2020 Volume 2 Issue 4 Pages 211-217
2020 Volume 2 Issue 4 Pages 211-217
Vascular remodeling (e.g., intimal thickening) is necessary for complete closure of the ductus arteriosus (DA). Smooth muscle cells are reported to contribute to DA remodeling. In contrast, the contribution of endothelial cells remains largely unknown. Recent data showed that tissue-type plasminogen activator (t-PA) was highly expressed in the endothelial cells of rat and human DA. It is well known that t-PA is an activator of the blood fibrinolytic system, but t-PA-induced localized proteolysis has been reported to play an important role in vascular development. We found that t-PA-induced plasminogen-plasmin conversion promoted matrix metalloproteinase-2 activation in endothelial cells of rat DA. Gelatinase activity was noted at the internal elastic laminae (IEL) of rat and human DA on in situ gelatin zymography. The in vivo injection of plasminogen to pre-term rats increased gelatinase activation, IEL disruption, and the subsequent intimal thickening formation in the pre-term rat DA. Human DA results partly supported the rat DA findings, suggesting that t-PA-mediated DA remodeling may also be present in the human DA. Current pharmacotherapy for patent DA (PDA) mainly focuses on increasing vascular constriction. Elucidating the molecular mechanisms of DA remodeling may help to expand the range of therapeutic strategies for PDA.
The ductus arteriosus (DA) is a fetal blood vessel connecting the pulmonary artery and aorta, and is indispensable for maintaining fetal life. In utero, the DA diverts the right ventricular blood away from the high-resistance pulmonary circulation and into the systemic circulation where gas is exchanged with maternal blood. Closure of the DA is normally completed in the 3 days after birth, but pre-term infants often have a patent DA after birth. Persistent patent DA (PDA) occurs in more than half of pre-term infants,1 and is related to blood flow imbalance (e.g., pulmonary congestion, and hypo-perfusion of systemic circulation) and subsequent mortality.2,3 Although approximately 50% of extremely pre-term infants receive pharmacotherapy with cyclooxygenase inhibitors, this involves a risk of renal dysfunction, spontaneous intestinal perforation and necrotizing enterocolitis.4 Thus, the current treatment strategies for PDA still need improvement.5
Complete closure of the DA involves 2 processes: functional and anatomical closure. Functional closure is a vasoconstriction induced by contraction of smooth muscle cells (SMC); anatomical closure is a form of vascular remodeling represented by intimal thickening.
Vasoconstriction of the DAPostnatal DA constriction is triggered by increased arterial pO2, decreased blood pressure in the DA lumen, decreased circulating prostaglandin E2 (PGE2) level, and a decreased number of PGE2 receptors.6–8
The postnatal increase of pO2 inhibits the ductal smooth muscle voltage-dependent potassium channels, such as Kv1.5 and Kv2.1,9,10 which causes membrane depolarization and calcium influx via the voltage-dependent calcium channels. This is followed by DA SMC contraction.11 Voltage-dependent calcium channel and potassium channel genes are upregulated in the mature DA.12,13 In addition to calcium influx via voltage-dependent calcium channels, oxygen-induced activation of the Rho/Rho-associated coiled-coil containing protein kinase (Rho/ROCK) pathway is associated with postnatal DA constriction.14
Reduced circulating PGE2 is related to postnatal DA constriction. The fetal DA is dilated by PGE2, which is derived from the placenta.15 After birth, the loss of the placenta and the increased flow of the lung, which is the major site of PGE catabolism, cause a decline in circulating PGE2.16 In addition, PGE2 receptors are decreased after birth.17 These changes promote DA contraction.
Furthermore, we recently reported that serum osmotic change and glutamate are related to postnatal DA constriction.18,19 We found that premature infants did not have a physiological decrease in serum osmolarity after birth, and hypo-osmolality contributes to postnatal DA contraction via the transient receptor potential melastatin3 (TRPM3) channel, which is one of the osmotic sensors.18 We also found that the glutamate plasma concentration was decreased in human premature infants with PDA, and that glutamate promoted the DA contraction through glutamate inotropic receptor α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type subunit 1 (GluR1)-mediated noradrenaline production.19
Intimal Thickening of the DAIn addition to contraction of the DA, DA remodeling (e.g., intimal thickening) has been suggested to be essential for the complete closure of the DA.20–27 In human DA, intimal thickening occurs gradually in mid-gestation and becomes prominent at full term (Figure 1A). Intimal thickening is not complete in pre-term infants,27–29 and it is weakened in full-term infants with PDA.26 The formation of intimal thickening in the DA involves some serial steps that occur toward birth (Figure 1B).20 The disruption of the internal elastic lamina (IEL) and the detachment of endothelial cells from the IEL are the early steps in the formation of intimal thickening in the DA.27 These processes are followed by the migration of SMC into the subendothelial region.27,28 PGE2 receptor ‘EP4’ signaling then promotes the production of hyaluronan in the subendothelial region and the subsequent migration of SMC toward the DA lumen, resulting in intimal thickening.21 The role of SMC in DA remodeling is therefore important.

Vascular remodeling of the ductus arteriosus (DA). (A) The human DA of a full-term infant shows prominent intimal thickening. Yellow lines, border of each layer. Scale bars, 1.0 mm. (B) Schematic diagram of the developmental morphologic changes of the DA. ECM, extracellular matrix; IEL, internal elastic laminae.
Disruption of the IEL is another hallmark of DA remodeling.28,30,31 The IEL of the DA is constructed similarly to the adjacent aorta in fetal development. Then, IEL disrupts only in the DA in mid-gestation. In humans, the IEL disruption of the DA starts at approximately 17 weeks of gestation and becomes progressively obvious at 22–26 weeks of gestation.27 The disruption of the IEL is followed by the migration of SMC into the subendothelial region, and intimal thickening becomes visible at the site of the disrupted IEL.28,29,32 Then, the IEL is disrupted more frequently between 27 and 34 weeks’ gestation,27 and the further migration of SMC promotes narrowing of the DA lumen during late gestation. From these histological observations of human DA, the disruption of the DA IEL is suggested to be incomplete in pre-term infants born at <27 weeks of gestation, who often have PDA. Gittenberger-de Groot reported that human PDA tissue lacked IEL disruption and subsequent intimal thickening, while normal human DA tissue had this remodeling.26
The functional role of the IEL as a barrier to the migration of SMC into the subendothelial region was also elucidated by using SMC- and endothelial cell-specific elastin knockout mice.33 Lin et al demonstrated that IEL was derived from both SMC and endothelial cells, and that areas of neointimal hyperplasia correlated with local IEL disruption in the aorta.33 In addition to the role of the IEL as a barrier to SMC migration, elastin itself affects the phenotype of vascular SMC. Mice with the global knockout of elastin have supravalvular aortic stenosis due to the accumulation of SMC;34 and tropoelastin, which is a monomer of elastin, inhibits the proliferation and migration of SMC.35
In our previous study, PGE2-EP4 signaling prevented elastogenesis in the tunica media of the DA via the degradation of lysyl oxidase, which catalyzes elastin cross-links.36 Unlike the sparse elastic fibers in the DA tunica media, IEL is first constructed and then progressively disrupted toward birth. Therefore, the signaling responsible for the disruption of the IEL is unclear.
Role of Endothelial Cells in the DAAlthough we and others have reported that SMC have an important role in the formation of intimal thickening,21,22,37,38 the role of the endothelial cells adjacent to the IEL is largely unknown. DA endothelial cells contribute to the DA dilatation via the production of endothelial nitric oxide synthetase,39 and endothelial cells also promote the proliferation of SMC and subsequent intimal thickening via the production of vascular endothelial growth factor.40–42 However, many points concerning the function of the DA endothelial cells are unclear. Recently, we found that endothelial cells play an important role in DA remodeling via the marked expression of tissue-type plasminogen activator (t-PA), which mediates matrix metalloproteinase-2 (MMP-2) activation, IEL disruption, and subsequent intimal thickening.43 We herein introduce our recent findings.43
Expression of t-PA in DA Endothelial CellsWe previously reported on the microarray analysis of rat DA and aorta,44,45 in which we found that t-PA was dominantly expressed in the endothelial cells of the DA.44 We also confirmed the expression of t-PA on quantitative polymerase chain reaction using isolated DA endothelial cells and aortic endothelial cells from full-term rat fetuses (embryonic day 21).43 In accordance with our previous microarray data, t-PA (Plat) mRNA was highly expressed in DA endothelial cells compared with aortic endothelial cells (Figure 2A).43
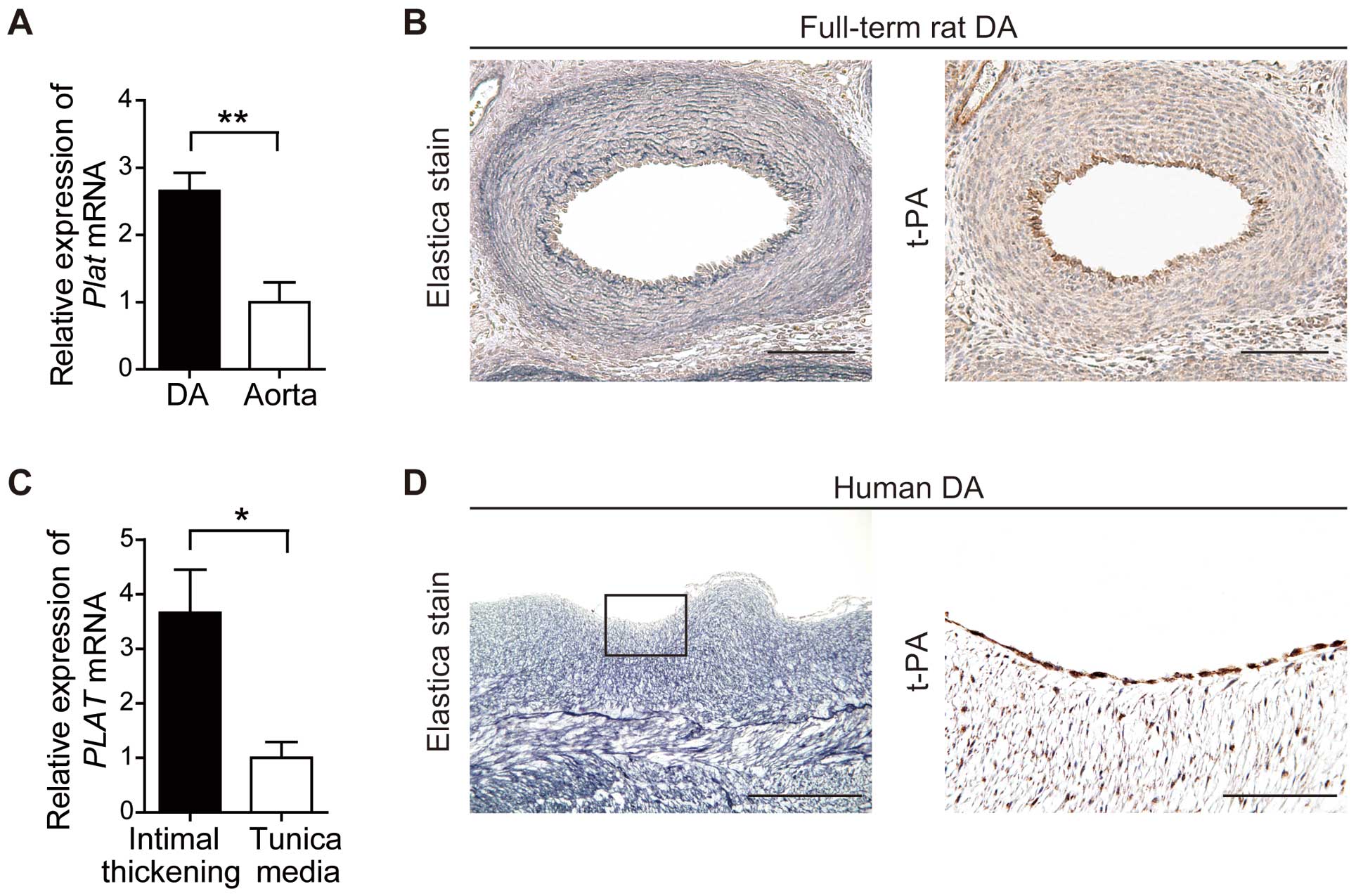
Expression of tissue-type plasminogen activator (t-PA) in the ductus arteriosus (DA). (A) Rat (n=6) and (C) human (n=5) Plat mRNA expression on quantitative reverse transcription-polymerase chain reaction. (B,D) Elastica van Gieson staining to visualize elastic fibers (Left, dark purple) and immunohistochemical staining of t-PA in serial sections (Right, brown) in (B) rat DA on embryonic day 21 (scale bars, 100 µm) and (D) human infant DA (scale bars: Left, 500 µm; Right, 100 µm). *P<0.05; **P<0.01. Reproduced with permission from Saito J, et al.43
We further examined the expression of t-PA in human DA.43 Although RNA sequencing of the human DA was recently reported,46 there has been no transcriptional profiling of the human DA intimal thickening region that contains DA endothelial cells. We collected human DA from neonatal patients with congenital heart disease and examined the expression of t-PA in the intimal thickening region compared with the tunica media. Although these patients had congenital heart disease, they required PGE1 to maintain patency of the DA, and all DA had remarkable intimal thickening, suggesting that these DA had normally developed until sample collection. We found that the t-PA (PLAT) mRNA expression in the intimal thickening region was 3.6-fold higher than that in the tunica media (Figure 2C), and on immunohistochemistry for t-PA using rat and human DA, t-PA was markedly expressed in the endothelial cells of both the rat and human DA (Figure 2B,D).
It is well known that t-PA is an activator of the blood fibrinolytic system, but t-PA-induced localized proteolysis has been reported to play an important role in vascular development.47 Levin et al investigated the developmental changes of locally expressed t-PA in the rat fetus and found that t-PA expression was dynamically regulated during vascular development.47 On immunohistochemical staining of t-PA using time-series rat fetuses, the t-PA was initially expressed in endothelial cells of the dorsal aorta and extended to the large arteries on embryonic day 13. The expression of t-PA was mostly decreased in the aortic endothelial cells by embryonic day 15. Alternatively, it appeared in the pulmonary, subclavian, vertebral and basilar arteries. After t-PA expression disappeared in the great arteries, t-PA expression was found in the smaller arterial branches.47 The authors suggested that the primary function of locally expressed t-PA may be localized proteolysis to promote vascular remodeling during fetal development, rather than systemic vascular fibrinolysis.47
Gelatinase Activity in the DAt-PA converts plasminogen into plasmin,48 which activates gelatinase MMP-249 and MMP-9;50 and elastic laminae are mainly degraded by MMP-2 and MMP-9.50 Our initial data showed the high expression of t-PA in DA endothelial cells of both rats and humans.43 Thus, we aimed to elucidate whether DA endothelial cell-induced t-PA plays a role in disruption of the IEL through the activation of MMP.43 We confirmed the gelatinase activity using isolated DA endothelial cells and aortic endothelial cells from rats (Figure 3A).43 On gelatin zymography the pro-MMP-2 level in rat DA endothelial cells was higher than in aortic endothelial cells under basal conditions (Figure 3B, control).43 Plasminogen supplementation increased MMP-2 activation in DA endothelial cells in comparison with aortic endothelial cells (Figure 3B, plasminogen). Neither DA endothelial cells nor aortic endothelial cells had detectable MMP-9 expression.43 This suggested that the conversion of plasminogen to plasmin in DA endothelial cells promotes the activation of MMP-2.43

Gelatinase activity in the ductus arteriosus (DA). (A) Schematic diagram of the activation of matrix metalloproteinase (MMP). t-PA, issue-type plasminogen activator. (B) MMP activity in conditioned culture medium with rat DA endothelial cells and aortic endothelial cells (embryonic day 21). Active-MMP-2, 62 kDa; pro-MMP-2, 72 kDa; and MMP-9, 92 kDa. (C,D) Elastica van Gieson staining and in situ gelatin zymography of (C) rat DA on embryonic day 21 and (D) human DA. (C) High gelatinase activity (green) is seen in the internal elastic laminae (IEL; arrows), whereas ethylenediamine tetra-acetic acid (EDTA) supplementation inhibited the gelatinase activity (Nuclei, blue). Scale bars, 50 µm. (D) High gelatinase activity is seen in the intimal thickening region and in the IEL. DQ-gelatin, Fluorogenic Dye-quenched gelatin. Scale bars, 200 µm. Reproduced with permission from Saito J, et al.43
Next, we confirmed the gelatinase activity in the DA tissue of rats and humans. In addition to gelatin zymography, prominent gelatinase activity was seen on in situ zymography in the intimal thickening region, which contains endothelial cells, and in the disrupted IEL of the DA (Figure 3C,D). This gelatinase activity was attenuated by the MMP inhibitor ethylenediamine tetra-acetic acid (EDTA).
Disruption of Elastic Laminae via MMP ActivationAlthough the t-PA-mediated activation of MMP is suggested to be involved in the remodeling of the vascular system,51 it is difficult to demonstrate the direct effect of t-PA on elastic laminae in vitro because cultured cells on a plastic plate cannot show the disruption of elastic fibers. We previously reported a 3-D vascular model that contains multilayered SMC and elastic laminae.52,53 In our recent study, we seeded endothelial cells on the top of the multilayered SMC and confirmed the effect of endothelial cell-derived t-PA on the layered elastic fibers (Figure 4A).43 In untreated vascular models, layered elastic laminae were confirmed under endothelial cells (Figure 4B, Left). In contrast, supplementation of plasminogen (a substrate of t-PA) increased the gelatinase activation and the disruption of the elastic laminae (Figure 4B, Right). This gelatinase activation was attenuated by the MMP inhibitor EDTA. We also found that t-PA-targeted siRNA in endothelial cells attenuated the gelatinase activation and subsequent disruption of the elastic laminae.43 These observations suggest that endothelial cell derived-t-PA involves the activation of MMP and subsequent disruption of elastic laminae.

Plasminogen-promoted internal elastic laminae (IEL) disruption, gelatinase activation and subsequent intimal thickening formation. (A) Schematic diagram of a 3-D model of vasculature. MMP, matrix metalloproteinase. (B) High gelatinase activity (green) is seen in the plasminogen-treated 3-D vascular model (Upper panel). Layered elastic fibers are seen on elastica van Gieson (EVG) staining (dark purple); this was disrupted in the plasminogen-treated 3-D vascular model (Lower right). Scale bars, 50 µm. (C) Time course of the in vivo experiment. E, embryonic day; IT, intimal thickening. (D) Schematic diagram of the injection of plasminogen into the rat fetus. PBS, phosphate-buffered saline. (E) Plasminogen increased the gelatinase activity (Upper), and disrupted the internal elastic laminae (IEL; arrows) and increased the intimal thickening in the plasminogen-treated rat DA (Lower). Scale bars, 50 µm. Reproduced with permission from Saito J, et al.43
A 3-D vascular model showed that plasminogen supplementation facilitated the disruption of elastic laminae via MMP activation. We then investigated whether plasminogen injection increased the IEL disruption and intimal thickening in the pre-term rat DA in vivo. On histology the IEL was almost completely intact on embryonic day 19 and became highly fragmented on embryonic day 21.43 We also confirmed the high expression of t-PA in DA endothelial cells on embryonic day 19 on immunohistochemistry.43 We then injected plasminogen on embryonic day 19 and analyzed the fetuses on embryonic day 20 (24 h after injection; Figure 4C). We injected the plasminogen i.p. into rat fetuses (Figure 4D). In accordance with the 3-D in vitro vascular model, on in situ gelatin zymography the gelatinase was highly activated in the plasminogen-injected rat DA (Figure 4E, Upper). The IEL disruption and intimal thickening were moderate and limited in controls (Figure 4E, Lower left). In contrast, the injection of plasminogen increased the elastic laminae disruption and the subsequent intimal thickening (Figure 4E, Lower right).
In our present study,43 we were unable to measure the serum plasminogen concentration in rat fetuses due to the limited amount of serum, but it has been reported that human infants have lower blood plasminogen concentration than adults.54,55 Andrew et al reported that the serum plasminogen concentration in normal human full-term infants is 60% of that in adults,54 and is lower in pre-term infants due to prematurity of liver function.55 Furthermore, the activity of fetal plasmin was also lower than that of the adult type.56 This indicates that infants physiologically have lower blood plasminogen level and plasmin activity; thus, the supplementation of plasminogen may help the t-PA-mediated IEL disruption in the DA.
Although plasminogen is associated with a possible unfavorable effect (bleeding tendency due to activation of fibrinolytic system), in clinical trials of plasminogen injection to premature infants, no apparent adverse effects were reported.57 Before 1984, when surfactant therapy was started clinically for premature infants with respiratory distress syndrome (RDS),58 several trials were performed to treat RDS infants, and one of those trials evaluated the effect of plasminogen on RDS. Pathologically, RDS is considered to be a hyaline-membrane disease. The hyaline membrane is composed of fibrin, which is derived from leaked fibrinogen from the pulmonary alveolus. This fibrin inhibits gas exchange and the activity of endogenous surfactant in the alveolus. Plasminogen was used to dissolve the fibrin of the alveolus in premature infants with RDS. Ambrus et al reported that the use of plasminogen in premature infants (1,000–2,500 g, at 28–40 weeks of gestation) decreased respiratory distress and mortality without obvious adverse effects in their double-blind, randomized clinical trials.57 t-PA may promote IEL disruption of the DA without major adverse effects, but we must carefully consider the possibility of plasminogen injection in the treatment of premature infants with PDA.
Rat and human DA endothelial cells had high t-PA expression. Endothelial cell-derived t-PA facilitated IEL disruption through MMP-2 activation, which may aid in the formation of intimal thickening in the DA (Figure 5). The human DA results partly supported the rat DA results, suggesting that t-PA-mediated DA remodeling may also be present in human DA. The elucidation of the molecular mechanisms of DA remodeling may help to expand the range of treatment strategies for pre-term infants with PDA.
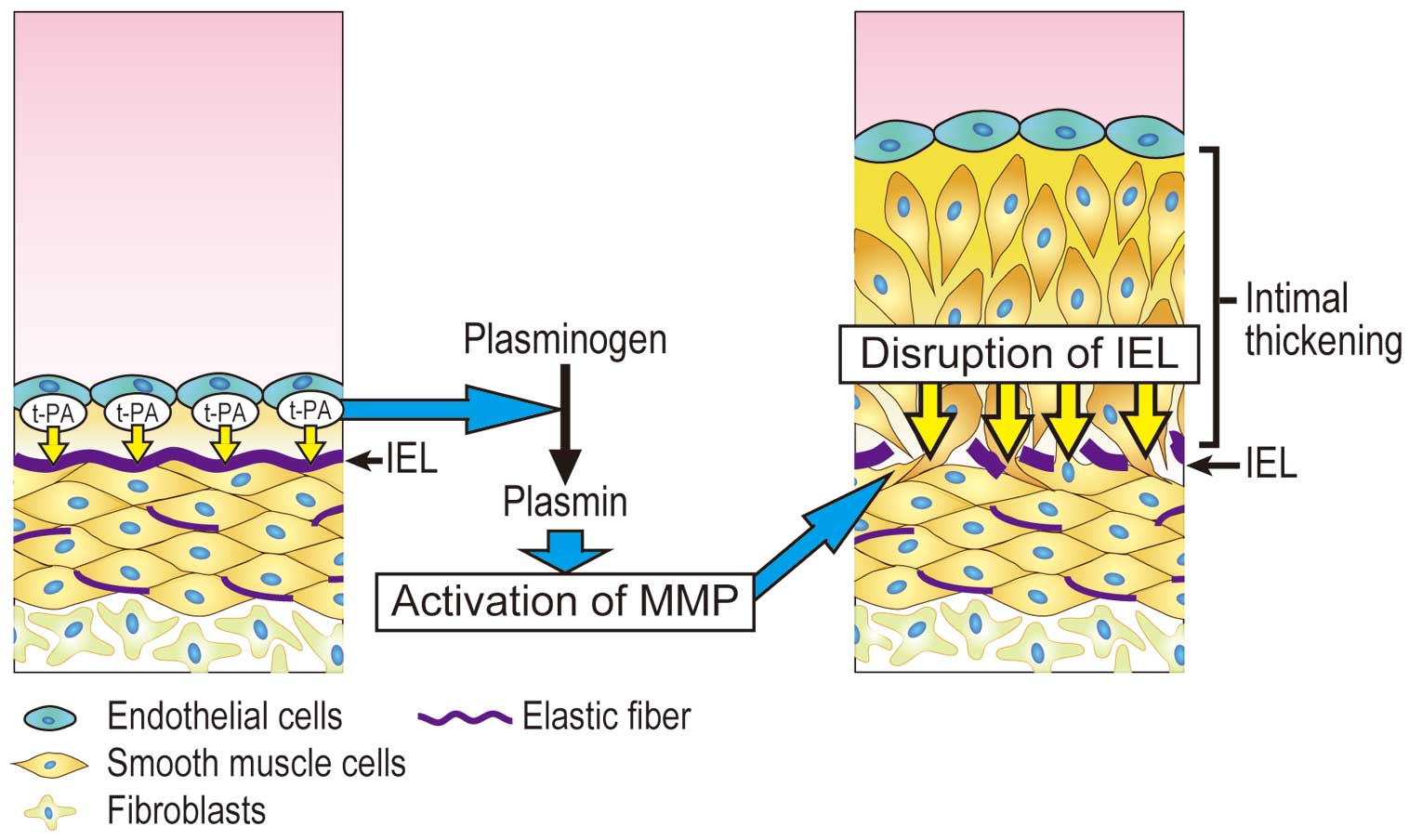
Schematic model of tissue-type plasminogen activator (t-PA)-mediated ductus arteriosus remodeling. IEL, internal elastic laminae.
The authors are grateful to Toshihide Asou (Department of Cardiovascular Surgery, Kanagawa Children’s Medical Center) and Munetaka Masuda (Department of Surgery, Yokohama City University) for providing human DA samples.
This work was supported by JSPS KAKENHI (J.S., JP18K15681, JP16H07107; U.Y., JP16H05358, JP15H05761, JP17K19403; Y.I., JP24390200, JP25670131), MEXT (Y.I., 22136009), and AMED (Y.I., 66890005, 66890011, 66890001, 66890023). These funders had no role in this study.
Data sharing is not applicable to this article given that no datasets were generated or analyzed during the current study.
The authors declare no conflicts of interest.
Approval for the human study was obtained from the human subject committees at Yokohama City University (B150305001) and Kanagawa Children’s Medical Center (1502-05). Approval for the animal study was obtained from the Institutional Animal Care and Use Committees of Yokohama City University (F-A-16-010).