I. General Outline
1. History of Heart Disease Screening in Schools
In Japan, the first heart disease screening program for children in schools was implemented in four schools in Fujiidera City, Osaka Prefecture as an epidemiological survey in 1954.
In 1958, the School Health Law, the Enforcement Ordinance of the School Health Law, and the Enforcement Regulation of the School Health Law were established to mandate health check-up to screen for cardiovascular diseases and abnormalities at entering school and select conduct additional clinical examination for those suspected to have heart disease to find it.
In 1973, the Enforcement Regulation of the School Health Law was revised to require heart disease screening as part of regular health check-up in schools. However, the Regulation does not define how to screen for heart disease, and allows the education committee of each local government to determine the contents of screening for children in state schools and the founders of private schools to determine the contents for their students. In order to standardize heart disease screening in schools, the Japanese Society of Pediatric Cardiology and Cardiac Surgery and the Japanese Society of School Health led discussions to prepare the Heart Disease Pocket Guide and the Heart Disease Management Guidance Form and to establish codes for electrocardiogram (ECG) abnormalities in heart disease screening in schools and categories of allowable intensity of exercise and school activities for children with arrhythmia, those with congenital heart disease, and those after cardiac surgery.
In December 1994, the Enforcement Regulation of the School Health Law was revised to mandate all students in the first year of elementary school, junior high school and senior high school to undergo an ECG test from 1995 on.
2. System of School Health Screening
2.1 Flow of Heart Disease Screening
After ECG became a mandate for students in the first year of elementary, junior high, and senior high schools in 1995, primary and secondary heart diseases screening were conducted as illustrated in
Figure 1.2
2.2 Primary Heart Disease Screening
Primary heart disease screening is performed every year for students in the first year of elementary, junior high, and senior high schools. The purpose of primary heart disease screening is not to diagnose heart diseases but to screen healthy students for the presence or suspected presence of heart diseases. The screening should consist of simple, convenient and cost-effective examinations that provide reliable results without causing mental or physical stress amongst participants.2
2.3 Primary Heart Disease Screening System
Figure 2
outlines primary heart disease screening in Tokyo. The first step of screening is identifying those who should undergo primary heart disease screening. In some areas, this selection process is referred to as the “primary screening” and electrocardiography (ECG) as the “secondary screening.”
In Tokyo, those who are found to show serious ECG findings are encouraged to visit a cardiology clinic without waiting for the secondary screening.
2.4 Subjects of Primary Screening
a. Those Who Are Legally Required to Undergo Primary Screening
- All students in primary, junior-high, and senior-high schools.
Those in the first year of each school should undergo ECG in schools.
*However, those who regularly visit the clinic for their underlying heart conditions may not undergo screening if they submit their School Activity Management Table.
b. Those Under Follow-up
- Those who were encouraged at last year’s screening to “undergo heart disease screening again next year”
- In some areas, those under follow-up may include students who regularly visit the clinic for their underlying heart conditions.
c. Those Selected by School Physicians Through Medical Examination
- Those in whom abnormal cardiac sounds or murmurs are audible, and those with arrhythmias
- Those with typical physical findings (e.g., physical constitution, facial appearance and chest shape)
d. Those Selected by the Teacher in Charge, Physical Education Teacher, or School Nurse Through Daily Activity Monitoring
- Those with a history of palpitations, shortness of breath, or fatiguability, amongst others
- Those with short- or long-term change in general impressions such as facial expression, bodily movement and way of speaking
e. Those Selected Through Questionnaires (e.g., Health Survey and Heart Disease Screening Questionnaire)
- Those who have a history of heart disease including Kawasaki disease with no clear documentation of medical management by their attending physicians
- Those who were suspected to have heart disease but have not visited the clinic yet
- Those with symptoms suggestive of heart disease
- Those who have a family history of sudden death in childhood and are suspected to have genetic heart disease or other conditions
*During selection, the school should contact parents or other family members of students who may need heart disease screening to confirm whether they are willing to undergo the screening if necessary.
Heart disease screening forms differ among areas, and may consist of different items.
2.5 Items of Primary Heart Disease Screening
a. Medical Examination by the School Physician
Those suspected to have heart disease based on cardiac murmurs and arrhythmias or visible signs/symptoms should be selected for ECG monitoring.
b. Heart Disease Screening Form
Parents or other family members are requested to describe the child’s current or past history and family history of cardiac diseases.3
c. ECG Examination
An ECG can detect many types of cardiac diseases in children and young people such as hereditary arrhythmia and cardiomyopathy. A 12-lead ECG rather than simple 4-lead ECG should be recorded to ensure accurate screening.4
d. Other Clinical Examinations
i. Phonocardiography
Those with cardiac murmurs are suspected to have organic heart disease. In schools, a large number of students should be screened in a short period of time, and it is difficult and costly to invite physicians with expertise in auscultation to school screenings. Therefore, phonocardiography rather than auscultation is used in some areas.
However, children with almost all congenital heart conditions that cause audible murmurs are often diagnosed before entering school. Phonocardiography for school children is now considered useful only in detecting innocent murmurs and some types of cardiac diseases such as atrial septal defect.5
ii. Others
In some areas, ECG and blood pressure are recorded simultaneously to select those who should undergo secondary screening.
e. Effective and Efficient Methods for Heart Disease Screening in Schools in Future
Phonocardiography may be effective in correctly selecting those who should undergo a 12-lead ECG to examine for lethal arrhythmias and myocardial disorders, identifying those with possible atrial septal defect, diagnosing innocent murmurs, and addressing challenges associated with medical examinations by the school physician.
2.6 Urgent Measures to Be Taken for Those Showing Serious ECG Abnormalities in Primary Screening
When ECG findings that may reflect serious abnormalities are found in primary heart disease screening, the record should be sent to an expert via FAX or email for review without delay. When the expert determines that the student needs urgent measures, he/she should be tentatively rated at an appropriate category of allowable intensity of exercise and daily activities until a final diagnosis is reached, and the school nurse should be informed of the fact.
Figure 3
summarizes the flow of urgent measures. The child and his/her parents should be instructed to visit a specialist clinic without delay.
2.7 Rating Criteria for Primary Screening and the Use of Screening Information Cards
In some areas, screening information cards are used in trial basis to record individual longitudinal data including a past history of heart diseases and Kawasaki disease, types of signs/symptoms, ECG findings in previous screenings, evaluation results, and ratings of allowable intensity of exercise and daily activities.
The results of primary screening should be rated as follows:7
1. No abnormal findings.
2. No management required: Abnormal findings are present but no restriction of activities of daily life or exercise is required.
3. Follow-up required: The child should undergo heart disease screening next year (and should be classified into an appropriate category of allowable intensity of exercise and daily activities).
4. Medical management required: The child should be managed by his/her attending physician.
5. Secondary screening required: The child should undergo secondary screening.
6. Detailed examination required: The child should visit a cardiology clinic without delay.
2.8 Flow of Examination in Secondary Screening and Thereafter
The secondary heart disease screening should consist of a 12-lead ECG and examination by an expert. Additional examination should be performed according to the results and findings of the secondary screening and/or later examination.
Children who are determined to need treatment, those in whom correct diagnosis is difficult to achieve, and those for whom allowable intensity of exercise and daily activities are difficult to determine should be rated as “detailed examination required,” and should be referred to a cardiology clinic (Figure 4).
2.9. Evaluation in Secondary Screening and Later Examination
The results of secondary screening should be recorded as follows:
1. No abnormal findings.
2. No management required: Abnormal findings are present but no restriction of activities of daily life or exercise is required.
3. Follow-up required: The child should undergo heart disease screening next year (and should be classified into an appropriate category of allowable intensity of exercise and daily activities).
4. Medical management required: The child should be managed by his/her attending physician.
5. Detailed examination required: The child should visit a cardiology clinic (and should be tentatively classified into an appropriate category of allowable intensity of exercise and daily activities) A patient referral document should be issued.
6. Not examined yet: The child has not undergone secondary screening or later examination, and should visit a clinic to receive instructions on the extent of allowable exercises and activities in school.
Urgent reporting:
When a student is rated as follows at the secondary screening or later examination, the healthcare profession in charge should contact his/her school nurse via phone or other methods to inform the results without delay:
(1) When the student should be referred to a cardiology clinic for detailed examination.
(2) When the student is rated categorized into one of the categories in School Activity Management Table where exercise should be restricted.
The school nurse should communicate with other members of the school, inform the teacher in charge and physical education teacher of the fact to take necessary measures to prevent hazards during exercise or other school activities until detailed examination are completed.
Those who have not undergo secondary screening or later examination should be instructed to visit a cardiology clinic and provide School Activity Management Table.
2.10 The Importance of Screening Those Who Need Follow-up and the Method of Implementation
In order to fulfill the purpose of heart disease screening in schools, a sufficient follow-up system should be established to prevent withdrawals. Those rated as “follow-up required” in school heart disease screening should undergo follow-up examination for changes in symptoms and physical conditions to confirm whether their rating of allowable intensity of exercise and daily activities is appropriate or not.
Information on such follow-up may not be shared appropriately in school in circumstances when the school nurse is transferred to another school, and therefore some students requiring follow-up may be missed. To avoid such circumstances, medical institutions that screen students should manage data and appear on a list of those who require follow-up in each school, and provide each school with a list of students who need follow-up at an appropriate timing. Satisfactory data management by medical institutions is critical to ensure follow-up examination are conducted with no omissions.
2.10.1 Conducting Follow-up Examination
Whenever necessary, follow-up examination should be implemented as follows:
Timing: Two styles are available: (1) Students requiring follow-up may be evaluated in one place in March, one month before starting a new school year, or at the beginning of a new school year. (2) They may join heart disease screening for new first-year students.
Methods: In the first style, those who need follow-up are treated similarly to those undergoing secondary mass screening, and may be examined according to their individual conditions. In the second style, healthcare professionals visit each school to evaluate them together with first-year students. They should undergo the same primary screening evaluation. When they are known to need additional examination, they will undergo a secondary screening, and will be managed accordingly (Figure 5).
3. Rating Criteria for Primary Screening
In primary heart disease screening in schools, all students undergo heart disease screening questionnaires and ECG, and some of them may also undergo phonocardiography and blood pressure monitoring whenever necessary. The results of primary screening are rated as follows: No abnormal findings, no management required, follow-up required, medical management required, secondary screening required, and detailed examination required.6
3.1 Evaluation of Heart Disease Screening Questionnaire6,7
The advancement of pediatric cardiology and healthcare systems for children have enabled almost all children with congenital heart diseases to be diagnosed before the reach school age. As the diagnosis and acute phase treatment of Kawasaki disease have advanced substantially, cardiac sequelae of Kawasaki disease have become less prevalent. An important aspect of the heart disease screening program in schools is grasping the history of these diseases in order to ensure an appropriate rating in terms of allowable intensity of exercise and daily activities. The presence/absence of common signs/symptoms of cardiovascular diseases and family history of sudden death in childhood are all important information as these suggest the presence of cardiac disease that runs in families.
These pieces of information can be obtained through the heart disease screening questionnaire. As the questionnaire investigates these important matters that may be known by either the parents or other family members who know the student’s growth history, rather than the student, should complete the questionnaire form.
Findings by the school physician, school nurse, teacher in charge, and physical education teacher are important information as well. Therefore, some questionnaire forms include columns for school physician’s findings, and those for physical findings and activities in school. These findings should be reviewed together with screening findings.
The School Heart Disease Screening Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery has reported a model questionnaire form in 2004, and recommended that each area should modify the form according to the age range of participants and geological characteristics before use (Table 1).7
Table 1.
Example of Heart Disease Screening Questionnaire (1)
| Heart disease screening questionnaire |
Dear parents,
The following questions are important to screen your child for heart diseases. Parents should complete all columns. Do not allow your child
to complete the form. Please fill in the blanks, and circle the items that apply |
| School name Grade Class No. |
| Name M/F Birth date (year/month/date) (age: years) |
| Q1. Has your child ever been diagnosed with heart disease? (yes, no) |
| If yes, please answer the following questions |
| The disease is (congenital heart disease, arrhythmia, myocardial disease, or others) |
| Diagnosis is , and the hospital name is . Has your child undergone surgery? (yes, no) |
| If yes, |
| Age:
years months Hospital: |
| If your child has been told they have heart murmurs, please answer the following questions |
| Did the doctor say that the murmurs are functional (innocent) and not related to heart disease? (yes, no) |
| Has your child been told they have rheumatic heart disease? (yes, no) |
| Q2. Has your child ever suffered from Kawasaki disease? (yes, no) |
| If yes, please answer the following questions |
| Kawasaki disease developed (at the age of years and months). Diagnosis was made at hospital |
| Has your child undergone two-dimensional echocardiography (cardiac ultrasonography) within 1 month after the onset? (yes, no) |
| Has the doctor said that your child has coronary artery aneurysm (cardiac sequelae)? (yes, no) |
| Has the doctor said that your child still has sequelae? (yes, no) |
| Q3. Has your child complained of the followings? |
| (1) Palpitations (2-fold increase in heart rate) develop suddenly (yes, no) |
| (2) Pulse-skipping occurs (yes, no) |
| (3) Fainting, rather than lightheadedness or seizures, occurs at rest, during exercise, or immediately after exercise (yes, no) |
Q4. Has any of your family and relatives (e.g., parents, siblings, grandparents, aunts and uncles, etc.) died suddenly from heart disease or
with unknown cause under 40 years of age? (yes, no) |
(Source: Baba K, et al. 2004.7)
In this questionnaire survey, each student is assessed based on his/her history of cardiac disease and current signs/symptoms as well as his/her family history. The items of evaluation are selected by each area according to its circumstances.8 Table 2
summarizes how to assess findings reported through the heart disease screening questionnaire that was prepared by authors of this guideline document.9
Table 2.
Guideline for Assessment of the Heart Disease Screening Questionnaire
| · A history of congenital heart disease, repair surgery for congenital heart disease, myocardial disease or other heart disease is reported |
| Receiving periodic medical assessment |
Management category is determined at specialist clinic |
| Not receiving periodic medical assessment |
The child should visit the clinic where he/she was diagnosed and treated to
determine exercise management category. When the child cannot visit the clinic,
he/she will undergo secondary screening and later examinations |
If the heart disease has cured due to spontaneous
closure, or when the child underwent repair surgery for
patent ductus arteriosus
If the child underwent cardiac intervention and the
attending physician determined that no management is
required |
On the basis of the results of primary screening, the child is rated as “no abnormal
findings” or “no management required or secondary screening and further
examination required whenever necessary” |
| · A history of arrhythmia and abnormal ECG findings is reported |
| Receiving periodic medical assessment |
Management category is determined at specialist clinic |
| Not receiving periodic medical assessment |
The child should visit the clinic where he/she was diagnosed and treated to
determine exercise management category. When the child cannot visit the clinic,
he/she will undergo secondary screening and later examination |
When the child no longer has such findings and the
attending physician rated the child as “no management
required” or when findings have been found and
followed up only in school heart disease screening |
On the basis of the results of primary screening, the child is rated as, “no abnormal
findings” or, “no management required or secondary screening and further
examination required whenever necessary” |
| · A history of Kawasaki disease is reported |
| Receiving periodic medical assessment |
Management category is determined at specialist clinic |
When the child is not currently followed up at clinic, and
sequelae are present or unknown |
The child should visit the clinic where he/she was diagnosed and treated to
determine exercise management category. When the child cannot visit the clinic,
he/she will undergo secondary screening and later examination |
When the child is not currently followed up at clinic, and
the attending physician denied cardiac sequelae |
The child should be rated as, “no management required” if ≥5 years have
passed since onset. Otherwise he/she should visit the clinic where he/she was
diagnosed and treated to determine exercise management category in priciple |
| · Findings from school physician, subjective complaints or a family history of sudden death at <40 years of age is reported |
| Receiving periodic medical assessment |
Management category is determined at specialist clinic |
| Not receiving periodic medical assessment |
The child should be rated considering symptoms, family history and ECG findings,
and should undergo secondary screening and further examination whenever
necessary |
(Tabulated based on the Tokyo Medical Association. 2009.9)
In the current heart disease screening program in schools, all students in the first year of elementary school, junior high school and senior high school should undergo an ECG in school. ECG findings are the only objective data obtained in this program. High-resolution ECG recordings should be used. Before ECG recording, healthcare professionals should explain the purpose and methods of ECG recording to students to ease their anxieties, and use appropriate measures such as recording ECG at rest, ensuring that the ECG instrument is grounded appropriately and leads are placed in appropriate positions, and avoiding filters during ECG recording whenever possible. At least 8 to 10-second ECG recording should be obtained from each participant. When arrhythmias are detected during recording, at least 2-fold duration of ECG recording is required.
ECG data should be reviewed by physicians with expertise in interpretation of ECG records in children and young people. When an automated ECG analysis program is used, the program should be adjusted for age and gender to ensure appropriate interpretation of ECG records in this population. Programs for adults should not be used.
When participants show any ECG findings that may indicate serious problems during primary screening, they should be considered for referral to a cardiology clinic for detailed examination.
Table 3
outlines ECG findings that require detailed examination.
Table 413
indicates classification codes of ECG findings in primary screening.
Table 3.
Examples of ECG Findings That Prompt Detailed Examination
| Findings |
Condition |
| QS pattern |
QS pattern when initial R-wave is present in adjacent right precordial leads |
| In any of lead I, II, and/or V6 (III and aVF) |
| Found in all leads of V1 to V4 |
| Definite finding of right ventricular hypertrophy |
≥5 points in the point-based criteria for right ventricular hypertrophy |
| Definite finding of left ventricular hypertrophy |
≥5 points in the point-based criteria for left ventricular hypertrophy |
| Severe ST depression |
ST-J depression ≥0.2 mV, and negative or biphasic T wave with ≥0.5 mV negative
deflection (any of lead I, II, aVL, aVF, V1 to V6, V3 to V6 for T wave) |
| Negative T waves in left precordial leads |
If present in V3 to V6 leads (in V4 to V6 leads in elementary school students) |
| Second-degree atrioventricular block |
Mobitz type II atrioventricular block |
| 2:1 block |
| Third-degree atrioventricular block |
Including advanced atrioventricular block |
| Complete left bundle branch block |
ECG changes consistent with complete left bundle branch block |
| Polymorphic premature ventricular contractions |
Polymorphic premature ventricular contractions are present |
Couplet or short run of premature ventricular
contractions |
Couplet or short run of premature ventricular contractions develop |
Premature ventricular contractions with R on T
phenomenon |
Premature ventricular contractions with R on T phenomenon are observed |
Premature ventricular contractions followed by
abnormal T waves |
Premature ventricular contractions followed by abnormal T waves are observed |
| Ventricular tachycardia |
Including polymorphic ventricular tachycardia |
| Atrial flutter/fibrillation |
ECG changes consistent with atrial flutter or fibrillation |
| Supraventricular tachycardia |
ECG changes consistent with supraventricular tachycardia |
| Sinoatrial block, severe bradycardia |
ECG changes consistent with sinoatrial block or severe bradycardia |
| QT prolongation |
If corrected QT interval (sec) determined using the tangent method and Fridericia
correction exceeds 0.43 in boys and girls in the first year of elementary school, 0.44 in
boys and girls in the first year of junior high school, 0.44 in boys and 0.45 in girls in the
first year of senior high school |
| Brugada-type ECG |
ST elevation of >0.2 mV at the J point, and coved or saddleback ST-T change was
observed in any of right precordial leads V1, V2 or V3 |
| Others |
The interview questionnaire and other findings indicating a risk of sudden death |
(Tabulated based on the Japanese Society of School Health. 2013,6
the Tokyo Medical Association. 2009,9
Baba K, et al. 2006.10)
Table 4.
Classification Codes of ECG Findings in Primary Screening
Source: Guidelines for ECG assessment in primary screening to select those needing secondary heart disease screening in school published
by the Japanese Society of Pediatric Cardiology and Cardiac Surgery (JSPCCS 2006)
(Those with ECG findings listed in Table 3 should be considered for detailed examination without delay) |
| Category |
| Group A: Findings requiring secondary screening/further examination |
| Group B: Findings not requiring secondary screening if no other findings are present |
| Group C: Findings that may not be focused in heart disease screening in schools |
| I. Q Wave |
| Category |
Code No. |
Findings |
| 1. Broad Q wave |
| A |
1-1-1 |
|Q|/R ≥1/3 and Q ≥0.03 sec (any one of lead I, II, V2 to V6) |
| 1-1-2 |
Q ≥0.04 sec (any one of lead I, II, V1 to V6) |
| 1-1-4 |
QIII ≥0.05 sec, and |Q aVF| ≥0.1 mV |
| 1-1-5 |
Q aVF ≥0.05 sec |
| B |
1-1-3 |
Q aVL ≥0.04 sec, and R aVL ≥0.3 mV |
| 1-2-2 |
0.03≤Q<0.04 sec (any one of lead I, II, V2 to V6) |
| 1-2-4 |
0.04≤QIII<0.05 sec, and |Q aVF| ≥0.1 mV |
| 1-2-5 |
0.04≤Q aVF<0.05 sec |
| C |
1-2-1 |
|Q|/R ≥1/3, and Q ≥0.02 and <0.03 sec (any one of lead I, II, V2 to V6) |
| 1-3-1 |
|Q|/R ≥1/5 and <1/3, and 0.02≤Q<0.03 sec (any one of lead I, II, V2 to V6) |
| 1-3-3 |
0.03≤Q aVL<0.04 sec, and R aVL ≥0.3 mV |
| 1-3-4 |
0.03≤QIII<0.04 sec, and |Q aVF| ≥0.1 mV |
| 1-3-5 |
0.03≤Q aVF<0.04 sec |
| 2. QS pattern |
| A |
1-1-6 |
QS pattern when initial R-wave is present in adjacent right precordial leads (any one of lead V2 to V6) |
| 1-1-7 |
QS pattern (all leads in V1 to V4, or all leads in V1 to V5) |
| 1-1-8 |
QS pattern (lead V6) |
| 1-2-3 |
QS pattern (lead I or II) |
| 1-2-7 |
QS pattern (all leads in V1 to V3) |
| 1-3-6 |
QS pattern (leads III and aVF) |
| C |
1-3-2 |
QS pattern (leads V1 and V2) |
| 3. Deep Q wave |
| A |
1-4-1 |
|QV5| < |QV6|, and |QV6| ≥0.5 mV |
| B |
1-2-6 |
|Q| ≥0.5 mV (lead III or aVF) |
| 4. Other Q wave findings |
| A |
1-5-1 |
qR(S) pattern (lead V1) |
| II. QRS Electrical Axis |
| Category |
Code No. |
Findings |
| B |
2-1-0 |
−90°<QRS axis≤−30° |
| 2-4-1 |
−180°<QRS axis≤−90° |
| C |
2-1-1 |
−30°<QRS axis≤0° |
| 2-2-1 |
+135°≤QRS axis<180° |
| 2-2-2 |
+120°≤QRS axis<+135° |
| 2-3-0 |
+90°≤QRS axis<+120° |
| 2-5-0 |
Indeterminate axis (90° in the frontal plane) |
| III. R Wave and S Wave |
| Ventricular hypertrophy: According to the point-based ECG assessment criteria for Ventricular hypertrophy in children (See Attachment 1) |
| IV. ST Junction and ST segment |
| Category |
Code No. |
Findings |
| A |
4-1-1 |
ST-J depression ≥0.2 mV, and flat or down-sloping ST segment (any one of lead I, II, aVL, aVF, or V1 to V6) |
| 4-1-2 |
0.1≤ST-J depression<0.2 mV, and flat or down-sloping ST segment (any one of lead I, II, aVL, aVF, or V1 to V6) |
| 4-2-1 |
0.05≤ST-J depression<0.1 mV and flat or down-sloping ST segment (any one of lead I, II, aVL, aVF, or V1
to V6) (girls in junior and senior high schools with this finding only in lead aVF are categorized to Group B) |
| B |
4-3-1 |
ST-J depression <0.05 mV, down-sloping ST segment, and depression of ≥0.05 mV from baseline at ST segment
or the lowest point of T wave (any one of lead I, II, aVL, or V2 to V6) |
| 4-4-1 |
ST-J depression >0.2 mV, and up-sloping or U-shaped ST segment (any one of lead I, II, aVL, or V1 to V6) (girls
in junior and senior high schools with this finding only in lead aVF are categorized to Group C) |
| C |
4-4-2 |
ST-J depression >0.1 mV, and up-sloping or U-shaped ST segment (any one of lead I, II, aVL, or V1 to V6) |
| V. T Wave |
| Category |
Code No. |
Findings |
| A |
5-1-1 |
Negative, or biphasic T wave with negative deflection ≥0.5 mV (any one of lead I, II, aVL [R ≥0.5 mV], aVF
[mainly positive QRS], or V3 to V6) (any one lead of V4 to V6 in precordial leads in elementary school students) |
| 5-2-1 |
Negative, or biphasic T wave with negative deflection ≥0.1 mV and <0.5 mV (any one of lead I, II, aVL [R ≥0.5 mV],
aVF [mainly positive QRS], or V4 to V6) (those with findings only in lead aVF are categorized to Group B) |
| B |
5-3-1 |
Flat (0), negative, or biphasic (± type) T wave with negative deflection <0.1 mV (flat or down-sloping ST segment)
(any one of lead I, II, aVL [R ≥0.5 mV], V5, or V6) (girls in junior or senior high schools with this finding are
categorized to Group C) |
| 5-6-1 |
Positive TV1, RV1 ≥ |SV1| (only in students in the first year of elementary school or younger) |
| C |
5-4-1 |
Positive T wave, 1/20 >T/R and R ≥1.0 mV (any one of lead I, II, aVL, V5 or V6) |
| VI. Atrioventricular Conduction |
| Category |
Code No. |
Findings |
| 1. Complete atrioventricular block |
| A |
6-1-0 |
Third degree (complete) atrioventricular block |
| 2. Second degree atrioventricular block |
| A |
6-2-1 |
Second degree atrioventricular block (Mobitz type II) |
| 6-2-2 |
Second degree atrioventricular block (2:1) |
| 6-2-3 |
Second degree atrioventricular block (Wenckebach type) |
| 3. PR (PQ) interval |
| A |
6-3-0 |
PR interval >0.28 sec |
| 6-3-1 |
PR interval >0.24 sec (only in elementary school students; Junior/senior high school students with the same
finding are categorized to Group B) |
| C |
6-3-3 |
PR interval ≥0.20 sec |
| 6-5-1 |
PR interval <0.08 sec |
| 4. WPW syndrome |
| A |
6-4-1 |
WPW-type ECG: PR interval <0.12 sec and QRS duration ≥0.12 sec and VAT >0.06 sec (any one of lead I, II,
aVL, V4, V5, or V6) |
| 6-4-2 |
WPW-type ECG: PR interval <0.10 sec and QRS duration ≥0.10 sec and VAT >0.05 sec (any one of lead I, II,
aVL, V4, V5, or V6) (only in elementary school students) |
| 6-4-3 |
WPW-type ECG (intermittent): |
| 5. Aberrant conduction |
| C |
6-6-0 |
Aberrant conduction |
| 6. Artificial pacemaker |
| A |
6-8-0 |
Artificial pacemaker |
| VII. Intraventricular Conduction |
| Category |
Code No. |
Findings |
| 1. Complete left bundle branch block |
| A |
7-1-1 |
Complete left bundle branch block: QRS duration ≥0.12 sec, and VAT ≥0.06 sec (any one of lead I, II, aVL, V5,
or V6) with no Q wave |
| 7-1-2 |
Complete left bundle branch block: QRS duration ≥0.10 sec, and VAT ≥0.05 sec (any one of lead I, II, aVL, V5,
or V6) with no Q wave (only in elementary school students) |
| 7-1-3 |
Intermittent complete left bundle branch block |
| 2. Complete right bundle branch block |
| A |
7-2-1 |
Complete right bundle branch block: QRS duration ≥0.12 sec, and R’>R and VAT ≥0.06 sec (lead V1, or V2) |
| 7-2-2 |
Complete right bundle branch block: QRS duration ≥0.10 sec, and R’>R and VAT ≥0.05 sec (lead V1, or V2)
(only in elementary school students) |
| 7-2-3 |
Intermittent complete right bundle branch block |
| 3. Incomplete right bundle branch block |
| A |
7-3-1 |
Incomplete right bundle branch block: 7-3-0 and R’V1≥|SV1| (only in junior and senior high school students) |
| 7-3-3 |
Incomplete right bundle branch block: 7-3-2 and R’V1≥|SV1| |
| B |
7-3-0 |
Incomplete right bundle branch block: QRS duration <0.12 sec, and R’>R (lead V1, or V2) (only in junior and
senior high school students) |
| 7-3-2 |
Incomplete right bundle branch block: QRS duration <0.10 sec, and R’>R (lead V1, or V2) |
| C |
7-5-0 |
QRS duration <0.12 sec, R-R’ type, and R’≤R (lead V1, or V2) (only in junior and senior high school students) |
| 7-5-1 |
QRS duration <0.10 sec, R-R’ type, and R’≤R (lead V1, or V2) |
| 7-5-2 |
7-5-0 or 7-5-1, and R’V1 ≥0.5 mV and RV1 ≥SV1 |
| 4. Intraventricular conduction disturbance |
| A |
7-4-0 |
Intraventricular conduction disturbance: QRS duration ≥0.12 sec |
| 7-4-1 |
Intraventricular conduction disturbance: QRS duration ≥0.10 sec (only in elementary school students) |
| 5. Incomplete left bundle branch block |
| A |
7-6-0 |
Incomplete left bundle branch block: 0.10≤QRS duration<0.12 sec, and R-R’ type and R’≥R (lead V5, or V6) with
no Q wave |
| 7-6-1 |
Incomplete left bundle branch block: QRS duration <0.10 sec, and R-R’ type and R’≥R (lead V5, or V6), with no
Q wave (only in elementary school students) |
| 6. Left anterior fascicular block |
| A |
7-7-0 |
Left anterior fascicular block: QRS duration <0.12 sec, and QI ≥0.025 mV, QI duration <0.03 sec, and left axis
deviation of −45° or more |
| 7-7-1 |
Left anterior fascicular block: QRS duration <0.10 sec, and QI ≥0.025 mV, QI duration <0.03 sec, and left axis
deviation of −30° or more (only in elementary school students) |
| 7. Bifascicular block |
| A |
7-8-0 |
Bifascicular block: 7-2-1 and left axis deviation of −45° or more |
| 7-8-1 |
Bifascicular block: 7-2-1 and left axis deviation of −30° or more (only in elementary school students. Junior and
senior high school students with this finding are categorized to Group C) |
| VIII. Rhythms |
| Category |
Code No. |
Findings |
| 1. Premature supraventricular contractions |
| A |
8-1-4 |
Multiform premature supraventricular contractions |
| B |
8-1-1 |
Monomorphic premature supraventricular contractions (Sporadic cases are categorized to Group C) |
| 2. Premature ventricular contractions |
| A |
8-1-2 |
Monomorphic premature ventricular contractions |
| 8-1-3 |
8-1-1 plus 8-1-2 |
| 8-1-5 |
Polymorphic premature ventricular contractions |
| 8-1-6 |
Premature ventricular contraction coupletes |
| 8-1-7 |
R on T type ventricular premature contraction |
| 8-1-8 |
Premature ventricular contractions followed by abnormal T waves |
| 3. Ventricular tachycardia |
| A |
8-2-1 |
Ventricular tachycardia |
| 4. Idioventricular rhythm |
| A |
8-2-2 |
Idioventricular rhythm |
| 5. Atrial fibrillation |
| A |
8-3-1 |
Atrial fibrillation |
| 6. Atrial flutter |
| A |
8-3-2 |
Atrial flutter |
| 7. Atrial flutter/fibrillation |
| A |
8-3-3 |
Atrial flutter/fibrillation |
| 8. Supraventricular tachycardia |
| A |
8-4-1 |
Supraventricular tachycardia |
| 9. Sinus arrest or sinoatrial block |
| A |
8-5-1 |
Sinus arrest or sinoatrial block |
| 10. Junctional rhythm |
| B |
8-6-1 |
Junctional rhythm |
| 11. Atrioventricular dissociation |
| B |
8-6-2 |
Atrioventricular dissociation |
| 12. Escape beats or escape rhythm |
| B |
8-6-3 |
Escape beats or escape rhythm |
| 13. Sinus tachycardia |
| A |
8-7-1 |
Heart rate (≥200 bpm) |
| 8-7-2 |
Heart rate (≥180 bpm) |
| B |
8-7-3 |
Heart rate (≥150 bpm) |
| 8-7-4 |
Heart rate ≥140 bpm (only in junior and senior high school students. Elementary school students with this finding
are categorized to Group C) |
| C |
8-7-5 |
Heart rate (≥130 bpm) |
| 8-7-6 |
Heart rate (≥100 bpm) |
| 14. Sinus bradycardia |
| B |
8-8-1 |
Heart rate (<40 bpm) |
| 8-8-2 |
Heart rate <45 bpm (only in elementary school students. Junior and senior high school students with this finding
are categorized to Group C) |
| C |
8-8-3 |
Heart rate (<50 bpm) |
| 8-8-4 |
Heart rate (<60 bpm) |
| 15. Other arrhythmias |
| A |
8-9-9 |
Arrhythmias not otherwise specified |
| C |
8-9-1 |
Sinus arrhythmia |
| IX. Others |
| Category |
Code No. |
Findings |
| 1. Low voltage |
| B |
9-1-0 |
Low voltage: QRS <0.5 mV (all leads of I, II, and III) or QRS <1.0 mV (all leads of V1 to V6) |
| 2. Left atrial load |
| B |
9-3-1 |
P ≥0.30 mV (any one of lead II, III, aVF, or V1) |
| 9-3-3 |
P duration ≥0.12 sec (any one of lead I, II, or aVL) |
| 9-3-4 |
P duration ≥0.10 sec (any one of lead I, II, or aVL) (only in elementary school students. Junior and senior high
school students with this finding are categorized to Group C) |
| 9-3-5 |
9-3-3 or 9-3-4 (only in elementary school students), biphasic P wave and positive duration < negative duration
(in lead V1 or V2) |
| C |
9-3-2 |
P ≥0.25 mV (any one of lead II, III, aVF, or V1) |
| 3. Dextrocardia |
| A |
9-6-1 |
Dextrocardia |
| 4. QT prolongation |
| A |
9-7-1 |
QT prolongation: There have been no screening criteria for QT prolongation by using automatic ECG analysis
regarding school screening. In this guideline document, QT prolongation is defined as a QTc of ≥0.45
sec obtained using the Fridericia correction. (QTc obtained in automatic ECG analysis is often about 20 msec longer
than that obtained with the tangent method.) If QT prolongation is suspected in automatic analysis, ECG should
be reviewed manually using the tangent method (see the following table)
The morphology of T wave should be considered as well
Table: Criteria of QT prolongation in the tangent method
· Boys and girls in 1 st year of elementary school: ≥0.43 sec
· Boys and girls in the 1st year of junior high school: ≥0.44 sec
· Students in the 1st year of senior high school: ≥0.44 sec in boys and 0.45 sec in girls
No data are available for other grades. Refer to the above figures for other grades
(Tabulated based on Hazeki D, et al. 2010.80) |
| 5. Reexamination is required |
| A |
9-8-0 |
ECG is unreadable due to baseline drift, alternate current interference, artifacts from electromyogram or other
technical errors |
| 6. Negative U wave |
| B |
9-9-1 |
Negative U wave |
| 7. Others
|
| A |
9-2-2 |
Brugada type ECG: Coved ST elevation in right precordial leads (ST elevation ≥0.2 mV at the J point in any one
of lead V1, V2 or V3, and coved type ST-T cahnge) |
| B |
9-2-3 |
Brugada type ECG: Saddleback ST elevation in right precordial leads (ST elevation ≥0.2 mV at the J point in
any of leads V1, V2 or V3, and saddleback type ST-T change) |
| C |
9-2-1 |
ST elevation ≥0.2 mV (any one of lead II, III, aVF, V5 or V6) (Ignore this finding when 6-4 or 7-1 is present) |
| 9-5-1 |
T >1.2 mV (any one of lead II, III, aVF or V6) (Ignore this finding when 6-4, 7-1 or 7-2 is present) |
| 9-7-2 |
VAT V6 ≥0.06 sec (Ignore this finding when 6-4 or 7-1 is present) |
| 9-7-3 |
VAT V6 ≥0.05 sec (Ignore this finding when 6-4 or 7-1 is present) |
| 9-7-4 |
VAT V1 ≥0.035 sec (Ignore this finding when 6-4, 7-2 or 7-3 is present) |
(1) Those with the finding 1-2 or 1-3 should be carefully examined for codes starting with 4 and 5. Myocardial ischemia and myocardial disease should be carefully ruled out in those with 1-2 or 1-3 plus codes starting with 4 or 5. (2) Those with the finding 7-3 or 7-5 should be examined carefully for heart sounds (phonocardiogram). (3) Those with tendency towards tachycardia or bradycardia should be examined carefully for cardiac rhythm disorder. (4) Those with severe QRS electrical axis deviation should be observed carefully for other findings. (5) Some ECG findings may need prompt management. (Source: Baba K, et al. 200610 with modifications)
Attachment 1.
Point-Based ECG Assessment Criteria for Ventricular Hypertrophy in Children
When ECG analysis is difficult due to baseline drift, alternate current interference, electromyogram interference, wrong lead placement, and other technical errors, a repeat ECG should be performed for the affected participants. Those in whom a repeat ECG is difficult should undergo a second screening or later examination in which a 12-lead ECG at rest should be recorded without fail.
3.3 ECG Review in Adults
Adults over 20 years of age may undergo screening for senior-high school students. Their ECG records should be assessed according to the criteria for adults.
3.4 Assessment of Phonocardiogram
Phonocardiography is still included in primary heart disease screening in many areas. A phonocardiogram helps physicians evaluate cardiac murmurs and abnormal heart sounds more objectively during auscultation and accurately detect heart diseases including those not causing abnormal ECG findings. Phonocardiography is also useful in diagnosing innocent heart murmurs. However, children with cardiac diseases causing murmurs and abnormal heart sounds are often diagnosed through screening programs during infancy and young childhood, therefore phonocardiography in school health screening is of less significance nowadays.
A typical method in the heart disease screening in schools is two-point phonocardiography recorded with a microphone positioned over the upper left sternal border and another microphone over the apex of the heart.
The phonocardiogram should be rated according to the “criteria for the assessment of two-point phonocardiograms in children” proposed by the Japanese Society of Pediatric Cardiology and Cardiac Surgery.14
3.5 Comprehensive Evaluation
Even when no definite abnormalities are found in any single one of the primary screening items of questionnaire, ECG or phonocardiogram, some participants should be selected for secondary screening and later examination according to the results of comprehensive evaluation. Such comprehensive evaluation is especially important to select those suspected to have catecholamine-induced polymorphic ventricular tachycardia, cardiomyopathy, myocarditis, congenital heart disease, valvular diseases, Kawasaki disease, and idiopathic pulmonary arterial hypertension, among others. The reader is advised to refer to the relevant section of disease-specific management for their characteristic findings at screening.
4. Evaluation in Secondary Screening and Later Examination
In secondary screening and later examination, those selected through the primary screening are assessed in detail for the presence/absence of cardiac diseases in order to diagnose the disease, assess its severity, and obtain information to be used to determine their allowable intensity of exercise and daily activities.
Secondary screening and later examination should consist of (1) medical examination by a specialist, (2) chest X-ray, (3) ECG, (4) exercise ECG, and (5) echocardiography. However, these examinations are not necessarily required for all students selected with the primary screening. Type of examinations should be selected individually according to their symptoms and findings in the primary screening15
(Table 5).
Table 5.
Examinations in Secondary Screening and Further Examination
| Criteria for primary screening |
Conditions and diseases requiring
consideration in secondary
screening and later examination |
Items of secondary screening and
later examination
[Examination by specialists and 12-lead
ECG are essential. Items in parentheses
should be conducted whenever necessary] |
| Screening questionnaire |
A history of congenital heart disease, repair
surgery for congenital heart disease or
arrhythmias |
Diagnosis, periodic follow-up at clinic |
Chest X-ray, echocardiography, (exercise ECG) |
| History of myocardial disease |
|
Chest X-ray, echocardiography |
| History of Kawasaki disease |
Presence/absence of cardiac sequelae,
periodic follow-up at clinic |
Chest X-ray, echocardiography |
History of hypertension and rheumatic fever
Any symptoms suggestive of heart disease |
Diagnosis, symptoms and ECG findings
that prompt secondary screening and later
examination |
Chest X-ray, echocardiography, (exercise ECG) |
| Findings noted in school |
Irregular heart sounds and murmurs that
prompt secondary screening and later
examination |
(Chest X-ray, echocardiography, exercise ECG) |
| ECG |
| Q wave |
Myocardial disease, myocardial ischemia,
ventricular overload, malposition |
Chest X-ray, echocardiography, exercise ECG |
| R wave, S wave |
Ventricular hypertrophy or other causative disease |
Chest X-ray, echocardiography |
ST-T change
T wave |
Myocardial disease, myocardial ischemia,
ventricular overload, myocardial ischemia |
Chest X-ray, echocardiography, (exercise ECG)
Chest X-ray, echocardiography, (exercise ECG) |
| Atrioventricular conduction |
Cardiac symptoms, underlying heart
disease |
Exercise ECG, (chest X-ray, echocardiography) |
Bundle branch block,
intraventricular conduction delay |
Cardiac symptoms, underlying heart
disease, history of cardiac surgery |
(Chest X-ray, echocardiography, exercise ECG) |
| Rhythm |
Cardiac symptoms, underlying heart
disease |
(Chest X-ray, echocardiography, exercise ECG) |
| Others |
Cardiac symptoms, underlying heart
disease |
(Chest X-ray, echocardiography, exercise ECG) |
The results of the secondary screening and later examination are rated as follows: “No abnormal findings,” “No management required,” “Follow-up required,” “Medical management required,” “Detailed examination required,” and “Not examined yet.” The final rating should be made after all necessary examinations have been conducted. Evaluation of findings and determining allowable intensity of exercise and daily activities should be made according to the sections of disease-specific management in Chapter II of the present guidelines, the “Guidelines for School Life and Exercise in Pupils and Students with Congenital Heart Disease (JSPCCS 2012),”16
the “Guidelines for School Life and Exercise in Pupils and Students with Arrhythmias without Underling Heart Diseases (JSPCCS 2013)”,11
and the “Guidelines for Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease (JCS 2013)”,17
and other appropriate documents.
Some students selected from the primary screening may need Holter ECG, CT/MRI, nuclear imaging and/or cardiac catheterization. These examinations may not necessarily be included in the secondary screening or later programs. Rather, those who are rated as “detailed examination required” may be referred to special clinics where these examinations may be conducted.
5. Examination at a Cardiology Clinic and Follow-up Examination
In primary or secondary screening or later examination, some students may be found to need accurate diagnosis and treatment. Others may not be correctly diagnosed or rated in terms of allowable intensity of exercise and daily activities only on the basis of finding obtained with the screening system. These participants should be rated as “detailed examination required” and be recommended to visit a cardiology clinic.6
Those who fall into any of the items below should be instructed to visit a cardiology clinic:
(1) Those with “ECG findings for which prompt detailed examination should be considered” (Table 3).
(2) Those who may be rated as “E-Prohibited” or more strict categories.
(3) Those who need treatment.
(4) Those who need examination in addition to those conducted at the time of secondary screening in order to determine their allowable intensity of exercise and daily activities.
(5) Those for whom currently available findings are insufficient for accurate diagnosis.
(6) Those who should visit a cardiology clinic more than twice a year for follow-up examination and management.
When visiting a cardiology clinic, a patient referral document that describes the reason for referral and screening findings, and, if possible, a copy of ECG chart and report forms for describing findings. Senior high school students over 20 years of age should be referred to an internal medicine clinic or a cardiovascular clinic. Students rated as “detailed examination required” should be tentatively classified into an appropriate category of allowable intensity of exercise and daily activities, and they and their parents should be instructed to visit a cardiology clinic as soon as possible. The school should ask the cardiology clinic to provide a final report on each referred student, and should retain the final reports as data for summarization and post-hoc analysis.
Follow-up evaluation is important for those who are rated as “management required” at the final evaluation of heart disease screening in schools. The pathophysiology and manifestations of heart diseases in children and young people may change over time and with age, and the intensity of activities in school and home may change over time. Accordingly, the school, parents, family physician, and specialist should share information and evaluate the student and revise the category of allowable intensity of exercise and daily activities periodically. For this reason, those requiring management, i.e., those who are classified into any categories other than “no management required” or “E-allowed” should be followed up periodically at a cardiology clinic. (See “
6. School Activity Management Table
” for categories of allowable intensity of exercise and daily activities). On the other hand, those with mild heart disease or those after cardiac surgery who are rated at the “E” category and are recommended to undergo a follow-up evaluation once a year may be rated as “follow-up evaluation required”, and may be followed up by general partitioners or through heart disease screening programs in schools. For those rated “follow-up evaluation required”, follow-up evaluation sessions should be planned according to the schedule of physicians and items to be evaluated. During follow-up evaluation sessions, physicians should review their history of conditions and past records before determining their allowable intensity of exercise and daily activities. Physicians should also determine the timing of the next follow-up evaluation, and record the reasons for the current follow-up evaluation, items evaluated, rating, and instructions given to help future reviews.6
In order to ensure consistent management, each student should continue to undergo follow-up evaluations at the same clinic whenever possible.
6. School Activity Management Table
The School Activity Management Table is issued for each of those who have been found to have abnormal findings or disease during heart disease screening in schools and those who have been diagnosed as having heart disease or are under treatment for it. The Table contains instructions by the attending physician or screening physician on his/her allowable intensity of exercise and daily activities to ensure his/her healthy school life. The types and names of sport and exercise activities differ between elementary schools and junior and senior high schools, different school activity management tables are used for elementary school students and junior and senior high school students. In 2011, the School Activity Management Table was revised to make the following changes:6
1. A new column of “Other cautions” is added to allow the attending physician and school physician to describe their opinions.
2. The previous version of the School Activity Management Table tended to overly restrict exercise and daily activities. The new version encourages students to join gym classes at their appropriate exercise intensity.
3. The School Activity Management Table for Elementary School Children indicates exercise intensity for each grade level.
6.1 Contents of School Activity Management Table
(1) Diagnosis: The name of illness or findings.
(2) Category of allowable intensity of exercise and daily activities: Rated as “No management required” or “Management required,” which is rated from A to E. The categories A to E are defined as follows:
A: Requires treatment at home or in hospital
B: Goes to school but must avoid exercise
C: Can do exercise that is mild intensity for average students at the same age
D: Can do exercise that is moderate intensity for average students at the same age
E: Can do exercise that is vigorous intensity for average students at the same age
(3) Whether school sport club activity is allowed or prohibited; Name of club.
(4) Next visit.
6.2 Definition of Exercise Intensity
a. Mild Exercise
Physical activities that do not increase respiratory rate in average students at the same age. Ball sports without foot-work. Resistance (isometric) exercise is not defined as mild exercise.
b. Moderate Exercise
Physical activities that increase respiratory rate without causing shortness of breath in average students of the same age. Players may talk easily with partners, if any, during exercise. No close body contact. Moderate exercise includes resistance (isometric) exercise without full-strength.
c. Intense Exercise
Physical activities that increase respiratory rate and cause shortness of breath in average students at the same age. Intense exercise includes isometric exercise associated with teeth clenching, shouting, facial redness during and after movement, and rapid breathing.
School sport club activities should be allowed or prohibited based individualized evaluation separate from A-E categories. The instruction consists of a code for allowable intensity of exercise and daily activities described in Section 6.1 (2) and a guide for school sport club activity (allowed or prohibited), for example, “D-prohibited (Moderate exercise is allowed but school sport club activity is prohibited)” and “E-allowed (Intense exercise and school sport club activity are allowed)” School sport club activities do not necessarily mean doing intense exercise to become a player or an athlete, but rather do include joining a club as a manager or a scorer. In the latter case, a note should be added in the parentheses. Instructions with no clear indication of “allowed” or “prohibited” should be read as “prohibited.” When it takes time to compete all necessary examinations to make a final rating, a tentative rating is made in the School Activity Management Table.
6.3 Exercise Intensity and School Activities
Table 618 and Table 719 summarize examples of mild exercise (allowed for children in Categories C, D, and E), moderate exercise (allowed for children in Categories D and E), and intense exercise (allowed only for children in Category E).
Table 6.
School Activity Management Table (for Elementary School Children)
Table 7.
School Activity Management Table (for Junior and Senior High School Students)
The heart disease screening system for school students consists of the primary and secondary screening programs. This section describes the usefulness of non-invasive tests used in the primary and secondary screening programs.
7.1 Tests Used in Heart Disease Screening
7.1.1 Electrocardiogram (ECG)
The 12-lead ECG is a standard diagnostic tool used throughout the world, and the induction method is standardized. In heart disease screening for schools, a 12-lead ECG is taken to detect heart diseases, and is useful in detecting and diagnosing severe arrhythmia, cardiomyopathy, and primary pulmonary hypertension. Although a simple 4-lead ECG (I, aVF, V1 and V6) is taken in some screening programs, a 12-lead ECG is preferable to ensure correct diagnosis. For example, a simple 4-lead ECG may not be used to detect T-wave changes in V3–V4 in patients with atrial septal defect or a notch at the end of QRS in V1–V3, which represents ventricular late potentials in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), or to assess ST elevation in V1–V3 in patients with Brugada syndrome or the shape of T wave that differs among different types of gene mutations in patients with long QT syndrome.
7.1.2 Phonocardiogram
Phonocardiography in school heart disease screening is beneficial in (1) improving accuracy in the diagnosis of congenital heart disease, (2) reducing the burden of school physicians who auscultate students, and (3) reducing the number of students sent to secondary screenings and later examination and thereby reducing the cost.
However, phonocardiography is not used widely in school health screening as most children with major congenital heart disease are diagnosed before school age and physicians with expertise in phonocardiography are small in number. Phonocardiograms taken in primary heart disease screening in schools should be assessed according to the “criteria for the assessment of two-point phonocardiograms in children” proposed by the Japanese Society of Pediatric Cardiology and Cardiac Surgery (Table 8).14
Table 8.
Assessment of Two-Point Phonocardiographic Findings in Children
| When experts assess two-point phonocardiogram, the presence or absence of the following murmurs should be assessed carefully |
| 1. Significant systolic murmurs |
| 2. Diastolic murmurs |
| 3. Continuous murmurs |
| 4. Abnormal heart sounds |
| Notes for assessments are as follows. Signals the medium frequency range should be mainly assessed |
| 1. Systolic murmurs: Significant murmurs are defined as follows |
| (1) Plateau-like, crescendo and decrescendo murmurs that begin after the main component of S1 |
| (2) Crescendo-decrescendo systolic murmurs |
| 1. Systolic murmurs continue through ≥80% of the entire systolic period |
| 2. Systolic murmurs continue through 60 to <80% of the entire systolic period, and |
| i) Those where the amplitude reaches its peak in the later period |
| ii) Those with high frequency |
| iii) Those with large amplitude |
| iv) Those with wide S2 split |
| (3) Murmurs that start well after S1 and continue to S2 |
| 2. Diastolic murmurs: Murmurs are considered as significant when diagnostic murmurs are present |
| (1) Early diastolic murmurs |
| Begin after S2 and often have high amplitude |
| They often appear on the medium frequency range over the third left intercostal space (3LIS) |
| (2) Mid-diastolic murmurs |
| Mid-diastolic murmurs are recorded on the medium frequency range of the 3LIS and apical phonocardiograms for a duration of ≥60 msec |
| Only changes in the low frequency range are not significant |
| (3) Presystolic murmurs |
| Presystolic murmurs occur from S4 to S1 |
| 3. Continuous murmurs: Murmurs are considered as significant when continuous murmurs are present |
| Continuous murmurs may reach peak amplitude slightly before S2. They are commonly recorded over 3LIS |
| 4. Abnormal heart sounds |
| (1) Loud S2 |
| S2 with very high amplitude is considered significant |
| (2) Wide split S2 |
| Murmurs with a time interval between the components IIa and IIp of ≥40 msec and fixed splitting are considered significant |
| (3) Loud S3 |
| Murmurs where S3 amplitude is similar or larger than S2 amplitude should be assessed according to the ventricular load estimated from ECG |
| (4) Loud S4 |
| Clear (high amplitude) S4 in medium frequency is considered significant |
| (5) Loud heart sounds |
1. Ejection sound: A short, high-amplitude sound that recorded 20 to 60 msec from the peak of R wave on ECG, and began with a certain
interval after the main component of S1 is considered significant |
| 2. Apical mid-systolic click: An apical mid-systolic click without systolic murmurs is not considered significant |
| 3. Atrioventricular opening snap: This is irrelevant in heart disease screening in children and young people |
| Note: |
1. Phonocardiogram should be assessed for beat-to-beat repeatability. Non-repeatable signals are highly likely caused by respiratory sounds
or exogenous noise |
2. First heart sound (S1): The main component of S1 is mainly associated with the closure of the atrioventricular valve, starts around the end
of the QRS wave on ECG, and lasts for 40 to 60 msec |
3. Second heart sound (S2): The main component of S2 is mainly associated with the closure of the aortic valve (IIa) and the closure of the
pulmonary valve (IIp), and is commonly recorded at around the end of T wave on ECG. IIa comes first, and IIb comes next. Respiratory
splitting is often noted in children |
| 4. Third heart sound (S3): S3 usually occurs 100 to 150 msec after S2 |
5. Fourth heart sound (S4): S4 is a small-amplitude sound recorded at around or slightly later than the peak of R wave on ECG, and lasts for
20 to 30 msec |
6. Time phase: For the purpose of this table, “systole” is defined as the period from the main component of S1 to the main component of S2,
and “diastole” is defined as the period from the main component of S2 to the main component of S1 |
7. Loudness of murmurs: It is difficult to set objective measures for the loudness of murmurs. “Large crescendo-decrescendo systolic
murmurs” are defined as those with a maximum amplitude similar to or larger than S2 |
8. Frequency of murmurs: In 2-point phonocardiogram, it is difficult to analyze the frequency of murmurs. High-frequency heart sounds define as dense
signals with variable amplitudes on phonocardiogram in 2-point phonocardiogram |
(Source: Okuni M, et al. 1994.14)
In heart disease screening in schools, the heart in chest X-ray should be assessed in the order of the following (1) to (9): (1) Superior vena cava: Enlargement of the superior vena cava may reflect the presence of conditions that increase right atrial pressure (e.g., total anomalous pulmonary venous connection). Hypoplasia of the superior vena cava may indicate deficiency or a persistent left superior vena cava; (2) Right atrium: Dilated right atrium may indicate volume overload (e.g., tricuspid valve insufficiency and Ebstein’ disease or other diseases) or pressure overload (an increase in right ventricular pressure due to tricuspid atresia or pulmonary hypertension); (3) Pulmonary arterial trunk: Protruding pulmonary arterial trunk may indicate an increase in pulmonary arterial pressure (pulmonary hypertension) or volume overload (due to diseases causing an increase in pulmonary blood flow); (4) Left atrium; (5) Left ventricle; (6) Aorta; (7) Pulmonary artery shadows; (8) Presence/absence of chest bone defects; and (9) Presence/absence of calcification.
7.1.4 Exercise Electrocardiography
Exercise ECG in heart disease screening in schools is conducted to (1) diagnose arrhythmias, assess their severity and prognosis, and determine their allowable intensity of exercise and daily activities; (2) diagnose pediatric ischemic heart disease including coronary sequelae of Kawasaki disease, and assess their severity and prognosis; (3) assess the prognosis of children after surgery and determine their allowable intensity of exercise and daily activities.
Holter ECG and exercise ECG are important tools for the diagnosis of ischemia and arrhythmias.
Table 920,21,21a,21b
and
Table 1022
summarize criteria for the assessment of exercise ECG. As any of these criteria may cause false negative or positive results, exercise ECG findings should be assessed carefully together with clinical findings and the results of other tests.
Table 9.
Criteria for Assessment of Exercise ECG (for Adults)
| Master’s criteria |
| 1. Single Master’s exercise test |
| 1) ST depression ≥0.05 mV |
2) Conversion of an upright T wave to an isoelectric or inverted T wave,
excluding cases where this finding is limited to lead III |
| 3) Conversion of a flat or negative T wave to a positive T wave (leads other than III) |
| 4) Premature contractions or marked arrhythmia: |
A wider QRS wave, intraventricular conduction disturbance or bundle branch block, deep Q wave,
PR prolongation, atrioventricular block |
| 2. Double Master exercise test |
| 1) Ischemic ST depression of ≥0.05 mV |
| 2) Junctional ST depression with QX/QT ≥50% or QT ratio ≥1.07 |
| 3) ST depression of ≥0.2 mV, regardless of its type |
4) ST elevation, occurrence of transient Q waves, transient left bundle branch block, U wave conversion,
severe arrhythmias (occurrence of transient ventricular tachycardia, complete and incomplete atrioventricular
block, atrial tachycardia, atrial fibrillation, or multifocal or short run of premature ventricular contractions) |
5) T wave inversion: Conversion of a positive T wave of ≥0.15 mV to a negative T wave of ≥0.15 mV,
or conversion from a negative T wave to a positive T wave of ≥0.15 mV |
(Source: Asai T. 2000,20 Master AM, et al. 1942,21 Master AM, et al. 1944,21a Master AM. 1968.21b)
Table 10.
Criteria for Ischemia on Exercise ECG
| Definite criteria |
| ST depression |
| A flat or down-sloping ST depression of ≥0.1 mV measured at 0.06 sec or 0.08 sec after the J point |
| ST elevation |
| ≥0.1 mV |
| ST depression at rest |
| A flat or down-sloping ST depression by ≥0.2 mV |
| Reference findings |
| Up-sloping ST depression |
| Relatively flat ST segment depression (≤1 mV/sec) of ≥0.1 mV |
| Conversion of a positive U wave to a negative U wave |
| Potentially false-positive findings |
| A counterclockwise rotation of HR-ST loop |
| Gradual conversion of up-sloping ST depression during exercise to long-lasting flat or down-sloping ST depression |
(Source: The Japanese Circulation Society. 2000.22)
In heart disease screening in schools, echocardiography at the secondary screening is positioned as a critically important test. Echocardiography helps physicians assess the anatomy, function and blood flow of the heart and large vessels in a real-time manner, and provides information important for the diagnosis of almost all underling heart diseases. It is important to equalize the precision of echocardiography to expand the use of this technique in secondary heart disease screening. As the necessity of echocardiography was found during primary screening, a sufficient number of clear echocardiogram images must be obtained during secondary screening to ensure accurate assessment.
In heart disease screening in schools, echocardiography is often used as a detailed examination for those with ECG findings of incomplete right bundle branch block to determine whether they have atrial septal defect or diseases causing right ventricular volume overload, Kawasaki disease, or cardiomyopathy.
7.1.6 Holter ECG
Holter ECG is used to (1) diagnose arrhythmias and assess the severity and variability of arrhythmias to specify the type, time of occurrence, condition at onset, frequency and relationship with symptoms; (2) assess symptoms to determine a relationship between symptoms (e.g., chest pain, syncope, palpitations and dizziness) with heart disease; (3) evaluate the efficacy of antiarrhythmic treatment; and (4) assess pacemaker function.
7.1.7 Event Recording23
Event recording is an effective tool for patients complaining of chest symptoms in determining whether their symptoms are caused by arrhythmia or myocardial ischemia.
Recently a number of reports have been published on the usefulness of event recording in schools.24
As the event recorder can record ECG data associated with different school activities, the data can be used to follow up students with heart problems and examine ECG patterns during exercise without difficulty. It has been reported that cardiac event recording used as a non-continuous monitor helps physicians follow up with students with QT prolongation or Brugada pattern on ECG. It has also been reported that event recorders are useful for students in whom school heart disease screening revealed ECG findings suggestive of mild heart disease. By assessing ECG data before, during and after exercise to observe for the occurrence of arrhythmia, physicians can determine their allowable intensity of exercise and school activities. As cardiac event recorders can monitor ECG signals during different school activities, they may be especially useful in following up with students who showed abnormal ECG patterns in primary screening.
8. Diseases That May Lead to Sudden Death
Data published by the Fire and Disaster Management Agency and the Injury and Accident Mutual Aid Benefit System of the Japan Sport Council have used to estimate diseases that caused deaths in students in Japan.25,26 Sudden death is defined as any death that occurs less than 24 hours after the onset of intrinsic cardiac arrest, and cardiovascular diseases are considered to account for about 70% of their causes.26 Table 11 lists cases of cardiac sudden deaths in school students in Japan in 2005 to 2009, and this list indicates potential causes of sudden death in school. Typical causative heart diseases are described in the following sections.
Table 11.
Causative Diseases of Out-of-Hospital Cardiogenic* Cardiac Arrest in Children (2005 to 2009)
| |
Cases in the school setting
(n=32) |
Cases outside the school setting
(n=26) |
| After diagnosis (n=28) (Under follow-up) |
| Exercise-related |
12 cases |
3 cases |
| Causative disease |
Hypertrophic cardiomyopathy (4) |
Congenital heart disease (7) |
| Congenital heart disease (3) |
Hypertrophic cardiomyopathy (2) |
| Long QT syndrome (3) |
Long QT syndrome (1) |
| Dilated cardiomyopathy (3) |
Dilated cardiomyopathy (1) |
| Noncompaction of left ventricular myocardium (1) |
Restrictive cardiomyopathy (1) |
| After myocarditis (1) |
|
| WPW syndrome (1) |
|
| Unknown (2) |
|
| Undiagnosed at onset (30 cases) (No follow-up) |
| Exercise-related |
15 cases |
8 cases |
| Causative disease |
Congenital coronary anomalies (5) |
Long QT syndrome (3) |
| Hypertrophic cardiomyopathy (2) |
Congenital coronary anomalies (2) |
| Long QT syndrome (2) |
Noncompaction of left ventricular myocardium (2) |
| Idiopathic ventricular fibrillation (3) |
Acute myocarditis (2) |
| CPVT (2) |
Idiopathic ventricular fibrillation (1) |
| Dilated cardiomyopathy (1) |
CPVT (1) |
| Unknown (1) |
Unknown (3) |
CPVT, catecholaminergic polymorphic ventricular tachycardia. (Source: Mitani Y, et al. 2014.25)
*Each year, several cases of ’non-cardiogenic’ sudden death in children due to CNS events (e.g., Subarachnoid hemorrhage, intracerebral hemorrhage, and cerebral infarction) have been reported.
Note: In a study of sudden cardiac death in the school setting,26
aortic dissection was reported in 2 children with known heart disease, and 4 children in whom heart disease were not found until autopsy. One possible case of commotio cordis has been reported. Cases of congenital heart disease were as follows:
· Aortic arch interruption+single ventricle+pulmonary vascular obstructive disease
· Single atrium+single ventricle+pulmonary artery atresia
· Post repair surgery of tetralogy of Fallot
· Ventricular septal defect
· Asplenia syndrome+atrioventricular septal defect
· Aortic arch interruption
· Single ventricle
· Double outlet right ventricle (+pacemaker)
· Transposition of the great arteries+pacemaker
Most sudden deaths in children and young people with a history of congenital heart diseases have occurred among those with surgically corrected complex cyanotic heart disease. Among those with uncorrected congenital heart disease, those with uncorrected moderate or severe aortic stenosis are at a risk of sudden death.
b. Cardiomyopathy
Hypertrophic cardiomyopathy accounts for 70 to 80% of children with cardiomyopathy. Cases of dilated cardiomyopathy, restrictive cardiomyopathy, noncompaction of left ventricular myocardium, and arrhythmogenic right ventricular dysplasia have also been reported. All these types of cardiomyopathy may cause sudden death, and account for about 20 to 30% of sudden deaths in children and young people. Sudden deaths due to cardiomyopathy have occurred in those who were diagnosed in school heart disease screening and had been followed up thereafter and in those in whom the disease was not diagnosed but was found in autopsy. Recent reports have described that students with sudden cardiac arrest were successfully treated with an automated external defibrillator (AED) and emergency medical treatment, and returned school with implantable cardioverter defibrillator (ICD) devices.26–28
c. Acute Myocarditis
Sudden deaths due to acute myocarditis often develop within hours or days after viral infection. The direct cause of these deaths is myocardial contractile dysfunction or severe arrhythmias, and the cause is often found at autopsy. It is impossible to find acute myocarditis in school heart disease screening, but children and young people with frequent extrasystoles should be suspected to have acute myocarditis.
d. Marfan Syndrome
Some individuals with Marfan syndrome, who are often tall and have long extremities, participate actively in sport activities but are at a higher risk of sudden death due to aortic dissection or rupture. Chest compressions and AED are usually ineffective for in this situation. Students with Marfan syndrome should undergo periodic evaluation at a cardiology clinic to receive instruction for allowable exercise intensity. Prophylactic surgery is recommended for those with the aorta more than 45 to 50 mm in diameter. Similar reports have been published for Ehlers-Danlos syndrome.
e. Anomalous Origin or Course of the Coronary Arteries
Among cases of anomalous origin or course of the coronary arteries, sudden deaths have been reported most frequently among patients with anomalous left coronary artery originating from the right sinus of Valsalva and coursing between the aorta and pulmonary artery that died due to compression of the left coronary artery during exercise or hypoplastic left coronary artery. It is difficult to detect these coronary anomalies including cases of coronary arteries originating from a single coronary ostium only on the basis of ECG at rest. These coronary anomalies cause significant morbidity only when they are under intense exercise stress, and are often not found until autopsy. Appropriate screening methods should be developed to examine athletes for coronary anomalies.
f. Sequelae of Kawasaki Disease
The advancement of acute-phase treatment and anticoagulant therapies for Kawasaki disease have dramatically reduced the prevalence of sudden deaths in those under 18 years of age. However, some adult patients with sequelae of Kawasaki disease are not properly managed by specialists, and cardiac accidents have frequently occurred in this population.
g. Pulmonary Hypertension
The advancement of treatments and management methods of pulmonary hypertension, including idiopathic disease and pulmonary hypertension caused by other diseases, have reduced the number of sudden deaths in school-aged children and young people.
8.2 Arrhythmias and Abnormal ECG Findings
a. Wolff-Parkinson-White (WPW) Syndrome (Accessory Pathway Syndrome)
The prevalence of paroxysmal supraventricular tachycardia in children with asymptomatic WPW syndrome found during heart disease screening is 10 to 20%, which is lower than in adult patients. The incidence of sudden deaths due to supraventricular tachycardia is low. When atrial fibrillation develops in patients with a short refractory period of the accessory pathway, it may lead to pseudo-ventricular tachycardia, ventricular fibrillation, and finally sudden death. These events most commonly occur in boys in junior or senior high school during substantially intense exercise. Currently available criteria for ECG assessment in heart disease screening in schools or childhood screening programs do not contain clear enough criteria for ECG findings that poses an increase in the risk of sudden death in those in WPW syndromes. In the United States, children with WPW syndrome associated with organic heart disease such as Ebstein’ disease and cardiomyopathy are at a higher risk of sudden death.29
b. Long QT Syndrome
Long QT syndrome is an inherited disorder characterized by delayed cardiac repolarization. Torsade de pointes (TdP), which may lead to syncope and sudden death may develop in this patient population. Family members have often experienced similar symptoms. In Japan, 1 or 2 sudden deaths in the school setting are reported each year. To date, a total of 16 genetic mutations have been reported to be associated with long QT syndrome.30
LQT1, LQT2 and LQT3 account for about 90% of all patients with Long QT syndrome. Patients with LQT1 often experience cardiac events during swimming. However, other types of exercise may induce TdP, and sudden deaths have been reported. In patients with LQT2, syncope is triggered by mental stress, auditory stimuli, or pregnancy/childbirth. In patients with LQT3, a condition caused by a genetic mutation that leads to Brugada syndrome, cardiac events develop during sleep. As some students who have been diagnosed with epilepsy may actually have long QT syndrome, detailed ECG assessment is important.
c. Multiple Premature Ventricular Contractions, Ventricular Tachycardia
Cases of sudden death in the school setting have been reported at a frequency of 1 case in several years among children under follow-up for premature ventricular contractions and ventricular tachycardia.
d. Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
CPVT more commonly affects children over 7 years of age and young people. Although it is difficult to detect this condition through ECG screening, some children and young people with CPVT are at risk of developing fatal arrhythmias. It has been demonstrated that CPVT is caused by mutations in genes coding for ryanodine receptor type 2 (RyR2) and calsequestrin 2 (CASQ2).31
RyR2 gene mutation is also known to cause arrhythmogenic right ventricular dysplasia.
e. Idiopathic Ventricular Fibrillation
There have been reported cases of cardiac arrest in children and young people without underlying heart diseases including hereditary arrhythmias in whom the AED detected ventricular fibrillation and delivered a shock. In many cases, cardiac arrest developed during intense exercise. Further investigation should be made to clarify its pathophysiology.
f. Commotio Cordis
Each year, one or two students have ventricular fibrillation after a blunt chest trauma by a baseball ball, a hockey puck, or sometimes a fist. These cases are a good indication for AED, and their lives have been saved in some cases.
g. Other Hereditary Arrhythmias
Recently, Brugada syndrome and short QT syndrome have attracted attention as underlying causes of sudden deaths, but sudden deaths in children with these diseases have rarely been reported. Further investigation should be made to take appropriate measures.
8.3 Summary
The above-mentioned diseases are classified into to the following categories in terms of the possibility of being found during heart disease screening in schools.
(1) Conditions that can be found during screening and for which the risk of sudden death can be estimated: Congenital heart diseases, myocardiopathy in those under follow-up, Marfan syndrome, WPW syndrome, long QT syndrome, and multiple or multifocal extrasystoles.
(2) Conditions that cannot be found during screening and may be diagnosed at autopsy or after the occurrence of a cardiac event: Myocarditis, congenital coronary anomalies, aortic dissection, undiagnosed cardiomyopathy, and commotio cordis.26
(3) Conditions that may not be found even at autopsy: Lethal arrhythmias such as catecholamine-induced polymorphic ventricular tachycardia (CPVT) and idiopathic ventricular tachycardia.
Conditions listed in (1) should be continuously screened in schools. Students with these conditions should be assessed in detail to clarify their risk factors. Conditions listed in (2) and (3) are difficult to detect using the current screening programs. Further investigations should be made to consider additional examination and their cost-effectiveness.
9. Statistics and Circumstances of Cardiopulmonary Resuscitation (CPR) in Schools
9.1 Deaths in the School Setting
According to the data published by the Japan Sport Council, the number of deaths of children or young people in the school setting, i.e., in elementary schools, junior high schools, senior high schools, technical colleges, special needs schools, kindergartens, and day-care centers was 135 in 1999,32 119 in 2003,32 68 in 2009,33 and 63 in 2013,34 showing a decreasing tendency. The number of sudden deaths (i.e., unexpected intrinsic deaths within 24 hours after onset) ranged from 35 to 83 from 1999 to 2008 and from 23 to 39 from 2009 to 2013. Sudden deaths accounted for 57% and 50% in all deaths in 1999–2008 and 2009–2013, respectively. The number of sudden deaths in 100,000 students in elementary schools, and junior and senior high schools has decreased over time (Figure 6). The decreased incidence of sudden deaths in schools may be explained with the advancement of heart disease screening programs in schools, the widespread use of AEDs and the advancement of surgical and medical treatment strategies. Since 1995, all students in the first year of elementary school, junior high school and senior high school must undergo an ECG test. Around year 2000, studies on inherited lethal arrhythmias including long QT syndrome have advanced the understanding of disease mechanisms and helped increase the detection rate of these conditions. In Japan, emergency medical technicians were able to use AEDs without direction by a physician in 2003, and the use of AEDs by laypersons were allowed in July 2004. In 2009, AEDs were placed in schools throughout Japan. It has been reported that bystander CPR using AEDs in schools contributes to the prevention of cardiac sudden deaths in students in schools.32 It is recommended that registry studies be conducted.
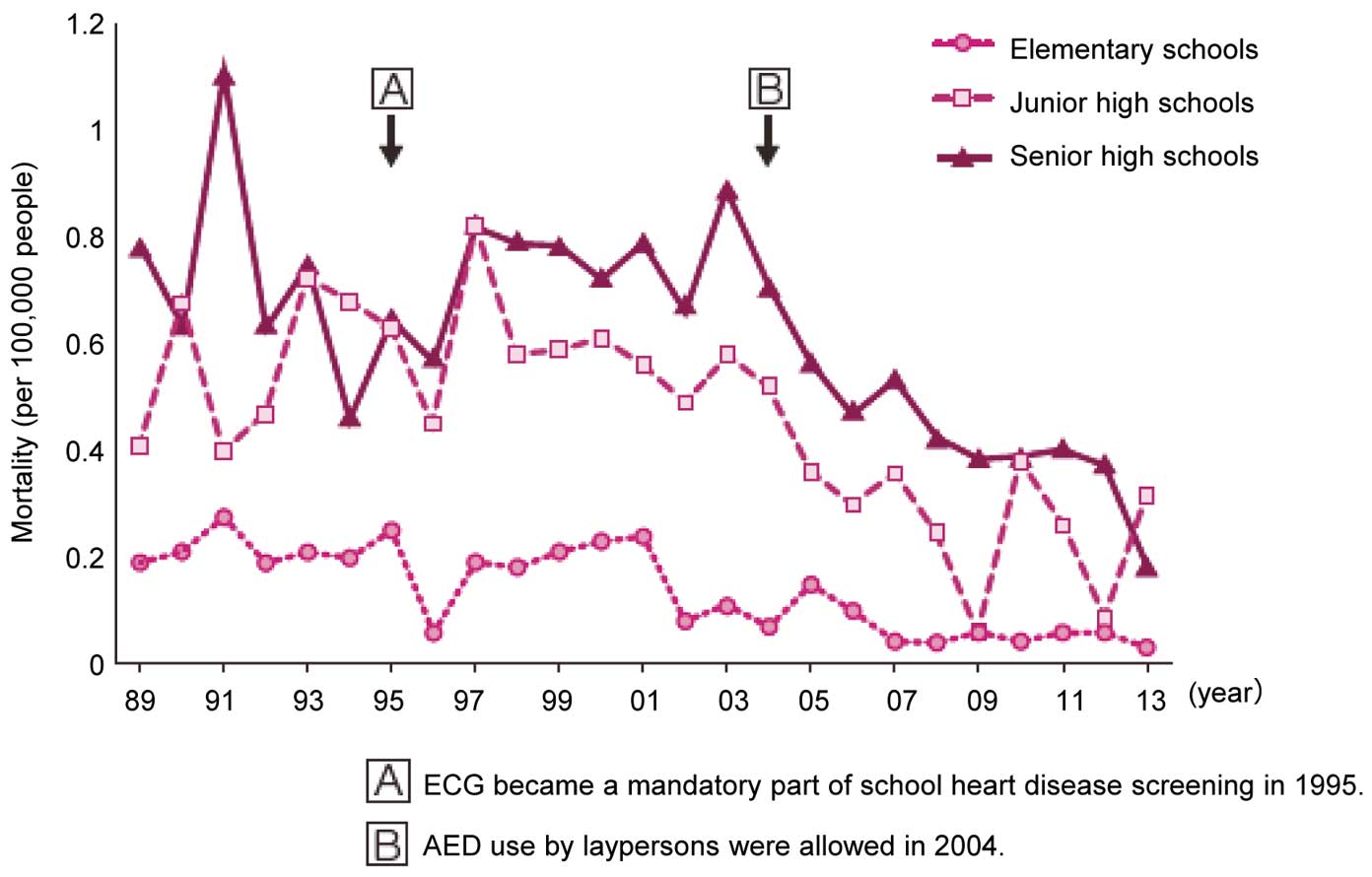
Since the introduction of AEDs in schools, few reports had been published on the statistics of CPR and AED use in schools. However, in 2013, the Ministry of Education, Culture, Sports, Science and Technology and the Japanese Society of School Health conducted, “A survey on health management in school life,” a nation-wide survey on heart disease screening, urinalysis, and allergic disorders,35
which included questions about CPR and AED use in schools. In this survey, a questionnaire and a computer-scored answer sheet were sent to all state elementary, junior high and senior high schools in Japan, and participating schools were instructed to fill out the sheet and return it completed by mail. The questions were about: (1) the number of cases of CPR and AED use for students in the school setting during 5 years from 2008 to 2012; (2) whether or not those treated with CPR and AEDs were known to have underlying diseases or abnormal ECG findings, or whether or not cardiac arrests were caused by injuries or other extrinsic factors; and (3) how the school educates students about CPR and AED use. The survey in 2008 included schools with a total of 9,258,357 students. Answers were returned from 81.3% of the elementary schools (16,904/20,677 schools), 81.2% of the junior high schools (7,885/9,707 schools), and 85.0% (2,969/3,481 schools), and valid responses describing the total number of students accounted for 92% of the responses. Duplicated answers and missing answers were removed from the data. The survey revealed that a total of 604 students received CPR, and 293 of them were treated with an AED in schools during the 5 years. The number of students treated with CPR and an AED were 224 (0.9/100,000 person-years) and 96 (0.4/100,000 person-years) in elementary schools, 218 (1.8/100,000 person-years) and 115 (0.9/100,000 person-years) in junior high schools, and 157 and (1.7/100,000 person-year) and 82 (0.9/100,000 person-year) in senior high schools. The incidence rates of events requiring CPR or AEDs in junior high schools and senior high schools were more than two-fold that in elementary schools. Health students who had cardiac accidents with no external factors accounted for 23.8%, 33.6%, and 40.5% of the cases receiving CPR or AEDs in elementary schools, junior high schools, and senior high schools, respectively, indicating an increasing tendency with age. Cases of CPR or AED use (including cases where AED pads were applied but a shock was not delivered) in these school categories occurred at a 2 to 4-fold higher incidence than those of deaths in the school setting each year. It is unclear whether or not all students who died in the school setting were treated with CPR or an AED, but the above survey data indicate that CRP and AEDs are widely conducted in schools and contribute to a decrease in the number of deaths in schools.
10. Prevention of Sudden Death in the School Setting
In April 2003, emergency medical technicians were able to use AEDs without direction by a physician. In July 2004, the use of AEDs by laypersons was also allowed. The following sections describe (1) the use of AEDs for out-of-hospital cardiac arrest (OHCA) in schools, and (2) the effects of AEDs on the outcome of OHCA.
10.1 Use of AEDs
AEDs are available in many public places such as medical institutions, fire stations, schools, and public facilities. After laypersons were allowed to use AEDs in 2004, the number of AEDs increased over time. As of 2008, it is estimated that 200,000 AEDs are in place throughout Japan.36
The MHLW has reported that as of the end of March 2011, AEDs are in place in 96.4% of all elementary schools and in 98.8% of all junior high schools.37
10.2 Effects of AEDs on Sudden Deaths
In a well-known survey of cardiac arrests in casinos in Las Vegas, the survival rate was 74% and 49% for those who received their first defibrillation by untrained bystanders within 3 minutes and more than 3 minutes after onset respectively. The survival rate in those who received defibrillation was significantly higher than that in victims with unwitnessed cardiac arrest, suggesting the efficacy of bystander AED use.38
According to the emergency resuscitation statistics published by the Fire and Disaster Management Agency, one-month survival rates in patients with cardiogenic cardiac arrest who received defibrillation compared to those who did not receive bystander defibrillation was 43% and 8%, respectively. The survival rate was significantly higher in those receiving bystander AED, which suggest the efficacy of AED use.39
It has also been reported that the availability of AEDs in public places throughout Japan has increased the use of AED defibrillation and elevated the social rehabilitation rate of cardiac arrest survivors through shortening the time from onset to defibrillation.40
After activating the EMS system, it takes 9 minutes for emergency responders to arrive on the scene and start CPR. In order to start CPR as soon as possible and thereby raise the percentage of those discharged alive, the cooperation of bystanders is essential. Therefore, the role of teachers as bystanders who witness cardiac arrest is critically important.
10.3 Effects of Bystander Defibrillation on the Outcome of Cardiogenic Cardiac Arrests in the School Setting
According to the data from the Injury and Accident Mutual Aid Benefit System by the Japan Sport Council,32
the number of sudden deaths in the school setting has tended to decrease. The numbers per 100,000 person-years of sudden death in elementary schools, junior high schools, and senior high schools were 0.21, 0.59, and 0.78 in 1999, and 0.04, 0.25 and 0.42 in 2008.
The Fire and Disaster Management Agency of Japan conducted a nationwide, population-based Japanese Utstein registry study of consecutive OHCA cases in elementary and junior high schools,27
and the Japanese Society of Pediatric Electrocardiography conducted in a nationwide survey to investigate circumstances and outcomes of OHCA cases in schools.25
Both studies indicated a relationship between the outcome of children with OHCA and bystander AED use. The rate of successful resuscitation among people with non-family-witnessed OHCA of cardiac origin who received bystander AED was 4% in 2005 and 37% in 2009, and the survival with favorable neurological outcome has improved as well. The time interval between cardiac arrest and defibrillation by bystander AED use was significantly shorter than that by the emergency medical service, and this time interval was an independent variable for survival with favorable neurological outcome. During the study period, the incidence rate of OHCA of cardiac origin did not change significantly. These findings indicate that the increased bystander AED use has improved the outcome of non-family-witnessed OHCA of cardiac origin in children and thereby decreased the incidence of sudden cardiac deaths.25
As schools are places where students stay and perform various activities during the day, most cases of AED use for children and young people with cardiac arrests have carried out in the school setting. A total of 55% of OHCA cases have occurred in schools, and 84% of them were related with sport activities and occurred on the playground or in the swimming pool or gym. Those under follow-up accounted for 48% of children and young people with OHCA, which indicates that they are at a higher risk of OHCA. Among the remaining 52% who represented those with no prior conditions indicated for follow-up, 47% of them were found to have coronary artery anomalies, catecholaminergic polymorphic ventricular tachycardia, polymorphic ventricular tachycardia, or other conditions which cannot be detected with resting ECG. These three conditions are found in 68% of those with OHCA with no prior conditions indicated for follow-up. As these three conditions are often induced by intense exercise, optimizing safety measures in exercise facilities may help prevent sudden deaths from OHCA. As exercise-induced OHCA accounted for about 50% of OHCA cases in children and young people who were under follow-up for the management of congenital heart disease or hypertrophic cardiomyopathy, schools should have correct information on underlying heart conditions for their students, and ensure safety measures are exercised in all areas of schools not limited to exercise facilities.25
10.4 Possible Effects of Resuscitation Guidelines for AEDs on School Health
The International Liaison Committee on Resuscitation (ILCOR) produces a consensus document on resuscitation including chest compression by rescuers, and AED defibrillation every five years as the basis for the local guidelines. In Japan, the Japan Resuscitation Council (JRC) Resuscitation Guidelines 2015 were published.41
In the JRC guidelines, a unified algorithm is proposed for CPR for children and that for adults in order to facilitate the wider dissemination of basic life support skills to laypersons. Recently, the Japanese Circulation Society and the MHLW have proposed where AEDs should be placed, and included schools in the lists of areas where an AED unit should be placed.42,43
As AEDs in schools are highly effective in saving lives, the Japanese Circulation Society has published a proposal to reduce the cases of cardiac sudden deaths further.44
Schools should ensure appropriate measures to prevent cardiac arrest through school heart disease screening and also establish effective first-aid using an AED to save the lives of those with cardiac arrest. Both approaches should be regarded as the two wheels of a cart for preventing sudden cardiac death in school children.
11. Heart Disease Screening in Universities
11.1 Health Check-ups in Universities
In universities, health check-ups are conducted at the time of admission according to the School Health Act, and Chapter 1 “Health check-ups at admission” and Chapter 2 “Health check-ups for students” of the Enforcement Regulation of the School Health Act. The laws and regulations request students in schools undergo heart disease screening with ECG and other clinical laboratory examinations for heart diseases and anomalies, but describe that ECG is not necessary for university students.
11.2 Primary Screening
A questionnaire survey of health administration facilities of national university corporations in Japan45
in 2015 has revealed that all 70 national universities that provided valid responses conducted a medical examination, 45 universities examine all students each year, 15 universities examine students in the first year only, and 5 universities only examine those who want to have a check-up. The questionnaire also revealed that the primary screening program in 2 universities does not contain chest auscultation, and one of the 2 universities uses simple phonocardiogram instead.
A survey of 34 major private universities with a student population of over 10,000 have revealed that 9 universities examine all students each year, 4 universities examine students in the first year only, and 2 universities only examine those who want to have a check-up. Chest auscultation is conducted in all students in 8 universities, and in the first year only in 3 universities.
11.3 Electrocardiography (ECG)
ECG is not an essential component of heart disease screening in university students. In a survey of national universities in Japan it was reported in the White Paper on Student Health 2005,46
44 of the 75 (58.7%) that national universities that provided valid responses conducted ECG in 32,620 of the 408,119 (8.00%) students in these universities. Students 18 years of age accounted for 46.7% of those assessed with ECG (15,591), which indicate that only first year students (freshman) undergo ECG.
According to a questionnaire survey in 2015, 53 of the 70 responding national universities conduct ECG in some way on their students, 2 universities conduct ECG for all students, 22 universities for students in the first year, and 28 universities for students involved in sports (e.g., those in the physical education department, members of sports clubs, those using sports facilities, and participants of school sports events). The mean number of students undergoing ECG per university was 629±997 (range: 7 to 4,200).
Among 34 major private universities with a student population of over 10,000, 26 universities conduct ECG in some way in their students, no universities conduct ECG for all students. Eight universities conduct ECG for students in the first year, and 28 universities for students involved in sports (e.g., those in the physical education department, members of sports clubs, those using sports facilities, and participants of school sports events). The average number of students undergoing ECG per university was 1,968±2,403 (range: 8 to 9,000).
11.4 Results of Primary Screening With ECG
According to the White Paper on Student Health 2005,46
abnormal ECG findings were found in 14.00% of respondents (16.80% in males and 9.60% in females).
11.5 Results of Secondary Screening With ECG
Some students with abnormal ECG findings in the primary screening are instructed to undergo detailed examination. However, the rating criteria differ among universities, and no standard criteria are available.46
Detailed examinations such as ultrasonography, Holter ECG and exercise ECG are conducted in students with abnormal ECG findings. However, no reports have been published on the details or results of these examinations in universities.
11.6 Management Policy
In universities, there are no standard policies for heart disease screening or no management forms corresponding to the School Activity Management Table for elementary and high schools. Each university’s health management center or other relevant organization use their own policies for the management of students with underlying heart diseases according to the Guidelines for Exercise Eligibility at Schools, Work-Sites, and Sports in Patients with Heart Diseases (JCS 2008)47
proposed by the Japanese Circulation Society or other relevant documents. This may be because many students with arrhythmias,11
congenital heart diseases16
and hereditary arrhythmic syndromes are expected to have been diagnosed and be managed before entering university through systematic heart disease screening programs from infancy to senior high school, or that ECG screening in universities is not requested in the School Health Law and its enforcement regulation. A rapidly increasing number of foreign students, especially students from developing countries, are studying in universities and graduate schools in Japan, and some of them have been diagnosed as having untreated congenital heart disease or hereditary arrhythmias after entering schools in Japan for the first time. Appropriate measures should be taken to address this issue.
12. Cost-Effectiveness of Heart Disease Screening in Schools
The cost-effectiveness of heart disease screening in schools may be assessed by calculating the cost for screening, and by comparing the total cost with screening and that without it. The latter method is required to assess the true cost-effectiveness of heart disease screening in schools.
12.1 Calculating the Cost for Screening
The calculated cost for screening and further examination is a measure of cost-effectiveness. Fuller et al in the United States assumed that among athletes found to have a risk of sudden cardiac death in screening, 90 and 10% of them can live for an additional 20 and 40 years, and calculated the costs per year of life saved for the preparticipation cardiovascular screening examinations.48
Tanaka et al used this method to calculate the cost-effectiveness of three different cardiovascular screening methods examined by Fuller et al, i.e., (1) interview and medical examination recommended by the American Heart Association, (2) ECG, and (3) echocardiography, as well as the standard screening method in Kagoshima.49
The cost per year of life saved was 8,800 dollars for the method in Kagoshima, which was substantially lower than the costs in the United States (44,000 to 200,000 dollars).48
A large part of this difference may reflect the difference in cost of laboratory examinations in Japan and the United States, but the results indicate that heart disease screening in schools in Japan is a cost-effective method.
12.2 Models to Calculate Cost-Effectiveness for Screening Methods
The Markov state-transition model is often used to calculate cost-effectiveness for screening children at a risk of sudden cardiac death.50–53
The reader should refer to these references for detailed description of the model. The cost-effectiveness of heart disease screening in schools may be evaluated by comparing the costs for screening, detailed examination for selected students, and treatment of patients who are found to have cardiovascular diseases with the burden of sudden cardiac deaths that may occur in the absence of heart disease screening in schools. This comparison needs many variables,50–53
but an evaluation is currently underway. To date, the following variables have been obtained.
12.2.1 Effect of Heart Disease Screening in Schools in Preventing Sudden Death
The law requesting schools to screen students with ECG was established in 1995. The incidence of sudden cardiac deaths (including sudden deaths from aortic causes) in the school setting was 0.45 per 100,000 people from 1989 to 1993, and 0.16 per 100,000 people in 2009 to 2012, which indicating a 64% decrease.
12.2.2 Selection of Diseases to Be Assessed and Their Prevalence Rates
Long QT syndrome (LQTS) and hypertrophic cardiomyopathy (HCM) are often selected as index diseases to assess the cost-effectiveness of heart disease screening.50,52,53
The cost for detailed examination for those selected through screening and the cost for treatment for those diagnosed as having an index disease may differ substantially by estimated prevalence rates of the diseases.
Many recent reports use the prevalence of LQTS at a 1 in every 2,000 people reported by Schwartz et al54
in the cost-effectiveness assessment for this disease. This frequency is similar to the prevalence of neonates with mutations responsible for LQTS. As mutations responsible for LQTS are detected in 60 to 70% of patients,55
it is estimated that long QT ECG is found in one in every 1,200–1,400 students. In fact, Fukushige et al has reported that one in about 1,200 students in the first year in junior high schools show long QT ECG in heart disease screening in schools.56
In Kagoshima, long QT ECG findings that met the criteria for diagnosis of LQTS in the HRS/EHRA/APHRS Expert Consensus57
were found in 1 in about 3,300 children in the first year of elementary schools, and 1 in about 1,300 students in the first year of junior high schools58
during the period from 2008 to 2013. Accordingly the prevalence of long QT ECG is estimated at 1 in every 1,200–1,300 among students in the first year of junior high school.
The reported prevalence of HCM in children and young people differ substantially between Western countries and Japan. Many reports from Western countries have described that HCM is found in 1 in every 500 children and young people.59
Although nationwide data are currently under analysis, the prevalence of HCM diagnosed in heart disease screening in schools in five regions in Japan was 1 in about 140,000 students in the first year of elementary schools, and 1 in about 20,000 students in the first year of junior high schools (unpublished data). The expensive cost in Western countries may be explained in part by the high prevalence of HCM.
12.2.3 Percentage of Those Who Need Treatment, Timing to Start Treatment, and Incidence of Sudden Death
Since researchers in Western countries often assess the cost-effectiveness of screening for LQTS in symptomatic children and young people who visit the clinic, a large percentage of the population need to start treatment without delay. When symptomatic students are assumed to need treatment, it is estimated that among those who were found to have LQTS during heart disease screening in schools in Japan, 25 to 30% of them experience symptoms sometime during their school years.
The incidence of sudden death in those with LQTS during treatment differs between Western countries and Japan as well. In Western countries, it has been estimated that the incidence of sudden death in patients receiving treatment for LQTS is 1 in 100 person-years. Although the corresponding data in Japan have not yet been accumulated, the incidence is likely to be substantially lower in Japan than Western counterparts.
Further investigations are required to clarify the number of children and young people who have been detected to have HCM in heart disease screening in schools and require treatment, timing to start treatment, and incidence of sudden death among them.
12.2.4 Costs for Examinations and Treatment
The cost-effectiveness of heart disease screening substantially depends on cost for examinations and treatment. Examinations are more affordable in Japan than in the United States. For example, the cost for echocardiography in Japan is about an eighth of that in the U.S. (at an exchange rate of 1 dollar=120 yen). On the other hand, implantable cardioverter defibrillators (ICDs) and other imported medical devices are more expensive in Japan than in Western countries, and the cost for ICD implantation is 1.6-fold that in the U.S. (Table 12).50,53
Cost for treatment is likely to affect the cost-effectiveness of heart disease screening more substantially in Japan than in Western countries.
Table 12.
Cost of Examination and Treatment in Different Countries
| |
United States53 |
Japan* |
Italy50 |
| First visit fee |
$147 |
¥2,820 |
|
| Follow-up consultation fee |
$81 |
¥720 |
|
| ECG at rest |
$33 |
¥1,300 |
€12 |
| Treadmill exercise test |
$254 |
¥8,000 |
€56 |
| Holter ECG |
$189 |
¥15,000 |
€62 |
| Echocardiography |
$599 |
¥8,800 |
€52 |
| ICD implantation |
$27,783 |
¥5,432,048# |
€12,000 |
*Costs in Japan are based on the National Health Insurance prices in 2014. #Mean costs in 6 patients with Hypertrophic cardiomyopathy (based on data provided by Prof. Kiyohide Fushimi at Tokyo Medical and Dental University, and Assoc. Prof. Kunio Ota at Kanazawa University)
Table 13
summarizes ECG-based heart disease screening methods used in Western countries and latest data on their cost-effectiveness. The cost effectiveness differs by the number of children and young people covered and diseases to be screened. We are planning to assess data from heart disease screening in schools to assess the cost-effectiveness of screening for different diseases.
Table 13.
Cost-Effectiveness of ECG-Based Heart Disease Screening in Western Countries
| Authors |
Year of
publication |
Subjects |
Cost effectiveness
calculation method |
Target diseases |
Cost per year of life
saved |
| Quaglini S, et al.50 |
2006 |
Infants |
ECG screening |
LQTS & CHDs |
€7,400 to €20,400 |
| Wheeler MT, et al.51 |
2010 |
Athletes 14 to 22 years of age |
Screening with
H & PE & ECG |
All CVDs |
$76,100 (95%CI
$62,400 to $130,000) |
| Leslie LK, et al.52 |
2012 |
8-year old children with ADHD/
Athletes 14 years of age |
Screening with
H & PE & ECG |
LQTS, HCM, WPW |
$91,000 to $204,000 |
| Anderson BR, et al.53 |
2014 |
All children 12 years of age |
Screening with
H & PE & ECG |
LQTS, HCM |
$55,000 |
CHDs, congenital heart disease; ADHD, attention-deficit/hyperactivity disorder; CVDs, cardiovascular diseases; HCM, hypertrophic cardiomyopathy; H & PE & ECG, history taking, physical examination, and ECG; LQTS, long QT syndrome; WPW, WPW syndrome.
(Results of a nation-wide survey by the Ministry of Education, Culture, Sports, Science and Technology and the Japanese Society of School Health in 2013.)
13.1 Introduction
In 1973, heart disease screening was added as an essential part of regular health check-ups in schools in Japan. In 1998, the Ministry of Education, Culture, Sports, Science and Technology and the Japanese Society of School Health conducted the first survey on the implementation of heart disease screening in schools and reported the results in, “A survey report on heart disease screening and urinalysis in school children and adolescents.” They conducted the second survey in 2013 where the implementation of heart disease screening was assessed as a part of a survey on health management in schools.35
According to the results of the two surveys, the current practice of heart disease screening in schools is described in the following sections.
13.2 About the Survey on Health Management in Schools
The survey on health management in schools was conducted by the Ministry of Education, Culture, Sports, Science and Technology and the Japanese Society of School Health. In this survey, a questionnaire and a computer-scored answer sheet were sent to the education committees of prefectural and municipal governments, and all state elementary, junior high and senior high schools, and secondary education schools in Japan. In schools, school nurses were asked to answer the questionnaire. Respondents were asked to provide information as of May 1, 2012. Answers were returned from 97.9% (46/47) of the prefectural education committees, 76.4% (1,330/1,741) of the municipal education committees, 81.8% (16,904/20,677) of the elementary schools, 81.2% (7,885/9,707) of the junior high schools, and 85.0% (2,959/3,481) of the senior high schools, 92.9% (26/28) of the secondary education schools. Valid responses describing the total number of students accounted for 92% of the responses. The survey covered 9,258,357 students in 25,552 schools, including 2,209,237 students in the first year of each school.
13.2.1 Primary Screening
The specialty of physicians who mainly assessed ECG in primary screening was pediatrics in over 20% of all categories of schools, and internal medicine in over 40% of elementary schools and junior high schools and in over 60% of senior high schools (Figure 7). The percentage of elementary schools where ECG was reviewed mainly by pediatricians differed substantially among prefectures (Figure 8).

The heart disease screening questionnaire was administered to all applicable students in 91% of the schools. However, this percentage differed among areas, and was 40–50% in three prefectures. Phonocardiogram was conducted in 25% of the schools, and auscultation by school doctors in 79% of the schools. Overall, simple 4-lead ECG (I, aVF, V1 and V6) was conducted in 36% of the schools, and 12-lead ECG in 60% of the schools. Simple 4-lead ECG was conducted in 40% of the elementary and junior high schools and 13% of the senior high schools, and the remaining schools used 12-lead ECG. The percentage of schools conducting 12-lead ECG differed substantially by prefectures (Figure 9).
13.2.2 Secondary Screening and Further Examination
In 2012, the percentage of students who were determined at primary heart disease screening to need further examination was 3.3% in all schools, and 3.0%, 3.6% and 3.4% in elementary, junior high, and senior high schools respectively. The percentage of students in whom detailed examination revealed conditions requiring medical management was 0.99% in all schools, and 0.9%, 1.0% and 1.0% in elementary, junior high, and senior high schools. These figures appeared reasonable as compared with other available data. The analysis by prefecture revealed that the percentage of those rated as “detailed examination required” was more than 5% and that of those rated as “medical management required” was more than 2% in some prefectures.
A comparison of the percentages of students rated as “detailed examination required” between prefectures where 12-lead ECG was conducted in <30% of the schools and those where it was conducted in ≥70% revealed that the percentage of those rated as “detailed examination required” was significantly higher in the prefectures in lower frequency of 12-lead ECG. The percentage of those rated as “medical management required” tended to be higher in these prefectures (Figure 10).

To the question of who evaluate students rate at “detail examination required” during primary screening, 48% of the respondents answered that parents determined where to go. To the question for municipal education committees on the activity of their heart disease screening review committee, 0–20% of the committees in each prefecture answered that the review committee has been established and is conducting review meeting(s) every year, 30–40% answered that cases are reviewed in other committee meeting(s), and 50–60% answered that they were not aware of the activity of the committee.
13.2.3 School Activity Management Table
Overall, 98.7% of the schools answered that the School Activity Management Table was completed by the physician who examined the student or his/her attending physician, and 2.6% of the schools also answered that it was completed by the student or his/her parents. To the question whether the School Activity Management Table is used for students with heart disease or renal disease, 87.5% and 58.6% of the schools answered that they use the table for those with heart disease and those with renal disease, but 10.6% of the schools answered they do not use the table at all. The survey also revealed that 75.5% of the schools request all students rated as “detailed examination required” to submit their Tables, 60.7% of the schools request all students who regularly visit the clinic to submit their Tables, and 7.1% of the schools allow their parents to decide whether they should submit their Tables to the school or not. The contents of the Tables submitted were shared with all relevant teachers and other staff members in 97% of the schools.
13.3 Summary
Forty years have passed since the heart disease screening program was introduced to schools in Japan, but the methods and accuracy of heart disease screening still differs substantially among geographical areas. It is especially of concern that about 40% of schools use simple 4-lead ECG and that a large percentage of those undergoing 4-lead ECG are rated as requiring detailed examination. The above findings indicate that more pediatricians should be involved in heart disease screening in schools where many internal medicine specialists and cardiologists are working, and that the role of pediatric cardiologists is important in enduring the accuracy of heart disease screening in this age population.
14. Future Perspective of School Health Screening
All those who are involved in heart disease screening in schools believe that this is an essential system for the prevention of sudden cardiac deaths in school students. This had not received worldwide recognition, but is now attracting international attention.59a
A European group led by Italian researchers is investigating the efficacy of ECG screening to prevent sudden cardiac deaths in young people and athletes,60
while researchers in the United States have questioned the significance of athlete screening with ECG.61
However, medical societies in the United States have proposed criteria for the assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people.62
In order to raise an international awareness of the importance of heart disease screening for school students, we must continue to improve our system, and publish our methodologies and results internationally.
14.1 Points for Improvement of Screening System
14.1.1 Data Collection and Accuracy Management63
More and more municipal medical associations have established school (heart) screening committees, and have reported the results of heart disease screening, screening-positive rate in primary screening, and the results of detailed examination. Mean screening-positive rate in primary screening is 3.0% (range: 1.1 to 8.3%) in elementary schools, 3.7% (1.1 to 10.0%) in junior high schools, and 3.5% (0.7 to 9.2%) in senior high schools, and the rates differ substantially between areas. The accuracy of heart disease screening schools is expected to improve further if the JCS and JSPCCS collaborate with local medical association to accumulate data on screening-positive rate from all over the country, specify and address area-specific challenges, and change the system accordingly. In the future, the JCS and JSPCSS should determine probability of diagnosing cardiac diseases in school children and adolescence,30
and propose expected percentages of students selected for secondary screening as benchmarks in order to encourage the School Heart Disease Screening Committee in each area to ensure the accuracy of screening.
14.1.2 ECG in Primary Screening
Since heart disease screening in schools was originally introduced to detect students with rheumatic heart disease, and each school physician had to examine a large number of students in a limited length of time, the screening program consisted of simple 4-lead ECG and phonocardiogram in most areas.
Nowadays, the purpose of heart disease screening in schools is shifting to the prevention of sudden cardiac deaths. School heart disease screening should include 12-lead ECG considering that T wave notching in long QT syndrome can be detected clearly in V3–V5, ST elevation in the right chest leads in Brugada syndrome may develop only in V2 and V3, and high R waves, abnormal ST segment, and abnormal T waves in hypertrophic cardiomyopathy may be observed clearly in V2–V3. As of 2013, primary heart disease screening includes 12-lead ECG in 55.9%, 59.2% and 84.6% of the elementary schools, junior high schools, and senior high schools in Japan. However in an analysis by prefecture, the percentage of elementary and high schools using 12-lead ECG differs substantially by prefecture and ranges from 20.3% to 98.1%. Schools and areas still using simple 4-lead ECG should take every opportunity to ask the relevant authorities to introduce 12-lead ECG.
14.1.3 Results of Detailed Examination
If it is assumed that the prevalence of each target diseases does not differ among different areas of Japan, the percentage of students who are newly diagnosed as having the target disease in detailed examination for schools in each year should be similar among areas. Nationwide analysis should be conducted to manage accuracy of the screening system.
14.1.4 Management After Diagnosis
The Guidelines for School Life and Exercise in Pupils and Students with Congenital Heart Disease (JSPCCS 2012)16
and the Guidelines for School Life and Exercise in Pupils and Students with Arrhythmias without Underlying Heart Diseases (JSPCCS 2013)11
recommend how to manage students with congenital heart disease and arrhythmias. Physicians involved in heart disease screening in schools should refer to these guidelines to ensure appropriate management of students diagnosed as having heart diseases through screening program described in this guideline document.
14.2 Reconstruction of Data System
In each municipal area, heart disease screening in schools is conducted by organization(s) that have completed a service agreement with the municipal government. When a local medical association conduct screening under an agreement with the municipal government, the association establishes the school (heart) screening committee, and monitors the screening system for improvement. However, as screening programs by local governments are outsourced through open tendering, private companies may conduct heart disease screening in schools. In order to obtain standardized screening data from all areas of Japan, local governments should inform applicants of detailed specifications for heart disease screening programs in schools, such as ECG recording methods, criteria for primary screening, and how to report screening-positive rates. Screening data are important to improve and reconstruct the screening system.
II. Disease-Specific Management
1. Management of Arrhythmias
Arrhythmias are most commonly observed abnormal ECG findings found during heart disease screening in schools. The criteria for primary screening and management of children and young people with positive screening results, and the criteria for secondary screening including examinations at a cardiology clinic and management of those diagnosed to have arrhythmias (Tables 14, 15, and
16) have been developed according to the Guidelines for School Life and Exercise in Pupils and Students with Arrhythmias without Underlying Heart Diseases (JSPCCS 2013).11
Table 14.
Arrhythmias: Criteria for Primary Screening and Selection Categories
| Arrhythmia |
Criteria for primary screening |
Selection
category* |
Premature
supraventricular
contractions |
Multiform premature supraventricular contractions, frequent occurrence, couplets |
A |
| Monomorphic premature supraventricular contractions (Sporadic cases are categorized to Group C) |
B |
Supraventricular
tachycardia |
Supraventricular tachycardia |
A |
| WPW syndrome |
WPW-type ECG:PR interval <0.12 sec and QRS duration ≥0.12 sec and VAT >0.06 sec (any one of
lead I, II, aVL, V4, V5 or V6) |
A |
WPW-type ECG:PR interval <0.10 sec and QRS duration ≥0.10 sec and VAT >0.05 sec (any one of
lead I, II, aVL, V4, V5 or V6) (only in elementary school students)
WPW-type ECG (intermittent): |
| Atrial flutter |
Atrial flutter |
A |
| Atrial fibrillation |
Atrial fibrillation |
A |
| Junctional rhythm |
Junctional rhythm |
B |
Premature ventricular
contractions |
Monomorphic premature ventricular contractions |
A |
Presence of monomorphic premature supraventricular contractions and monomorphic premature
ventricular contractions |
| Polymorphic premature ventricular contractions |
| Premature ventricular contraction couplets |
| R on T type ventricular premature contraction |
| Premature ventricular contractions followed by abnormal T waves |
| Ventricular parasystole |
Treat similarly to those with premature ventricular contractions |
A |
Accelerated
idioventricular rhythm |
Accelerated idioventricular rhythm |
A |
| Ventricular tachycardia |
Ventricular tachycardia |
A |
Complete right
bundle branch block |
Complete right bundle branch block: QRS duration ≥0.12 sec, and R’ > R and VAT ≥0.06 sec (lead
V1, or V2) |
A |
Complete right bundle branch block: QRS duration ≥0.10 sec, and R’ > R and VAT ≥0.05 sec (lead
V1, or V2) (only in elementary school students) |
| Intermittent complete right bundle branch block |
Complete left bundle
branch block |
Complete left bundle branch block: QRS duration ≥0.12 sec, and VAT ≥0.06 sec (any one of lead I,
II, aVL, V5, or V6) with no Q wave |
A |
Complete left bundle branch block: QRS duration ≥0.10 sec, and VAT ≥0.05 sec (any one of lead I,
II, aVL, V5, or V6) with no Q wave (only in elementary school students) |
| Intermittent complete left bundle branch block |
| Sick sinus syndrome |
Sinus arrest or sinoatrial block |
A |
| Sinus bradycardia (<40 bpm) |
B |
Sinus bradycardia (<45 bpm) (only in elementary school students. Junior/senior high school
students with the same finding are categorized to Group C) |
First-degree
atrioventricular block |
PR interval >0.28 sec |
A |
PR interval >0.24 sec (only in elementary school students. Junior/senior high school students with
the same finding are categorized to Group B) |
Second-degree
atrioventricular block |
Second-degree atrioventricular block (Mobitz type II) |
A |
| Second-degree atrioventricular block (2:1 atrioventricular block) |
| Second-degree atrioventricular block (Wenckebach type) |
Advanced or complete
atrioventricular block |
Third degree (complete) atrioventricular block |
A |
*Selection category. Group A: Findings requiring secondary screening/further examination. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 15.
Arrythmias: Conditions and Categories for Exercise Management, and Follow-up Interval
| Arrhythmia |
Conditions |
Management category |
Follow-up
interval |
Premature
supraventricular
contractions |
1. Infrequent occurrence |
No management required |
|
2. Frequent occurrence but with no increase during
exercise |
E-allowed |
1 year |
3. Couplets, multiform or an increase the occurrence
during exercise |
E-allowed |
6 months to
1 year |
Supraventricular
tachycardia |
[Not induced by exercise] |
1. Short-lasting, asymptomatic or causing only mild
symptoms, and no cardiac systolic dysfunction |
E-allowed |
6 months to
1 year |
2. Long-lasting, asymptomatic, and no cardiac systolic
dysfunction |
E-prohibited or E-allowed |
6 months to
1 year |
3. Long-lasting, symptomatic, and cardiac systolic
dysfunction |
(1) Drug treatment is effective, and symptoms
and cardiac systolic dysfunction disappear after
treatment |
D, E-prohibited or E-allowed |
1 to 6 months |
| (2) Drug treatment is not effective |
B or C |
As required |
4. Successfully treated with radiofrequency catheter
ablation with no complications |
E-allowed or No management
required |
1–3 years |
| [Induced by exercise] |
1. Relatively slow supraventricular tachycardia develops
during exercise, but terminates shortly thereafter |
E-prohibited |
3 to 6 months |
Several consecutive supraventricular premature
beats develop but terminate spontaneously |
E-allowed |
6 months to
1 year |
| 2. Exercise induces persistent tachycardia |
D or E-prohibited |
1 to 6 months |
| 3. Drug treatment is effective |
D, E-prohibited or E-allowed |
1 to 6 months |
4. Drug treatment is not effective, but cardiac systolic
dysfunction or symptoms do not develop |
D or E-prohibited |
1 to 6 months |
5. Drug treatment is not effective, and cardiac systolic
dysfunction and symptoms develop |
B or C |
As required |
6. Successfully treated with radiofrequency catheter
ablation with no complications |
E-allowed or No management
required |
1 to 3 years |
7. When exercise induces other types of arrhythmias,
follow the description on the relevant type of arrhythmia |
|
|
| WPW syndrome |
1. No tachycardia episodes, normal heart contraction
and structure |
E-allowed |
1 to 3 years |
| |
Those under long-term follow-up
may be rated at “no management
required” |
|
| 2. Tachycardia episodes develop |
Treat similarly to those with supraventricular
tachycardia |
| Atrial flutter/fibrillation |
1. Drug therapy can control heart rate |
C or D |
As required |
| 2. Drug therapy is not effective |
A, B or C |
As required |
3. A history of syncope, or a marked increase in heart rate
during exercise |
A, B or C |
As required |
4. Successfully treated with radiofrequency catheter
ablation with no complications |
E-allowed or No management
required |
1 to 3 years |
| Junctional rhythm |
1. Heart rate at rest <80 bpm, occurrence of sinus rhythm
and an appropriate increase in ventricular rate during
exercise |
No management required |
|
| 2. Heart rate at rest ≥80bpm |
Treat similarly to those with supraventricular
tachycardia |
| 3. Poor increase in sinus rate during exercise |
Treat similarly to those with sinus node dysfunction |
Premature ventricular
contractions (PVCs) |
1. Rare and isolate monomorphic PVCs, a disappearance,
decrease or no change of PVCs during exercise |
E-allowed. Those with decreasing
tendency or no change may be
rated as “No management required” |
1 to 3 years |
2. An increase in monomorphic PVCs or occurrence of
two consecutive PVCs during exercise
(Holter ECG is recommended)
*When heart rate does not reach a heart rate of
≥150 bpm during Master test, a treadmill should be
used to achieve a heart rate of ≥150bpm |
D, E-prohibited or E-allowed |
1 to 6 months |
| 3. Polymorphic PVCs are documented |
D, E-prohibited or E-allowed
(Requiring detailed examination
by a specialist) |
|
| Ventricular parasystole |
Treat similarly to those with premature ventricular contractions |
Accelerated
idioventricular rhythm |
1. Heart rate ≤60 bpm |
No management required |
|
| 2. Heart rate 60 to <100 bpm |
E-allowed or No management
required |
1 to 3 years |
| 3. Treat similarly to those with ventricular tachycardia when ventricular rate exceeds 100 bpm |
Nonsustained
monomorphic ventricular
tachycardia |
1. A disappearance or marked decrease the occurrence
during exercise |
E-allowed or E-prohibited |
6 months to
1 year |
2. No change or an increase the occurrence during
exercise Those with high heart rate during tachycardia
should be examined carefully |
D or E-prohibited |
3 to 6 months |
3. Those with nonsustained polymorphic ventricular
tachycardia during exercise, should be examined by
a specialist |
Treat similarly to those with polymorphic ventricular
tachycardia |
Monomorphic sustained
monomorphic ventricular
tachycardia |
1. Sustained ventricular tachycardia with a low ventricular
rate, asymptomatic, and a disappearance during
exercise |
E-prohibited or E-allowed |
1 to 6 months |
2. Those with a history of sustained ventricular
tachycardia but no history of syncope or cardiac
systolic dysfunction, controlled with drug treatment,
and not induced during exercise ECG |
E-prohibited or E-allowed |
1 to 6 months |
3. Those with a history of syncope or cardiac systolic
dysfunction, but controlled with drug treatment, and
not induced during exercise ECG |
C, D or E-prohibited |
1 to 3 months |
4. Those without a history of syncope or cardiac systolic
dysfunction, but induced during exercise ECG |
C, D or E-prohibited |
1 to 3 months |
5. Occurrence of syncope or cardiac systolic dysfunction,
and not controlled with drug treatment |
A or B |
1 to 3 months |
| 6. Successfully treated with catheter ablation |
E-allowed or No management
required |
1 to 3 years |
Polymorphic ventricular
tachycardia |
1. Asymptomatic, no family history, no cardiac systolic
dysfunction, slow ventricular rate, and a disappearance
during exercise |
E-prohibited or E-allowed |
1 to 6 months |
| 2. High heart rate, asymptomatic, and no family history |
D |
1 to 6 months |
| 3. High heart rate with syncope or other symptoms |
Refer to sections of long QT
syndrome and new hereditary
arrhythmias |
1 to 6 months |
| 4. CPVT is diagnosed or suspected |
Treat similarly to those with CPVT |
|
Complete right bundle
branch block |
1. Without left axis deviation |
No management required |
|
2. Occurrence of Bifascicular block, i.e., complete right
bundle branch block and left axis deviation (left anterior
fascicular block) |
E-allowed or No management
required |
1 year |
Complete left bundle
branch block |
1. No organic heart disease exists |
No management required |
|
| Sick sinus syndrome |
[Criteria for activity management before pacemaker implantation] |
1. Asymptomatic, slight tendency towards bradycardia,
and an appropriate increase in ventricular rate during
exercise |
E-prohibited or E-allowed |
3 to 6 months |
2. Asymptomatic but a poor increase in ventricular rate
during exercise |
D or E-prohibited |
As required |
3. Occurrence of dizziness, syncope or cardiac systolic
dysfunction associated with bradycardia |
A, B or C |
As required |
| [Criteria for activity management after pacemaker implantation] |
| 1. After implantation |
D, E-prohibited or E-allowed |
3–6 months or
as required |
First-degree
atrioventricular block |
1. PR interval of ≤0.24 sec (elementary school students),
≤0.28 sec (junior or senior high school students) |
No management required |
|
| 2. A normalization of PR interval during exercise |
No management required |
|
| 3. No normalization of PR interval during exercise |
E-allowed |
1 year |
4. Second degree or more severe atrioventricular block
develops during exercise |
See the relevant columns |
|
Second degree
atrioventricular block |
[Wenckebach type] |
| 1. Occurring only at night or at rest |
No management required |
|
2. A normalization of atrioventricular conduction during
exercise |
No management required |
|
3. Occurrence of first degree atrioventricular block
during exercise |
E-allowed |
1 to 3 years |
4. A persistence of second degree atrioventricular block
during exercise |
E-prohibited or E-allowed |
6 months to
1 year |
5. Occurrence of advanced or complete atrioventricular
block during exercise |
Treat similarly to those with advanced
atrioventricular block |
| [Mobitz type II or 2:1 atrioventricular block] |
| Treat similarly to those with advanced atrioventricular block |
Advanced or complete
atrioventricular block |
[Criteria for activity management before pacemaker implantation] |
1. Asymptomatic, a two-fold increase in ventricular rate
(or an increase in ventricular rate to ≥100 bpm) during
exercise |
D, E-prohibited or E-allowed |
3 to 6 months |
2. Asymptomatic, no two-fold increase in ventricular rate
(or an increase in ventricular rate to ≥100 bpm) during
exercise |
C or D |
3 to 6 months |
3. Asymptomatic, but occurrence of premature ventricular
contractions or ventricular tachycardia during exercise |
C or D |
As required |
4. Occurrence of dizziness, syncope or cardiac systolic
dysfunction associated with arrhythmia |
B or C |
As required |
| [Criteria for activity management after pacemaker implantation] |
| 1. After implantation |
D, E-prohibited or E-allowed |
3 to 6 months
or as required |
CPVT, catecholamine-induced polymorphic ventricular tachycardia.
Table 16.
Arrythmias: Diagnostic Examinations Required at Secondary Screening and Further Examination, and Findings That Require Referral to a Specialist
| Arrhythmia |
Items to be examined at secondary
screening/further examination |
Secondary screening findings
requiring specialist referral |
Premature supraventricular
contractions |
12-lead ECG, exercise ECG, Holter ECG |
None |
| Supraventricular tachycardia |
12-lead ECG, chest X-ray, exercise ECG, Holter
ECG |
When heart failure is present, or when persistent
supraventricular tachycardia is present |
| WPW syndrome |
12-lead ECG, exercise ECG, echocardiography |
When atrial fibrillation is also observed |
| Atrial flutter |
12-lead ECG, exercise ECG, Holter ECG,
echocardiography, electrophysiological testing |
All need specialist referral |
| Atrial fibrillation |
12-lead ECG, exercise ECG, Holter ECG,
echocardiography, electrophysiological testing |
All need specialist referral |
| Junctional rhythm |
12-lead ECG, exercise ECG, Holter ECG |
None |
Premature ventricular
contractions (PCVs) |
12-lead ECG, exercise ECG, Holter ECG |
When many PCVs are found on 3-minute ECG |
| Ventricular parasystole |
12-lead ECG, exercise ECG, (Holter ECG) |
Treat similarly to those with premature ventricular
contractions |
| Accelerated idioventricular rhythm |
12-lead ECG, exercise ECG, Holter ECG |
Heart rate ≥100 bpm |
Nonsustained monomorphic
ventricular tachycardia |
12-lead ECG, exercise ECG, Holter ECG |
Symptomatic, high heart rate, relatively persistent |
Monomorphic sustained
monomorphic ventricular
tachycardia |
12-lead ECG, exercise ECG, Holter ECG, event
recorder, echocardiography |
Those with syncope or cardiac systolic dysfunction
need prompt treatment. Those with exercise-
induced form, multiform, or a ventricular rate of
≥150 bpm during tachycardias should be referred
to a specialist clinic without delay |
Polymorphic ventricular
tachycardia |
12-lead ECG, exercise ECG, Holter ECG |
All need specialist referral |
| Complete right bundle branch block |
12-lead ECG, exercise ECG, echocardiography |
Those with organic heart disease |
| Complete left bundle branch block |
12-lead ECG, exercise ECG, echocardiography |
Those with organic heart disease |
| Sick sinus syndrome |
12-lead ECG, exercise ECG, Holter ECG,
electrophysiological testing |
Those requiring electrophysiological testing,
those with frequent sinoatrial block, those with a
maximum PP interval of ≥3 sec, and those with
bradycardia-tachycardia syndrome |
First-degree atrioventricular
block |
12-lead ECG, exercise ECG, Holter ECG |
When Mobitz type II or more severe
atrioventricular block develops |
Wenckebach type atrioventricular
block |
12-lead ECG, exercise ECG, Holter ECG |
When Mobitz type II or more severe
atrioventricular block develops |
Mobitz type II or 2:1 atrioventricular
block |
Treat similarly to those with advanced atrioventricular block |
Advanced or complete
atrioventricular block |
12-lead ECG, exercise ECG, Holter ECG |
All need specialist referral |
Exercise electrocardiogram should be conducted during exercise at a heart rate of ≥150 beats/min. Regardless of the loading methods utilized, healthcare professionals should observe precautions for exercise ECG65
to conduct the test safely. Children and young people with a history of syncope should undergo exercise ECG under continuous ECG monitoring.
a. Premature Supraventricular Contractions66
Premature supraventricular contractions are a type of arrhythmia characterized by atrial beats occurring at a rate faster than the sinus rate.
b. Supraventricular Tachycardia
For the purposes of this document, supraventricular tachycardia is defined as three or more consecutive premature supraventricular contractions.
c. Wolff-Parkinson-White (WPW) Syndrome
WPW syndrome is characterized by the presence of an accessory conduction pathway between the atria and ventricles (the bundle of Kent) that causes short PR interval, delta waves and broad QRS complexes on ECG.
d. Atrial Flutter, Atrial Fibrillation
Atrial flutter is a type of tachycardia caused by a re-entry circuit in the atria with an atrial rate of ≥240 beats/min. Atrial fibrillation is an abnormal fast irregular heartbeat with an atrial rate of ≥300 beats/min. Both atrial flutter and atrial fibrillation are very rare among children. Children and young people with atrial flutter and atrial fibrillation should be carefully examined for underlying conditions.67–69
Atrial flutter detected by ECG at heart disease screening typically has an arterial rate of around 300 beats/min with a ventricular rate that depends on the capacity of the atrioventricular nodal conduction. When atrioventricular conduction approaches 1:1 during exercise, the ventricular rate increases, which may cause serious symptoms such as syncope and shock.
e. Junctional Rhythms
Junctional rhythms originate from the atrioventricular junction at a rate of 30 to 60 beats/min. Junctional rhythms may appear during sleep or other times of increased vagal tone, and are often observed in athletes. It usually returns to the sinus rhythm during exercise testing.
f. Premature Ventricular Contractions
Premature ventricular contractions (PVCs) are a type of arrhythmia that is relatively common among children. Ventricular beats occur at a rate faster than the sinus rate. PVCs may often originate from the right ventricular outflow tract, the left ventricular septum or the apex.
g. Ventricular Parasystole
Ventricular parasystole is a dual rhythm in which an ectopic ventricular focus activates the ventricles independent of the sinus rhythm.
h. Accelerated Idioventricular Rhythm
Accelerated idioventricular rhythm is a ventricular rhythm with a rate of <120 beats/min due to enhanced ventricular automaticity.
i. Nonsustained Monomorphic Ventricular Tachycardia
Nonsustained monomorphic ventricular tachycardia is present when at least three consecutive PVCs (lasting for less than 30 seconds and <100 consecutive PVCs) at a ventricular rate of about ≥120 beats/min.
j. Sustained Monomorphic Ventricular Tachycardia
Sustained monomorphic ventricular tachycardia is defined as PVCs lasting for more than 30 seconds or at least 100 consecutive PVCs, or as PVCs lasting for <30 seconds but requiring electrical cardioversion.
k. Polymorphic Ventricular Tachycardia
Polymorphic ventricular tachycardia is defined as 3 or more consecutive QRS complexes with more than one QRS morphology.
l. Complete Right Bundle Branch Block70–72
Complete right bundle branch block is defined as an rsR’ or rSR’ pattern in leads V3R to V2 with a QRS interval of ≥0.10 sec in children in elementary school or younger, and ≥0.12 sec in those in junior high school or older.
m. Complete Left Bundle Branch Block73
Complete left bundle branch block is defined as the absence of Q waves, a slurring of the upward slope of the R wave, and a QRS interval of ≥0.10 sec in children in elementary school or younger and ≥0.12 sec in those in junior high school or older.
n. Sick Sinus Syndrome
Sick sinus syndrome includes sinus arrest, sinoatrial block, and bradycardia-tachycardia syndrome.
o. Atrioventricular Block
Atrioventricular block is classified according to its severity, and each type needs different management.
i. First-Degree Atrioventricular Block
First-degree atrioventricular block is defined as prolongation of the PR interval.
ii. Second-Degree Atrioventricular Block (Wenckebach Type)
Wenckebach type atrioventricular block is characterized by progressive prolongation of the PR interval and an interruption of conduction from the atria to the ventricles.
iii. Mobitz Type II or 2:1 Atrioventricular Block
Mobitz type II atrioventricular block is characterized by an interruption in atrial to ventricular conduction without prolongation of the PR interval, and 2:1 atrioventricular block by two atrial beats for each ventricular beat.
iv. Advanced or Complete Atrioventricular Block
Advanced atrioventricular block is defined as an interruption of conduction from the atria to the ventricles in at least 2 out of every 3 atrial beats. Complete atrioventricular block is defined as the total interruption of conduction from the atria to the ventricles.
2. Management of Hereditary Arrhythmias
a. Description
Hereditary arrhythmias are caused by mutations in genes that encode ion channels controlling cardiac excitation and conduction or their regulatory proteins, and directly cause ventricular tachycardia/fibrillation, atrial tachycardia/fibrillation, or lethal bradycardia that occur in families. In general, hereditary arrhythmias are not related to organic heart disease. The following sections describe congenital long QT syndrome, one of the commonly observed hereditary arrhythmias in school-aged children, as well as catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada syndrome, short QT syndrome, and familial sick sinus syndrome.
b. Long QT Syndrome
i. Congenital Long QT Syndrome: Outline
Congenital long QT syndrome (LQTS) is a familial disease due to mutations of genes coding ion channels of cardiomyocytes, which cause delayed repolarization, a long QT interval, and ventricular arrhythmias on ECG. The hallmark of LQTS is syncope and eventually sudden death in people with no organic heart disease.74
To date, a total of 16 gene mutations responsible for LQTS have been found. However, mutations are identified only in 70% of patients with LQTS undergoing examinations, and about 90% of patients with identified mutations have LQT1 to LQT3 (LQT1:LQT2:LQT3 is roughly equal to 4:3:2).74
The trigger of cardiac events includes exercise and swimming in those with LQT1, sudden noises (e.g., starter pistol sound and alarm clock sound) and before or after waking in those with LQT2, and rest/sleep, and increased vagal tone in those with LQT3.
Table 17
outlines diagnostic criteria for LQTS.74,74a
The following section describes LQT7 that occurs more frequently than other LQTS types and its phenotype slightly differs from others.
Table 17.
Risk Score and Diagnostic Criteria of Long QT Syndrome
| |
Points |
| 1. ECG findings# |
| A. QTc^ |
| ≥480 msec |
3 |
| 460 to 479 msec |
2 |
| 450 to 459 msec (in males) |
1 |
| B. QTc^ 4th minute of recovery from exercise stress test ≥480 msec |
1 |
| C. Torsade de pointes* |
2 |
| D. T wave alternans |
1 |
| E. Notched T wave in 3 leads |
1 |
| F. Low heart rate for age** |
0.5 |
| 2. Clinical history |
| A. Syncope* |
| With stress |
2 |
| Without stress |
1 |
| B. Congenital deafness |
0.5 |
| 3. Family history |
| A. Family members with definite LQTS*** |
1 |
| B. Unexplained sudden cardiac death below age 30 among immediate family members*** |
0.5 |
#In the absence of medications or disorders known to affect these ECG features. ^QTc calculated by Bazett’s formula where  . *Mutually exclusive. **Resting heart rate below the 2nd percentile for age. ***The same family member cannot be counted in A and B. Total score: ≥3.5 points, high probability; 1.5 to 3.0 points, intermediate probability; ≤1.0 point, low probability of LQTS. (Source: Schwartz PJ, et al. 2011.74a
)
. *Mutually exclusive. **Resting heart rate below the 2nd percentile for age. ***The same family member cannot be counted in A and B. Total score: ≥3.5 points, high probability; 1.5 to 3.0 points, intermediate probability; ≤1.0 point, low probability of LQTS. (Source: Schwartz PJ, et al. 2011.74a
)
LQT7 (Andersen-Tawil syndrome) is an autosomal-dominant disorder characterized by (1) enlarged U waves, a prolonged QT interval (or QU interval) and bidirectional ventricular tachycardia on ECG, (2) skeletal anomalies (short stature and characteristic facial features), and (3) periodic quadriplegia. Mutations in the
KCNJ2
gene are found in about 50% of patients with LQT7. This genotype is referred to as ATS type 1 (ATS1).74
In general, patients with ATS1 tend to have premature ventricular contractions more frequently at rest. The rate of bidirectional ventricular tachycardia in this patient population is relatively slower than those with CPVT, and the incidences of syncope and sudden death are low. On the other hand, in those with CPVT, LQT1 and LQT2, ventricular tachycardia is infrequent at rest, and tends to develop during exercise or under mental stress. The rate of ventricular tachycardia in patients with ATS is high, and patients show characteristic bidirectional or polymorphic ventricular tachycardia including torsade de pointes (TdP), which may lead to ventricular fibrillation and sudden death. ATS1 and these disorders can be differentiated by these points.75
c. Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a familial arrhythmia characterized by occurrence of polymorphic or bidirectional ventricular tachycardia or fibrillation which is sometimes combined with atrial flutter/fibrillation and may lead to syncope, seizures, cardiac arrest or sudden death during exercise or under mental stress. Patients with CPVT have a poor prognosis and become symptomatic at the age of 10 years on average, and go into cardiac arrest in about 50% of patients by the age of 20–30 years. Other than the tendency of showing sinus bradycardia on ECG at rest, resting ECG findings are generally normal. Many patients with syncope may be misdiagnosed with other types of arrhythmias, vagal reflex or epilepsy.76
Those suspected to have CPVT should be assessed by a stress ECG and detailed history taking including family history.
d. Brugada Syndrome
Brugada syndrome is an inherited arrhythmia characterized by coved or saddleback ST-segment elevations in the right precordial leads (V1–V3) in ECG. Syncope and sudden death due to ventricular fibrillation or polymorphic ventricular tachycardia may occur at rest or at night.74
Brugada syndrome is prevalent in young Asian males mainly in South East Asian with an average age of diagnosis of 41±15 years. Symptomatic children are rare and are found in 1 out of about 10,000 children. Fever may trigger typical ST-T waves and ventricular arrhythmia.77
e. Short QT Syndrome
Short QT syndrome is a familial arrhythmia characterized by a short QT interval on ECG, and may cause atrial fibrillation, ventricular tachycardia/fibrillation and sudden death.
The short QT syndrome diagnostic criteria proposed by Gollob et al have been used (Table 18).78
Table 18.
Diagnostic Criteria for Short QT Syndrome (Gollob Score)
| |
Points |
| QTc (msec) |
| <370 |
1 |
| <350 |
2 |
| <330 |
3 |
| J point-T peak interval <120 msec |
1 |
| Clinical history* |
| History of sudden cardiac arrest |
2 |
| Polymorphic ventricular tachycardia or ventricular fibrillation |
2 |
| Unexplained syncope |
1 |
| Atrial fibrillation |
1 |
| Family history* |
| First- or second-degree relative with high-probability Short QT syndrome |
2 |
| First- or second-degree relative with autopsy-negative sudden cardiac death |
1 |
| Sudden infant death syndrome |
1 |
| Genotype* |
| Genotype positive |
2 |
| Mutation of undetermined significance in a culprit gene |
1 |
≥4 points, high probability; 3 points, intermediate probability; ≤2 points, low probability of short QT syndrome. *A minimum of 1 point must be obtained in the ECG section in order to obtain additional points. (Source: Gollob MH, et al. 2011.78 https://www.sciencedirect.com/science/article/pii/S0735109710047212?via%3Dihub)
Familial sick sinus syndrome is characterized by pathological sinus bradycardia, chronotropic incompetence, sinus arrest, sinoatrial block, and bradycardia-tachycardia syndrome associated with tachycardia such as atrial fibrillation/flutter that occur sporadically in families.74,79
g. Diagnostic Criteria and Management
Table 19
lists the criteria for selection classification in primary screening.
Table 20
summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period.
Table 21
lists assessment and examinations required in secondary screening and thereafter and findings requiring referral to a specialist.
Table 19.
Hereditary Arrhythmias: Criteria for Primary Screening and Selection Categories
| Hereditary arrhythmias |
Criteria for primary screening |
Selection category* |
| Long QT syndrome type 1 to 3 |
QT prolongation: There have been no screening criteria for QT prolongation by using
automatic ECG analysis regarding school screening. In this guideline document, QT
prolongation is defined as a QTc of ≥0.45 sec obtained using the Fridericia correction.
(QTc obtained in automatic ECG analysis is often about 20 msec longer than that obtained
with the tangent method.) If QT prolongation is suspected in automatic analysis, ECG
should be reviewed manually using the tangent method (see the following table). The
morphology of T wave should be considered as well.
Table. Criteria of QT prolongation using the tangent method
· Boys and girls in the 1st year of elementary school: ≥0.43 sec
· Boys and girls in the 1st year of junior high school, ≥0.44 sec
· Boys and girls in the 1st year of senior high school, ≥0.44 in boys and 0.45 sec in girls
No data are available for other grades. Refer to the above figures for other grades.
(Tabulated based on Hazeki D, et al. 2010.80) |
A |
LQT7 (Andersen-Tawil
leftsyndrome) |
Bidirectional ventricular tachycardia or large U wave |
A |
| Short QT |
QTc value (obtained using Bazett’s formula) <0.33 sec (tentative criteria) |
A |
Catecholaminergic polymorphic
leftventricular tachycardia (CPVT) |
Exercise- or stress-induced polymorphic ventricular tachycardia with a history of syncope,
leftseizures, palpitations or chest pain, and/or a family history of syncope or sudden death in
leftyoung age is present |
A |
| Brugada-type ECG |
Brugada-type ECG: ST elevation ≥0.2 mV at the J point in any of right chest leads V1, V2
leftor V3 and coved or saddleback ST-T |
A |
*Selection category. Group A: Findings requiring secondary screening/further examination. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 20.
Hereditary Arrythmias: Conditions and Categories for Exercise Management and Follow-up Interval
Hereditary
arrhythmias |
Conditions |
Management
category |
Follow-up
interval |
| QT prolongation |
[When symptoms, TdP or ventricular tachycardia have been
documented]
The child and parents should be fully informed that medication non-
compliance may induce serious symptoms. Refer to a specialist |
|
|
| 1. Symptoms controlled with medication |
D or E-prohibited, no
swimming |
As required |
| 2. Symptoms persist even after considerable medical treatment |
C or D, no swimming |
As required |
| [No symptoms] |
1. Slight QTc prolongation at rest, no family history, and no QTc
prolongation during exercise |
E-prohibited or E-allowed,
requiring observation
during swimming |
6 months to
1 year |
| 2. Marked QTc prolongation at rest |
E-prohibited, no swimming |
As required |
| 3. Symptomatic, or a family history of TdP or ventricular tachycardia |
D, E-prohibited or
E-allowed, no swimming |
As required |
| 4. QTc prolongation during exercise |
D or E-prohibited, no
swimming |
As required |
| LQT3 |
Exercise induced ventricular tachycardia with a history of syncope or
ventricular tachycardia, or a family history of sudden death |
C-prohibited or
D-prohibited |
1 to 3 months
or as required |
| If none of the above presents |
D-prohibited, E-prohibited
or E-allowed |
As required |
| LQT7 |
Exercise-induced ventricular tachycardia with a history of syncope or
ventricular tachycardia or a family history of sudden death is present |
C-prohibited or
D-prohibited |
1 to 3 months
or as required |
| If none of the above presents |
D-prohibited, E-prohibited
or E-allowed |
3 to 6 months |
| CPVT |
CPVT is diagnosed or suspected, and drug treatment is effective |
C or D |
1 to 3 months
or as required |
| CPVT is diagnosed or suspected, and drug treatment is not effective |
B or C |
1 to 3 months
or as required |
| Short QT |
Diagnostic criteria in children have not been established
Children suspected with short QT syndrome should be referred to a
specialist |
B-prohibited, C-prohibited
or D-prohibited |
As required |
| Brugada-type ECG |
1. Asymptomatic, no family history, but suspected to have Brugada
syndrome by a specialist |
E-allowed |
1 year |
| 2. Those diagnosed as Brugada syndrome by an expert |
C, D, E-prohibited or
E-allowed |
As required |
Familial sick sinus
syndrome |
Asymptomatic, mild tendency toward bradycardia, and an appropriate
increase in ventricular rate during exercise |
E-prohibited or E-allowed |
6 months to
1 year |
| Asymptomatic but a poor increase in ventricular rate during exercise |
D or E-prohibited |
6 months to
1 year |
| Those with syncope, dizziness, or cardiac systolic dysfunction |
A, B or C |
1 to 3 months
or as required |
| After pacemaker implantation |
D, E-prohibited or
E-allowed |
3 to 6 months |
CPVT, catecholamineergic polymorphic ventricular tachycardia; TdP, torsade de pointes.
Table 21.
Hereditary Arrhythmias: Diagnostic Examinations Required at Secondary Screening and Further Examination, and Findings That Require Referral to a Specialist
Hereditary
arrhythmias |
Items to be examined at secondary
screening/further examination |
Secondary screening findings
requiring specialist referral |
Long QT syndrome
type 1 to 3 |
Auscultation, chest X-ray (Posterior-anterior view),
12-lead ECG, exercise ECG |
Long QT syndrome is suspected or diagnosed |
LQT7
(Andersen-Tawil
syndrome) |
12-lead ECG, exercise ECG, Holter ECG, blood
testing Electrolytes |
Nonsustained bidirectional ventricular tachycardia or polymorphic
ventricular tachycardia is documented on resting ECG; Similar
types of ventricular tachycardia is induced during exercise ECG;
characteristic facial features or short height are noted; or a
history of periodic quadriplegia is present |
| Short QT |
12-lead ECG, exercise ECG, Holter ECG |
Short QT on ECG is suspected, especially with a history of
syncope or a family history of sudden death |
Catecholaminergic
polymorphic
ventricular
tachycardia |
12-lead ECG, exercise ECG, Holter ECG,
echocardiography |
Those in whom ventricular arrhythmia, especially bidirectional,
multiform premature ventricular contractions, or ventricular
tachycardia was induced by exercise; those with a history of
syncope, seizure, palpitations, or chest pain during exercise or
under mental stress; or those with a family history of syncope
or sudden death (especially at a young age) |
| Brugada-type ECG |
12-lead ECG (if possible, additional ECG should
be recorded from a higher intercostal space),
Holter ECG, exercise ECG, if required
pharmacological provocation test using
sodium-channel blockers (especially Class IC or
Class IA drugs), electrophysiological testing |
Those diagnosed as having Brugada syndrome. Those with
‘suspicious’ ECG findings but with a history of syncope or a
family history of sudden death (especially in young age) |
Familial sick sinus
syndrome |
12-lead ECG, exercise ECG, Holter ECG,
electrophysiological testing |
Those with ECG findings suggesting sick sinus syndrome,
especially a history of syncope or decreased exercise capacity,
and those with a family history of sudden death or a pacemaker
implantation |
Table 22
lists the criteria for selection classification in primary screening.
Table 23
summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period.
Table 24
lists assessment and examinations required in secondary screening and thereafter and findings requiring referral to a specialist.
Table 22.
Cardiomyopathy and Myocarditis: Criteria for Primary Screening and Selection Categories
| Heart disease |
Criteria for primary screening |
Selection
category* |
Hypertrophic
cardiomyopathy |
Deep Q wave in leads II, III, aVF, and left precordial leads |
A |
| High R wave in leads V5 to V6, and deep S wave in lead V1 (Findings of left ventricular hypertrophy) |
Severe ventricular septal hypertrophy may lead to a decreased electrocardiographic force in left
ventricular free wall (poor R wave progression), which may cause findings of right ventricular
hypertrophy |
| Flat or negative T wave or giant negative T wave in left precordial leads |
| Pulmonary P wave or mitral P wave |
| Giant negative T wave in left precordial leads in patients with apical hypertrophic cardiomyopathy |
| Extrasystoles, ventricular tachycardia, atrial fibrillation, or WPW syndrome may be observed |
Those with palpitations, dyspnea, chest pain or exercise-induced syncope must be selected for
further screening/examination |
| A family history of hypertrophic cardiomyopathy or sudden death at young age |
| Dilated cardiomyopathy |
Findings of left ventricular hypertrophy, left bundle branch block, wide QRS duration, ST depression,
flat T wave |
A |
| Non-specific abnormal ST-T change in early stage |
| Mitral P wave and/or pulmonary P wave |
| Various types of arrhythmias |
Restrictive
cardiomyopathy |
Pulmonary P wave, and/or mitral P wave |
A |
| ST depression and negative T wave |
Noncompaction of
ventricular myocardium |
Marked left ventricular hypertrophy in infancy, and non-specific changes including ST-T changes
in early childhood |
A |
| Often associated with arrhythmia, and relatively frequently associated with WPW syndrome |
Arrhythmogenic right
ventricular
cardiomyopathy |
Negative T waves in right precprdial leads V1–V3 |
A |
| A notch (ε wave) after QRS wave in leads V1–V3 |
| Left bundle branch block-type ventricular tachycardia is present |
| Myocarditis |
When cardiac symptoms such as palpitations, arrhythmia, edema, dyspnea, decreased
consciousness, syncope, or cardiomegaly develop during or after the common cold, the
pupil/student should refrain from sports activities, and visit a cardiologist |
A |
Sinus tachycardia, weak heart sounds, and gallop rhythm are audible on chest auscultation.
Those with complete atrioventricular block have serious bradycardia |
On ECG, ST-T changes, abnormal Q wave, low voltage, bundle branch block, atrioventricular
block, multiple premature ventricular contractions, and ventricular tachycardia or other various
findings may appear and these findings may change one after another |
*Selection category. Group A: Findings requiring secondary screening/further examination. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 23.
Cardiomyopathy and Myocarditis: Conditions and Categories for Exercise Management, and Follow-up Interval
| Heart disease |
Conditions |
Management
category |
Follow-up
interval |
| Hypertrophic cardiomyopathy |
Asymptomatic |
D |
6 to 3 months |
Those with chest pain, syncope or other symptoms, and those with
obstructive cardiomyopathy |
B or C |
1 month |
| High-risk children |
A, B or C |
1 month |
| Dilated cardiomyopathy |
Asymptomatic |
D |
6 to 3 months |
| Symptomatic |
C |
1 month |
| Restrictive cardiomyopathy |
Asymptomatic |
D |
1 to 3 months |
| Symptomatic |
C |
1 month |
Noncompaction of ventricular
myocardium |
Asymptomatic, normal cardiac function |
E-prohibited or
E-allowed |
1 to 3 years |
| Cardiac dysfunction, asymptomatic |
D |
3 to 6 months |
| Cardiac dysfunction, symptomatic |
C |
1 month |
Arrhythmogenic right
ventricular cardiomyopathy |
Exercise is contraindicated |
C |
1 to 3 months |
| Myocarditis |
Disappearance of disease activity after acute phase. Asymptomatic |
D |
3 to 6 months |
| Disappearance of disease activity after acute phase. Symptomatic |
C |
1 to 3 months |
| Chronic myocarditis, asymptomatic |
D |
3 to 6 months |
| Chronic myocarditis, symptomatic |
C |
1 to 3 months |
Table 24.
Cardiomyopathy and Myocarditis: Diagnostic Examinations Required at Secondary Screening and Further Examination, and Findings That Require Referral to a Specialist
| Heart disease |
Items to be examined at secondary
screening/further examination |
Secondary screening findings
requiring specialist referral |
Hypertrophic
cardiomyopathy |
History taking, auscultation, 12-lead
ECG, echocardiography |
Left ventricular hypertrophy |
| Ventricular septal hypertrophy (asymmetric septal hypertrophy, ASH) |
| Left ventricular outflow tract obstruction |
| Systolic anterior motion (SAM) of the anterior mitral valve or other findings |
Dilated
cardiomyopathy |
History taking, auscultation, 12-lead
ECG, echocardiography |
S3, S4, gallop rhythm, and apical regurgitation sounds due to mitral valve
insufficiency |
| Serious ventricular or atrial arrhythmias |
Dyspnea, palpitations, chest compression, cough, cold extremities, edema, or
fatiguability during exercise |
Definitive findings include enlarged left ventricular cavity, decreased contraction,
mitral regurgitation, and myocardial thinning |
| Cardiomegaly on chest X-ray |
Restrictive
cardiomyopathy |
History taking, auscultation, 12-lead
ECG, echocardiography |
Chest X-ray: Double shadow due to left atrial enlargement, mild to moderate
cardiomegaly, findings of pulmonary congestion |
Symptoms: Cough due to pulmonary congestion, fatiguability and dyspnea on
effort are typical |
Physical findings: Findings of heart failure such as S3, edema, hepatomegaly,
and ascites |
Noncompaction of
ventricular
myocardium |
History taking, auscultation, 12-lead
ECG, echocardiography |
Echocardiography: Similarly to dilated cardiomyopathy, enlarged left ventricular
cavity, decreased contraction, mitral regurgitation, and myocardial thinning
are observed. Prominent and excessive myocardial trabeculations in ventricular
wall with deep intertrabecular spaces are found in one or more ventricular
wall segment. A noncompacted to compacted myocardial (NC/C) ratio in end
diastole is ≥2 |
| MRI and CT findings: Distribution of trabeculations may be assessed |
Arrhythmogenic
right ventricular
cardiomyopathy |
History taking, auscultation, 12-lead
ECG, echocardiography |
The following ECG findings are observed: |
| 1) Negative T waves in right precordial leads V1–V3 |
| 2) A notch (epsilon wave) after QRS wave in leads V1–V3 |
| 3) Left bundle branch block type ventricular tachycardia is present |
Echocardiography: Specific findings including right ventricular aneurysm,
prominent trabeculations, wall bulging and poor contraction of the right ventricle |
| Myocarditis |
History taking, auscultation, 12-lead
ECG, echocardiography |
ECG: Bundle branch block, atrioventricular block, frequent extrasystoles and
ventricular tachycardia, ST-T changes, abnormal Q waves, and low voltage |
Questionnaire: Relatively sudden onset symptoms such as chest pain,
palpitations and syncope |
Hypertrophic cardiomyopathy (HCM) is a disease characterized by an increased myocardial mass with a resultant small ventricular cavity, which causes diastolic dysfunction. HCM includes symmetric septal hypertrophy (non-obstructive hypertrophic cardiomyopathy), asymmetric septal hypertrophy (obstructive hypertrophic cardiomyopathy) and apical hypertrophic cardiomyopathy.81,82
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities82
The following examinations are required to assess the risk of cardiac events.
(1) Family history taking in sudden death at young age and myocardial disorder.
(2) History taking in syncope (especially during exercise), seizures, chest pain, cardiac arrest and sustained ventricular tachycardia.
(3) Echocardiography.
(4) Holter ECG.
(5) Exercise testing.
3.2 Dilated Cardiomyopathy
a. Description
Dilated cardiomyopathy is characterized by systolic dysfunction and cardiomegaly, which causes progressive heart failure with a poor prognosis.83,84
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
The following examinations are required to assess the risk of cardiac events.
(1) Detailed history taking including family history.
(2) 12-lead ECG.
(3) Echocardiography.
(4) Holter ECG.
(5) Exercise testing, catheterization, or other appropriate diagnostic methods.
Echocardiography is useful in the diagnosis and severity assessment of dilated cardiomyopathy. It reveals an enlargement of the left ventricular cavity, a decrease in left ventricular ejection fraction, and the presence of mitral valve insufficiency. Cardiac indices such as the mitral inflow E/A ratio and Tei index are useful in the assessment of prognosis.
3.3 Restrictive Cardiomyopathy
a. Description
Restrictive cardiomyopathy is characterized by a decrease in ventricular compliance and diastolic dysfunction without ventricular dilation, hypertrophy, or impaired myocardial contractility.85,86
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
The following examinations are required to assess the risk of cardiac events.
(1) Echocardiography: Marked atrial enlargement is observed. The ventricular size is reduced or normal without ventricular wall thickness. Systolic function is normal. Doppler echocardiography shows ventricular inflow obstruction. It is important to differentiate from constrictive pericarditis.
(2) Detailed history taking including family history.
(3) 12-lead ECG, Holter ECG.
(4) Chest X-ray.
Prognosis is very poor when the patient has findings of pulmonary congestion visible on chest X-ray, or suggestive of myocardial ischemia such as a history of chest pain or syncope and ST changes on ECG. As the disease may progress rapidly, all children and young people diagnosed with restrictive cardiomyopathy are at high risk of cardiac events.
3.4 Noncompaction of Ventricular Myocardium
a. Description
Noncompaction of ventricular myocardium is a cardiomyopathy where genetic factors are likely to play a major role in its pathogenesis, and is characterized by prominent and excessive myocardial trabeculations in ventricular wall with deep intertrabecular spaces. This disease typically develops in neonatal and infancy periods and leads to heart failure and death and is indicated for heart transplantation. This disease may also be coincidentally found in asymptomatic children and young people on heart disease screening. Many cases in pre-school age children and adults have been reported.87
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
(1) Prognosis is poor when echocardiography shows cardiac dysfunction.
(2) Prognosis factors include cardiac function at the initial visit and presence/absence of heart failure.
(3) About 50% of those with cardiac dysfunction die or need heart transplantation in 5 years.
3.5 Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
a. Description
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a progressive disease characterized by progressive myocardial degeneration, fatty infiltration and fibrosis mainly in the right ventricle or sometimes both ventriculi. Right ventricular dilation or contractile dysfunction, and arrhythmias originating from the right ventricle. This disease often runs in families. ARVC develops in adolescence or later, and most often develops in people 20–40 years of age. This is an important cause of sudden death in young people, and sudden death may develop as the first manifestation of this disease. Patients may have palpitations and fatigue, but some people are asymptomatic. Exercise-induced ventricular tachycardia and frequent persistent ventricular tachycardia may lead to syncope and sudden death.88
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
(1) Detailed history taking including family history.
(2) 12-lead ECG, 24-hour or 48-hour Holter ECG, chest X-ray, or other appropriate examinations.
(3) Echocardiography: Specific findings such as right ventricular aneurysm, prominent trabeculations, wall bulging and poor contraction of the right ventricular wall are observed.
(4) MRI: Right ventricular aneurysm, poor contraction of the right ventricle, fatty infiltration, and delayed enhancement are observed.
(5) Myocardial biopsy: Marked fatty infiltration and fibrosis.
(6) Predictors of poor prognosis include onset at young age, being an athlete, a family history of sudden death, extensive right ventricular lesions or left ventricular involvement, a history of syncope, and ventricular tachycardia.
(7) Children with ARVC are at high risk of cardiac events.
3.6 Myocarditis
a. Description
Acute myocarditis is an inflammatory disease characterized by myocardial infiltration with inflammatory cells and myocardial damage. The most common cause is infection with enteroviruses such as group B coxsackieviruses. Acute myocarditis is an important cause of sudden death in young people. According to annual rare disease surveillance reports published by the Japanese Society of Pediatric Cardiology and Cardiac Surgery, a total of 478 cases of acute myocarditis in children were reported and about 10% of them died during 9 years of surveillance.
Myocarditis often develops several days after acute upper respiratory or gastrointestinal infection, and typically manifests with nonspecific symptoms such as generalized malaise, bad mood and dyspnea. It may also manifest with arrhythmias due to atrioventricular conduction abnormalities such as cause Stokes-Adams attacks caused by complete atrioventricular block, ventricular tachycardia or supraventricular tachycardia.
Chronic myocarditis is rare in children and young people, but those with myocarditis lasting at least a few months may experience sudden death, congestive heart failure, arrhythmias, persistent fatiguability, dyspnea and poor weight gain, and show typical signs and symptoms of dilated cardiomyopathy. Patients with chronic myocarditis include those in whom the disease developed without clinical signs and symptoms without clear date of onset, and those in whom acute myocarditis persisted. Chronic myocarditis is an intractable condition with poor prognosis.
Myocarditis should be suspected as a possible cause of arrhythmias and myocardial damage that are found in previously healthy children and young people in heart disease screening.82,89
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
Those who are suspected to have acute myocarditis should be hospitalized to undergo following examinations:
(1) Echocardiography: To examine for ventricular or atrial enlargement, decreased wall motion, pericardial effusion, transient myocardial thickening, and atrioventricular valve regurgitation due to papillary muscle dysfunction.
(2) Cardiac nuclear imaging: Those in the acute phase should undergo 67Ga myocardial scintigraphy or 99 mTc myocardial scintigraphy for detection of positive findings, and 111In-antimyosin antibody imaging to detect and locate the accumulation of labeled antibodies. Those in the acute to chronic phase should undergo 201Tl myocardial scintigraphy to examine for defects and locate lesions.
(3) MRI: Findings consistent with edema are observed in areas of reduced wall motion and indicate reduced intracardiac blood flow.
(4) Subendocardial biopsy: Biopsy samples should be examined for infiltration of inflammatory cells, lysis, denaturation, rupture or loss of cardiomyocytes, and interstitial edema or fibrosis.
Those with no active myocarditis should undergo echocardiography to assess cardiac function and Holter ECG and exercise electrocardiogram to assess for arrhythmia to determine their allowable intensity of exercise.
4. Management of Left-to-Right Shunt Heart Disease
a. Diagnostic Criteria and Management
Table 25
lists the criteria for selection classification in primary screening.
Table 26
summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period.
Table 27
lists assessment and examinations required in secondary screening and thereafter and findings requiring referral to a specialist.
Table 25.
Left-to-Right Cardiac Shunt: Criteria for Primary Screening and Selection Categories
| Heart disease |
Criteria for primary screening |
Selection
Category* |
| Atrial septal defect |
Questionnaire: A history of atrial septal defect but a possible lack of appropriate management |
A |
Auscultation: Systolic murmurs over the second left intercostal space, fixed splitting of S2, and
diastolic rumble over the fourth left intercostal space |
ECG: incomplete right bundle branch block, right axis deviation, first degree atrioventricular block, T
wave inversion in V4 lead should be considered |
Ventricular septal
defect |
Questionnaire: A history of ventricular septal defect but a possible lack of appropriate management |
A |
Auscultation: Holosystolic murmurs at the left sternal border, loud S2 and apical diastolic rumble
(These findings are rarely found in screening) |
ECG: Mild cases show normal ECG. Moderate or severe cases show findings of left ventricular
hypertrophy due to left ventricular volume overload and findings of right ventricular hypertrophy due to
the progression of pulmonary hypertension |
Patent ductus
arteriosus |
Questionnaire: Cases where patent ductus arteriosus was suspected but no detailed examination was
made. In some cases where patent ductus arteriosus was diagnosed and treated but residual shunt or
aortic anomalies may be suspected in auscultation or ECG, which require periodic follow-up |
A |
Auscultation: Cases with continuous murmurs at the left sternal border, or systolic murmurs (excluding
innocent heart murmurs) should be selected. Apical diastolic rumble, loud S2 |
| Palpation: Bounding pulse |
ECG: Mild left ventricular hypertrophy or right ventricular hypertrophy ECG findings are within normal
range in cases of mild patent ductus arteriosus |
Atrioventricular
septal defect |
Questionnaire: Cases with a history of atrioventricular septal defect should be selected |
A |
Auscultation: Those with murmurs at the upper left sternal border, or holosystolic murmur and
diastolic rumbles at the lower left sternal border, apical holosystolic murmur, or apical diastolic rumble
should be selected. Careful examination for loud S2 should also be required |
ECG: Those with incomplete right bundle branch block associated with left axis deviation should be
selected. It should be noted that some of those with incomplete atrioventricular septal defect may
show left axis deviation without incomplete right bundle branch block, and may be overlooked. Children/
adolescents with left axis deviation should be assessed on the basis of questionnaire and auscultation,
and those with a long PQ interval or findings of left ventricular
hypertrophy or right ventricular hypertrophy should be selected for further screening. |
*Selection category. Group A: Findings requiring secondary screening/further examination. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 26.
Left-to-Right Cardiac Shunt: Conditions and Categories for Exercise Management, and Follow-up Interval
| Heart disease |
Conditions |
Management
category |
Follow-up
interval |
| Atrial septal defect |
Those before or after treatment, positive for pulmonary hypertension |
E-prohibited |
1 to 3 months |
Those before or after treatment, negative for pulmonary hypertension and
arrhythmia |
E-allowed |
1 to several
years |
| The condition has been treated and no problems are noted |
No management
required |
|
Ventricular septal
defect |
Those before or after treatment, positive for pulmonary hypertension |
E-prohibited |
1 to 3 months |
Those before or after treatment, negative for pulmonary hypertension and
arrhythmia |
E-allowed |
1 to several
years |
| The condition has been treated and no problems are noted |
No management
required |
|
Patent ductus
arteriosus |
Those before or after treatment, positive for pulmonary hypertension |
E-prohibited |
1 to 3 months |
| Those before or after treatment, negative for pulmonary hypertension |
E-allowed |
1 to several
years |
| The condition has been treated and no problems are noted |
No management
required |
|
Atrioventricular
septal defect |
1. Those associated with left atrioventricular valve insufficiency |
1) Echocardiography reveals left ventricular enlargement and severe
regurgitation |
C |
1 to 3 months |
2) Echocardiography reveals left ventricular enlargement and moderate
regurgitation |
D |
1 to 3 months |
3) Echocardiography reveals no left ventricular enlargement and mild
regurgitation |
E-allowed or
E-prohibited |
6 months to
1 year |
| 2. Those associated with left ventricular outflow tract obstruction |
1) Doppler echocardiography or cardiac catheterization reveals a pressure
gradient of <20 mmHg |
E-allowed |
6 months to
1 year |
2) Doppler echocardiography or cardiac catheterization reveals a pressure
gradient of 20 mmHg to <40 mmHg |
E-prohibited |
3 to 6 months |
3) Doppler echocardiography or cardiac catheterization reveals a pressure
gradient of ≥40 mmHg |
C or D |
1 to 3 months |
| 3. Those before or after treatment, positive for pulmonary hypertension |
E-prohibited |
1 to 3 months |
4. Those before or after treatment, negative for pulmonary hypertension;
those after intracardiac repair, negative for significant persistent lesions |
E-allowed |
1 year |
Table 27.
Left-to-Right Cardiac Shunt: Diagnostic Examinations Required at Secondary Screening and Further Examination, and Findings That Require Referral to a Specialist
| Heart disease |
Items to be examined at secondary
screening/further examinations |
Secondary screening findings
requiring specialist referral |
| Atrial septal defect |
Auscultation, 12-lead ECG, chest X-ray
(Posterior-anterior view), echocardiography |
ECG: Right ventricular hypertrophy |
Chest X-ray: Protrusion of the left second arch (pulmonary artery
enlargement), protrusion of the right second arch (right atrial
enlargement), and increased pulmonary vascularity |
Echocardiography: A clearly visible defect between the atriums, right
ventricular dilatation, paradoxical movement of ventricular septum, mitral
valve prolapse, and partial anomalous pulmonary venous drainage |
Ventricular septal
defect |
Auscultation, 12-lead ECG, chest X-ray
(Posterior-anterior view), echocardiography |
ECG: Left ventricular hypertrophy, right ventricular hypertrophy |
| Chest X-ray: Cardiomegaly, increased pulmonary vascularity |
Echocardiography: A moderate to large-sized defect, left ventricular
enlargement, pulmonary hypertension, aortic valve prolapse, and
aortic valve insufficiency |
Patent ductus
arteriosus |
Auscultation, palpation, 12-lead ECG, chest
X-ray, echocardiography |
All need specialist referral |
Atrioventricular
septal defect |
Auscultation, chest X-ray (Posterior-anterior
view), 12-lead ECG, echocardiography |
All need specialist referral |
Atrial septal defect is classified by the location of the defect in the atrial septum into ostium primum type, ostium secundum type, sinus venous type, single atrium type and coronary sinus type.
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities16
Management should differ depending on the presence or absence of pulmonary hypertension (Note 1) and arrhythmia (Note 2). Findings of right ventricular hypertrophy on ECG, and the position and size of the defect and the level of right heart enlargement on echocardiography are useful in the assessment of disease progression.
(Note 1) For the purposes of this guideline, pulmonary hypertension is defined as a mean pulmonary artery pressure at rest of ≥25 mmHg.
(Note 2) Mild arrhythmia considered not related to surgery should be regarded as negative.
4.2 Ventricular Septal Defect
a. Description
Ventricular septal defect is classified into infundibular, perimembranous, inflow, and muscular ventricular septal defect. Ventricular septal defect is commonly found after detection of cardiac murmurs in health check-ups for infants and young children, and is rare to remain unfound until heart disease screening in schools.
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities17
Ventricular overload does not usually recognized in mild cases, but left heart enlargement sometimes develops in moderate or severe cases. Management should differ depending on the presence or absence of pulmonary hypertension (Note 1) and arrhythmia (Note 2). Findings of right ventricular hypertrophy on ECG, and the position and size of the defect and the level of left heart enlargement on echocardiography are useful in the assessment of disease progression.
(Note 1) For the purposes of this guideline, pulmonary hypertension is defined as a mean pulmonary artery pressure at rest of ≥25 mmHg.
(Note 2) Mild arrhythmia considered not related to surgery should be regarded as negative.
4.3 Patent Ductus Arteriosus
a. Description
Patent ductus arteriosus is defined as persistent patency of the ductus arteriosus, which stays open in the fetus, even after birth.
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
Those with untreated patent ductus arteriosus should be assessed with echocardiography and other examinations for the presence of pulmonary hypertension. Those with repaired patent ductus arteriosus should be assessed at the clinic where they were diagnosed or treated and be rated for allowable intensity of exercise and daily activities.
4.4 Atrioventricular Septal Defect
a. Description
Atrioventricular septal defect (AVSD) is classified into complete or incomplete form, depending on the presence of absence of a bridging tongue of tissue connecting the anterior and posterior common leaflets. In general, patients with partial AVSD have ostium primum atrial septal defect only, while those with complete AVSD have ostium primum atrial septal defects and common atrioventricular canal defect. Those after complete correction of AVSD should be carefully observed for the presence of residual shunt, atrioventricular valve dysfunction, left ventricular outflow tract obstruction, pulmonary hypertension, arrhythmias (e.g., supraventricular tachycardia, atrial fibrillation, atrial flutter and complete atrioventricular block),90–96
and need life-long follow-up.97
In many cases AVSD is diagnosed and treated before school age, but in rare cases incomplete AVSD is found in school health screening.
b. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
Children and young people who are found to have untreated incomplete AVSD should be managed in terms of exercise intensity similarly to those with atrial septal defect, but additional assessment based on atrioventricular valve function is required. They should be assessed by an expert to determine allowable exercise intensity. Those after correction of incomplete AVSD should be managed on the basis of the presence or absence of residual shunt, atrioventricular valve dysfunction, pulmonary hypertension, left ventricular outflow tract obstruction, and arrhythmias, among other conditions. Those with repaired AVSD should be assessed at the clinic where they were diagnosed or treated and be rated for allowable intensity of exercise and daily activities.
5. Management of Right-to-Left Shunt Heart Disease
a. Description
Many children born with congenital heart disease with a right-to-left shunting (i.e., cyanotic congenital heart disease) have undergone intracardiac repair during infancy or pre-school age, and have no cyanosis at school age. However, since those following corrective surgery may have arrhythmias, heart failure, thromboembolism or other complications months and years post-surgery, they will require periodic follow-up and management of exercise intensity in school. Those with uncorrected cyanotic congenital heart disease and those in whom cyanosis persists even after corrective surgery have low exercise capacity, and need detailed activity management. Those who underwent the Fontan surgery as the final palliative surgical procedure must undergo periodic assessment by experts who understand their complex hemodynamics.
b. Diagnostic Criteria and Management
Table 28
lists the criteria for selection classification at primary screening.
Table 29
summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period.
Table 30
lists assessment and examinations required at secondary screening and thereafter and findings requiring referral to a specialist.
Table 28.
Right-to-Left Cardiac Shunt: Criteria for Primary Screening and Selection Categories
| Heart disease |
Criteria for primary
screening |
Selection
category* |
| Tetralogy of Fallot |
All patients |
A |
Complete transposition of the
great arteries |
All patients |
A |
Functional single ventricle
(after the Fontan procedure) |
All patients |
A |
Persistent right-to-left shunt
(hypoxemia) |
All patients |
A |
*Selection category. Group A: Findings requiring secondary screening/further examinations. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 29.
Right-to-Left Cardiac Shunt: Conditions and Categories for Exercise Management, and Follow-up Interval
| Heart disease |
Conditions |
Management
category |
Follow-up
interval |
| Tetralogy of Fallot |
Asymptomatic; none or mild pulmonary valve regurgitation/ tricuspid valve
regurgitation; none to moderate right ventricular enlargement with intact
right ventricular systolic function; normal or slightly increased right ventricular
pressure; intact left ventricular systolic function; and no induction of
tachyarrhythmia during exercise |
E-allowed |
6 months to
1 year |
Asymptomatic but significant pulmonary valve regurgitation/tricuspid valve
regurgitation/right ventricular enlargement; or significant right ventricular
outflow tract stenosis with an increase in right ventricular pressure and a
right/left systolic pressure ratio of ≤50%; or tachyarrhythmia controlled with
drug therapy or catheter ablation |
D, E-prohibited
or E-allowed |
3 to 6 months |
Symptomatic, mild to moderate decrease in exercise capacity, moderate
or severe pulmonary valve regurgitation and right ventricular enlargement;
moderate or severe right ventricular outflow tract stenosis with a right/left
systolic pressure ratio of ≥50% (≥70% if left ventricular dysfunction is
present); or exercise-induced tachyarrhythmia not controllable with any
treatment |
C-prohibited or
D-prohibited |
1 to 3 months |
Complete
transposition of the
great arteries |
Asymptomatic, good exercise capacity, no residual abnormality with good
right and left ventricular function, and no induction of tachycardia during
exercise |
E-allowed |
6 months to
1 year |
Asymptomatic, mild residual abnormality (e.g., small ventricular septal
defect, mild stenosis/regurgitation of new aortic/pulmonary valves, or mild
arrhythmia such as isolated extrasystoles), and no abnormal ECG findings
during exercise |
E-prohibited or
E-allowed |
3 to 6 months |
Significant residual abnormality (right ventricular outflow tract stenosis
≥30 mmHg, significant regurgitation of new aortic valves), significant left/right
ventricular hypertrophy, left/right ventricular dysfunction, or tachyarrhythmia |
D or E-prohibited |
1 to 3 months |
Moderate or severe right ventricular outflow tract/pulmonary artery stenosis
with a right/left ventricular systolic pressure ratio of ≥50%, moderate or
severe new aortic valve regurgitation, or induction of tachyarrhythmia or
ST depression during exercise ECG |
B, C or D-prohibited |
1 to 3 months |
Functional single
ventricle (after the
Fontan procedure) |
The patient should be assessed comprehensively for arrhythmia, ventricular
function, valve function, oxygen saturation and exercise capacity, and
should be allowed to participate in daily physical activities and physical
education to a certain extent, under circumstances where the patient
can take a rest whenever necessary |
B, C, D or E-prohibited,
in some cases
E-allowed (adjust
intensity individually) |
3 to 6 months |
Persistent right-to-left
shunt (hypoxemia) |
Determine the allowable exercise intensity according to a comprehensive
assessment of underlying disease and test results |
B, C, D or
E-prohibited |
3 to 6 months
or as required |
Table 30.
Right-to-Left Cardiac Shunt: Diagnostic Examinations Required at Secondary Screening and Further Examination, and Findings That Require Referral to a Specialist
| Heart disease |
Items to be examined at secondary
screening/further examinations |
Secondary screening
requiring specialist
referral |
| Tetralogy of Fallot |
12-lead ECG, Holter ECG, exercise ECG (using a treadmill or ergometer to achieve a heart
rate of >150 bpm), chest X-ray, and echocardiography (to assess right and left ventricular
function, right ventricular outflow tract pressure gradient, and pulmonary/tricuspid/aortic
valve function, and aortic root enlargement)
Examinations that should be conducted whenever possible: Cardiac MRI (to assess right
ventricular volume/function, and pulmonary valve regurgitation), and 6-minute walk test or
cardiopulmonary exercise test (to assess peak V̇O2 and V̇E/V̇CO2 and others) |
All need specialist
referral |
Complete
transposition of the
great arteries |
12-lead ECG, Holter ECG, exercise ECG (using a treadmill or ergometer to achieve a heart
rate of >150 bpm to examine for occurrence of arrhythmia and ST-T changes), chest X-ray,
and echocardiography (to assess left ventricular function, right ventricular outflow tract
stenosis, aortic regurgitation, and aortic root enlargement) Examinations that should be
conducted whenever possible: Cardiac MRI (to assess right and left ventricular function,
right ventricular outflow tract/pulmonary artery stenosis/regurgitation), myocardial
scintigraphy (to assess blood flow and fatty acid metabolism), and 6-minute walk test or
cardiopulmonary exercise test (to assess peak V̇O2 and V̇E/V̇CO2 and others) |
All need specialist
referral |
Functional single
ventricle (after the
Fontan procedure) |
12-lead ECG, Holter ECG, exercise ECG, chest X-ray, cardiac CT image, and
echocardiography (to assess ventricular function and atrioventricular valve regurgitation)
Examinations that should be conducted whenever possible: Cardiac MRI (to assess
systemic ventricular volume/function and pulmonary artery/atrioventricular valve
regurgitation), cardiopulmonary exercise test (to assess peak V̇O2 and V̇E/V̇CO2 and
others) or 6-mininute walk test, and pulmonary perfusion scintigraphy |
All need specialist
referral |
Persistent right-to-left
shunt (hypoxemia) |
12-lead ECG, Holter ECG, exercise ECG, chest X-ray, and echocardiography. Examinations
that should be conducted whenever possible: Cardiac MRI, cardiopulmonary exercise test
(to assess peak V̇O2 and V̇E/V̇CO2 and others) or 6-mininute walk test, cardiac CT image,
pulmonary perfusion scintigraphy, and cardiac catheterization |
All need specialist
referral |
Tetralogy of Fallot is a typical disease causing a right-to-left shunt, comprised of a ventricular septal defect, right ventricular outflow tract stenosis, overriding of the aorta, and right ventricular hypertrophy. Almost all patients with tetralogy of Fallot have undergone corrective surgery pre-school age, but may have complications years later following surgery. Postoperative complications may include pulmonary valve insufficiency, tricuspid valve insufficiency, right ventricular enlargement/dysfunction, right heart failure, left ventricular dysfunction, left heart failure, persistent right ventricular outflow tract stenosis, persistent peripheral pulmonary artery stenosis, aortic dilatation, tachyarrhythmia, and sudden death. Severe pulmonary valve insufficiency may be associated with increases in the right ventricular end-diastolic volume and stroke volume, a decrease in cardiac output, and a tendency toward decreasing exercise capacity over time,98
and increased risk of ventricular tachycardia. Mean peak oxygen uptake (peak V̇O2) in those after corrective surgery are 70 to 80% that of healthy children.99
In order to ensure a safe school life, they should be assessed for signs and symptoms of heart failure and exercise capacity, and undergo exercise ECG to examine whether exercise induces tachyarrhythmia. Risk factors for the development of ventricular tachycardia in those after surgical correction of tetralogy of Fallot include the number of cardiac surgery procedures, QRS interval (>180 msec) on ECG, and left ventricular diastolic function.100
5.2 Complete Transposition of the Great Arteries
a. Description
Complete transposition of the great arteries is a congenital heart defect in which the aorta originates from the right ventricle and the pulmonary artery originates from the left ventricle. Severe hypoxemia develops immediately after birth. Surgical procedures and clinical course after surgery may vary by cardiac morphology, many patients undergo arterial switch operation (Jatene procedure) during neonatal period. The arterial switch operation is superior to conventional procedures to redirect blood flow at the atrial level such as Mustard and Senning procedures because the new procedure may preserve sinus node function and allow the left ventricle and mitral valve to support the systemic circulation. Clinical course after surgery is also better in those undergoing the arterial switch operation.101
Those having received the arterial switch operation tend to have lower exercise capacity compared with healthy counterparts, but their exercise capacity is higher than children after surgical correction of other types of cyanotic congenital heart diseases.99
Exercise capacity reduces when right ventricular outflow tract obstruction progresses years after the arterial switch operation.102
5.3 Functional Single Ventricle (After Fontan Procedure)
a. Description
The Fontan operation is the final palliative surgical procedure for the correction of complex congenital heart diseases that results in only one functional pumping ventricle, and is used for the treatment of tricuspid atresia, single ventricle, hypoplastic right heart syndrome (pulmonary atresia with intact ventricular septal defect), and hypoplastic left heart syndrome, and others. In the Fontan-type circulation, systemic venous blood is delivered to the lungs passively through an increase in central venous pressure and intrathoracic negative pressure during inspiration without a support of right ventricular pumping function. Exercise capacity improves significantly after the Fontan operation compared with the preoperative condition with cyanosis,103
but is still lower than in healthy people. The most common cause of their limited exercise capacity is a limited increase in ventricular stroke volume during exercise. A limited increase in heart rate and persistent cyanosis also affects their exercise capacity.104
However, appropriate exercise training is not risky in children and young people after the Fontan operation, and is expected to improve their exercise capacity and QOL.105
Aerobic exercise is known to be effective in improving exercise capacity in those with the Fontan circulation, but resistance training is also effective in improving ventricular stroke volume and exercise capacity in this patient population by enhancing peripheral muscle pump function and thereby increasing preload.106
Accordingly, students after the Fontan operation should be individually assessed for their allowable exercise intensity to avoid unnecessary exercise restriction. On the other hand, appropriate management should be taken to prevent arrhythmias since tachyarrhythmia especially supraventricular tachycardia and bradyarrhythmia such as sinus node dysfunction and atrioventricular block may worsen hemodynamics and cause sudden death.
(Note 1) Children and young people who have permanent pacemakers or other implantable devices and are on anticoagulant therapy should refrain from sports that cause a high level of impact on body, injuries, and rough physical contacts with others.107
(Note 2) The patient, teachers and administrator should understand signs and symptoms for which the patient should stop his/her exercise and take a rest. These signs and symptoms may include palpitations, chest pain, tachycardia, respiratory distress and/or dyspnea not expected from the intensity of exercise, nausea, dizziness, and syncope.107
5.4 Persistent Right-to-Left Shunt (Hypoxemia)
a. Description
Persistent right-to-left shunt may result from the following three conditions.
(1) Congenital heart disease that has not been treated with intracardiac repair (corrective surgery) or still causes persistent right-to-left shunt in the cardiac or aortic level after surgery.
(2) Eisenmenger syndrome.
(3) Heart diseases associated with pulmonary arteriovenous fistula.
6. Management of Valve Disease, Aortic Coarctation, and Marfan Syndrome16,47,108
a. Diagnostic Criteria and Management
Table 31 lists the criteria for selection classification at primary screening. Table 32 summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period. Table 33 lists assessment and examinations required at secondary screening and thereafter and findings requiring referral to a specialist.
Table 31.
Valve Disease, Coarctation of the Aorta, and Marfan Syndrome: Criteria for Primary Screening and Selection Categories
| Heart disease |
Criteria for primary screening |
Selection
category* |
| Mitral valve insufficiency |
Apical regurgitant systolic murmurs, S3, left atrial/ventricular overload on ECG |
A |
| Mitral valve stenosis |
Apical diastolic rumble, opening snap, left atrial overload on ECG, and findings of right
ventricular hypertrophy, right atrial overload, and right axis deviation that reflect pulmonary
hypertension |
A |
| Aortic valve insufficiency |
Regurgitant diastolic murmurs, and ECG findings of left ventricular hypertrophy |
A |
| Aortic valve stenosis |
Ejection systolic murmurs, and ECG findings of left ventricular hypertrophy |
A |
| Tricuspid valve insufficiency |
Volume overload in the right atrium and ventricle on ECG |
A |
| Tricuspid valve stenosis |
ECG findings of right atrial overload, diastolic rumble over the fourth left intercostal space
enhanced by inspiration, tricuspid opening snap |
A |
| Pulmonary artery stenosis |
Ejection systolic murmurs, ECG findings of right ventricular hypertrophy and right axis
deviation |
A |
Pulmonary valve
insufficiency |
Regurgitant diastolic murmurs and systolic ejection murmurs due to relative pulmonary
valve stenosis (to-and-fro murmur), and ECG findings of right ventricular volume overload |
A |
| Coarctation of the aorta |
Vascular bruits, hypertension and ECG findings of left ventricular hypertrophy |
A |
| Marfan syndrome |
Characteristic skeletal features, joint disorder, family history, murmurs due to valve disorder |
A |
*Selection category. Group A: Findings requiring secondary screening/further examinations. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 32.
Valve Disease, Coarctation of the Aorta, and Marfan Syndrome: Conditions and Categories for Exercise Management, and Follow-up Interval
| Heart disease |
Conditions |
Management
category |
Follow-up
interval |
Mitral valve
insufficiency |
Mild (In UCG, the percentage of the regurgitant jet area to left atrial area is <20%. No left
atrial enlargement is observed. VC* is >0.3 cm) |
E-allowed |
1 year |
Moderate (In UCG, the percentage of the regurgitant jet area to left atrial area is 20 to
40%. Mild left atrial enlargement is observed. VC* is 0.3 to <0.7 cm) |
E-prohibited or
higher category |
3 to 6
months |
Severe (In UCG, the percentage of the regurgitant jet area to left atrial area is >40%.
Moderate left atrial enlargement is observed. VC* is ≥0.7 cm) |
C or higher
category |
1 to 3
months |
Mitral valve
stenosis |
Mild (mean pressure difference <5 mmHg, pulmonary artery systolic pressure <30 mmHg,
valve orifice area >1.5 cm2) |
E-allowed |
1 year |
Moderate (mean pressure difference 5 to 10 mmHg, pulmonary artery systolic pressure 30
to 50 mmHg, valve orifice area 1.0 to 1.5 cm2) |
D |
3 months to
1 year |
Severe (mean pressure difference >10 mmHg, pulmonary artery systolic pressure
>50 mmHg, valve orifice area <1.0 cm2) |
C |
1 to 6
months |
Aortic valve
insufficiency |
Mild (In UCG, the percentage of the color regurgitant jet width to left ventricular outflow
tract width is <25%, and the jet reaches until the anterior mitral valve. VC* is <0.3 cm.
Ascending aortic angiography reveals first-degree regurgitation) |
E-allowed |
1 year |
Moderate (In UCG, the percentage of the color regurgitant jet width to left ventricular outflow
tract width is 25 to 64%. VC* is 0.3 to 0.6 cm. Ascending aortic angiography reveals
second-degree regurgitation) |
E-prohibited or
higher category |
3 months to
1 year |
Severe (In UCG, the percentage of the color regurgitant jet width to left ventricular outflow
tract width is >65%. VC* is >0.6 cm. Findings of left ventricular enlargement such as a left
ventricular end-systolic diameter of >50 mm or a left ventricular end-diastolic index of
25 mm/m2, are observed. At the proximal end of the abdominal aorta, holodiastolic reversal
flow is noted. Ascending aortic angiography reveals third or fourth-degree regurgitation) |
C or higher
category |
1 to 6
months |
Aortic valve
stenosis |
Mild (In Doppler echocardiography, the peak velocity (Vp) at the aortic valve is <3 m/sec.
Pressure difference between the aorta and the left ventricle is <20 mmHg) |
E-allowed |
1 year |
| Moderate (Pressure difference between the aorta and the left ventricle is 20 to 39 mmHg) |
E-prohibited or
higher category |
6 months to
1 year |
Severe (The Vp at the aortic valve is >4 msec. Pressure difference between the aorta and
the left ventricle is ≥40 mmHg) |
C or higher
category |
3 to 6
months |
Tricuspid
valve
insufficiency |
Mild (The right atrium, right ventricle and inferior vena cava are normal in size) |
E-allowed |
1 year |
Moderate (The inferior vena cava has normal size or mild enlargement, with respiratory
variation in diameter. Hepatic venous blood flow shows systolic blunting. VC* <0.7 cm) |
E-prohibited or
higher category |
6 months to
1 year |
Severe (The inferior vena cava is enlarged with no respiratory variation in diameter.
Hepatic venous blood flow shows systolic reverse flow. VC* is ≥0.7 cm) |
E-prohibited or
higher category |
3 to 6
months |
Tricuspid
valve
stenosis |
Mild (The right atrium, right ventricle and inferior vena cava are normal in size) |
E-allowed |
1 year |
| Moderate (Slight enlargement of the right atrium and inferior vena cava) |
E-prohibited or
higher category |
6 months to
1 year |
Severe (Enlargement of the right atrium and inferior vena cava is noted. Pressure half-time
at the tricuspid valve obtained with the continuous wave Doppler method, which represents
time to decrease the right atrium-ventricular pressure difference to half, is ≥190 msec. The
valve orifice area is <1 cm2) |
D or higher
category |
3 to 6
months |
Pulmonary
artery
stenosis |
Mild (Pressure difference between the right ventricle and the pulmonary artery is <40 mmHg.
In UCG continuous wave Doppler, the peak velocity at the main pulmonary artery is <3.5 msec) |
E-allowed |
1 year |
| Moderate (right ventricular pressure: 50 mmHg to systemic blood pressure) |
E-prohibited
or higher category |
6 months to
1 year |
| Severe (right ventricular pressure ≥ systemic blood pressure) |
D or higher
category |
3 to 6
months |
Pulmonary
valve
insufficiency |
Mild |
E-allowed |
1 year |
| Moderate (right ventricular enlargement) |
E-prohibited or
higher category |
6 months to
1 year |
Severe (A regurgitant jet reaching the entire right ventricular outflow tract, paradoxical
movement of the ventricular septum, and a significantly dilated right ventricle and reduced
left ventricle in size) |
D or higher
category |
3 to 6
months |
Coarctation
of the aorta |
Mild (pressure difference across the stenosis <20 mmHg) |
E-allowed |
1 year |
| Moderate (pressure difference across the stenosis 20 to <40 mmHg) |
E-prohibited or
higher category |
6 months to
1 year |
| Severe (pressure difference across the stenosis ≥40 mmHg) |
D or higher
category |
3 to 6
months |
Marfan
syndrome |
No abnormal cardiovascular findings |
E-allowed |
1 to 3 years |
For aortic valve insufficiency and mitral valve insufficiency, see the criteria for the relevant
condition |
|
|
| Advanced aortic dilatation or aortic dissection |
C or higher
category |
1 to 6
months |
UCG, ultrasonic echocardiography. *The vena contracta (VC) size should be used for junior-high-school or later school students in a near adult or adult-sized body.
Table 33.
Valve Diseases, Coarctation of the Aorta, and Marfan Syndrome: Diagnostic Examinations Required at Secondary Screening and Further Examinations, and Findings That Require Referral to a Specialist
| Heart disease |
Items to be examined at secondary
screening/further examinations |
Secondary screening findings
requiring specialist referral |
Mitral valve
insufficiency |
Auscultation, chest X-ray (two views should be taken), 12-lead ECG,
echocardiography, BNP assay (whenever possible) |
All need specialist referral |
Mitral valve
stenosis |
Auscultation, chest X-ray (two views should be taken), 12-lead ECG,
echoocardiogram, BNP assay (whenever possible) |
All need specialist referral |
Aortic valve
insufficiency |
Auscultation, chest X-ray (two views should be taken), 12-lead ECG,
echocardiography, BNP assay (whenever possible) |
All need specialist referral |
Aortic valve
stenosis |
Auscultation, chest X-ray (two views should be taken), 12-lead ECG,
echocardiography, BNP assay (whenever possible) |
All need specialist referral |
Tricuspid valve
insufficiency |
Auscultation, chest X-ray (posterior-anterior view), 12-lead ECG,
echocardiography |
All need specialist referral |
Tricuspid valve
stenosis |
Auscultation, chest X-ray (posterior-anterior view), 12-lead ECG,
echocardiography |
All need specialist referral |
Pulmonary artery
stenosis |
Auscultation, chest X-ray (posterior-anterior view), 12-lead ECG,
echocardiography |
All need specialist referral |
Pulmonary valve
insufficiency |
Auscultation, chest X-ray (posterior-anterior view), 12-lead ECG,
echocardiography |
All need specialist referral |
Coarctation of the
aorta |
Auscultation, chest X-ray, 12-lead ECG, echocardiography, cardiac
catheterization/angiography, CT and MRI |
All need specialist referral |
| Marfan syndrome |
Visual inspection, auscultation, chest X-ray, 12-lead ECG, echocardiography,
CT or MRI |
All need specialist referral |
BNP, brain natriuretic peptide.
Mitral valve insufficiency is caused by abnormalities of the mitral valve and its apparatus (e.g., annulus, leaflets, chordae tendineae, and papillary muscles), leading to regurgitation of blood from the left ventricle to the left atrium during systole. Mitral valve regurgitation is classified as primary and secondary. Primary regurgitation is caused by abnormal leaflets or chordae tendineae. Secondary regurgitation is caused by left ventricular enlargement or annulus dilation. As mitral valve insufficiency causes volume overload of the left ventricle and atrium, an increase in left atrial pressure, and a decrease in left ventricular afterload, this eventually leads to symptoms of pulmonary congestion and decreased cardiac output such as shortness of breath on effort, dyspnea, palpitation and fatiguability.
b. Mitral Valve Stenosis
Mitral valve stenosis is a narrowing the mitral valve opening, and restricts blood flow from the left atrium to the left ventricle. Mitral valve stenosis in children is often caused by congenital anomaly, and accounts for 0.2 to 0.3% of all cases of congenital heart diseases. It causes pulmonary edema and pulmonary hypertension due to pulmonary venous congestion, inadequate body weight gain, and frequent respiratory infection. When advanced, it causes decreased cardiac output and right heart failure. Arrhythmias such as atrial fibrillation may also develop.
6.2 Aortic Valve Diseases
a. Aortic Valve Insufficiency
Aortic valve insufficiency is a congenital heart condition that causes regurgitation of blood from the aorta to the left ventricle due to anomaly of aortic valves. In patients with bicuspid aortic valve, the risk of aortic valve insufficiency increases with age. Aortic valve insufficiency may develop in patients with congenital heart diseases such as tetralogy of Fallot and those after the arterial switch operation for the correction of transposition of the great arteries, and those with connective tissue disorder such as Marfan syndrome. Aortic valve insufficiency does not cause symptoms in mild cases, but causes left ventricular dilatation in advanced cases. When left ventricular dilatation progresses to a level where the compensation mechanism collapses, signs and symptoms associated with low cardiac output such as shortness of breath on effort, dyspnea, palpitation and fatiguability as well as angina develop.
Prevention of infective endocarditis is important in all patients. In aortic valve insufficiency, the vena contracta (VC) is defined as the narrowest part of the regurgitation jet just distal to the regurgitant orifice, and the width of the vena contracta is obtained using color Doppler from a parasternal long-axis view.
b. Aortic Valve Stenosis
Aortic valve stenosis is a heart disease in which the aortic valve is narrowed and disturbs blood ejection from the left ventricle to the aorta. Bicuspid aortic valve is found in 2 to 3% of the population. Some people with bicuspid aortic valve develop aortic valve stenosis in childhood. Severe heart failure develops in neonates with severe aortic valve stenosis. Mild aortic valve stenosis does not cause symptoms. The disease often progresses with age. Children and young people with aortic valve stenosis may experience shortness of breath during exercise, fatiguability, chest pain during exercise and syncope. Sudden death may occur in some cases.
6.3 Tricuspid Valve Diseases
a. Tricuspid Valve Insufficiency
Tricuspid valve insufficiency is a heart disease due to tricuspid valve anomaly, and causes blood regurgitation from the right ventricle to the right atrium. In some cases, tricuspid valve insufficiency is associated with Ebstein’ disease.
b. Tricuspid Valve Stenosis
Tricuspid valve stenosis is a rare congenital heart disease in which the tricuspid valve is narrowed and disturbs blood ejection from the right atrium to the right ventricle. Tricuspid valve stenosis rarely develops alone, and is often associated with hypoplastic right heart. Echocardiography reveals a dome-shaped valve and narrowed orifice of the tricuspid valve, fast inflow into the right ventricle, and an enlarged right atrium. Tricuspid valve stenosis does not cause symptoms in mild cases. However in severe cases, fatiguability and anorexia due to decreased cardiac output, vomiting, and right upper abdominal pain due to hepatomegaly may develop.
6.4 Pulmonary Valve Diseases
a. Pulmonary Artery Stenosis
Pulmonary artery stenosis is a heart disease caused by a narrowing of the pulmonary valve, and disturbed blood ejection from the right ventricle to the pulmonary artery. Pulmonary artery stenosis accounts for nearly 10% of all cases of congenital heart diseases. No symptoms develop in mild cases. Moderate or severe pulmonary artery stenosis progresses with age and can lead to heart failure and arrhythmias. The prognosis of isolated pulmonary artery stenosis is relatively favorable.
b. Pulmonary Valve Insufficiency
Pulmonary valve insufficiency is a heart disease due to pulmonary artery anomaly, and causes blood regurgitation from the pulmonary artery to the right ventricle. Pulmonary valve insufficiency is often associated with other congenital heart diseases, and may worsen after surgery. No symptoms develop and prognosis is favorable in mild cases. In severe cases, symptoms of right heart failure develop.
6.5 Coarctation of the Aorta
Coarctation of the aorta is a narrowing of the transition between the aortic isthmus and the descending aorta (i.e., the junction of the ductus arteriosus and the aorta), and causes pressure overload on the left ventricle. If the narrowing is severe, heart failure develops in infancy. Some patients have no symptoms until adulthood, hypertension in the upper extremities, cerebral hemorrhage, coronary atherosclerosis, and myocardial infarction may develop. The life expectancy is shorter than average. Patients need treatment and follow-up.
6.6 Marfan Syndrome
Marfan syndrome is an autosomal dominant mutation of gene encoding fibrillin-1 (FBN1) that provides connective tissue elasticity and strength, and fibrillin increases in a protein called transforming growth factor beta (TGF-β) that controls the growth and repair of tissues and organs throughout the body. These mutations are characterized by structural anomalies such as high height and long extremities, lens dislocation and cardiovascular complications. Cardiovascular findings include an enlarged sinus of Valsalva, dissecting aortic aneurysm including ascending aortic aneurysm, mitral valve prolapse, and enlargement and dissection of the descending aorta.
7. Management of Coronary Artery Anomalies
a. Description25,109–114
Cases of anomalous origin of the coronary artery where the right coronary artery originating from the left sinus of Valsalva or the left coronary artery from the right sinus of Valsalva account for 11 to 12% of all cases out-of-hospital cardiac arrest (OHCA) in young athletes or school children and young people. Abnormal origin of coronary artery does not induce symptoms, and is found incidentally during examinations for other problems, or during autopsy of sudden cardiac death, or in survivors of OHCA. About 30% of people with an abnormal origin of the coronary artery have syncope, chest pain, and/or palpitations during exercise. Males are more prevalent than females. Cardiac events develop mainly in those aged between 10 and 30 years during competitive exercise in the afternoon.
The prevalence of anomalous origin of the left coronary artery with an interarterial course is a sixth to tenth that of anomalous origin of the right coronary artery, but accounts for 85% of cases of cardiac arrest due to anomalous origin of the coronary artery, which indicates a higher risk of the former. The risk of cardiac arrest is also high in those with angiographically-evident abnormal coronary blood flow (e.g., intramural coronary arteries, acute angle origin, kinking, slit-like stenosis due to compression, flap-like closure of the coronary orifice, and other anomalies inducing coronary spasm).
b. Diagnostic Criteria and Management
Table 34
lists the criteria for selection classification at primary screening.
Table 35
summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period.
Table 36
lists assessment and examinations required at secondary screening and thereafter and findings requiring referral to a specialist.
Table 34.
Coronary Anomalies: Criteria for Primary Screening and Selection Categories
| Criteria for primary screening |
Selection
category* |
| Questionnaire: Syncope, chest pain, palpitations during exercise |
A |
| ECG: ECG findings during screening are within the normal range. It is difficult to detect abnormalities |
|
*Selection category. Group A: Findings requiring secondary screening/further examinations. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 35.
Coronary Anomalies: Conditions and Categories for Exercise Management, and Follow-up Interval
| Conditions |
Management
category |
Follow-up
interval |
Those at a high risk of fatal coronary events should be considered for surgical treatment without delay.
Competitive sports should be avoided before surgery |
C-prohibited |
1 to 3 months |
Those with anomalous left coronary artery originating from the right sinus of Valsalva with an interarterial
course may be considered for surgical treatment at 10 years of age or later regardless whether
examinations reveal ischemic findings. Competitive sports should be avoided before surgery |
C or D-prohibited |
1 to 6 months |
Those with anomalous right coronary artery originating from the left sinus of Valsalva with an interarterial
course may be considered for surgical treatment at 10 years of age or later if examinations demonstrate
myocardial ischemia and imaging reveals coronary circulation disorder. Competitive sports should be
avoided before surgery |
C or D-prohibited |
1 to 6 months |
Those without ischemia or coronary circulation disorder during examinations are often not indicated for
surgery. Some may not refrain from exercise |
E-allowed |
6 months to
1 year |
Table 36.
Coronary Anomalies: Diagnostic Examinations Required at Secondary Screening and Further Examinations, and Findings That Require Referral to a Specialist
Items to be examined at secondary
screening/further examinations |
Secondary screening findings
requiring specialist referral |
| Coronary CT image, coronary MRI, selective coronary angiography |
All need specialist referral |
Kawasaki disease is an acute febrile illness mainly in children ≤4 years of age, and is characterized by acute syndromes followed by cardiovascular complications.115
Since cardiac sequelae such as enlargement and aneurysms of the coronary arteries which may lead to coronary stenosis and myocardial infarction develop in the remote phase in about 3% of patients with Kawasaki disease.116,117
Children and young people with a history of Kawasaki disease should be examined for their condition to confirm the appropriateness of exercise and daily life management.
a. Diagnostic CRITERIA and Management
Table 37 lists the criteria for selection classification at primary screening. Table 38 summarizes the conditions for categories of allowable intensity of exercise and daily activities and follow-up period. Table 39 lists assessment and examinations required at secondary screening and thereafter and findings requiring referral to a specialist.
Table 37.
Kawasaki Disease: Criteria for Primary Screening and Selection Categories
| Criteria for primary screening |
Selection category |
| (1) Those receiving periodic medical assessment |
Exercise management category should be determined at the clinic |
(2) Those not receiving medical assessment with
no information on the presence/absence of
cardiac sequelae |
The child should visit the clinic where he/she was diagnosed and received the acute
phase treatment to determine exercise management category. When the child cannot
visit the clinic, he/she will undergo secondary screening and later examinations |
(3) Those who completed follow-up assessment,
and do not have cardiac sequelae |
Those in whom Kawasaki disease developed less than 5 years ago should visit the clinic
where he/she was diagnosed and treated to determine exercise management category.
When the child cannot visit the clinic, he/she will undergo secondary screening and later
examinations |
Most of those who reported a history of Kawasaki disease had the disease during infancy and do not have active disease. It is important to identify their current status based on the questionnaire survey. Age of onset, presence/absence of cardiac sequelae, status of medical management, and date of final medical assessment should be confirmed.47
Table 38.
Kawasaki Disease: Conditions and Categories for Exercise Management, and Follow-up Interval
| Conditions |
Management category |
Follow-up
interval |
(1) Those without cardiac sequelae (including those with transient dilatation
of coronary arteries which regresses after acute phase) |
|
|
| <5 years after onset |
E-allowed |
1 year |
| ≥5 years after onset |
No management required |
|
| (2) Those with cardiac sequelae |
|
|
i. Those with small aneurysms or dilatation: Those in whom coronary lesions
have disappeared should be treated as described in conditions (1),
Those who still have coronary lesions should be followed up at the clinic |
E-allowed |
6 months to
1 year |
| ii. Those with remaining medium aneurysms and other coronary lesions |
|
|
a. Those with no findings of stenosis or myocardial ischemia until the
lesions disappear, drug treatment and follow-up evaluation at least
annually should be continued |
E-allowed |
6 months to
1 year |
| Those with giant aneurysms |
D-prohibited |
3 to 6 months |
| Those after ≥1 year after onset who have persistent cardiac sequelae |
E-prohibited |
3 to 6 months |
| b. Those with findings of stenosis and myocardial ischemia |
D or higher category. School sport club
activity should be prohibited. A–D rating
should be based on findings of exercise
ECG and severity of myocardial ischemia |
1 to 6 months |
| The Management Table for those in the ‘a’ and ‘b’ categories should describe bleeding tendency due to drug treatment, if any |
| iii. Those with a history of myocardial infarction |
Rated at A to E according to the child’s
condition. Basically, sport club activities
should be prohibited |
1 to 6 months |
| The Management Table should describe bleeding tendency due to drug treatment, if any |
Table 39.
Kawasaki Disease: Diagnostic Examinations Required at Secondary Screening and Further Examinations, and Findings That Require Referral to a Specialist
Items to be examined at secondary
screening/further examinations |
Secondary screening findings
requiring specialist referral |
ECG, exercise ECG,
echocardiography |
Echocardiography reveals coronary lesions (e.g., dilatations and aneurysms) |
| ECG or exercise ECG reveal findings suggestive of ischemia |
Questionnaire reveals the presence of symptoms suggestive of ischemia
(e.g., chest pain and feeling of chest tightness) |
Those who meet the above three conditions should be assessed and managed by a specialist. Myocardial scintigraphy, coronary CT image, coronary MRI and/or coronary angiography should be added appropriately.
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive pulmonary vascular disorder with unknown cause characterized by a mean resting pulmonary arterial pressure of ≥25 mmHg due to endothelial dysfunction of pulmonary arterioles. It is important to differentiate IPAH from hereditary pulmonary arterial hypertension (HPAH) that is due to genetic mutations and runs in families, secondary PAH associated with other conditions, and pulmonary hypertension due to left heart disease, lung diseases and/or hypoxia, chronic thromboembolism, or other multiple factors. Prompt diagnosis and treatment is essential to improve outcome.
a. Diagnostic Criteria and Management
Table 40
lists the criteria for selection classification in primary screening.
Table 41
summarizes the conditions for categories of allowable intensity of exercise, daily activities and follow-up period.
Table 42
lists assessment and examinations required in secondary screening and thereafter, and findings requiring referral to a specialist.
Table 40.
Idiopathic Pulmonary Arterial Hypertension: Criteria for Primary Screening and Selection Categories
| Criteria for primary screening |
Selection
category* |
Questionnaire: Those with a family history of pulmonary hypertension or sudden death, and those with fatiguability on effort,
shortness of breath, chest pain or syncope |
A |
ECG: Those with a ≥3 points in the point-based ECG assessment criteria for right ventricular hypertrophy in children, and
those with a 1 or 2 points for right ventricular hypertrophy with clear findings of right atrial overload or a ’strain’ pattern
indicative of right ventricular hypertrophy in the ST-T wave in right precordial leads |
A |
*Selection category. Group A: Findings requiring secondary screening/further examinations. Group B: Findings not requiring secondary screening if no other findings are present. Group C: Findings that may not be focused in heart disease screening in schools.
Table 41.
Idiopathic Pulmonary Arterial Hypertension: Conditions and Categories for Exercise Management, and Follow-up Interval
| Conditions |
Management
category |
Follow-up
interval |
Mean pulmonary arterial pressure ≥25 mmHg, pulmonary artery wedge pressure ≤15 mmHg, and
pulmonary vascular resistance index >3 WU·m2 |
Start at B or C |
1 to 2 weeks |
Clinical course (based on symptoms, physical findings, and echocardiography and other
examinations) |
C to E-prohibited |
1 to 2 months |
Table 42.
Idiopathic Pulmonary Arterial Hypertension: Diagnostic Examinations Required at Secondary Screening and Further Examinations, and Findings That Require Referral to a Specialist
Items to be examined at secondary
screening/further examinations |
Secondary screening findings
requiring specialist referral |
Auscultation, chest X-ray (posterior-anterior view),
echocardiography |
Auscultation: Loud S2 |
Chest X-ray: Protrusion of the left second arch, protrusion of the left fourth arch and
the right second arch |
Echocardiography: A flat ventricular septum, right ventricular enlargement, increased
tricuspid regurgitation velocity (≥3 msec) |
Hypertensive heart diseases such as coronary artery disease, heart failure and cardiac hypertrophy are rare in children. However, evidence supports blood pressure tracking from childhood to adulthood.121
In Japan the definition of hypertension in children is not known widely. This chapter describe how to determine blood pressure in children and how to diagnose hypertension.
a. Methods for Blood Pressure Measurement122–125
Table 43
summarizes how to measure blood pressure in children. Blood pressure should be taken from the right upper arm since some children may have coarctation of the aorta.122
Table 43.
How to Determine Blood Pressure in Children and Adolescents
121–125
| 1. After sitting quietly for 5 minutes,122,123 determine sitting blood pressure from the right upper arm122 |
| 2. The cuff bladder length should cover 80% of the acromion-olecranon length122 |
| 3. Measure blood pressure more than once. Methods to calculate average blood pressure differed among studies |
(1) Calculate the average blood pressure using two stable measurements124
Stable measurements that differ by <5 mmHg should be used124 |
| (2) Measure blood pressure three times, and calculate the mean of the last two measurements125 |
The guidelines for the management of hypertension 2014 published by the Japanese Society of Hypertension proposed the criteria for hypertension in children as summarized in
Table 44.124
As described in the guidelines, relatively high values are used to define hypertension in children.124
The National High Blood Pressure Education Program (NHBPEP) in the United States classifies high blood pressure levels into three categories of prehypertension (the 90th to <95th percentile or >120/80 mmHg), Stage I hypertension (the 95th to <99th percentile plus 5 mmHg), and Stage II hypertension (greater than the 99th percentile plus 5 mmHg).122,126
In Japan, a similar category system is used in adults. In order to follow the NHBPEP criteria, the 90th, 95th and 99th percentiles of blood pressure in each year category should be determined.
Tables 45
and
46
tabulate the 90th, 95th and 99th percentiles of blood pressure in children and young people in Japan.127,128
Table 44.
Criteria of Hypertension in Children by Age Group
| |
Systolic blood
pressure (mmHg) |
Diastolic blood
pressure (mmHg) |
| Pre-school children |
≥120 |
≥70 |
| Elementary school |
| Lower grades |
≥130 |
≥80 |
| Upper grades |
≥135 |
≥80 |
| Junior high |
| Males |
≥140 |
≥85 |
| Females |
≥135 |
≥80 |
| Senior high |
≥140 |
≥85 |
(Source: The Japanese Society of Hypertension. 2014.124)
Table 45.
Statistics of Systolic Blood Pressure in Children and Adolescents
| |
Males (mmHg) |
Females (mmHg) |
Pre-
school
children |
1 to 2* |
3 to 4* |
5 to 6* |
Junior
high |
Senior
high |
Pre-
school
children |
1 to 2* |
3 to 4* |
5 to 6* |
Junior
high |
Senior
high |
| No. of subjects |
130 |
171 |
176 |
193 |
237 |
573 |
124 |
165 |
222 |
187 |
238 |
728 |
| Mean |
91 |
94 |
96 |
100 |
104 |
117 |
93 |
93 |
96 |
99 |
101 |
107 |
| Standard deviation |
8 |
9 |
10 |
10 |
10 |
10 |
9 |
9 |
9 |
9 |
9 |
9 |
| 90th percentile |
103 |
106 |
108 |
114 |
118 |
129 |
105 |
103 |
108 |
111 |
113 |
119 |
| 95th percentile |
108 |
110 |
114 |
117 |
122 |
132 |
107 |
105 |
110 |
115 |
116 |
123 |
| 99th percentile |
111 |
118 |
124 |
127 |
130 |
140 |
115 |
121 |
117 |
121 |
121 |
129 |
*1 to 2, 3 to 4, and 5 to 6 represent elementary school grade. (Source: Yoshinaga M. 2009,127 Yoshinaga M. 2015.128)
Table 46.
Statistics of Diastolic Blood Pressure in Children and Adolescents
| |
Males (mmHg) |
Females (mmHg) |
Pre-
school
children |
1 to 2* |
3 to 4* |
5 to 6* |
Junior
high |
Senior
high |
Pre-
school
children |
1 to 2* |
3 to 4* |
5 to 6* |
Junior
high |
Senior
high |
| No. of subjects |
130 |
171 |
176 |
193 |
237 |
573 |
124 |
165 |
222 |
187 |
238 |
728 |
| Mean |
51 |
53 |
54 |
55 |
56 |
63 |
50 |
53 |
54 |
56 |
55 |
62 |
| Standard deviation |
8 |
8 |
8 |
8 |
10 |
9 |
8 |
7 |
7 |
8 |
7 |
9 |
| 90th percentile |
61 |
64 |
64 |
66 |
67 |
75 |
62 |
61 |
63 |
65 |
65 |
73 |
| 95th percentile |
65 |
67 |
67 |
69 |
72 |
79 |
64 |
66 |
67 |
69 |
67 |
77 |
| 99th percentile |
69 |
71 |
77 |
72 |
87 |
83 |
70 |
73 |
72 |
77 |
74 |
83 |
*1 to 2, 3 to 4, and 5 to 6 represent elementary school grade. (Source: Yoshinaga M. 2009,127 Yoshinaga M. 2015. 128)
Children and young people whose blood pressure values meet the criteria in
Table 44
or exceed the NHBPEP criteria of Stage II hypertension more than once should be referred to a specialist.
d. Procedures and Information Required for the Determination of Allowable Intensity of Exercise and Daily Activities
Criteria have been revised according to the references 112, 126 and 129.
11. Management of Athletes
11.1 Students in Elementary, Junior High, and Senior High Schools
It is well known that periodic exercise favorably affects the cardiovascular system,130
but it is also known that competitive sports and recreational sports activities increase the risk of sudden cardiac death.131
Sudden death during exercise in children and young people is often associated with congenital heart disease, congenital coronary anomaly, myopathies, and arrhythmias caused by genetic factors.132
In Japan, heart disease screening including ECG has been conducted in all school children and young people for several years, and has improved in terms of quality and resource allocations over time. Accordingly, the present guideline document assumes that heart disease screening in school include interview, physical examination and ECG at rest.
Athletes who perform intense physical activities constantly often have structural adaptive changes of the heart.133–135
This may lead to ECG changes that are rare among non-athletes. These ECG finding may reflect physiological ECG changes do to physical training, but it is important to differentiate physiological changes from those not related to training.136
The European Society of Cardiology has published criteria for ECG interpretation in athletes.136
It is useful in differentiating potentially pathologic ECG changes from physiological ECG changes.137
The criteria was further revised, and was published as the ‘Seattle Criteria’ (Tables 47
and
48).138–141
Table 47.
Normal ECG Findings in Athletes
| 1. Sinus bradycardia (≥30 bpm) |
| 2. Sinus arrhythmia |
| 3. Ectopic atrial rhythm |
| 4. Junctional escape rhythm |
| 5. First-degree AV block (PR interval >200 msec) |
| 6. Mobitz type I (Wenckebach) second-degree AV block |
| 7. Incomplete right bundle branch block |
8. Isolateed QRS voltage criteria for LVH
Except: QRS voltage criteria for left LVH occurring with any non-voltage criteria for LVH such as left arterial enlargement,
left axis deviation, ST segment depression, T-wave inversion or pathological Q waves |
| 9. Early repolarisation (ST elevation, J point elevation, J-waves or terminal portion QRS slurring) |
| 10. Convex (domed) ST segment elevation combined with T-wave inversion in leads V1–V4 in black/African athletes |
These common training-related ECG alternations are physiological adaptations to regular exercise, considered normal variants in athletes and do not require further evaluation in asymptomatic athletes. AV, atrioventricular; LVH, left ventricular hypertrophy. (Source: Drezner JA, et al. 2013.138)
Note: Athletes are not defined in the reference document,138
but may be considered as those who perform sports activities daily basis and those participating in sports activities as recreation. One should consider that these training-related ECG alterations are rare among elementary school students and junior high school students.
Table 48.
Abnormal ECG Findings in Athletes
Abnormal ECG
findings# |
Definition |
| T-wave inversion |
>1 mm in depth in two or more leads V2–V6, II and aVF, or I and aVL (excludes lead III, aVR and V1) |
| ST depression |
≥0.5 mm in depth in two or more leads |
| Pathologic Q wave |
>3 mm in depth or >40 msec in duration in two or more leads (except for lead III and aVR) |
| Complete left bundle branch block |
QRS duration ≥120 msec, predominantly negative QRS complex in lead V1 (QS or rS), and upright
monophasic R wave in leads I and V6 |
| Intraventricular conduction delay |
Any QRS duration ≥140 msec |
| Left axis deviation |
−30° to −90° |
| Left atrial enlargement |
Prolonged P wave duration of >120 msec in leads I or II with negative portion of the P wave ≥1 mm in
depth and ≥40 msec in duration in lead V1 |
| Right ventricular hypertrophy pattern |
R-V1+S-V5 >10.5 mm and right axis deviation of >120° |
| Ventricular pre-excitation |
PR interval <120 msec with a delta wave (slurred upstroke in the QRS complex) and QRS duration
(>120 msec) |
| Long QT interval* |
QTc ≥470 msec (male), QTc ≥480 msec (female), QTc ≥500 msec (marked QT prolongation) |
| Short QT interval* |
QTc ≤320 msec |
| Brugada-like ECG pattern |
High take-off and downsloping ST segment elevation followed by a negative T wave in ≥2 leads in V1
to V3 |
| Profound sinus bradycardia |
<30 bpm or sinus pauses ≥3 sec |
| Supraventricular tachycardia |
Supraventricular tachycardia, atrial tachycardia, atrial-fibrillation, atrial-flutter |
| Premature ventricular contractions |
≥2 PVCs per 10 sec tracing |
| Ventricular arrhythmia |
PVC couplets, triplets and non-sustained ventricular tachycardia |
These ECG findings are unrelated to regular training or expected physiological adaptation to exercise, may suggest the presence of pathological cardiovascular disease, and require further diagnostic evaluation. *The QT interval corrected for heart rate is ideally measured with heart rates of 60–90 bpm. Consider repeating the ECG after mild aerobic activity for borderline or abnormal QTc values with a heart rate <50 bpm. (Source: Drezner JA, et al. 2013.138)
#Note: This criterion may not be applied to children before puberty.
The present guideline document does not provide screening criteria specific to athletes, and recommends to use general criteria to them. However, the contents of the Seattle Criteria in
Tables 47
and
48
are fairly consistent with our criteria. The general criteria described in this document will help schools select athletes who should undergo secondary screening.
11.2 University Students
Since it is difficult to maintain statistics about cardiovascular accidents associated with sports activities in university students, the actual incidence rate of sudden death during sports activities in this population is often unclear.
Rough incidence of sudden death during sports activities is higher in university students who perform more intense exercise than in high school students. There is a tendency towards increasing sudden death during sports activities in higher grade schools where students perform more intense sports activities after entering the next grade school. The incidence of sudden death during sports activities in males is several-fold that of females, which is consistent with the statistics in adults. The intensity of sports activities in university students is similar to that of adults including professional athletes, and university students should be managed similarly to adults. Guidelines for sports participation in Japan have been described in the recommendations,142
which are based on the Bethesda Conference guidelines in the United States, by the Internal Medicine Group in the Scientific Committee of the Japanese Society of Clinical Sports Medicine, and in the Guidelines for Exercise Eligibility at Schools, Work-Sites, and Sports in Patients with Heart Diseases47
published by the Japanese Circulation Society. One should refer to these documents to assess the eligibility of university athletes.
As ECG screening is not commonly conducted in universities, they should consider introducing ECG as an essential part of health check-up for new university students.














 . *Mutually exclusive. **Resting heart rate below the 2nd percentile for age. ***The same family member cannot be counted in A and B. Total score: ≥3.5 points, high probability; 1.5 to 3.0 points, intermediate probability; ≤1.0 point, low probability of LQTS. (Source: Schwartz PJ, et al. 2011.
. *Mutually exclusive. **Resting heart rate below the 2nd percentile for age. ***The same family member cannot be counted in A and B. Total score: ≥3.5 points, high probability; 1.5 to 3.0 points, intermediate probability; ≤1.0 point, low probability of LQTS. (Source: Schwartz PJ, et al. 2011.