study.
CS DiagnosisCS was diagnosed according to the Japanese Ministry of Health and Welfare guidelines modified in 2006 and the Japanese Circulation Society guideline revised in 2016.12–14 The diagnostic criteria of CS, including isolated CS, are shown in Supplementary Tables 1–3. In brief, CS is diagnosed on the basis of histological or clinical confirmation. Histological diagnosis is confirmed when endomyocardial biopsy demonstrates noncaseating epithelioid cell granulomas. In the absence of histological confirmation, clinical diagnosis is confirmed when extracardiac sarcoidosis is detected and clinical cardiac criteria are satisfied. Regarding these criteria, the Japanese Ministry of Health and Welfare guidelines require ≥2 of 4 major criteria: high-grade atrioventricular block, basal thinning of the ventricular septum, positive myocardial uptake of 67Ga citrate scintigraphy or 18F-FDG PET, and left ventricular (LV) contractile dysfunction (LV ejection fraction (EF) <50%), or 1 of 4 major criteria and ≥2 of 5 minor criteria: abnormal ECG findings, abnormal echocardiographic findings, perfusion defects on myocardial perfusion scintigraphy, delayed contrast enhancement on CMR, and monocyte infiltration and fibrosis on endomyocardial biopsy.12,13 The Japanese Circulation Society guideline requires ≥2 of 5 major criteria: high-grade atrioventricular block or fatal ventricular arrhythmia, basal thinning of the ventricular septum or abnormal ventricular wall anatomy, LV contractile dysfunction (LVEF <50%) or focal ventricular wall asynergy, positive myocardial uptake of 67Ga citrate scintigraphy or 18F-FDG PET, and delayed contrast enhancement on CMR, or 1 of 5 major criteria and ≥2 of 3 minor criteria: abnormal ECG findings, perfusion defects on myocardial perfusion scintigraphy, and monocyte infiltration and fibrosis on endomyocardial biopsy.14 Additionally, isolated CS is diagnosed by histological or clinical confirmation of sarcoidosis in the heart alone according to the Japanese Circulation Society guideline, requiring the following criteria: (1) no clinical findings characteristics of sarcoidosis are observed in any organs other than the heart; (2) 67Ga citrate scintigraphy or 18F-FDG PET reveals no abnormal tracer accumulation in any organs other than the heart; and (3) chest computed tomography scan reveals no shadow along the lymphatic tracts in the lungs and no hilar and mediastinal lymphadenopathy. Histological diagnosis is confirmed when endomyocardial biopsy demonstrates noncaseating epithelioid cell granulomas. Clinical diagnosis is confirmed when the criterion of positive myocardial uptake of 67Ga citrate scintigraphy or 18F-FDG PET and ≥3 of 4 other major criteria are satisfied: high-grade atrioventricular block or fatal ventricular arrhythmia, basal thinning of the ventricular septum or abnormal ventricular wall anatomy, LV contractile dysfunction (LVEF <50%) or focal ventricular wall asynergy, and delayed contrast enhancement on CMR.14,15
Clinical AssessmentBlood samples were obtained in the outpatient clinic when patients were in a clinically stable condition. The initial measurement of hs-cTnT was performed when prednisone had been tapered to a maintenance dose in patients receiving steroid treatment, and after medical therapies were introduced in patients not receiving steroid treatment. The schedule of hs-cTnT measurements during the follow-up period after the initial measurement was determined by the outpatient physician, and the levels of hs-cTnT levels were measured using the Elecsys Troponin T-high sensitive immunoassay (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The normal hs-cTnT levels is ≤0.014 ng/mL.
Serum angiotensin-converting enzyme levels and plasma B-type natriuretic peptide (BNP) levels were also measured. Transthoracic echocardiography was performed by sonographers to measure LV end-diastolic and end-systolic diameters. LVEF was calculated by the disc summation technique.
Study DesignThis was a prospective, observational study. Patients were divided into 2 groups according to hs-cTnT level: high hs-cTnT (>0.014 ng/mL; n=37), normal hs-cTnT (≤0.014 ng/mL; n=55). The primary endpoint was cardiac death, and the secondary endpoint was cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure. Ventricular fibrillation and sustained ventricular tachycardia were documented by ECG or implantable devices. Sustained ventricular tachycardia was defined as spontaneous ventricular tachycardia at a rate ≥120 beats/min that lasted ≥30 s. Patients were followed from the date of the initial measurement of hs-cTnT until the date of cardiac death, cardiac event, or the end of follow-up.
Statistical AnalysisData are presented as the mean±standard deviation or median for continuous variables, and as number and percentage for categorical variables. Differences between groups were analyzed using the t-test and the Mann-Whitney U test for continuous variables, and the χ2 test for categorical variables. The cumulative event rate was estimated by Kaplan-Meier analysis, and the difference was analyzed by log-rank test. Predictors were determined using Cox proportional hazard analysis. Variables for univariate and multivariate analyses included age, sex, LVEF, and hs-cTnT levels for the primary endpoint and age, sex, LVEF, BNP level, and hs-cTnT level for the secondary endpoint. Hazard ratios are presented with 95% confidence intervals. Statistical analysis was performed with JMP version 14.0 (SAS Institute Inc., Cary, NC, USA), and P<0.05 was considered statistically significant.
Of the 92 patients with CS, including 17 with isolated CS, 19 were diagnosed by histological confirmation and 73 were diagnosed by clinical confirmation. Lung involvement was observed in 54 patients, skin involvement in 17 patients, and eye involvement in 19 patients. The mean age was 63±11 years, and 39 (42%) were male. The median hs-cTnT level was 0.013 ng/mL (range: 0.003–0.135 ng/mL, mean: 0.020±0.021 ng/mL). At the time of hs-cTnT measurement, 75 (82%) patients had received steroid treatment. The mean dose of prednisone was 5.1±2.7 mg daily (range: 2.5–10 mg).
A comparison of the clinical characteristics of patients with high hs-cTnT or normal hs-cTnT levels is shown in Table 1. Patients with high hs-cTnT were older than those with normal hs-cTnT, and they had a higher prevalence of heart failure. Higher BNP levels and lower LVEF were more frequently observed in patients with high hs-cTnT. The presence of positive myocardial uptake of 67Ga citrate scintigraphy or 18F-FDG PET at the time of CS diagnosis did not differ between the patient groups, but right ventricular uptake was more frequently observed in patients with high hs-cTnT than in those with normal hs-cTnT (24% vs. 9%, P=0.04). The presence of delayed contrast enhancement on CMR did not differ between the patient groups. Beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, and diuretics were administered more frequently in patients with high hs-cTnT. Implantable cardioverter defibrillator and cardiac resynchronization therapies were applied more frequently in patients with high hs-cTnT.
Clinical Characteristics of Patients With High or Normal hs-cTnT Levels
| Variables | High hs-cTnT (n=37) |
Normal hs-cTnT (n=55) |
P value |
|---|---|---|---|
| Age (years) | 67±10 | 61±12 | 0.01 |
| Male sex | 20 (54%) | 19 (35%) | 0.06 |
| High-degree atrioventricular block | 13 (35%) | 26 (47%) | 0.25 |
| Ventricular tachyarrhythmias | 14 (38%) | 16 (29%) | 0.39 |
| Heart failure | 28 (76%) | 17 (31%) | <0.01 |
| New York Heart Association class I or II | 35 (95%) | 52 (95%) | 0.99 |
| New York Heart Association class III | 2 (5%) | 3 (5%) | |
| Angiotensin-converting enzyme (IU/L) | 8.5±5.3 | 9.7±4.2 | 0.25 |
| BNP (pg/mL) | 283±238 | 109±114 | <0.01 |
| hs-cTnT (ng/mL) | 0.035±0.026 | 0.009±0.004 | <0.01 |
| LV end-diastolic diameter (mm) | 58±8 | 52±10 | <0.01 |
| LV end-systolic diameter (mm) | 47±10 | 40±12 | <0.01 |
| LVEF (%) | 41±11 | 51±14 | <0.01 |
| Positive myocardial uptake of 67Ga citrate scintigraphy or 18F-FDG PET |
34 (92%) | 46 (84%) | 0.25 |
| Delayed contrast enhancement on CMR | 20 (54%) | 30 (55%) | 0.56 |
| Steroids | 30 (81%) | 45 (82%) | 0.93 |
| Prednisone (mg/day) | 5.3±2.9 | 5.0±2.7 | 0.64 |
| β-blockers | 34 (92%) | 39 (71%) | 0.01 |
| Angiotensin-converting enzyme inhibitor/ angiotensin-receptor blocker |
28 (76%) | 26 (47%) | <0.01 |
| Diuretics | 25 (68%) | 23 (42%) | 0.02 |
| Anti-arrhythmic drugs | 16 (43%) | 19 (35%) | 0.41 |
| Implantable cardioverter defibrillator | 26 (70%) | 24 (44%) | 0.01 |
| Cardiac resynchronization therapy | 17 (46%) | 11 (20%) | <0.01 |
Values are mean±standard deviation or number (%). BNP, B-type natriuretic peptide; CMR, cardiac magnetic resonance; 18F-FDG, fluorine-18 fluorodeoxyglucose; 67Ga, gallium-67; hs-cTnT, high-sensitivity cardiac troponin T; LVEF, left ventricular (LV) ejection fraction; PET, positron emission tomography.
Cardiac Death
During a median follow-up of 63 months (range: 1–116 months), there were 9 cardiac deaths: 7 (19%) patients with high hs-cTnT and 2 (4%) with normal hs-cTnT. Of the 7 patients with high hs-cTnT, 4 died suddenly and 3 died of heart failure. Of the 2 patients with normal hs-cTnT, 1 died suddenly and 1 died of heart failure. Kaplan-Meier analysis showed that the rate of cardiac death was significantly higher in patients with high hs-cTnT than in those with normal hs-cTnT (log-rank, P<0.01) (Figure 1). Cox proportional hazard analysis showed that high hs-cTnT was independently associated with cardiac death (Table 2).
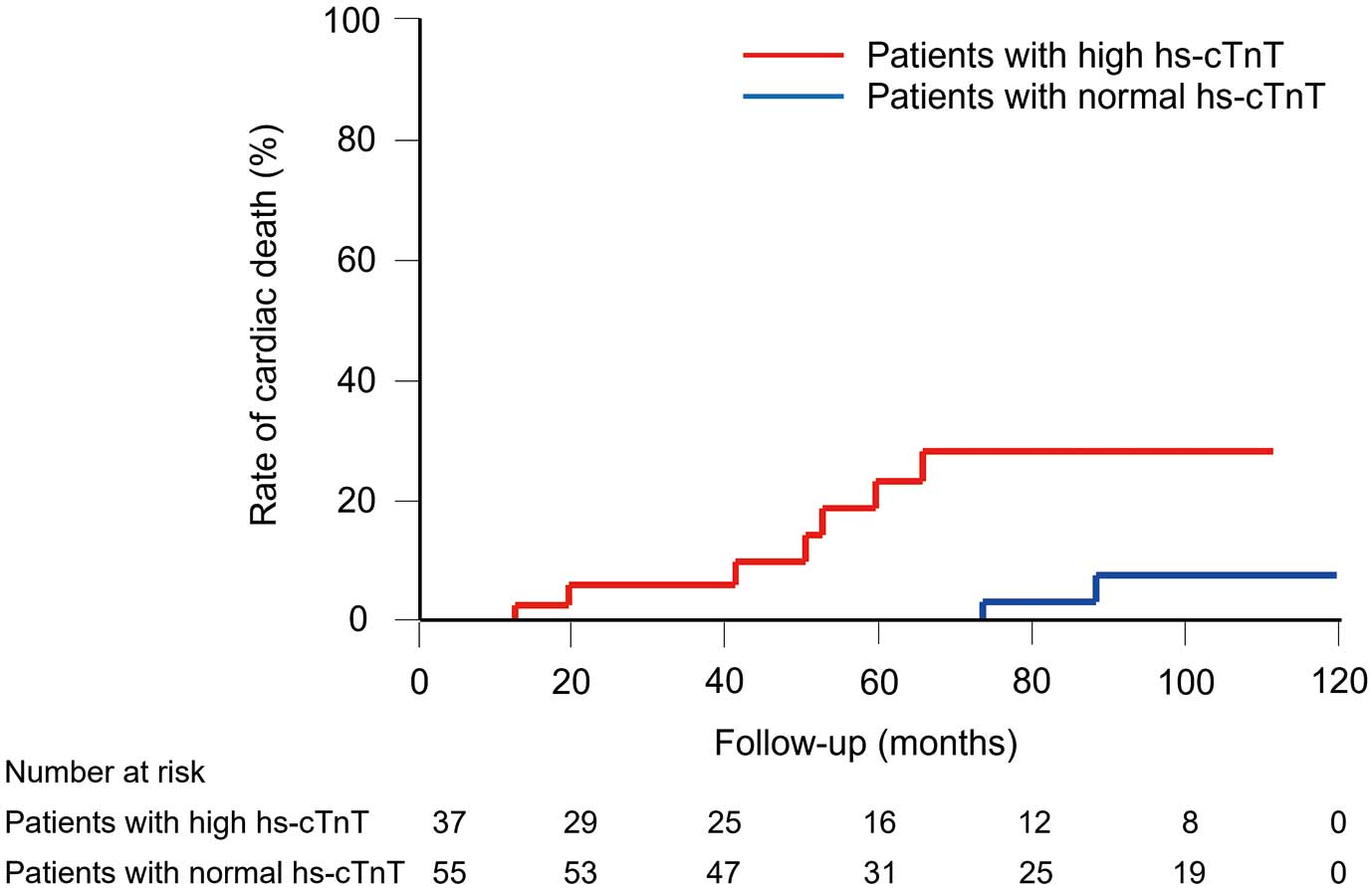
Rates of cardiac death in patients with high or normal hs-cTnT levels. Log-rank test, P<0.01. hs-cTnT, high-sensitivity cardiac troponin T.
Predictors of Cardiac Death
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age >60 years | 1.60 (0.39–10.8) | 0.54 | 1.03 (0.22–7.30) | 0.98 |
| Male | 6.11 (1.47–41.1) | 0.01 | 4.29 (1.03–29.0) | 0.04 |
| LVEF <40% | 5.02 (1.32–23.8) | 0.02 | 3.26 (0.81–16.4) | 0.10 |
| High hs-cTnT | 6.73 (1.62–45.2) | <0.01 | 4.79 (1.14–32.5) | 0.03 |
CI, confidence interval; HR, hazard ratio; hs-cTnT, high-sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction.
Cardiac Death, Ventricular Fibrillation, Sustained Ventricular Tachycardia, or Hospitalization for Heart Failure
During a median follow-up of 52 months (range: 1–116 months), 24 (65%) patients with high hs-cTnT and 11 (20%) patients with normal hs-cTnT died or experienced ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure. Ventricular fibrillation occurred in 1 patient with high hs-cTnT who had been treated with an implantable cardioverter defibrillator. Sustained ventricular tachycardia occurred in 12 patients with high hs-cTnT, including 11 who had been treated with an implantable cardioverter defibrillator, and 8 patients with normal hs-cTnT who had been treated with an implantable cardioverter defibrillator. Of the 12 patients with high hs-cTnT, 3 subsequently died suddenly. Of the 8 patients with normal hs-cTnT, 1 subsequently died suddenly. Hospitalization for heart failure occurred in 11 patients with high hs-cTnT and 3 patients with normal hs-cTnT. Of the 11 patients with high hs-cTnT, 1 subsequently died suddenly and 3 subsequently died of heart failure. Of the 3 patients with normal hs-cTnT, 1 subsequently died of heart failure.
Kaplan-Meier analysis showed that the rate of cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure was significantly higher in patients with high hs-cTnT than in those with normal hs-cTnT (log-rank, P<0.01) (Figure 2). More than half of patients with high hs-cTnT had these cardiac events within 2 years. Cox proportional hazard analysis showed that high hs-cTnT was independently associated with these cardiac events (Table 3).

Rates of cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure in patients with high or normal hs-cTnT levels. Log-rank test, P<0.01. hs-cTnT, high-sensitivity cardiac troponin T.
Predictors of Cardiac Death, Ventricular Fibrillation, Sustained Ventricular Tachycardia or Hospitalization for Heart Failure
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age >60 years | 2.11 (0.98–5.26) | 0.06 | 1.59 (0.72–4.03) | 0.26 |
| Male | 2.75 (1.40–5.64) | <0.01 | 1.58 (0.78–3.32) | 0.20 |
| LVEF <40% | 3.59 (1.83–7.14) | <0.01 | 1.70 (0.85–3.46) | 0.13 |
| BNP >100 pg/mL | 6.22 (2.75–16.7) | <0.01 | 4.24 (1.69–12.4) | <0.01 |
| High hs-cTnT | 5.58 (2.78–12.0) | <0.01 | 3.36 (1.56–7.60) | <0.01 |
Abbreviations as in Tables 1,2.
Regarding the frequency of ventricular tachyarrhythmias, ≥5 ventricular tachyarrhythmias occurred in 8 (22%) patients with high hs-cTnT and 4 (7%) patients with normal hs-cTnT. The rate of frequent ventricular tachyarrhythmias was higher in patients with high hs-cTnT than in those with normal hs-cTnT (P=0.04). Regarding rehospitalization for heart failure, ≥2 hospitalizations occurred in 7 (19%) patients with high hs-cTnT and 1 (2%) patient with normal hs-TnT. The rate of rehospitalization was higher in patients with high hs-cTnT than in those with normal hs-cTnT (P<0.01).
As fatal cardiac events, the endpoint was defined as cardiac death, ventricular fibrillation, or sustained ventricular tachycardia, including implantable cardioverter defibrillators. Kaplan-Meier analysis showed that the rate of fatal cardiac events was significantly higher in patients with high hs-cTnT than in those with normal hs-cTnT (log-rank, P<0.01) (Figure 3).

Rates of cardiac death, ventricular fibrillation, or sustained ventricular tachycardia in patients with high or normal hs-cTnT levels. Log-rank test, P<0.01. hs-cTnT, high-sensitivity cardiac troponin T.
Clinical Course of hs-cTnT
The clinical course of hs-cTnT levels in all the patients is shown in Figure 4. The mean hs-cTnT level was 0.020±0.021 ng/mL at the initial measurement and 0.022±0.020 ng/mL at the latest measurement. The hs-cTnT levels were not significantly different between the initial measurement and the latest measurement (P=0.42). Among the 37 patients with high hs-cTnT, there was no significant difference in hs-cTnT levels between the initial measurement and the latest measurement (0.035±0.026 ng/mL to 0.038±0.024 ng/mL, P=0.66). Among the 55 patients with normal hs-cTnT, the levels were higher at the latest measurement than at the initial measurement, but the increase was not remarkable (0.009±0.004 to 0.012±0.006 ng/mL, P<0.01).

Clinical course of hs-cTnT levels. hs-cTnT, high-sensitivity cardiac troponin T.
hs-cTnT and BNP
The median BNP level was 117 pg/mL. When patients were divided into 4 groups according to hs-cTnT and median BNP levels, the results were: high hs-cTnT + high BNP (n=27), high hs-cTnT + low BNP (n=10), normal hs-cTnT + high BNP (n=19), normal hs-cTnT + low BNP (n=36). Kaplan-Meier analysis showed that the rate of cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure was highest in patients with high hs-cTnT and high BNP, and lowest in patients with normal hs-cTnT and low BNP (log-rank, P<0.01) (Figure 5).

Rates of cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure according to hs-cTnT and BNP levels. Log-rank test, P<0.01. BNP, B-type natriuretic peptide; hs-cTnT, high-sensitivity cardiac troponin T.
The major findings of the present study were: (1) cardiac death occurred more frequently in patients with high hs-cTnT than in those with normal hs-cTnT; (2) patients with high hs-cTnT had cardiac death, ventricular fibrillation, sustained ventricular tachycardia, or hospitalization for heart failure more frequently than those with normal hs-cTnT; (3) ≥50% of patients with high hs-cTnT had cardiac events within 2 years; and (4) hs-cTnT was an independent predictor of adverse outcomes. To the best of our knowledge, this is the first study to show the efficacy of hs-cTnT for predicting adverse outcomes in patients with CS. The strengths of this study are that we included a relatively large number of patients with CS, despite it being a rare disease, and that we evaluated clinical outcomes during long-term follow-up.
CS causes myocardial inflammation and fibrosis, resulting in life-threatening ventricular tachyarrhythmias and severe heart failure due to LV dysfunction.1,16 Medical therapies, including steroid treatment, are important to prevent the progression of myocardial injury and to improve the prognosis.5,6,17–20 LV dysfunction, ventricular tachyarrhythmias, or heart failure symptoms have been reported as a risk factor of poor outcomes in patients with CS.5,7,21 CMR imaging or programmed ventricular stimulation is reportedly useful for risk stratification,8,22 but simple markers that can be assessed repeatedly in clinical practice are more useful for physicians to evaluate disease severity and determine the therapeutic strategy. To date, clinical biomarkers have not been utilized for patients with CS.
The cTnT level reflects the severity of ongoing myocardial damage and is elevated in many patients with heart failure. It has prognostic utility for the risk of cardiac events, independent of LV function or natriuretic peptides.23–25 A few studies have reported that the cTnT level is often elevated in patients with CS.10,11,26 One study showed that hs-cTnT correlated with abnormal accumulation of 18F-FDG PET, indicating the utility of hs-cTnT for evaluating the activity of CS.10 Elevated levels of cTnT were reported to normalize after steroid treatment in some patients.10,11 One case report showed the usefulness of hs-cTnT for the early detection of disease relapse after tapering of glucocorticoid therapy.27 These findings suggest the potential of hs-cTnT as a biomarker of the disease activity, reflecting inflammatory myocardial injury, as well as an indicator of the response to steroid treatment.10,11,26,27
As for prognostic effect, only one study reported that the cTnT level before steroid treatment was associated with the occurrence of ventricular tachyarrhythmias.28 Another study, which reported on the usefulness of cTnT as a marker of treatment response, indicated an association between the level at presentation before steroid treatment and poor outcomes.11 CS requires long-term continuous medical therapies, including steroid treatment, but none of the previous studies have evaluated the efficacy of hs-cTnT for predicting clinical outcomes in the chronic phase of CS after the initiation of medical therapies.
Our study results showed that patients with high hs-cTnT levels had major cardiac events at a high rate. Even a slight elevation of cTnT led to cardiac events. The hs-cTnT levels did not change significantly during the follow-up period, which might reflect chronic ongoing myocardial damage in cardiac failure. Our findings indicated that elevated hs-cTnT levels in the chronic phase of CS can predict adverse outcomes, so hs-cTnT may be a valuable biomarker for identifying patients at increased risk of life-threatening complications. For physicians, prognostic factors are important when considering therapeutic strategies during the chronic phase of CS. Our findings suggest that patients having high hs-cTnT levels that do not normalize after medical therapies, should be closely monitored with additional therapeutic management. Furthermore, BNP is a crucial biomarker of prognosis in cardiac diseases.29,30 In the present study, patients with high hs-cTnT and high BNP levels had cardiac events more frequently, whereas patients with normal hs-cTnT and low BNP had fewer cardiac events. This finding indicated that the combined measurement of hs-cTnT and BNP can be useful for risk stratification of patients with CS. Additionally, right ventricular uptake on 67Ga citrate scintigraphy or 18F-FDG PET at the time of CS diagnosis was frequently observed in patients with high hs-cTnT levels, reflecting the severity of inflammatory myocardial injury.
Study LimitationsFirst, this was a single-center study and larger studies are required to confirm our findings. Second, 67Ga citrate scintigraphy or 18F-FDG PET was not performed at the time of hs-cTnT measurement. Positive myocardial uptake was assessed at the time of CS diagnosis. This study could not investigate the association between hs-cTnT and myocardial inflammation at the same time. Additionally, this study could not compare the effectiveness of prognostic prediction between hs-cTnT and 67Ga citrate scintigraphy or 18F-FDG PET. It remains unclear whether hs-cTnT measurement provides additional value beyond assessing disease activity with 67Ga citrate scintigraphy or 18F-FDG PET. Third, this study did not assess the interaction of cardiac events with therapeutic management. Fourth, active inflammation was not fully suppressed by steroid treatment in some cases, which might have affected the association of hs-cTnT with cardiac events. Finally, CS was diagnosed by the guidelines of the Japanese Ministry of Health and Welfare and the Japanese Circulation Society, both of which include histological or clinical confirmation.12–14,31 Not all patients had CS confirmed by histological findings.
Elevated hs-cTnT levels in the chronic phase of CS after medical therapies were associated with adverse outcomes. Our findings suggested that hs-cTnT is effective for predicting adverse outcomes in patients with CS, and may be a valuable biomarker for risk stratification.
None declared.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The present study was approved by Okayama University ethics committee.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-24-0801