Article ID: CJ-25-0032
Article ID: CJ-25-0032
Background: Preoperative risk assessment is very important to ensure surgical safety and predict postoperative complications. However, no large-scale studies have evaluated the risk of perioperative cardiovascular events in Japan. This study evaluated perioperative cardiovascular events using real-world data. In addition, the applicability of machine learning to risk stratification was examined to develop a predictive model for perioperative cardiovascular events.
Methods and Results: This was an observational cohort study using the Japan Medical Data Center database, which includes claim and health examination data in Japan, between January 2005 and April 2021. In all, 133,634 gastrointestinal surgeries were included in the analysis. The primary outcome was 30-day risk of major adverse cardiovascular events (MACE). The 30-day MACE incidence rate following surgery was 3.8%. Machine learning was used to perform a binary classification task to predict MACE occurrence within 30 days after surgery. A clustering algorithm was developed based on the Shapley additive explanation values obtained from training data, and generalizability was evaluated using test data. Of the variables, age, history of ischemic heart disease or heart failure, history of stroke, diabetes, hypertension, atrial fibrillation, cases of malignancy, and pancreatic biliary surgery were identified as factors associated with MACE occurrence.
Conclusions: A machine learning model built from basic clinical information, comorbidities, and surgical information demonstrated the capacity to stratify MACE risk in patients undergoing gastrointestinal surgery.
Preoperative risk assessment is very important to ensure surgical safety and predict postoperative complications. A recent cohort study reported that approximately 3.0% of non-cardiac surgery patients experienced a major cardiac or cerebrovascular complication in the perioperative period.1 Perioperative complications are determined by the prevalence of comorbidities, the clinical condition of patients, the invasiveness of the procedure, and the surgical technique.2 Several scoring systems have been developed to assess perioperative complications from clinical information, such as the Revised Cardiac Risk Index (RCRI),3 the American College of Surgery National Surgical Quality Improvement Program surgical risk calculator,4 and the Cardiovascular Risk Index.5 The RCRI has long been used as an algorithm for perioperative risk assessment and has been validated in many cohorts.6–8 Unfortunately, no validation studies using large-scale data have been conducted in Japan, except for 1 single-center study with a relatively small sample size.9
The aim of this study was to clarify the incidence of perioperative cardiovascular events in patients undergoing gastrointestinal surgery using real-world data from Japan. Using machine learning algorithms, the aim was also to develop a predictive model for perioperative cardiovascular events. Using the constructed predictive model, risk stratification of patients was performed and its utility verified. Once validated and implemented, this predictive tool will enable surgeons or cardiologists to more accurately assess perioperative risk.
This research report followed the TRIPOD+AI statement10 on reporting clinical prediction models that use machine learning methods.
DataThe Japan Medical Data Center (JMDC) Claims Database was used for the analysis. This database contains medical claims and health examination data in Japan.11–16 This database, available for purchase from the JMDC, contains data of approximately 11.6 million individuals from January 2005 through April 2021. It includes diagnostic information based on the International Classification of Diseases, Tenth Revision (ICD-10), prescription data based on the Anatomical Therapeutic Chemical classification system, medical practice details based on electronic claims processing codes, and results of certain medical examinations.
ParticipantsFrom the dataset, only codes recorded during hospitalization were selected. K codes were used to extract surgical cases in which general anesthesia or epidural anesthesia were used. Only patients aged ≥18 years were included in the study. Patients who underwent any of the following thoracic or abdominal surgeries were excluded: non-gastrointestinal surgery, exploratory laparotomy, endoscopic surgery, gastrostomy and enterostomy, colostomy, and transplantation surgery. Surgeries that were performed in fewer than 5 patients were also excluded. Patients for whom the observation period was ≤30 days were excluded.
Data PreparationThe ICD-10 codes presented in Supplementary Table 1 were used to identify complications and comorbidities. The RCRI was calculated using serum creatinine levels from physical examination data performed within 180 days before surgery (serum creatinine data were available for only 31.0% of patients in the dataset). Emergency surgery was defined as any admission identified as “after hours,” “holiday,” or “urgent.”
Surgical procedures were classified as follows. Because K codes are unique to Japan, internationally used ICD-10 Procedure Coding System (PCS) codes17 were assigned. To improve the clinical interpretability and relevance of the individual features, the Agency for Healthcare Research and Quality/Healthcare Cost and Utilization Project’s Clinical Classifications Software Refined (CCSR)18,19 was used. The ICD-10-PCS codes were classified into CCSR categories using the list provided.20 This resulted in all gastrointestinal surgical procedures being grouped into 27 categories (Supplementary Table 2).
OutcomeThe primary endpoint was a major adverse cardiovascular event (MACE) within 30 days of surgery, defined as the rate of the composite of cardiovascular death, myocardial infarction, stroke, and heart failure. Previous reviews have shown that there is inconsistency in the definition of MACE across studies.21 In the present study, the MACE composite endpoint was defined based on ICD-10 codes, in accordance with the approach taken in previous studies (Supplementary Table 3).22–25
PredictionsIn this study, machine learning was used to perform a binary classification task to predict MACE occurrence within 30 days after surgery. The variables used for machine learning models were age, sex, 10 comorbidities, 3 surgical information categories (open/laparoscopic, malignancy surgery, emergency surgery), and 27 surgical methods (CCSR categories). Of the variables used, age was a continuous variable and the others were categorical.
Sample SizeThe sample size required to minimize the problem of overfitting26 was calculated, and this size was found to be sufficient to answer the research questions.
Missing DataThere were no missing values for any of the variables.
Analytical MethodsThe dataset was split randomly into a 7 : 3 ratio of training set to test set. Models were trained on the training data using logistic regression, random forest,27 extreme gradient boosting (XGBoost),28 category boosting (CatBoost),29 and light gradient boosting machine (LightGBM)30 algorithms.
Optuna, an automatic hyperparameter optimization software framework, was used to optimize the hyperparameters of each model.31 The objective function was set to maximize the Matthews correlation coefficient (MCC) and the number of trials was 100. Details of the hyperparameters optimized in each model are presented in Supplementary Table 4. The model was divided into 5 sections of training data, and cross-validation was used to assess the model’s performance. Confidence intervals were generated using block bootstrapping of the predictions in the test set. The model’s prediction performance was internally validated with 2,500 bootstrap resamples. The machine learning model was developed in Python 3.8.8 using the scikit-learn library. The versions of the XGBoost, CatBoost, and LightGBM libraries were 2.1.0, 1.2.5, and 4.0.0, respectively.
Class ImbalanceThe number of patients in the postoperative MACE group was much smaller than the number of patients in the group without postoperative MACE, leading to extreme class imbalance. For logistic regression, random forest, and LightGBM, the hyperparameter “class_weight” was set to “balanced” to automatically correct for imbalances. In XGBoost and CatBoost, “pos_weight” was set to 1 to adjust the imbalance between positive and negative samples.
FairnessIdeally, the data should include information from individuals of different ages, sexes, races/ethnicities, health status and comorbidities, and geographic locations.10 However, due to the nature of the database, the main limitations of this study in terms of fairness were that the dataset included patients aged less than 75 years, and the data were primarily from Japanese patients.
Model OutputTo evaluate model performance, each model was applied to the test dataset to predict MACE occurrence within 30 days after surgery. Receiver operating characteristic (ROC) and precision recall (PR) curves were generated for each model. In addition, isotonic regression was used to perform probability calibration, and the Brier score was calculated. The performance of each model was compared using the bootstrap method, but no statistically significant differences were observed. In this study, the area under the PR curve (PR-AUC) was selected as the primary metric for model comparison and selection.
Training Versus EvaluationThe Shapley additive explanation (SHAP)32 values of the training data were calculated using the optimal model, and the data were clustered into 3 clusters using the K-means method27 based on the SHAP values. The elbow method was used to determine the optimal number of clusters in the K-means algorithm. For the test data, the same model and method were used to cluster the data into 3 clusters. The clustered training and test data were compared and evaluated for MACE incidence. Kaplan-Meier curves were used to assess the cumulative incidence of MACE within each cluster.33
Statistical AnalysisContinuous variables with a normal distribution are expressed as the mean±SD. Categorical variables are expressed as percentages (%), unless indicated otherwise. Baseline characteristics were compared using the Chi-squared or Fisher’s exact test for categorical variables, and Student’s or Welch’s t-test after testing that the continuous variables were normally distributed. Significance was defined as P<0.05, and statistical analyses were performed using R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical ConsiderationsThis study was conducted in accordance with the Declaration of Helsinki. Although this study used anonymized data and was outside the scope of the guidelines for research involving human subjects in Japan, the study was conducted after approval was obtained from the Ethics Committee of The University of Tokyo Hospital (Approval no. 2024105NIe).
In all, 133,634 surgeries were included in the analysis (Figure 1). Patient characteristics in the original dataset are presented in the Table. Within 30 days of surgery, 506 (0.38%) patients experienced a MACE. Supplementary Table 5 presents baseline characteristics of patients with and without MACE. Figure 2 shows the incidence of MACE by surgical category classified according to the CCSR. Gastroenterostomy and duodenojejunal anastomosis had the highest event rates, followed by esophagectomy, generalized peritonitis and abdominal abscess surgery, and pancreatectomy. The medium-risk category included liver resection, colon resection, cholecystectomy (without stones), etc. The low-risk categories included inguinal hernia, femoral hernia, appendectomy, abdominal wall surgery, etc. (for details, see Supplementary Table 6). Figure 3 shows the accuracy of the prediction models for each method. Calibration curves to assess the goodness of fit of the models are shown in the Supplementary Figure. All models achieved high areas under the ROC curve (ROC-AUC), with values ranging from 0.831 to 0.856; the LightGBM model showed the largest PR-AUC, MCC, and F1 values (Supplementary Table 7). RCRI, calculated using a subset of the dataset, had an ROC-AUC of 0.684, which was lower than the machine learning model.

Cohort study design and study flowchart. JMDC, Japan Medical Data Center.
Patient Characteristics
| Variable | Overall | Training set | Test set | P value |
|---|---|---|---|---|
| No. patients | 133,634 | 93,543 | 40,091 | |
| Male sex | 89,063 (66.6) | 62,331 (66.6) | 26,732 (66.7) | 0.879 |
| Age (years) | 48.5±12.9 | 48.5±12.9 | 48.5±12.9 | 0.693 |
| Comorbidities | ||||
| Ischemic heart disease | 9,695 (7.3) | 6,755 (7.2) | 2,940 (7.3) | 0.476 |
| Heart failure | 7,667 (5.7) | 5,352 (5.7) | 2,315 (5.8) | 0.713 |
| Stroke | 3,772 (2.8) | 2,670 (2.9) | 1,102 (2.7) | 0.294 |
| Diabetes | 24,878 (18.6) | 17,399 (18.6) | 7,479 (18.7) | 0.819 |
| Hypertension | 33,933 (25.4) | 23,808 (25.5) | 10,125 (25.3) | 0.454 |
| Dyslipidemia | 37,227 (27.9) | 26,141 (27.9) | 11,086 (27.7) | 0.276 |
| Chronic kidney disease | 2,128 (1.6) | 1,482 (1.6) | 646 (1.6) | 0.735 |
| Atrial fibrillation | 2,688 (2.0) | 1,880 (2.0) | 808 (2.0) | 0.963 |
| Heart valve disease | 3,219 (2.4) | 2,240 (2.4) | 979 (2.4) | 0.619 |
| Chronic obstructive pulmonary disease | 2,240 (1.7) | 1,553 (1.7) | 687 (1.7) | 0.501 |
| Surgical information | ||||
| Laparoscopic/thoracoscopic surgery | 60,379 (45.2) | 42,127 (45.0) | 18,252 (45.5) | 0.099 |
| Open surgery | 73,255 (54.8) | 51,416 (55.0) | 21,839 (54.5) | 0.099 |
| Malignancy | 27,712 (20.7) | 19,272 (20.6) | 8,440 (21.1) | 0.064 |
| Emergency surgery | 7,803 (5.8) | 5,465 (5.8) | 2,338 (5.8) | 0.950 |
| Surgical method (CCSR category) | ||||
| Abdominal wall procedures | 172 (0.1) | 118 (0.1) | 54 (0.1) | 0.752 |
| Abdominal wall repair | 1,491 (1.1) | 1,044 (1.1) | 447 (1.1) | 1.000 |
| Anorectal repair | 3,074 (2.3) | 2,146 (2.3) | 928 (2.3) | 0.833 |
| Appendectomy | 16,998 (12.7) | 11,916 (12.7) | 5,082 (12.7) | 0.761 |
| Biliary and pancreatic calculus removal | 24,714 (18.5) | 17,386 (18.6) | 7,328 (18.3) | 0.187 |
| Cholecystectomy | 195 (0.1) | 141 (0.2) | 54 (0.1) | 0.532 |
| Colectomy | 9,343 (7.0) | 6,494 (6.9) | 2,849 (7.1) | 0.286 |
| Diaphragmatic hernia repair | 8 (0.0) | 6 (0.0) | 2 (0.0) | 1.000 |
| Duodenal resection | 30 (0.0) | 18 (0.0) | 12 (0.0) | 0.319 |
| Esophagectomy | 396 (0.3) | 279 (0.3) | 117 (0.3) | 0.886 |
| Exploration of peritoneal cavity | 27 (0.0) | 14 (0.0) | 13 (0.0) | 0.065 |
| Gastrectomy | 6,020 (4.5) | 4,198 (4.5) | 1,822 (4.5) | 0.656 |
| GI system drainage | 2,140 (1.6) | 1,520 (1.6) | 620 (1.5) | 0.306 |
| GI system lysis of adhesions | 2,026 (1.5) | 1,430 (1.5) | 596 (1.5) | 0.581 |
| GI system repair | 733 (0.5) | 493 (0.5) | 240 (0.6) | 0.113 |
| Hepatobiliary and pancreatic drainage | 13 (0.0) | 12 (0.0) | 1 (0.0) | 0.126 |
| Hepatobiliary and pancreatic procedures | 158 (0.1) | 103 (0.1) | 55 (0.1) | 0.218 |
| Hepatobiliary resection and ablation | 2,738 (2.0) | 1,879 (2.0) | 859 (2.1) | 0.118 |
| Inguinal and femoral hernia repair | 10,426 (7.8) | 7,332 (7.8) | 3,094 (7.7) | 0.458 |
| Omentectomy or peritoneum resection | 553 (0.4) | 395 (0.4) | 158 (0.4) | 0.491 |
| Other peritoneal cavity procedures | 1,390 (1.0) | 955 (1.0) | 435 (1.1) | 0.303 |
| Pancreatectomy | 2,410 (1.8) | 1,706 (1.8) | 704 (1.8) | 0.406 |
| Proctectomy or anal resection | 6,900 (5.2) | 4,758 (5.1) | 2,142 (5.3) | 0.054 |
| Retroperitoneal procedures | 483 (0.4) | 339 (0.4) | 144 (0.4) | 0.968 |
| Saphenous vein harvest and other therapeutic vessel removal | 36,817 (27.6) | 25,805 (27.6) | 11,012 (27.5) | 0.661 |
| Small bowel resection | 3,852 (2.9) | 2,692 (2.9) | 1,160 (2.9) | 0.890 |
| Upper GI therapeutic procedures | 527 (0.4) | 364 (0.4) | 163 (0.4) | 0.675 |
| RCRI scoreA | 1.2±0.5 | 1.2±0.5 | 1.2±0.5 | 0.480 |
| RCRI score ≥2A | 2,999 (12.3) | 2,095 (12.3) | 904 (12.5) | 0.622 |
| MACE | 506 (0.4) | 354 (0.4) | 152 (0.4) | 0.430 |
An=24,286. Categorical variables are expressed as n (%) and continuous variables are presented as the mean±SD. CCSR, Clinical Classifications Software Refined; GI, gastrointestinal; MACE, Major adverse cardiac events; RCRI, Revised Cardiac Risk Index.
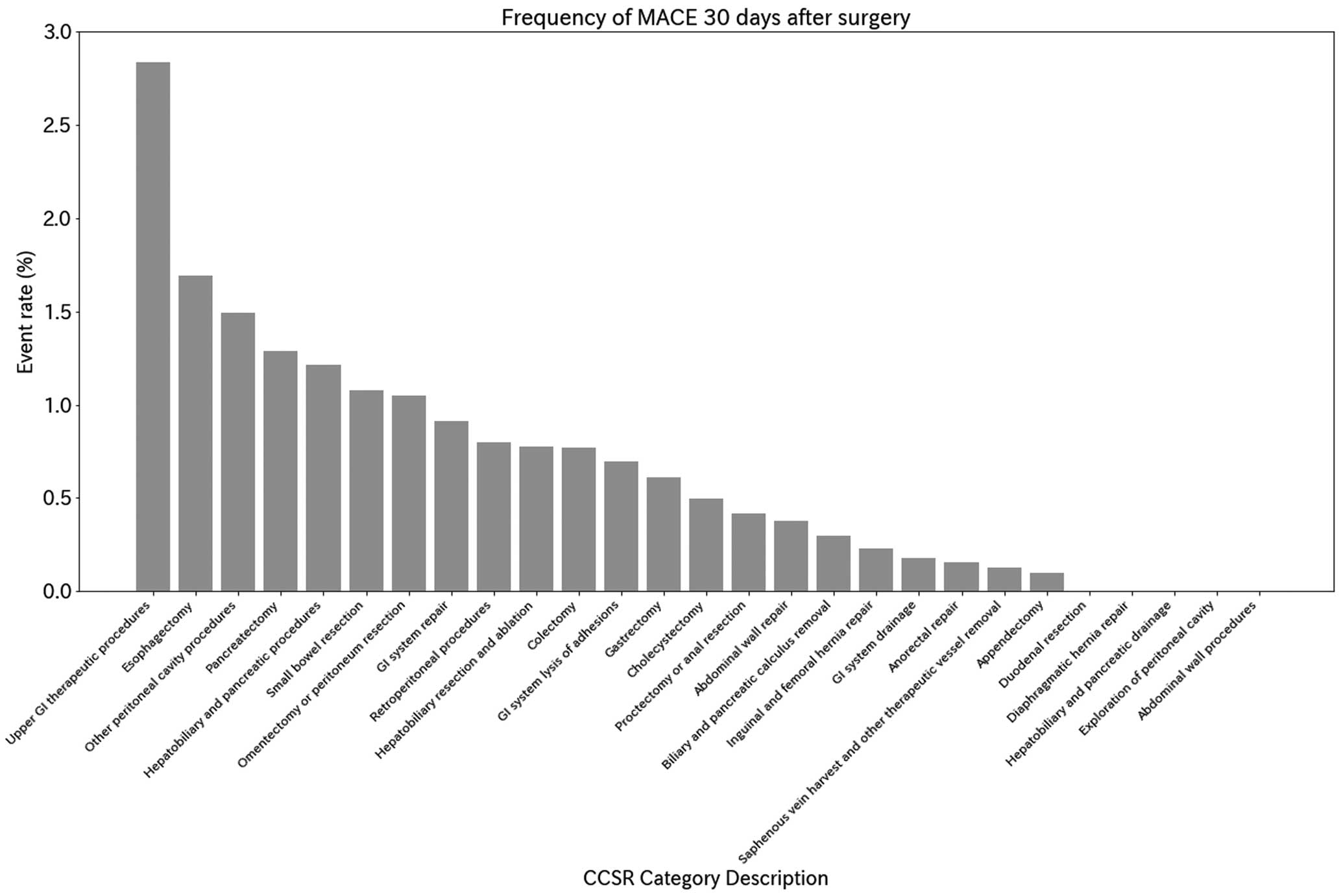
Frequency of major adverse cardiovascular events (MACE) 30 days after surgery. CCSR, Clinical Classifications Software Refined; GI, gastrointestinal.

Metrics for evaluating test sets: (A) Receiver operating characteristic (ROC) and (B) precision recall curves. AUC, area under the curve; RCRI, Revised Cardiac Risk Index.
Of the features used to predict the outcomes, age had the highest impact (Figure 4A). The summary plot shows that higher age and the presence of a history of hypertension, malignancy, and heart failure contributed to MACE occurrence. In contrast, hemorrhoid surgery (variable name: saphenous vein harvest and other therapeutic vessel removal), appendicectomy, and surgery for inguinal and femoral hernias showed negative correlations as predictors of a lower MACE incidence (Figure 4B).
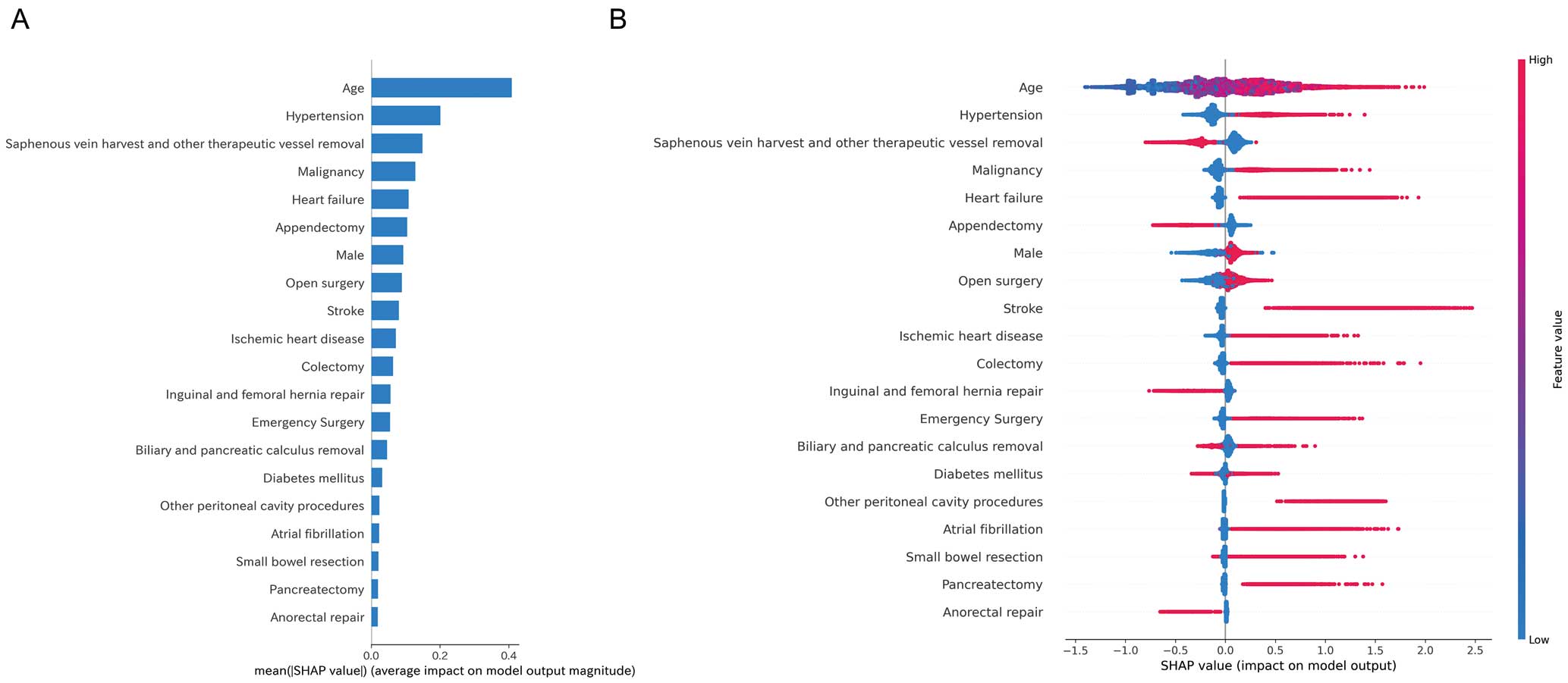
Feature importance based on Shapley additive explanations (SHAP) values. (A) Mean absolute SHAP values are plotted to illustrate global feature importance. (B) SHAP summary plot to illustrate the effects of global features on the major adverse cardiovascular events (MACE) outcome. Red dots represent higher feature values, and blue dots represent lower feature values.
Figure 5A shows the MACE rates for each cluster of the training data clustered into 3 clusters using the K-means method. Clustering the test data using the same model yielded similar results, as shown in Figure 5B. Figure 6 shows the Kaplan-Meier curves for 30-day MACE after surgery for each cluster. Patients in Clusters 1 and 2 had significantly higher MACE rates than those in Cluster 0 (P<0.001).

Major adverse cardiovascular events (MACE) rates for each cluster of (A) the training data and (B) the test data clustered into 3 clusters using the K-means method. Data are the mean±SD. (A) The mean (±SD) MACE incidence was 0.0023±0.0023%, 0.47±0.033%, and 2.1±0.17% for Clusters 0, 1, and 2, respectively. (B) The mean (±SD) MACE incidence was 0.098±0.0023%, 0.36±0.043%, and 2.3±0.28% for Clusters 0, 1, and 2, respectively.

Kaplan-Meier curves for 30-day major adverse cardiovascular events after surgery for each cluster.
Figure 7 shows the percentage distribution of the variables in each cluster. Each variable was standardized from 0 to 1. The results showed that the high-risk group was characterized by older age, a history of ischemic heart disease or heart failure, a history of stroke, history of diabetes, a history of hypertension, a history of atrial fibrillation, cases of malignancy, and pancreatic biliary surgery. Details of the characteristics by cluster for the training data are presented in Supplementary Table 8.

Cluster characteristics heat map visualizing the probability distribution of variables in each cluster in the training data. GI, gastrointestinal.
In summary, Cluster 0 (low risk) is characterized by younger age, lower rates of comorbidity, the CCSR categories of “appendectomy” and “saphenous vein harvest and other therapeutic vessel removal” (such as hemorrhoid surgery), and other benign procedures. Conversely, Cluster 2 (high risk) is characterized by older age, a higher proportion of comorbidities, a higher proportion of malignancy surgery, and “hepatobiliary resection and ablation” and “pancreatectomy” in the CCSR category. Cluster 1 (intermediate risk) was an intermediate patient background. In the present study, emergency surgery and laparoscopic or open surgery did not contribute significantly to risk estimation.
In this study, the perioperative MACE risk in gastrointestinal surgery was evaluated using real-world data from Japan, and a machine learning prediction model was developed. Several important findings were obtained. First, gastrointestinal anastomosis, duodenojejunal anastomosis, esophagectomy, generalized peritonitis surgery, abdominal abscess surgery, and pancreatectomy were identified as high-risk procedures. These surgeries are considered high MACE risk because they require advanced techniques and management of the patient’s general condition. These results were comparable to those of the estimated surgical risk according to type of surgery in previous large studies,34,35 and several clinical guidelines, such as the American College of Cardiology and American Heart Association,36 the European Society of Cardiology,2 and the Japanese Circulation Society.37 However, the present study showed the risks of more segmented and refined surgical procedures.
Second, the accuracy of each model was found to be higher than that of the previously used RCRI, with LightGBM in particular demonstrating superior performance. In a previous Japanese study,9 it was unclear whether the RCRI score can identify patients at higher MACE risk. In the present study, the visualization of high-impact variables using SHAP showed factors that are important in prediction. In particular, age, medical history (ischemic heart disease, heart failure, stroke, diabetes, hypertension, and atrial fibrillation), presence of malignancy, and type of surgery emerged as important predictors.
Peng et al. presented the findings of a machine learning model that predicted postoperative MACE risk in approximately 5,700 elderly patients at a single institution.38 In that study, New York Heart Association functional class and biomarkers, such as B-type natriuretic peptide and troponin-T levels, were identified as important variables in the model.38 The machine learning model developed from general patient background, comorbidities, and surgical procedure information demonstrated satisfactory performance in the present study.
Conversely, all models showed low PR-AUC, MCC, and F1 values as a consequence of pronounced outcome imbalances. Furthermore, the adjustment of hyperparameters did not lead to any discernible enhancement. Nevertheless, the integration of the clustering method with the K-means method based on SHAP values may facilitate the stratification of risk and the identification of lower-frequency event occurrence risks. The high-risk group includes a significant proportion of patients who are older and have multiple cardiovascular diseases and malignancies. These patients require particularly careful preoperative evaluation and postoperative management.
A comparison of the clustering results and MACE incidence for the test data with those for the training data showed similar outcomes, thereby confirming the high degree of generalizability exhibited by the predictive model.
This suggests that the constructed model is likely to be of significant utility in actual clinical practice. This predictive tool demonstrates superior accuracy in risk stratification compared to the RCRI. Ideally, hospitalists would provide comprehensive support to ensure high-quality care.39 However, the shortage of physicians and cardiologists, particularly in small and medium-sized hospitals, may make it difficult to undertake adequate perioperative risk assessment.40 The concept proposed in the present study has the potential to assist surgeons in accurately assessing surgical risk. In addition, validation and clinical implementation could provide a practical solution to the shortage of hospital doctors.
Study LimitationsThis study has several potential limitations. First, due to the nature of the database, the surgeries analyzed did not include cases in which the patient’s age at the time of surgery was ≥75 years. Furthermore, the lack of availability of medical history between the time of medical insurance enrollment and the time of surgery resulted in variations in the duration of observation of the medical history. Second, because the data were derived from receipts, it was not possible to ascertain whether an event occurred before or after the surgery on the day of the surgery. Consequently, such events were not included as outcomes. Therefore, there may be a degree of bias in this study regarding the occurrence of cardiovascular events due to these factors. Furthermore, due to the nature of the database, mortality data are available only on a monthly basis, so the analysis was conducted using mortality data obtained from the disease outcome with date information. Accordingly, some reliability concerns remain for the mortality information. Third, the model had low F1 values due to class imbalance and dataset specific characteristics. This may lead to misclassification of high-risk patients representing the minority class. There are limitations to improving the accuracy of rare events. Conversely, efforts to increase risk stratification capacity are important to improve practicality. Finally, the study was based on data from receipts, and the analysis relied on information recorded for the purpose of reimbursement, which may introduce bias regarding the reflection of actual clinical conditions. Despite these limitations, the study provides valuable insights into the risk assessment of perioperative cardiovascular events in gastrointestinal surgery using real-world data. Future studies should integrate diverse data sources to enhance the accuracy and applicability of the prediction model. Prospective studies are also necessary to validate the operational feasibility and usefulness of the model for application in actual clinical practice.
A machine learning model built from basic clinical information, comorbidities, and surgical information demonstrated its ability to stratify MACE risk in patients undergoing gastrointestinal surgery.
The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing.
This study was funded by the Progress of the Next Cross-ministerial Strategic Innovation Promotion Program (SIP) on “Integrated Health Care System” (Grant no. JPJ012425). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
There are no financial conflicts of interest to disclose concerning this study.
This study was approved by the Ethics Committee of The University of Tokyo Hospital (Approval no. 2024105NIe).
The relevant analytical protocol and code underlying this analysis are available upon request to the corresponding author. The JMDC database is a commercial database and cannot be accessed through this request.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-25-0032