Abstract
Background:
The relationship between outcome and trigger in takotsubo syndrome (TTS) has been recently discussed, but the data are still limited.
Methods and Results:
We enrolled 745 consecutive patients with TTS from the Tokyo Cardiovascular Care Unit Network registry. The patients were divided into 4 groups based on trigger: (1) medical illness, 202 (27%); (2) physical activity, trauma and injury, 54 (7%); (3) emotional trigger, 199 (27%); and (4) unidentifiable trigger, 290 (39%). Compared with other groups, the medical illness group had the lowest percentage of female patients (68%, 85%, 89%, and 79%, respectively; P<0.001) and the highest mean patient age (75±11 years, 72±11, 73±12, and 75±11 years, respectively; P=0.02). In-hospital all-cause mortality was higher (11%) in this group (0%, 2%, and 2%, respectively; P<0.001). On multivariate logistic regression analysis, the medical illness group independently predicted all-cause death (OR, 4.73; 95% CI: 1.33–16.87); although there was no significant difference in cardiac deaths between the 4 groups.
Conclusions:
TTS has a wide spectrum of outcome depending on the trigger. The medical illness trigger was a powerful predictor of outcome but the main cause of death is not cardiac complication.
Takotsubo syndrome (TTS) is an acute cardiac syndrome characterized by transient reversible systolic dysfunction of the left ventricle (LV) in the absence of culprit coronary artery disease (CAD).1
Since its first description in Japan in 1990, TTS has been acknowledged worldwide and diagnosed in approximately 2% of patients with suspected acute coronary syndrome (ACS) on troponin elevation.2,3
Emotional or physical triggers can precede TTS, although this is not vital.4
Such triggers induce the pathogenesis of TTS, wherein the potential role of excess catecholamine has long been debated.5
Early in the study of TTS, emotional triggers were reported as mainly preceding TTS, but subsequent studies have clarified that physical triggers also significantly precede TTS. Moreover, TTS can occur even without a trigger.4
The recognition of mortality due to TTS has also changed.6
Mortality due to TTS had been initially reported as very low, and TTS was considered as a benign disorder, but it has now been found that mortality and severe complications due to TTS are similar to those of ACS.1,7
Moreover, the relationship between TTS outcome and trigger has been recently reported from Western countries, but such data still remain limited, and there are no data available from Japan.8,9
Therefore, the aim of this study was to investigate the relationship between the outcome of TTS and the trigger.
Methods
Subjects
Data from the Tokyo Cardiovascular Care Unit (CCU) Network registry between 2010 and 2015 were analyzed. The Tokyo CCU Network is a well-organized network for acute cardiovascular care, consisting of 71 cardiovascular centers in the metropolitan area of Japan, in collaboration with the Tokyo Fire Department. The registry was registered under the University Hospital Medical Information Network Clinical Trials Registry (UMIN000013128) and is described in detail in another study.10
Since the establishment of the Takotsubo Cardiomyopathy Study Group of the Tokyo CCU Network Scientific Committee in 2010, inpatient data on TTS were collected retrospectively between 2010 and 2012. Since 2013, inpatient data on TTS have been collected prospectively. Diagnoses were made based on modified Mayo Clinic criteria by individual physicians at each hospital.11
Patients were excluded if either they did not undergo coronary angiography (CAG) or they had missing data on the trigger.
The requirement of informed consent was waived because all data were anonymized. Ethics approval was obtained from the institutional review board of the Tokyo CCU Network Scientific Committee (approval no. 16-002), and the study design conformed to the principles of the Declaration of Helsinki.
Patient Characteristics vs. TTS Trigger
The following data were collected from uniform case report databases: age, sex, body mass index (BMI) on admission, place of onset, comorbidity, type of TTS, trigger of TTS, coexistence of coronary stenosis with TTS, vital signs, shock on arrival, respiratory support, Killip class, length of hospital stay, and laboratory data on admission. Brain natriuretic peptide (BNP) or N-terminal prohormone of BNP (NTproBNP) were evaluated based on the upper limit of normal.1
Renal dysfunction was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2.12
Shock on arrival was defined as systolic blood pressure (SBP) <90 mmHg,13
and tachycardia on arrival was defined as heart rate (HR) >100 beats/min in the emergency room.14
Respiratory support was defined as mechanical ventilation or non-invasive positive-pressure ventilation. Data on LV ejection fraction (LVEF) were obtained from transthoracic echocardiogram on admission and reduced EF was defined as LVEF <40%.15
Data on CAD were obtained from CAG based on narrowing of the lumen diameter in a major epicardial artery or one of its major branches (≥75% for left anterior descending artery, left circumflex artery, right coronary artery, or left main trunk). Type of TTS was diagnosed on left ventriculography and transthoracic echocardiogram.
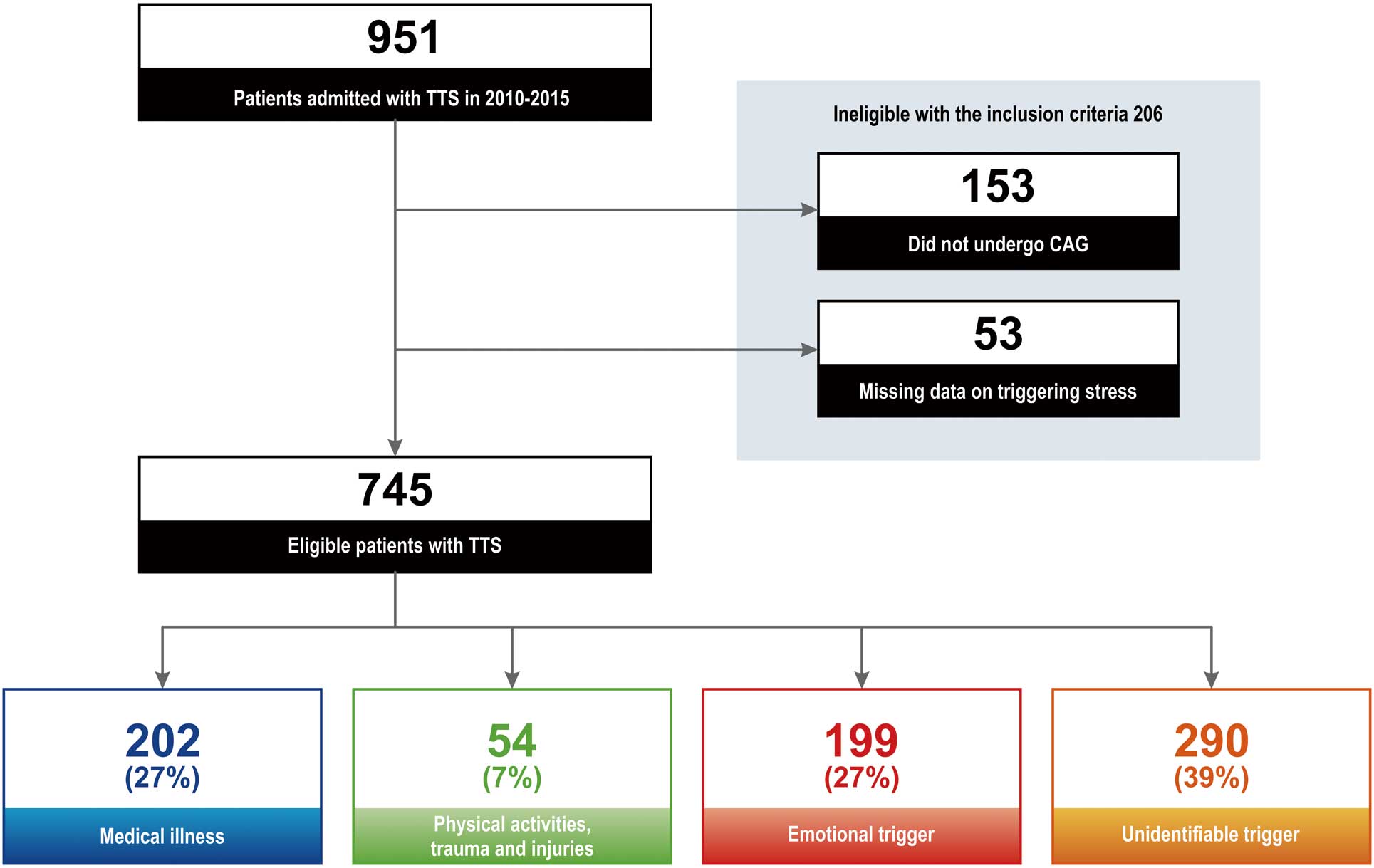
We divided the patients into 4 groups according to TTS trigger (Figure 1).16,17
In the first group (medical illness group) TTS was precipitated by internal medical illness, and was not related to physical activity (e.g., snow shoveling or sports), trauma or injury. In this first group, we diagnosed the medical illness as the primary trigger according to the patient’s history, and further classified the trigger into the following subgroups: central nervous system, cardiovascular, respiratory, gastrointestinal, hematologic, endocrine and metabolic, allergic, otolaryngologic, urological, and gynecologic dysfunction. In the second group (physical activity, trauma and injury group) TTS was precipitated by physical activity (e.g., snow shoveling or sports), trauma or injury, and was not related to internal medical illness conditions. In the third group (emotional trigger group) TTS was precipitated by stressful emotional events such as anger/frustration, grief/loss, panic/fear/anxiety; and the last group (unidentifiable trigger group) had TTS without any precipitating events. When patients had ≥2 trigger types, they were categorized based on the main trigger selected by the attending physician.
We determined in-hospital all-cause death and cardiac death according to the definition of the American College of Cardiology Foundation/American Heart Association.18
Cardiac death was defined as death from heart failure, myocardial infarction (MI), sudden cardiac death, or other cardiac death. Non-cardiac death was defined as death other than cardiac death, and details of the cause of death were recorded individually.
Statistical Analysis
Continuous variables are presented as mean±SD or median (IQR), and categorical variables as n (%). Categorical variables were assessed using the chi-squared test. Continuous variables were compared using Student’s t-test or the Mann-Whitney U-test for analysis between 2 groups, or the 1-way analysis of variance or Kruskal-Wallis test for analysis between 3 or more groups. Post-hoc test with Bonferroni correction was performed to detect the difference between 2 groups after analysis of variance between 3 or more groups. Multiple logistic regression analysis was performed to determine whether the trigger was independently associated with all-cause death and cardiac death. Age, sex, and other variables with P<0.20 on univariate analysis, such as renal dysfunction, shock on arrival, tachycardia on arrival, reduced EF, creatine kinase (CK), white blood cell (WBC) count, and BNP/NTproBNP, were included in a multiple logistic regression model. The selection of variables for univariate analysis was based on clinical importance and on previous studies.1,6,10,19,20
Data on WBC, CK and BNP/NTproBNP were divided into 3 groups based on tertiles.
Due to missing values in multiple logistic regression analysis, multiple imputation by multivariate normal regression method was performed, and thus, multiple logistic regression analysis was conducted using the imputation dataset.
Adjusted OR and 95% CI were estimated using logistic regression models. All statistical analyses were performed using SPSS Statistics 23.0 (IBM, Armonk, NY, USA) and Stata 14 (Stata Corp., College Station, TX, USA), and a 2-sided P<0.05 was considered statistically significant.
Results
Patients
Between 2010 and 2015, 951 consecutive patients with TTS were admitted to the cardiovascular centers of the Tokyo CCU Network. Of these, 745 were eligible for inclusion in the study (Figure 1). Mean age was 73±11 years, and 79% were women. Medical illness as the trigger was identified in 202 patients (27%), and physical activity, trauma and injury in 54 patients (7%). An emotional trigger was present in 199 patients (27%), and TTS occurred without any precipitating events (unidentifiable trigger) in 290 patients (39%).
Patient baseline characteristics are listed in
Table 1. The medical illness group had the lowest proportion of female patients (138, 68%) of the 4 groups (physical activity, trauma and injury group, n=46, 85%; emotional trigger group, n=178, 89%; and unidentifiable trigger group, n=229, 79%; P<0.001). The medical illness group and physical activity, trauma and injury group had older patients (75±11 years) than the other groups (emotional trigger group, 72±11 years, and unidentifiable trigger group, 73±12 years; P=0.02). The medical illness group was also more likely to have low BMI and a history of diabetes mellitus. According to
Table 1, the type of TTS and Killip class were similar in all groups. Meanwhile, the medical illness group had increased BNP/NTproBNP, WBC, and CRP; decreased LVEF; higher HR; and lower SBP/diastolic blood pressure. The medical illness group also had the highest number of patients with respiratory support (n=21, 10%) of the 4 groups (physical activity, trauma and injury group, n=5, 7%; emotional trigger group, n=12, 6%; and unidentifiable trigger group, n=11, 4%; P=0.03). Moreover, shock on arrival was most common (22, 11%) in the medical illness group compared with the other groups (physical activity, trauma and injury group, n=2, 4%; emotional trigger group, n=9, 5%; and unidentifiable trigger group, n=11, 4%; P=0.007). The number of patients presenting with in-hospital onset of TTS was the highest in the medical illness group (37 patients, 34%), followed by the physical activity, trauma and injury group (5 patients, 23%), unidentifiable trigger group (17 patients, 9%), and emotional trigger group (12 patients, 8%; P<0.001,
Table 1).
Table 1.
Subject Characteristics vs. TTS Trigger
| |
Medical illness
(n=202) |
Physical activity,
trauma and injury
(n=54) |
Emotional trigger
(n=199) |
Unidentifiable
trigger (n=290) |
P-value |
| Age (years) |
75±11 (n=202) |
75±11 (n=54) |
72±11 (n=199) |
73±12 (n=290) |
0.02 |
| Age >75 years |
117/202 (58) |
28/54 (52) |
83/199 (42) |
152/290 (52) |
0.01 |
| Female |
138/202 (68) |
46/54 (85) |
178/199 (89) |
229/290 (79) |
<0.001 |
| BMI (kg/m2) |
20±4 (n=179) |
22±4 (n=49) |
21±4 (n=185) |
21±4 (n=278) |
0.01 |
| Comorbidity/prior history |
| Hypertension† |
47/108 (44) |
11/21 (52) |
75/150 (50) |
99/181 (55) |
0.33 |
| Diabetes mellitus† |
23/108 (21) |
0/21 (0) |
19/150 (13) |
36/181 (20) |
0.03 |
| Dyslipidemia† |
23/108 (21) |
4/21 (19) |
40/150 (27) |
57/181 (32) |
0.23 |
| Hyperuricemia† |
4/108 (4) |
1/21 (5) |
6/150 (4) |
7/181 (4) |
1.00 |
| PAD† |
1/108 (1) |
0/21 (0) |
0/150 (0) |
2/181 (1) |
0.61 |
| Renal dysfunction‡‡‡ |
86/188 (46) |
13/52 (25) |
45/188 (24) |
106/274 (39) |
<0.001 |
| OMI† |
2/108 (2) |
0/21 (0) |
3/150 (2) |
4/181 (2) |
0.92 |
| Post-PCI† |
1/108 (1) |
0/21 (0) |
3/150 (2) |
7/181 (4) |
0.35 |
| Post-CABG† |
0/108 (0) |
0/21 (0) |
0/150 (0) |
3/181 (2) |
0.20 |
| Smoking† |
34/105 (32) |
3/19 (16) |
29/145 (20) |
51/177 (29) |
0.08 |
| In-hospital onset† |
37/108 (34) |
5/22 (23) |
12/152 (8) |
17/182 (9) |
<0.001 |
| Type of TTS |
|
|
|
|
0.87 |
| Apical |
184/200 (92) |
50/54 (93) |
179/197 (91) |
261/288 (91) |
|
| Mid |
10/200 (5) |
1/54 (2) |
11/197 (6) |
16/288 (6) |
|
| Basal |
2/200 (1) |
1/54 (2) |
5/197 (3) |
6/288 (2) |
|
| Other |
4/200 (2) |
2/54 (4) |
2/197 (1) |
5/288 (2) |
|
| Killip class |
|
|
|
|
0.15 |
| I |
97/150 (65) |
34/47 (72) |
107/135 (79) |
161/204 (79) |
|
| II |
30/150 (20) |
7/47 (15) |
15/135 (11) |
28/204 (14) |
|
| III |
12/150 (8) |
4/47 (9) |
8/135 (6) |
8/204 (4) |
|
| IV |
11/150 (7) |
2/47 (4) |
5/135 (4) |
7/204 (3) |
|
| Coexisting CAD‡,†† |
10/77 (13) |
6/29 (21) |
1/43 (2) |
10/102 (10) |
0.08 |
| ST elevation |
133/201 (66) |
41/53 (77) |
140/196 (71) |
194/285 (68) |
0.37 |
| Respiratory support |
21/202 (10) |
5/54 (7) |
12/199 (6) |
11/290 (4) |
0.03 |
| Shock on arrival§§ |
22/201 (11) |
2/52 (4) |
9/197 (5) |
11/282 (4) |
0.007 |
| HR (beats/min) |
96±24 (n=198) |
87±22 (n=53) |
86±20 (n=194) |
88±22 (n=280) |
<0.001 |
| Tachycardia on arrival¶¶ |
83/198 (42) |
12/53 (23) |
40/194 (21) |
69/280 (25) |
<0.001 |
| SBP (mmHg) |
127±32
(n=201) |
145±32
(n=52) |
136±29
(n=196) |
135±31
(n=281) |
0.001 |
| DBP (mmHg) |
76±20 (n=201) |
83±19 (n=52) |
81±18 (n=196) |
77±19 (n=281) |
0.01 |
| SpO2 (%) |
96±5 (n=192) |
96±5 (n=51) |
97±8 (n=190) |
96±9 (n=276) |
0.56 |
| WBC (/μL)‡‡ |
10,570±5,528
(n=199) |
9,548±3,391
(n=51) |
8,273±3,901
(n=194) |
8,636±3,460
(n=289) |
<0.001 |
| CRP (mg/dL)‡‡ |
5.0±6.6
(n=200) |
1.7±2.7
(n=52) |
1.2±3.2
(n=195) |
1.3±2.9
(n=284) |
<0.001 |
| BNP/NTproBNP (ULN)§,‡‡ |
24±25 (n=139) |
21±27 (n=41) |
16±21 (n=148) |
18±25 (n=222) |
0.05 |
| CK (IU/L)‡‡ |
97 (32–203)
(n=166) |
91 (30–220)
(n=41) |
120 (68–192)
(n=182) |
119 (60–196)
(n=257) |
0.29 |
| TTE LVEF (%)¶ |
47 (40–57)
(n=129) |
55 (47–63)
(n=28) |
54 (43–63)
(n=132) |
52 (42–62)
(n=227) |
0.01 |
| Reduced EF††† |
36/129 (28) |
3/28 (11) |
28/132 (21) |
41/227 (18) |
0.08 |
| Length of hospitalization (days) |
11 (6–17)
(n=157) |
11 (7–19)
(n=44) |
9 (6–13)
(n=184) |
10 (7–14)
(n=271) |
0.006 |
Data given as mean±SD, median (IQR) or n (%). †Data unavailable for TTS patients between 2010 and 2012. ‡Data unavailable for TTS patients between 2013 and 2014. §Ratio of BNP or NTproBNP to the ULN. ¶TTE LVEF data obtained from echocardiography on admission. ††≥75% narrowing of the lumen diameter in a major epicardial artery or 1 of its major branches. ‡‡Measured on admission. §§SBP <90 mmHg in the emergency room. ¶¶HR >100 beats/min in the emergency room. †††LVEF <40%. ‡‡‡eGFR <60 mL/min/1.73 m2. BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass graft; CAD, coronary artery disease; CK, creatine kinase; CRP, C-reactive protein; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HR, heart rate; LVEF, left ventricular ejection fraction; NTproBNP, N-terminal prohormone of brain natriuretic peptide; OMI, old myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SpO2, saturation of percutaneous oxygen; TTE, transthoracic echocardiogram; TTS, takotsubo syndrome; ULN, upper limit of normal; WBC, white blood cell.
Length of hospitalization was 11 days (IQR, 6–17) in the medical illness group, 11 days (IQR, 7–19) in the physical activity, trauma and injury group, 9 days (IQR, 6–13) in the emotional trigger group, and 10 days (IQR, 7–14) in the unidentifiable trigger group (P=0.006;
Table 1).
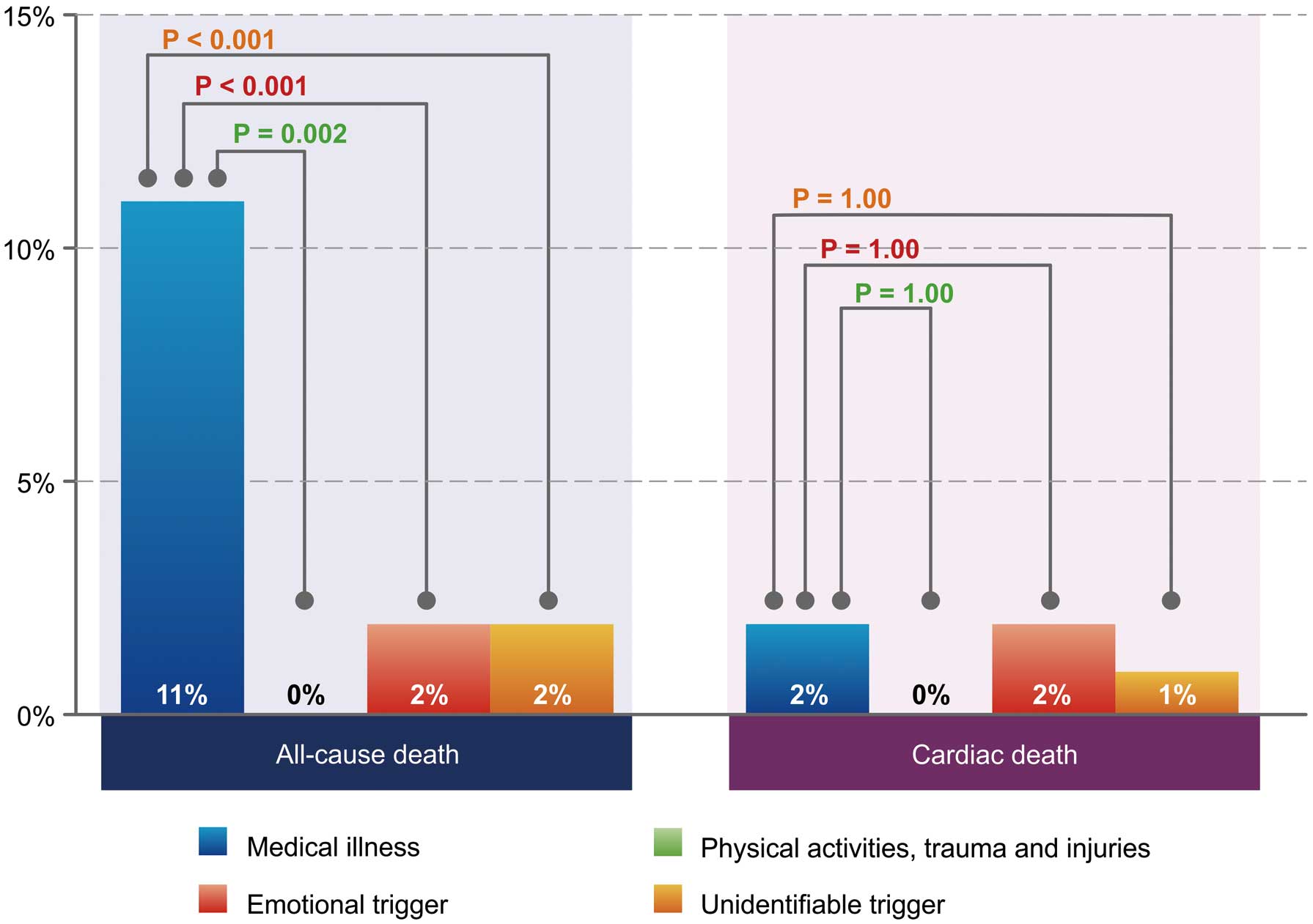
Figure 2
shows the univariate evaluation of in-hospital outcome. All-cause mortality was higher in the medical illness group (n=22, 11%) than in the other groups (physical activity, trauma and injury group, n=0, 0%; emotional trigger group, n=3, 2%; and unidentifiable trigger group, n=5, 2%; P<0.001). Post-hoc test results are also shown in
Figure 2. Meanwhile, there was no significant difference in cardiac deaths between the groups (medical illness group, n=4, 2%; physical activity, trauma and injury group, n=0, 0%; emotional trigger group, n=3, 2%; and unidentifiable trigger group, n=3, 1%; P=0.66). A total of 18 (9%) non-cardiac deaths occurred in the medical illness group, 0 (0%) in the physical activity, trauma and injury group, 0 (0%) in the emotional trigger group, and 2 (1%) in the unidentifiable trigger group (P<0.001).
Details of cause of cardiac death and non-cardiac death by patient group are listed in
Supplementary Tables 1,2. Causes of cardiac death in the medical illness group included heart failure 3/201 (2%), and MI 1/201 (0.5%), while the cause of cardiac death in the unidentifiable trigger group was heart failure 3/287 (1%).
Causes of non-cardiac death in the medical illness group included pneumonia (n=6, 3%), sepsis (n=4, 2%), cerebral infarction (n=1, 0.5%), cerebral hemorrhage (n=1, 0.5%), multiple organ failure (n=1, 0.5%), subarachnoid hemorrhage (n=1, 0.5%), acute cholangitis (n=1, 0.5%), and malignant tumor (n=1, 0.5%), while the causes of non-cardiac death in the unidentifiable trigger group included sepsis (n=1, 0.3%) and cerebral infarction (n=1, 0.3%). On subanalysis of the medical illness group, all-cause mortality tended to be higher for respiratory disease (n=7, 14%), although this was not statistically significant (P=0.88;
Supplementary Table 3). Additionally, no significant differences were found between medical illness subgroups for cardiac death (P=0.84;
Supplementary Table 2). On evaluation of in-hospital outcomes with multiple logistic regression analysis using the multiple imputation dataset, medical illness group was an independent predictor of all-cause death, but not an independent predictor of cardiac death (Table 2). In addition, shock on arrival emerged as an independent predictor of both all-cause and cardiac death. Even when the multiple logistic regression analysis was performed using the dataset without multiple imputation, the medical illness group was still an independent predictor of all-cause death but not an independent predictor of cardiac death (Table 2). In addition, reduced EF emerged as an independent predictor of all-cause death.
Table 2.
Predictors of All-Cause Death and Cardiac Death in TTS Patients
| Variables |
Univariate§ |
Multivariate (n=740) |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Predictors of all-cause death using the imputation dataset |
| Trigger |
| Emotional trigger |
1.00 (Ref.) |
|
1.00 (Ref.) |
|
| Medical illness |
8.03 (2.36–27.29) |
0.001 |
4.73 (1.33–16.87)* |
0.02 |
| Physical activity, trauma and injury |
– |
|
– |
|
| Unidentifiable trigger |
1.16 (0.27–4.90) |
0.84 |
0.96 (0.22–4.18) |
0.96 |
| Age >75 years |
2.33 (1.05–5.17) |
0.04 |
1.82 (0.77–4.24) |
0.17 |
| Male sex |
2.67 (1.26–5.66) |
0.01 |
1.90 (0.73–4.33) |
1.52 |
| Renal dysfunction§§ |
3.21 (1.45–7.12) |
0.004 |
2.09 (0.90–4.86) |
0.09 |
| Shock on arrival¶ |
5.68 (2.28–14.16) |
<0.001 |
3.67 (1.34–10.04)* |
0.01 |
| Tachycardia on arrival†† |
2.67 (1.28–5.57) |
0.009 |
1.53 (0.69–3.40) |
0.3 |
| Reduced EF‡‡ |
3.98 (1.54–10.29) |
0.004 |
2.46 (0.91–6.66) |
0.08 |
| CK, <83, 83–190, >190 IU/L¶¶ |
| <83 IU/L |
1.00 (Ref.) |
|
NA |
|
| 83–190 IU/L |
0.40 (0.14–1.16) |
0.92 |
NA |
|
| >190 IU/L |
0.84 (0.35–1.98) |
0.69 |
NA |
|
| WBC, <6,801, 6,801–10,000, >10,000 IU/L¶¶ |
| <6,800 IU/L |
1.00 (Ref.) |
|
NA |
|
| 6,800–10,000 IU/L |
0.81 (0.31–2.09) |
0.66 |
NA |
|
| >10,000 IU/L |
1.24 (0.52–2.92) |
0.63 |
NA |
|
| BNP/NTproBNP, <5.3, 5.3–19.5, >19.5 ULN†,¶¶ |
| <5.3 ULN |
1.00 (Ref.) |
|
NA |
|
| 5.3–19.5 ULN |
3.60 (0.74–17.6) |
0.11 |
NA |
|
| >19.5 ULN |
3.60 (0.74–17.6) |
0.11 |
NA |
|
| |
Univariate§ |
Multivariate (n=740) |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Predictors of cardiac death using the imputation dataset |
| Trigger |
| Emotional trigger |
1.00 (Ref.) |
|
1.00 (Ref.) |
|
| Medical illness |
1.33 (0.29–6.01) |
0.71 |
0.76 (0.15–3.92) |
0.74 |
| Physical activity, trauma and injury |
– |
|
– |
|
| Unidentifiable trigger |
0.69 (0.14–3.46) |
0.65 |
0.67 (0.13–3.53) |
0.64 |
| Age >75 |
1.46 (0.41–5.22) |
0.56 |
1.33 (0.36–4.91) |
0.66 |
| Male sex |
1.64 (0.42–6.43) |
0.48 |
1.45 (0.34–6.13) |
0.61 |
| Renal dysfunction§§ |
1.08 (0.26–4.56) |
0.92 |
NA |
|
| Shock on arrival¶ |
7.07 (1.76–28.33) |
0.006 |
6.53 (1.52–28.11)* |
0.01 |
| Tachycardia on arrival†† |
2.57 (0.74–9.03) |
0.14 |
2.17 (0.59–8.07) |
0.25 |
| Reduced EF‡‡ |
3.81 (0.76–19.15) |
0.10 |
2.34 (0.49–11.23) |
0.29 |
| CK, <83, 83–190, >190 IU/L¶¶ |
| <83 IU/L |
1.00 (Ref.) |
|
NA |
|
| 83–190 IU/L |
0.66 (0.11–3.99) |
0.65 |
NA |
|
| >190 IU/L |
1.36 (0.30–6.13) |
0.69 |
NA |
|
| WBC, <6,801, 6,801–10,000, >10,000 IU/L¶¶ |
| <6,800 IU/L |
1.00 (Ref.) |
|
NA |
|
| 6,800–10,000 IU/L |
1.02 (0.14–7.31) |
0.98 |
NA |
|
| >10,000 IU/L |
3.12 (0.62–15.59) |
0.17 |
NA |
|
| BNP/NTproBNP, <5.3, 5.3–19.5, >19.5 ULN†,‡,¶¶ |
| <5.3 ULN |
– |
– |
NA |
|
| 5.3–19.5 ULN |
1.00 (Ref.) |
|
NA |
|
| >19.5 ULN |
0.20 (0.02–1.70) |
0.14 |
NA |
|
| Variables |
Univariate§ |
Multivariate (n=466) |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Predictors of all-cause death using dataset without the multiple imputation |
| Underlying trigger |
| Emotional trigger |
1.00 (Ref.) |
|
1.00 (Ref.) |
|
| Medical illness |
8.03 (2.36–27.29) |
0.001 |
5.28 (1.10–25.42)* |
0.04 |
| Physical activity, trauma and injury |
– |
|
– |
|
| Unidentifiable trigger |
1.16 (0.27–4.90) |
0.84 |
0.55 (0.07–4.04) |
0.55 |
| Age >75 years |
2.33 (1.05–5.17) |
0.04 |
1.20 (0.42–3.44) |
0.74 |
| Male sex |
2.67 (1.26–5.66) |
0.01 |
2.19 (0.75–6.38) |
0.15 |
| Renal dysfunction§§ |
3.21 (1.45–7.12) |
0.004 |
1.51 (0.53–4.31) |
0.44 |
| Shock on arrival¶ |
5.68 (2.28–14.16) |
<0.001 |
3.07 (0.74–12.81) |
0.12 |
| Tachycardia on arrival†† |
2.67 (1.28–5.57) |
0.009 |
0.85 (0.28–2.56) |
0.78 |
| Reduced EF‡‡ |
3.98 (1.54–10.29) |
0.004 |
3.10 (1.09–8.83)* |
0.03 |
| CK, <83, 83–190, >190 IU/L¶¶ |
| <83 IU/L |
1.00 (Ref.) |
|
NA |
|
| 83–190 IU/L |
0.40 (0.14–1.16) |
0.92 |
NA |
|
| >190 IU/L |
0.84 (0.35–1.98) |
0.69 |
NA |
|
| WBC, <6,801, 6,801–10,000, >10,000 IU/L¶¶ |
| <6,800 IU/L |
1.00 (Ref.) |
|
NA |
|
| 6,800–10,000 IU/L |
0.81 (0.31–2.09) |
0.66 |
NA |
|
| >10,000 IU/L |
1.24 (0.52–2.92) |
0.63 |
NA |
|
| BNP/NTproBNP, <5.3, 5.3–19.5, >19.5 ULN†,¶¶ |
| <5.3 ULN |
1.00 (Ref.) |
|
NA |
|
| 5.3–19.5 ULN |
3.60 (0.74–17.6) |
0.11 |
NA |
|
| >19.5 ULN |
3.60 (0.74–17.6) |
0.11 |
NA |
|
| |
Univariate§ |
Multivariate (n=498) |
| OR (95% CI) |
P-value |
OR (95% CI) |
P-value |
| Predictors of cardiac death using dataset without the multiple imputation |
| Trigger |
| Emotional trigger |
1.00 (Ref.) |
|
1.00 (Ref.) |
|
| Medical illness |
1.33 (0.29–6.01) |
0.71 |
0.56 (0.07–4.67) |
0.60 |
| Physical activity, trauma and injury |
– |
|
– |
|
| Unidentifiable trigger |
0.69 (0.14–3.46) |
0.65 |
0.49 (0.0.6–3.83) |
0.50 |
| Age >75 |
1.46 (0.41–5.22) |
0.56 |
1.07 (0.21–5.56) |
0.94 |
| Male sex |
1.64 (0.42–6.43) |
0.48 |
4.17 (0.76–22.93) |
0.10 |
| Renal dysfunction§§ |
1.08 (0.26–4.56) |
0.92 |
NA |
|
| Shock on arrival¶ |
7.07 (1.76–28.33) |
0.006 |
2.93 (0.31–27.86) |
0.35 |
| Tachycardia on arrival†† |
2.57 (0.74–9.03) |
0.14 |
1.28 (0.22–7.50) |
0.79 |
| Reduced EF‡‡ |
3.81 (0.76–19.15) |
0.10 |
3.37 (0.66–17.29) |
0.15 |
| CK, <83, 83–190, >190 IU/L¶¶ |
| <83 IU/L |
1.00 (Ref.) |
|
NA |
|
| 83–190 IU/L |
0.66 (0.11–3.99) |
0.65 |
NA |
|
| >190 IU/L |
1.36 (0.30–6.13) |
0.69 |
NA |
|
| WBC, <6,801, 6,801–10,000, >10,000 IU/L¶¶ |
| <6,800 IU/L |
1.00 (Ref.) |
|
NA |
|
| 6,800–10,000 IU/L |
1.02 (0.14–7.31) |
0.98 |
NA |
|
| >10,000 IU/L |
3.12 (0.62–15.59) |
0.17 |
NA |
|
| BNP/NTproBNP, <5.3, 5.3–19.5, >19.5 ULN†,‡,¶¶ |
| <5.3 ULN |
– |
– |
NA |
|
| 5.3–19.5 ULN |
1.00 (Ref.) |
|
NA |
|
| >19.5 ULN |
0.20 (0.02–1.70) |
0.14 |
NA |
|
*P<0.05 (multivariate analysis). †Ratio of BNP or NTproBNP to ULN. ‡5.3–19.5 chosen as the reference in the univariate analysis of BNP/NTproBNP in cardiac death because there was no event in BNP/NTproBNP <5.3. §Selection of variables based on clinical importance and on previous studies.1,6,10,19,20 ¶SBP <90 mmHg in the emergency room. ††HR >100 beats/min in the emergency room. ‡‡LVEF <40%. §§eGFR <60 mL/min/1.73 m2. ¶¶Tertiles calculated in the analysis. NA, not applicable. Other abbreviations as in Table 1.
Discussion
The present study has demonstrated different types of trigger and highlighted the crucial relationship between medical illness and mortality in patients with TTS, using data from a multicenter registry. The present study is the first report from Japan on the relationship between TTS outcome and trigger. Intriguingly, the present all-cause mortality in the medical illness group was higher than that of ST-elevation MI in a previous study.21
The present medical illness group also had the highest proportion of male patients compared with the other groups, which is clearly different from the general presentation of TTS.1,3
Notably, medical illness was the strongest independent variable of mortality on multivariate analysis, and the main cause of death was non-cardiac death. This shows that the poor prognosis of TTS patients was not mainly due to cardiovascular complications but due to their underlying trigger medical conditions. We also noted a high prevalence of TTS even without any evidence of precipitating trigger. Moreover, the unidentifiable trigger group, together with the emotional trigger group and the physical activity, trauma and injury group, had favorable outcomes.
In a recent investigation, TTS began to be recognized as a non-benign disorder.19
The spectrum of TTS is wide and ranges from low to very high risk depending on the individual. This is because TTS potentially includes a wide range of pathologies and underlying mechanisms.1,22
Thus far, the triggers have generally been classified as emotional or physical. The presence of a physical trigger has also been reported to predict a higher incidence of worse outcomes.1,4,8,19
For the purposes of determining the relationship between TTS prognosis and trigger, however, categorization of TTS trigger into only the 2 groups of “physical” and “emotional” is insufficient because the baseline characteristics of TTS and its underlying trigger stress are multifactorial. Therefore, to distinguish the high-risk group, we categorized patients with TTS into 4 groups, as described. Subsequently, we were able to identify the group with the worst prognosis out of the 4. The present finding of medical illness as the main predictor of mortality is corroborated by a previous study that used data from the National Inpatient Sample (NIS) database in the USA.23
In that study, TTS triggered by critical illness tended to occur in male patients, with a mortality rate of up to 12%;23
a potential limitation of that study, however, was that the diagnosis was made based on the NIS database. In contrast, in the study, we assure the validity of mortality data from our registry, where all patients were diagnosed on CAG, and details of the triggers were evaluated. The present results are consistent with those of a recent study showing that physical trigger was a risk factor not only for TTS in the first 30 days but also for long-term outcomes of TTS, based on the viewpoint that medical illness as trigger is a crucial risk factor for mortality.6
That study, however, found that neurologic disorder was the strongest risk factor, whereas the present study found no significant difference between the subtypes of medical illness. First, this conflicting result can be explained by the method used to classify the physical trigger. The present subgroup analysis did not identify a significant difference because we compared the mortality in subgroups of medical illness after separating the medical illness group from the physical activity, trauma and injury group, which had the lowest mortality. Second, geographical differences could be another reason. The Japanese registry from the Tokyo CCU Network had older patients and more male patients compared with the registries from Europe and the USA, and distribution of the trigger was found to be different.1
A previous study from the Tokyo CCU Network showed that male patients with TTS had more serious cardiac complications, although there was no significant difference in mortality.20
This still suggests that male sex may be a risk factor for worse outcomes in patients with TTS.24,25
In the present study, however, male sex was not an independent predictor of mortality on multivariate analysis but was a strong confounder of medical illness group, although it was significant on univariate analysis.
We found that high mortality from TTS was associated with medical illness as the trigger. The mortality from TTS triggered by medical illness can be underestimated because of selection bias, known as the survivor-cohort effect.26
Diagnosis of TTS based on current diagnostic criteria has been restricted to only those who underwent CAG.11,16
Patients who did not survive prior to a definite diagnosis on CAG or those who could not undergo CAG due to serious triggering underlying medical illness conditions were not included.
Trauma and injury have previously been included in the same category as medical illness but we made a distinction between trauma/injury and medical illness and, interestingly, the trauma/injury group had very low mortality. In fact, the result from a recent study that noted TTS triggered by medical illness had higher mortality comparing to TTS triggered by surgical procedure including trauma and injury,9
was similar to the present study.
Study Limitations
This study has some limitations. First, the data have been collected prospectively since 2013, but were collected retrospectively between 2010 and 2012 and analyzed retrospectively. Second, data were missing in significant portions of several variables in the present study. Therefore, we performed multiple logistic regression analysis using the multiple imputation dataset, and confirmed this using the dataset without the multiple imputation. Third, we were unable to compare long-term prognosis between the groups because post-discharge data were unavailable. Finally, the present results may not be generalizable to other countries because clinical practice may vary from country to country.
Conclusions
TTS has a wide spectrum of outcome depending on the type of trigger. The medical illness trigger was a powerful predictor of outcome but the main cause of death was not cardiac complication.
Acknowledgments
This work was supported by the Tokyo Metropolitan Government. The funder had no role in the execution of this study or in the interpretation of the results.
Disclosures
The authors declare no conflicts of interest.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-19-0045
References
- 1.
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015; 373: 929–938.
- 2.
Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases. J Cardiol 1991; 21: 203–214.
- 3.
Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J 2006; 27: 1523–1529.
- 4.
Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010; 55: 333–341.
- 5.
Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539–548.
- 6.
Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol 2018; 72: 874–882.
- 7.
Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of takotsubo cardiomyopathy in the United States. Am Heart J 2012; 164: 66–71.
- 8.
Konstantinos G, El-Battrawy I, Schramm K, Uzair A, Hoffmann U, Martin B, et al. Comparison and outcome analysis of patients with takotsubo cardiomyopathy triggered by emotional stress or physical stress. Front Psychol 2017; 8: 527.
- 9.
Yerasi C, Koifman E, Weissman G, Wang Z, Torguson R, Gai J, et al. Impact of triggering event in outcomes of stress-induced (takotsubo) cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2017; 6: 280–286.
- 10.
Yamaguchi T, Yoshikawa T, Isogai T, Miyamoto T, Maekawa Y, Ueda T, et al. Predictive value of QRS duration at admission for in-hospital clinical outcome of takotsubo cardiomyopathy. Circ J 2016; 81: 62–68.
- 11.
Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J 2008; 155: 408–417.
- 12.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–S266.
- 13.
Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013; 369: 1726–1734.
- 14.
Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132(18 Suppl 2): S444–S464.
- 15.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al; Authors/Task Force Members; Document Reviewers. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975.
- 16.
Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, et al. Current state of knowledge on takotsubo syndrome: A position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016; 18: 8–27.
- 17.
Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and management. Eur Heart J 2018; 39: 2047–2062.
- 18.
Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015; 132: 302–361.
- 19.
Isogai T, Yoshikawa T, Ueda T, Yamaguchi T, Imori Y, Maekawa Y, et al. Apical takotsubo syndrome versus anterior acute myocardial infarction: Findings from the Tokyo Cardiovascular Care Unit network registry. Eur Heart J Acute Cardiovasc Care 2019; 8: 86–95.
- 20.
Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, et al. Gender differences in patients with takotsubo cardiomyopathy: Multi-center registry from Tokyo CCU Network. PLoS One 2015; 10: e0136655.
- 21.
Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L; SWEDEHEART/RIKS-HIA. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011; 305: 1677–1684.
- 22.
Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, et al. Natural history and predictors of mortality of patients with Takotsubo syndrome. Int J Cardiol 2018; 267: 22–27.
- 23.
Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: A study of the National Inpatient Sample 2008 to 2009. Am Heart J 2012; 164: 215–221.
- 24.
Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J 2016; 172: 53–63.
- 25.
Singh K, Carson K, Shah R, Sawhney G, Singh B, Parsaik A, et al. Meta-analysis of clinical correlates of acute mortality in takotsubo cardiomyopathy. Am J Cardiol 2014; 113: 1420–1428.
- 26.
Löwel H, Lewis M, Hörmann A. Prognostic significance of prehospital phase in acute myocardial infarct. Results of the Augsburg Myocardial Infarct Registry, 1985–1988. Dtsch Med Wochenschr 1991; 116: 729–733.



